Abstract
Boronic acids that change fluorescent properties upon sugar binding are very important reporter units for the development of small molecule lectin mimics (boronolectins). Aimed at developing long wavelength fluorescent boronic acid reporter compounds, we have designed and synthesized a series of boronic acid analogs 2a-d with an extended π conjugation. Such designs are based on earlier fluorescent boronic acids that change fluorescent properties upon sugar binding. Compared with the corresponding parent chromophores, these new compounds with extended conjugations show longer excitation and emission wavelengths as designed. The patterns of fluorescent changes for the new compounds are also different from that of the corresponding parent compounds.
Keywords: Boronic acids, π Conjugated system, Fluorescence, Sensing, Sensor
1. Introduction
Lectin-carbohydrate binding plays very important roles in biological and pathological processes such as cell-cell adhesion,1-4 inflammation reactions,5-7 cancer metastasis,8,1,9-13 etc. Therefore, small molecule mimics of lectins that can recognize certain carbohydrate biomarkers have the potential to be developed as therapeutic and diagnostic agents. Along this line, we have reported the first lectin mimic, termed as boronolectin,14 which can recognize cell-surface carbohydrate biomarkers for cell labelling and recognition applications.15,16 In preparing small molecule lectin mimics, the boronic acid functional group is especially useful because of its intrinsic affinity for the diol functional group,17-20 which is commonly found on carbohydrates. The intrinsic affinity of boronic acids for the hydroxyl and diol groups has been used in the preparation of boronic acid-based sugar sensors,21-33 carbohydrate transporters,34-36 drug delivery materials,37,38 and other pharmaceutical agents.39,40,14
In the preparation of boronolectins, water-soluble boronic acids that change fluorescent properties upon diol binding are very useful for two reasons.14 First, the fluorescent property changes upon binding are of tremendous help in screening combinatorial libraries. Second, they allow for the development of new boronic acid-based sensors and diagnostics without the need of incorporating a separate signalling moiety. Along this line, we41,42,14,43,44,40,45,46 and others24 have developed several such boronic acid fluorescent reporters. In our continuing effort to develop boronolectins and fluorescent boronolectins, we are interested in examining whether adding conjugation to such known boronic acid fluorescent reporters would result in more favorable spectroscopic properties in terms of wavelength and magnitude of fluorescent property changes. For this, we have selected four representative compounds (1a-d, Figure 1). Three of them are naphthylboronic acids (1a-c) that are positional isomers of each other and one is a quinolinylboronic acid (1d). Out of these four, 1a shows fluorescence intensity increases upon sugar binding;41 1b shows ratiometric fluorescent intensity changes at two wavelengths;47 1c shows fluorescent intensity decreases upon sugar binding;47 and 1d did not show significant fluorescent property changes upon sugar binding. These compounds were chosen for their (1) water soluble nature, (2) known fluorescent property changes upon binding(except for 1d), (3) chemical stability, and (4) anticipated ease of synthetic manipulation. With these reporters having diverse patterns of spectroscopic changes upon sugar binding, we reasoned that the results should allow us to do some representative sampling of what could be expected by extending the conjugation of similar boronic acid fluorescent reporters. Specifically, we are interested in learning the effect of such extended conjugations on (1) the excitation and emission wavelengths, (2) quantum yields of both the free boronic acids and their corresponding esters, (3) the pattern of fluorescent property changes, and (4) mechanism of fluorescent property changes upon sugar binding.
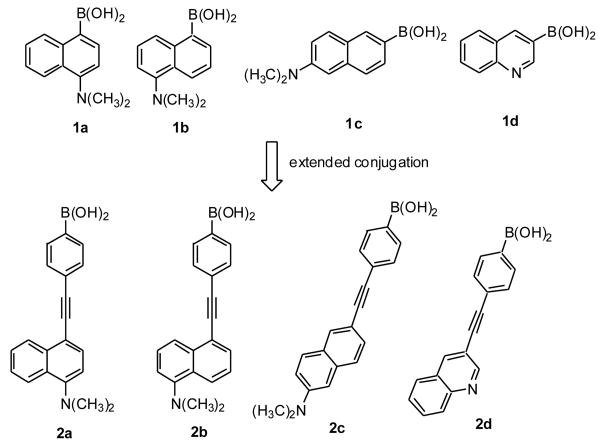
Figure 1.
Design of compounds 2a-d from 1a-d.
Phenylethynyl and ethynyl groups have received a great deal of attention as a linker for new materials with potential applications ranging from chemical sensing to molecular electronics.48-54 Such elongation affords the needed conjugation and only introduces one more rotational degree of freedom. Therefore such compounds are expected to have good fluorescent properties. After considering the ease of synthesis, potential effect on water solubility, chemical stability of the extended product, and the desire to minimize the degree of freedom in the new extended conjugation system, we decided to use an alkyne group as a way to extend the conjugation. Therefore, four new compounds (2a-d) with their conjugation extended by an alkyne group were designed (Figure 1). The results show that extended conjugation not only resulted in increased emission and excitation wavelength as expected, but also improved fluorescent property changes upon binding with interesting mechanistic implications.
2. Results and discussions
2.1. Synthesis
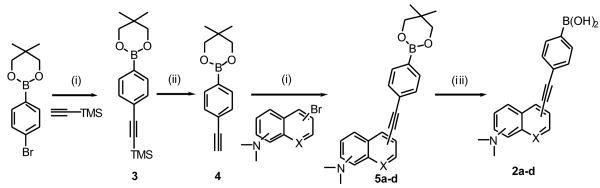
The designed 4-arylethynylphenyl boronic acids 2a-d were synthesized using the Sonogashira coupling reaction of arylbromides with 2,2-dimethylpropane-1,3-diyl-4-ethynylphenyl boronate (4) as the building block under microwave irradiation followed by the deprotection of neopentyl group with TFA in water. Specifically, Sonogashira coupling reaction of 2,2-dimethylpropane-1,3-diyl-4-bromophenyl boronate with trimethylsilyl acetylene under microwave irradiation gave 2,2-dimethylpropane-1,3-diyl[4-trimethylsilylethynylphenyl] boronate (3) in 98% isolated yield. This yield was much higher than that without using microwave.55 Deprotection of the trimethylsilyl group (TMS) of 3 with potassium carbonate afforded 4.55 It should be noted that the two key reactions for the synthesis of 3 and 5a-d were both microwave-facilitated Sonogashira coupling reaction and no Suzuki coupling side reactions were observed. In addition, the use of microwave was found to significantly shorten the reaction time.
2.2. Fluorescence studies
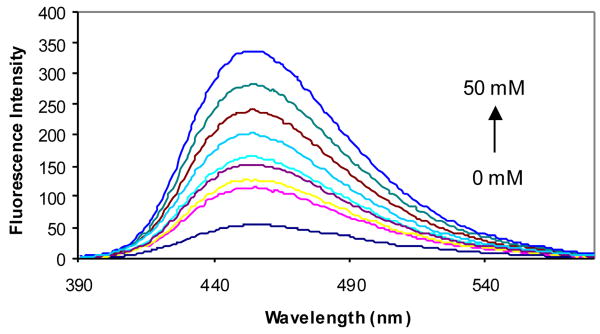
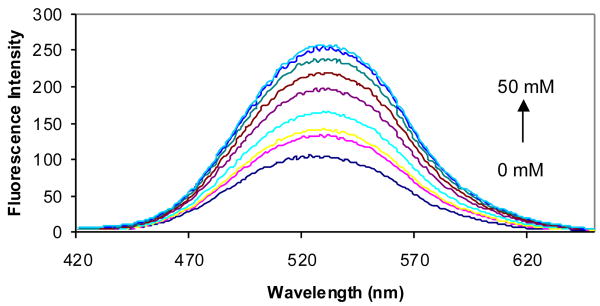
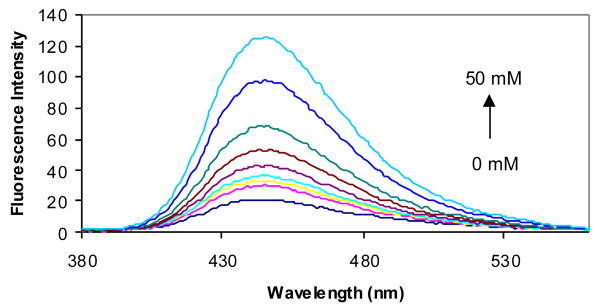
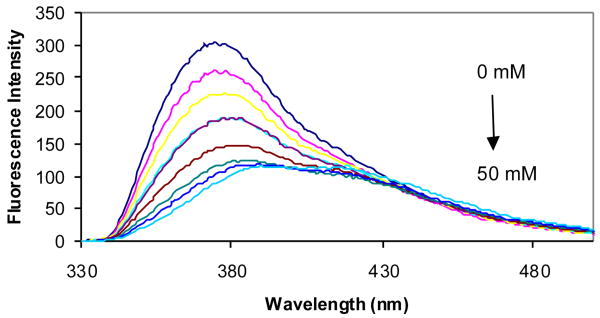
The fluorescent properties of compounds 2a-d in the presence and absence of sugars were examined in phosphate buffer (0.1 M, pH 7.4). Compound 2a showed an 8-fold fluorescence intensity increase upon addition of 50 mM D-fructose (Figure 2). It also showed significant fluorescence intensity increases upon addition of other sugars. Compared with compound 1a (λex = 300 nm and λem = 445 nm),41 the λex of 2a increased by about 40 nm to 340 nm and the λem increased by only about 10 nm to 454 nm (Table 1).
Figure 2.
Fluorescent spectral changes of 2a (1.0 × 10-5 M) with different concentrations of D-fructose (0-50 mM) in 0.1 M aqueous phosphate buffer at pH 7.4, λex = 340 nm.
Table 1.
Association constantsa and fluorescent properties of 1a-c and 2a-d with and without added sugar (50 mM fructose)
| Compd | λex | Without sugar | With Fructose | Ka (M-1) | |||||
|---|---|---|---|---|---|---|---|---|---|
| [nm] | λem [nm] | ΦF | λem [nm] | ΦF | sorbitol | fructose | galactose | glucose | |
| 1a41 | 300 | 445 | 0.01 | 445 | 0.42 | 226 ± 12 | 207 ± 8 | 12.0 ± 3.6 | 4.0 ± 2.1 |
| 2a | 340 | 454 | 0.013 | 454 | 0.096 | 619 ± 16 | 293 ± 19 | 8.1 ± 1.4 | 2.5 ± 1.6 |
| 1b56 | 300 | 513, 433 | 0.07 | 433 | 0.23 | 337 ± 11 | 311 ± 13 | 23.0 ± 5.2 | 3.6 ± 1.8 |
| 2b | 340 | 531 | 0.027 | 531 | 0.12 | 311 ± 9 | 178 ± 2.1 | 7.5 ± 0.8 | 7.0 ± 2.0 |
| 1c47 | 310 | 432 | 0.89 | -b | -b | 227 ± 10 | 120 ± 6 | 8.0 ± 1.5 | 2.4 ± 1.1 |
| 2c | 330 | 448 | 0.025 | 448 | 0.058 | 11.1 ± 0.3 | 132 ± 5 | 1.8 ± 0.6 | 0.1 ± 0.05 |
| 1d | 310 | -b | -b | 412 (weak) | 0.034 | -b | -b | -b | -b |
| 2d | 310 | 371 | 0.1 | 371, 430 | 0.08 | 822 ± 17 | 427 ± 18 | 49 ± 6.0 | 18 ± 3.0 |
Constants determined by fitting into a 1:1 binding model. Errors reported are two standard deviations; R2> 0.99 in all cases.
Not applicable.
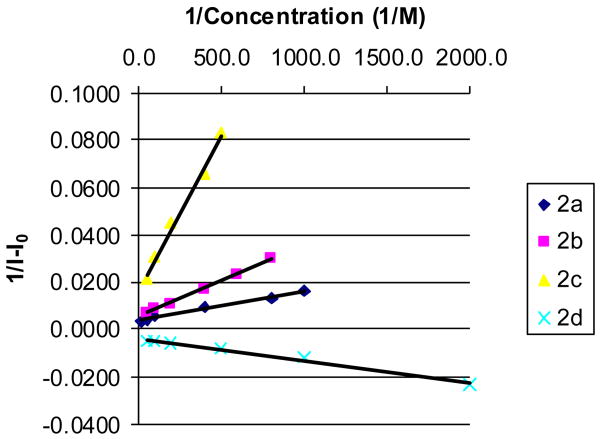
Binding constants for 2a as well as the other sensors (2b-d) with fructose and three other sugars were determined at pH 7.4 by fitting 1/ΔIf vs. 1/Con. into a 1:1 binding model. In all cases, R2 were > 0.99, indicating an excellent fit into a 1:1 binding model (Figure 3).
Figure 3.
Determination of the Ka for 2a-d with fructose in 0.1 M phosphate buffer at pH 7.4; R2> 0.99 in all cases.
The affinity trend with 2a followed that of 1a17,18 in the order of sorbitol > fructose > galactose > glucose with Ka (M-1) of 619, 293, 8.1 and 2.5, respectively (Table 1). As has been addressed before in the literature, sorbitol can engage in trivalent binding, which is the reason for its high affinity relative to other sugars.57,58,14
The fluorescence quantum yields (ΦF) of 2a were determined in 0.1 M phosphate buffer (pH 7.4) in the absence and presence of 50 mM fructose, with 8-quinolinc boronic acid (ΦF = 0.58 in 12 M H2SO4) as a reference compound.59 The results (Table 1) show that the ΦF (0.013) of 2a in the absence of a sugar is similar to that of 1a (0.01). However, after addition of 50 mM fructose, the ΦF of 2a increase to 0.096, which is lower than the ΦF (0.42) of 1a under the same conditions. Therefore, it seems that the increased wavelength came at the expense of a decreased quantum yield possibly due to the increased degree of freedom of rotation which allows for the irradiative decay of the excited state.
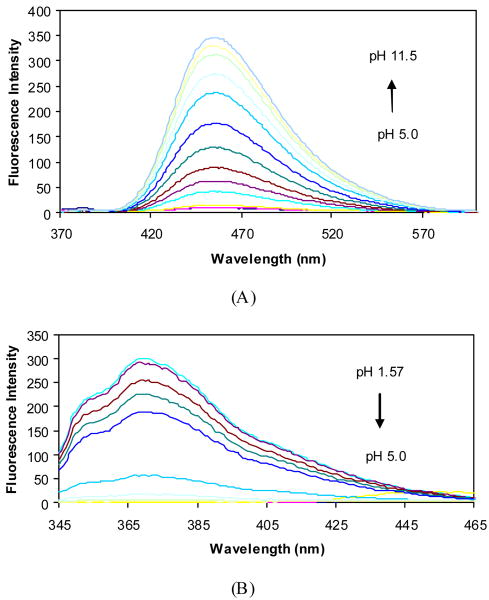
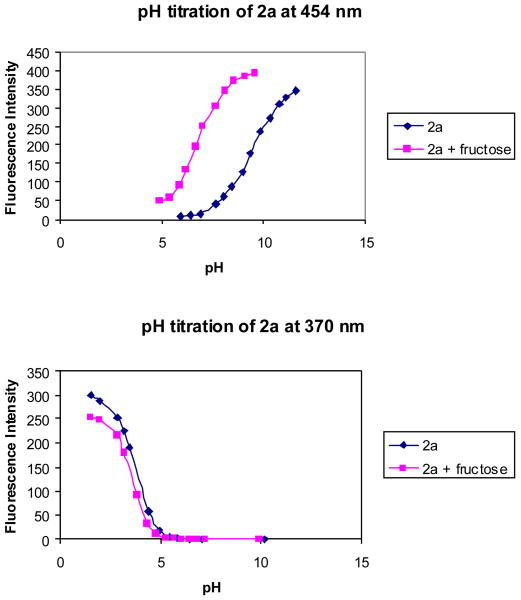
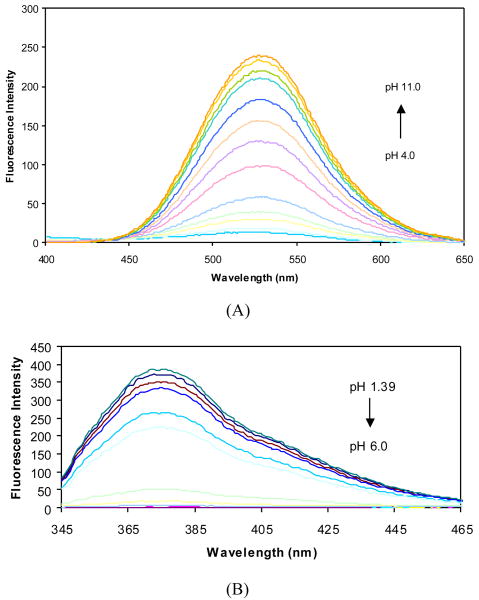
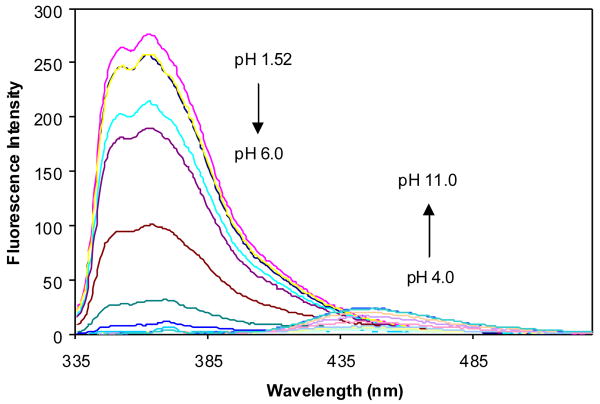
The pH induced fluorescence spectral changes of 2a in the absence and presence of fructose at a fixed concentration (50 mM) were studied to examine the relationship between the fluorescence intensity changes and the different ionization states. The fluorescence intensity of 2a at 370 nm decreased when pH increased from 1.57 to ∼5.0, and did not show further changes beyond 5.0. Almost no fluorescence was observed at 454 nm in the pH range 1.57-5.0, while a new peak increased very significantly at 454 nm as pH increased from 5.0 to 11.5 (Figures 4 and 5).
Figure 4.
Fluorescence spectral changes of 2a (1.0×10-5 M) in 0.1 M aqueous phosphate buffer at different pH, λex = 340 nm. (A) fluorescent intensity changes at 454 nm; (B) fluorescent intensity changes at 370 nm.
Figure 5.
pH titration of 2a (1.0 × 10-5 M) with or without sugar (50 mM fructose) in 0.1 M aqueous phosphate buffer at pH 7.4, λex = 340 nm.
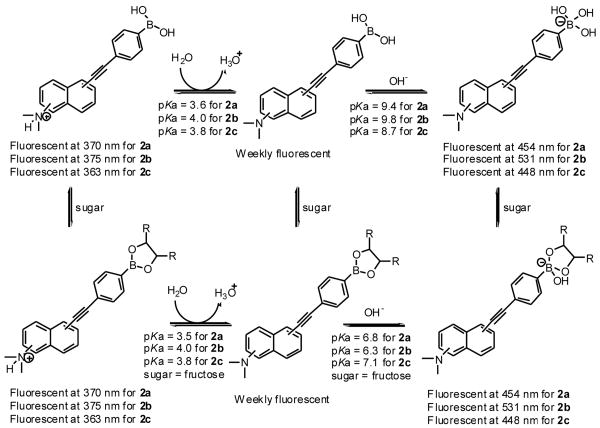
The pH-induced spectral changes at 370 nm in the absence of a sugar were similar to that in the presence of 50 mM fructose. A decrease in intensity (more than 99%) at 370 nm was observed when pH increased from 1.57 to 5.0. Plotting the intensity changes at 370 nm against pH revealed the first apparent pKa of 3.6 for 2a in the absence of sugar and 3.5 in the presence of fructose. The pH profiles of the fluorescence intensity of 2a at 454 nm revealed the second apparent pKa of 9.4 in the absence of a sugar and 6.8 in the presence of fructose (50 mM). It is reasonable to assign the first pKa to the aniline group and the second pKa to the boronic acid. Therefore, one can use Scheme 2 to show the various ionization states of 2a, which helps to understand the assignment of each fluorescent peak to a particular species.
Scheme 2.
Fluorescent properties of 2a, 2b and 2c in different ionization states in the absence and presence of a sugar in 0.1 M aqueous phosphate buffer
The pH-induced fluorescence changes of 2a and 1a have a similar trend. The free forms of 2a and 1a are weakly fluorescent and only the protonated form (370 nm) and the boron ionized form (454 nm) are fluorescent.
With compound 2b, its affinity trend also followed that of its parent compound, 1b, in the order of sorbitol > fructose > galactose > glucose with Ka (M-1) of 311, 178, 7.5 and 7.0, respectively (Table 1). However, compared with the situation for 2a, the extension of the conjugation of 5-N,N-dimethylaminonaphthylboronic acid (1b) gave some very noticeably different outcomes. Compound 2b exhibited very different fluorescence properties compared with its parent, 1b. Addition of fructose (50 mM) to a solution of 1b induced a 61% fluorescence intensity decrease at the longest wavelength, 513 nm, and a 36-fold increase at 433 nm.47,56 In contrast, 2b showed a 2.5-fold fluorescence intensity increase upon addition of 50 mM D-fructose (Figure 6) at 531 nm. With the added conjugation, the excitation wavelength of 2b increased by 40 nm to 340 nm and the highest emission wavelength changed from 513 to 531 nm. It should be noted that numerous literature precedents have shown that substitution patterns make a great difference in dictating the fluorescent properties of a boronic acid compound.41,56,47,46 Therefore, it is not surprising that positional isomers behave somewhat differently in their fluorescent responses upon elongated conjugation.
Figure 6.
Fluorescent spectral changes of 2b (1.0 × 10-5 M) with different concentrations of D-fructose (0-50 mM) in 0.1 M aqueous phosphate buffer at pH 7.4, λex = 340 nm.
The fluorescence quantum yields (ΦF) of 2b were determined in the presence and absence of a sugar using the same method as with 2a. The results (Table 1) show that ΦF of compounds 2b in the absence of a sugar was 0.027, which increased to 0.12 after addition of 50 mM fructose.
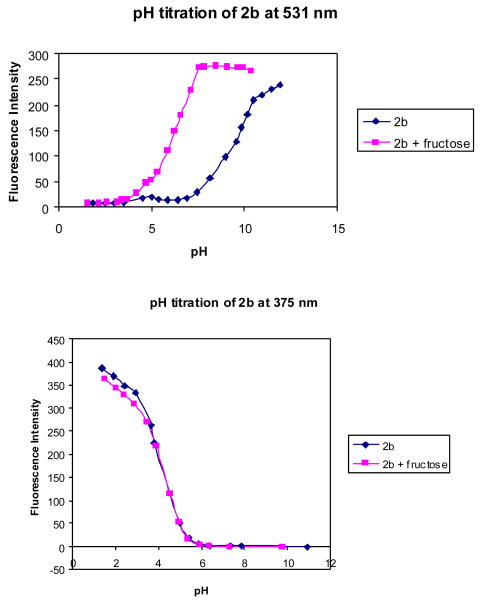
Figures 7 and 8 show the fluorescence intensity changes of 2b at different pH in the absence and presence of fructose (50 mM). It can be seen that the fluorescence intensity of 2b at 375 nm decreased when pH increased from 1.39 to ∼6.0, and did not show further changes beyond 6.0, while a new peak increased very significantly at 531 nm as pH increased from 4.0 to 11.0.
Figure 7.
Fluorescent spectral changes of 2b in 0.1 M aqueous phosphate buffer (1.0 × 10-5 M) in the absence of sugar at different pH, λex = 340 nm. (A) changes at 531 nm; (B) changes at 375 nm.
Figure 8.
pH titration of 2b (1.0 × 10-5 M) with or without fructose sugar (50 mM) in 0.1 M aqueous phosphate buffer, λex = 340 nm.
The pH-induced spectral changes at 375 nm in the absence of a sugar are similar to those in the presence of 50 mM fructose. A decrease in intensity (more than 99%) at 375 nm was observed when pH increased from 1.57 to 5.0. Plotting the intensity changes at 375 nm against pH revealed the first apparent pKa for 2b as 4.0 both in the absence and in the presence of fructose. The pH profiles of the fluorescence intensity of 2b at 531 nm revealed the second apparent pKa of 2b as 9.8 in the absence of a sugar and 6.3 in the presence of fructose. It is reasonable to assign the first pKa to the aniline group and the second pKa to the boronic acid. Scheme 2 shows the various ionization states of 2b and their relationship to various fluorescent peaks.
Compound 2c is similar to 2b, where elongation of the conjugation resulted in more than just an increase in excitation and emission wavelengths. It displayed different patterns of fluorescence intensity changes compared with its parent compound, 1c. With the addition of fructose to the solution of 1c, a significant 80% decrease in fluorescent intensity was observed at 432 nm.47 In contrast, 2c showed a 6-fold fluorescence intensity increase upon addition of 50 mM D-fructose (Figure 9) at 448 nm. The affinities of 2c for various sugars are in the order of fructose > sorbitol > galactose > glucose with Ka (M-1) of 132, 11.1, 0.2 and 0.1, respectively (Table 1). This order of affinity strength is different from that of its parent compound, 1c. It is very interesting to note the relatively low affinity of 2c for sorbitol, galactose, and glucose. It is not readily clear why this is the case. It should be noted that we have conducted an extensive evaluation of various factors that affect boronic acid-diol binding constants. Though pKa and pH values are two major factors, there are other factors that are not well understood at the present time. 17,18
Figure 9.
Fluorescence spectral changes of 2c (1.0 × 10-6 M) with different concentrations of D-fructose (0-50 mM) in 0.1 M aqueous phosphate buffer at pH 7.4, λex = 330 nm
The fluorescence quantum yields (ΦF) of 2c were determined in a similar fashion as for 2a and 2b. After addition of 50 mM fructose, the fluorescence quantum yield (ΦF) of 2c increased from 0.025 to 0.058 at 448 nm. As a comparison, the fluorescence quantum yield (ΦF) of 1c in the absence of a sugar was 0.89, and the quantum yield (ΦF) decreased after the addition of 50 mM fructose.
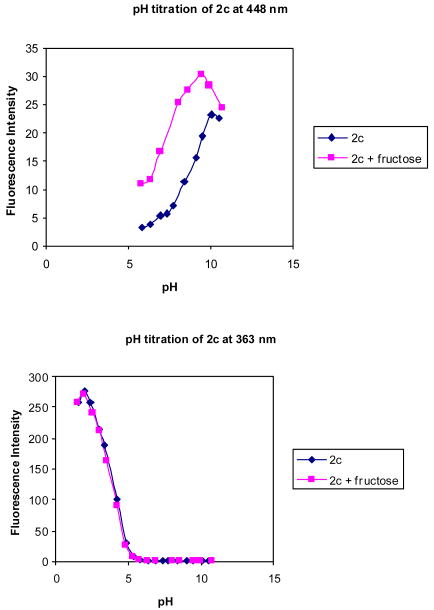
Figures 10 and 11 shows the fluorescence intensity changes of 2c at different pH in the absence and presence of fructose (50 mM). It can be seen that the fluorescence intensity of 2c at 363 nm decreased when pH increased from 1.52 to ∼6.0, while a new peak increased in intensity at 448 nm as pH increased from 4.0 to 11.0.
Figure 10.
Fluorescence spectral changes of 2c (1.0 × 10-6 M) in 0.1 M aqueous phosphate buffer at different pH, λex = 330 nm.
Figure 11.
pH titration of 2c (1.0 × 10-6 M) with and without fructose (50 mM) in 0.1 M aqueous phosphate buffer at pH 7.4, λex = 330 nm.
The pH-induced spectral changes of 2c at 363 nm in the absence of a sugar are similar to that in the presence of 50 mM fructose. A more than 200-fold intensity decrease at 363 nm was observed when pH increased from 1.52 to 6.0. Plotting the intensity changes at 363 nm against pH revealed the first apparent pKa as 3.8 for 2c both in the absence and presence of fructose. The pH profiles of the fluorescence intensity of 2c at 448 nm revealed the second apparent pKa of 2c as 8.7 in the absence of a sugar and 7.1 in the presence of fructose. It is reasonable to assign the first pKa to the aniline group and the second pKa to the boronic acid. Scheme 2 shows the various ionization states of 2c and their relationship to various fluorescent emissions.
It is also interesting to note that the pH-induced fluorescence changes of 2c are very different from that of 1c. The free form of 2c is only weakly fluorescent and only the protonated form and the boron ionized form are fluorescent (Scheme 2). In contrast, the free form of 1c is the fluorescent species, and neither the protonated form nor the boron ionized form of 1c is fluorescent.
The effect of conjugation elongation was most prominent with compound 2d, which showed a significant fluorescence intensity decrease upon addition of D-fructose (Figure 12) at 371 nm, while its parent compound, 1d, showed no significant fluorescence intensity changes upon addition of D-fructose and sorbitol.60 The affinity trend of 2d followed that of 1a-c, 2a and 2b in the order of sorbitol > fructose > galactose > glucose with Ka (M-1) of 822, 427, 49, and 18, respectively (Table 1). Compound 2d also showed the highest binding affinity with different sugars compared with all other water-soluble boronic acid sensors described in this study. This situation is very similar to 3-pyridine boronic acid, which also showed a high affinity for various sugars. The key reason seems to be due to the formation of zwitterionic complex, which favors binding.61
Figure 12.
Fluorescence spectral changes of 2d (1.0 × 10-5 M) with different concentrations of D-fructose (0-50 mM) in 0.1 M aqueous phosphate buffer at pH 7.4, λex = 310 nm
The fluorescence quantum yields (ΦF) of 2d were also determined in 0.1 M phosphate buffer (pH 7.4) in the absence of a sugar and in the presence of 50 mM fructose in a similar fashion as with 2a-c. The quantum yield of 2d slightly decreased from 10% to 8% after addition of 50 mM fructose.
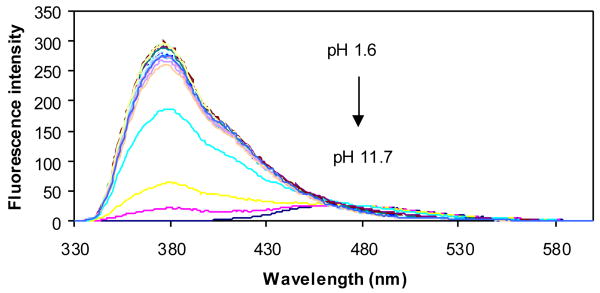
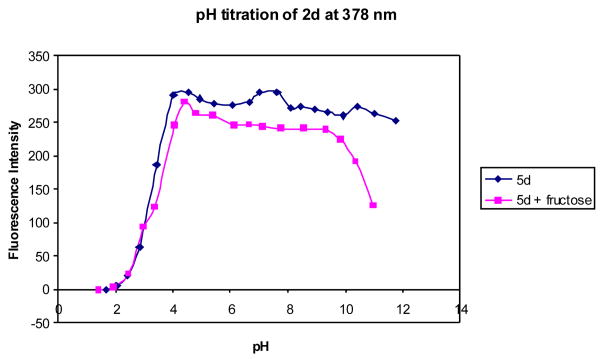
The pH induced fluorescence spectral changes of 2d in the absence of any sugar (Figures 13 and 14) showed only one apparent pKa at 3.3, while in the presence of 50 mM fructose, two apparent pKa values were found for 2d at 3.3 and 10. If the situation is similar to 3-pyridineboronic acid, the first pKa of the complex could be the boron and the second one could be due to the deprotonation of the quinoline nitrogen.
Figure 13.
Fluorescence spectral changes of 2d (1.0 × 10-5 M) in the absence of sugar at different pH, in 0.1 M aqueous phosphate buffer, λex = 310 nm
Figure 14.
pH titration of 2d (1.0 × 10-5 M) with or without a sugar (50 mM) in 0.1 M aqueous phosphate buffer at pH 7.4, λex = 310 nm.
3. Conclusion
A series of boronic acid analogs 2a-d with increased conjugation have been synthesized in four steps from the corresponding arylbromides and 2,2-dimethylpropane-1,3-diyl-4-ethynylphenylboronate. The key reaction was a microwave-facilitated selective Sonogashira reaction, which gave 47% ∼ 67% yields. Detailed fluorescent studies show that extending the conjugation with an alkynyl group (1) decreased quantum yield, (2) increased the excitation and emission wavelength by 20-40 and 9-16 nm respectively, (3) resulted in alterations in the patterns of the fluorescent property changes upon sugar binding, and (4) altered the pH profiles and thus the mechanism through which fluorescent properties change upon sugar addition. The results suggested that extending the conjugation of a known fluorescent boronic acid with an alkynyl moiety results in more than a simple increase in excitation and emission wavelength.
4. Experimental
4.1. General
All reagents were purchased from Acros and Aldrich. Microwave heating was performed in the single-mode microwave cavity of a Discover Synthesis System (CEM Co.), and all microwave-irradiated reactions were conducted in a heavy-walled glass vials sealed with Teflon septa. 1H-NMR, 13C-NMR and DEPT spectra were recorded at 400 and 100 MHz, respectively, on a Bruker Avance 400 NMR spectrometer. Mass spectra were performed by the Mass Spectrometry Facilities at Georgia State University. Fluorescence spectra were recorded on a Shimadzu RF-5301 PC spectrofluorometer. Absorption spectra were recorded on a Shimadzu UV-1700 UV-Visible spectrophotometer. All pH values were determined by a UB-10 Ultra Basic Benchtop pH meter (Denver Instrument).
4.2. Synthesis
(6-Bromo-naphthalen-2-yl)-methylamine,62 (6-bromo-naphthalen-2-yl)-dimethylamine,47 (4-bromo-naphthalen-1-yl)-dimethylamine,63 and (5-bromo-naphthalen-1-yl)-dimethylamine56 were synthesized following literature procedures.
2,2-dimethylpropane-1,3-diyl [4-trimethylsilylethynylphenyl] boronate (3).55
A mixture of 2,2-dimethylpropane-1,3-diyl-4-bromophenyl boronate (0.24 g, 0.9 mmol), Pd(PPh3)2Cl2 (32 mg, 0.05 mmol), CuI (9 mg, 0.05 mmol), triphenylphosphine (48 mg, 0.18 mmol), trimethylacetylene (0.14 mL, 1 mmol), and dimethylamine (1.5 mL, 13.6 mmol) in dimethylformamide (DMF, 0.5 mL) was irradiated with microwave at 120 °C for 20 min in a heavy-walled glass vial sealed with a Teflon septum. After irradiation, the solvent was concentrated under reduced pressure and the residue was purified by flash column chromatography to give 0.25 g (98%) of 2,2-dimethylpropane-1,3-diyl [4-trimethylsilylethynylphenyl] boronate (3). 1H-NMR (CDCl3): 7.65 (d, J = 8.0 Hz, 2H), 7.37 (d, J = 8.0 Hz, 2H), 3.67 (s, 4H), 0.93 (s, 6H), 0.17 (s, 9H). 13C-NMR (CDCl3): 133.5 (d), 131.0 (d), 125.1 (s), 105.4 (s), 95.0 (s), 72.3 (t), 31.8 (s), 21.9 (q), -0.05 (q). GC-MS (EI): 286 [M]+.
2,2-Dimethylpropane-1,3-diyl-4-ethynylphenyl boronate (4).55
To a solution of 2,2-dimethylpropane-1,3-diyl [4-trimethylsilylethynylphenyl] boronate (3) (0.14 g, 0.5 mmol) in methanol (10 mL), was added potassium carbonate (0.14 g, 1 mmol). The resulting mixture was stirred at room temperature for 0.5 h and then filtered. The filtrate was concentrated under reduced pressure. The crude residue was purified by flash column chromatography to afford 0.22 g (80%) of 2,2-dimethylpropane-1,3-diyl-4-ethynylphenyl boronate (4). 1H-NMR (CDCl3): 7.82 (d, J = 8.0 Hz, 2H), 7.54 (d, J = 8.0 Hz, 2H), 3.81 (s, 4H), 3.18 (s, 1H), 1.06 (s, 6H). 13C-NMR (CDCl3): 133.6 (d), 131.1 (d), 124.1 (s), 83.9 (s), 78.0 (d), 72.3 (t), 31.8 (s), 21.8 (q). GC-MS (EI): 214 (M+). HRMS (ESI-) calcd for C18H26BO4 [M+C5H12O2 (neopental glycol)-H]-: 317.1924. Found: 317.1929.
General procedure64,55 for the synthesis of compounds 5a-d by microwave-assisted Sonogashira coupling
A mixture of aryl bromide (0.9 mmol), Pd(PPh3)2Cl2 (0.05 mmol), CuI (0.05 mmol), triphenylphosphine (0.18 mmol), diethylamine (13.6 mmol) and 2,2-dimethylpropane-1,3-diyl-4-ethynylphenylboronate (1 mmol) was irradiated under microwave at 120 °C for 25 min. in a heavy-walled glass vial sealed with a Teflon septum. Then the reaction mixture was filtered and washed with dichloromethane. The filtrate was concentrated under reduced pressure and the residue was purified by flash column chromatography (silica gel 60) to give compound 5.
{4-[4-(5,5-Dimethyl[1,3,2]dioxaborinan-2-yl)-phenylethynyl]naphthalen-1-yl}dimethylamine (5a)
Yield: 51%. 1H-NMR (CDCl3): 8.44 (dd, J = 0.8 and 8.0 Hz, 1H), 8.22 (dd, J = 1.2 and 8.0 Hz, 1H), 7.81 (d, J = 8.0 Hz, 2H), 7.67 (d, J = 7.6 Hz, 1H), 7.61 (d, J = 8.4 Hz, 2H), 7.57 (m, 1H), 7.52 (m, 1H), 7.00 (d, J = 7.6 Hz, 1H), 3.78 (s, 4H), 2.92 (s, 6H), 1.03 (s, 6H). 13C-NMR (CDCl3): 151.7 (s), 134.5 (s), 133.7 (d), 130.8 (d), 130.5 (d), 128.3 (s), 126.8 (d), 126.5 (d), 125.9 (s), 125.5 (d), 124.6 (d), 114.9 (s), 113.3 (d), 93.6 (s), 89.1 (s), 72.3 (t), 45.0 (q), 31.9 (s), 21.9 (q). GC-MS (EI): 383 [M]+.
{5-[4-(5,5-Dimethyl[1,3,2]dioxaborinan-2-yl)-phenylethynyl]naphthalen-1-yl}dimethylamine (5b)
Yield: 49%. 1H-NMR (CDCl3): 8.27 (d, J = 8.0 Hz, 1H), 8.16 (d, J = 8.0 Hz, 1H), 7.84 (d, J = 8.0 Hz, 2H), 7.75 (dd, J = 0.8 and 7.2 Hz, 1H), 7.63 (d, J = 8.0 Hz, 2H), 7.51 (m, 1H), 7.46 (m, 1H), 7.13 (d, J = 7.2 Hz, 1H), 3.80 (s, 4H), 2.91 (s, 6H), 1.05 (s, 6H). 13C-NMR (CDCl3): 151.3 (s), 134.7 (s), 133.8 (d), 130.7 (d), 130.4 (d), 128.7 (s), 126.7 (d), 125.6(s), 125.1 (d), 124.4 (d), 121.1 (s), 121.0 (d), 114.6 (d), 94.4 (s), 89.0 (s), 72.3 (t), 45.3 (q), 31.9 (s), 21.9 (q). GC-MS (EI): 383 [M]+.
{6-[4-(5,5-Dimethyl[1,3,2]dioxaborinan-2-yl)-phenylethynyl]naphthalen-2-yl}dimethylamine (5c)
Yield: 47%. 1H-NMR (CDCl3): 7.90 (s, 1H), 7.78 (d, J = 8.4 Hz, 2H), 7.67 (d, J = 9.2 Hz, 1H), 7.59 (d, J = 8.4 Hz, 1H), 7.53 (d, J = 8.4 Hz, 2H), 7.46 (dd, J = 1.2 and 8.4 Hz, 1H), 7.16 (dd, J = 2.4 and 9.2 Hz, 1H), 6.87 (d, J = 2.4 Hz, 1H), 3.78 (s, 4H), 3.06 (s, 6H), 1.03 (s, 6H). 13C-NMR (CDCl3): 149.1 (s), 134.6 (s), 133.7 (d), 131.3 (d), 130.6 (d), 128.9 (d), 128.7 (d), 126.12 (d), 126.09 (s), 125.8 (s), 116.5 (d), 116.1 (s), 106.0 (d), 91.6 (s), 88.9 (s), 72.3 (t), 40.6 (q), 31.9 (s), 21.9 (q). GC-MS (EI): 383 [M]+.
3-[4-(5,5-Dimethyl[1,3,2]dioxaborinan-2-yl)phenylethynyl]quinoline (5d)
Yield: 67%. 1H-NMR (CDCl3): 9.00 (d, J = 2.0 Hz, 1H), 8.30 (d, J = 1.6 Hz, 1H), 8.10 (d, J = 8.4 Hz, 1H), 7.83∼7.78 (m, 3H), 7.72 (m, 1H), 7.59∼7.54 (m, 3H), 3.78 (s, 4H), 1.03 (s, 6H).13C-NMR (CDCl3): 152.1 (d), 146.8 (s), 138.3 (d), 133.8 (d), 130.8 (d), 130.0 (d), 129.4 (d), 127.6 (d), 127.3 (d), 124.5 (s), 117.5 (s), 93.0 (s), 87.5 (s), 72.3 (t), 31.9 (s), 21.9 (q). GC-MS (EI) : 341 [M]+. HRMS (ESI+) calcd for C22H21BNO2 [M+H]+ : 342.1665. Found: 342.1670.
General procedure for the preparation of compounds 2a-d by deprotection of the neopentyl group
A mixture of 5 (0.5 mmol) in 70% TFA was stirred at 60∼70 °C overnight. The reaction mixture was neutralized with saturated Na2CO3 and extracted with dichloromethane. The combined organic solution was dried over Na2SO4. After evaporation of solvent, the crude was purified on silica gel by flash column chromatography to afford 2.
Compound 2a
Yield: 43%. 1H-NMR (CDCl3): 8.48 (dd, J = 0.8 and 8.4 Hz, 1H), 8.25∼8.23 (m, 3H), 7.76∼7.72 (m, 3H), 7.60 (m, 1H), 7.54 (m, 1H), 7.03 (d, J = 7.6 Hz, 1H), 2.95 (s, 6H). 13C-NMR (CDCl3): 152.0 (s), 135.5 (d), 134.6 (s), 131.1 (d), 130.9 (d), 128.3 (s, two carbon overlap), 126.73 (d), 126.68 (d), 125.5 (d), 124.7 (d), 114.6 (s), 113.3 (d), 93.4 (s), 90.5 (s), 45.0 (q). MS (ESI-): 314.05 [M-H]-. HRMS (ESI-) calcd for C20H17BNO2 [M-H]-: 314.1352. Found: 314.1360.
Compound 2b
Yield: 36%. 1H-NMR (CDCl3): 8.30∼8.27 (m, 2H), 8.18 (d, J = 8.4 Hz, 1H), 7.81∼7.75 (m, 3H), 7.66 (m, 1H), 7.56 ∼ 7.46 (m, 2H), 7.16 (d, J = 7.2 Hz, 1H), 2.90 (s, 6H). MS (ESI-): 314.10 [M-H]-. HRMS (ESI-) calcd for C20H17BNO2 [M-H]-: 314.1352. Found: 314.1342.
Compound 2c
Yield: 41%. 1H-NMR (CDCl3): 8.22∼8.19 (m, 2H), 7.95 (s, 1H), 7.70∼7.67 (m, 3H), 7.62 (d, J = 8.8 Hz, 1H), 7.51 (dd, J = 1.6 and 8.4 Hz, 1H), 7.16 (dd, J = 1.6 and 8.8 Hz, 1H), 6.88 (s, 1H), 3.08 (s, 6H). 13C-NMR (CDCl3): 149.2 (s), 135.5 (d), 134.7 (s), 131.6 (d), 130.9 (d), 128.9 (d), 128.1 (s), 126.2 (d), 126.1 (s), 116.6 (d), 115.8 (s), 106.0 (d), 93.0 (s), 88.7 (s), 40.7 (q). HRMS (ESI-) calcd for C20H17BNO2 [M-H]-: 314.1352. Found: 314.1351.
Compound 2d
Yield: 47%. 1H-NMR (MeOD): 8.96 (s, 1H), 8.54 (s, 1H), 8.07 (d, J = 8.4 Hz, 1H), 7.99 (d, J = 8.0 Hz, 1H), 7.85∼7.82 (m, 3H), 7.69 (m, 1H), 7.59 (d, J = 7.6 Hz, 2H).13C-NMR (CDCl3): 151.8, 146.3, 138.9, 138.8, 134.5, 133.5, 131.0, 130.4, 129.0, 127.7, 127.5, 92.9, 87.6. HRMS (ESI+) calcd for C17H13BNO2 [M+H]+: 274.1039. Found: 274.1028.
4.3. Procedure for sugar-binding studies
The solutions of the sensors (1.0 × 10-5 or 1.0 × 10-6 M) were prepared in 0.1 M phosphate buffer at pH 7.4 and the solutions of sugars (1.0 × 10-1 M) were prepared in above solutions of the sensors at pH 7.4. Then, 3 mL of mixtures were prepared by mixing a sensor solution with a different ratio of sugar solution. After stirring for 20 min, the mixture was transferred into a 1 cm quartz cell and the fluorescence intensity was recorded immediately.
Scheme 1.
Synthesis of 4-aryethynylphenylboronic acids 2a-d: (i) PdCl2(Ph3P)2, CuI, Ph3P, 120 °C, microwave irradiation, 98% for 3 and 47% ∼ 67% for 5a-d; (ii) K2CO3, MeOH, rt, 80%; (iii) TFA, H2O, 36% ∼ 47%.
Acknowledgments
Financial support from the National Institutes of Health (CA123329, CA113917, CA88343, and NO1-CO-27184), the Georgia Cancer Coalition through a Distinguished Cancer Scientist Award, and the Georgia research Alliance through an Eminent Scholar endowment and an Eminent Scholar challenge grant is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yamada N, Chung YS, Sawada T, Okuno M, Sowa M. Dig Dis Sci. 1995;40:1005–1012. doi: 10.1007/BF02064189. [DOI] [PubMed] [Google Scholar]
- 2.O'Driscoll L, Linehan R, Liang YH, Joyce H, Oglesby I, Clynes M. Anticancer Res. 2002;22:3117–3125. [PubMed] [Google Scholar]
- 3.Yehiel Z, Eisenstein M, Goren RA, Hadari YR, Levy Y, Ronen D. Glycoconjugate J. 2004;19:517–526. doi: 10.1023/B:GLYC.0000014081.55445.af. [DOI] [PubMed] [Google Scholar]
- 4.Tei K, Kawakami-Kimura N, Taguchi O, Kumamoto K, Higashiyama S, Taniguchi N, Toda K, Kawata R, Hisa Y, Kannagi R. Cancer Res. 2002;62:6289–6296. [PubMed] [Google Scholar]
- 5.McEver RP. Glycoconjugate J. 1997;14:585–591. doi: 10.1023/a:1018584425879. [DOI] [PubMed] [Google Scholar]
- 6.Ley K. Trends Mol Med. 2003;9:263–268. doi: 10.1016/s1471-4914(03)00071-6. [DOI] [PubMed] [Google Scholar]
- 7.Young AR, Meeusen EN. Glycoconjugate J. 2004;19:601–606. doi: 10.1023/B:GLYC.0000014091.00844.0a. [DOI] [PubMed] [Google Scholar]
- 8.Kannagi R. Glycoconjugate J. 1997;14:577–584. doi: 10.1023/a:1018532409041. [DOI] [PubMed] [Google Scholar]
- 9.Kannagi R. Glycoconjugate J. 2004;20:353–364. doi: 10.1023/B:GLYC.0000033631.35357.41. [DOI] [PubMed] [Google Scholar]
- 10.Gabius HJ, Gabius S, editors. Lectins and Cancer. Springer-Verlag; New York: 1991. [Google Scholar]
- 11.Danguy A, Camby I, Kiss R. Biochem Biophys Acta-Gen Subj. 2002;1572:285–293. doi: 10.1016/s0304-4165(02)00315-x. [DOI] [PubMed] [Google Scholar]
- 12.Takenaka Y, Fukumori Y, Raz A. Glycoconjugate J. 2004;19:543–549. doi: 10.1023/B:GLYC.0000014084.01324.15. [DOI] [PubMed] [Google Scholar]
- 13.vanden Brule FE, Califice S, Vastronovo S. Glycoconjugate J. 2004;19:537–542. doi: 10.1023/B:GLYC.0000014083.48508.6a. [DOI] [PubMed] [Google Scholar]
- 14.Yan J, Fang H, Wang B. Med Res Rev. 2005;25:490–520. doi: 10.1002/med.20038. [DOI] [PubMed] [Google Scholar]
- 15.Yang W, Gao S, Gao X, Karnati VR, Ni W, Wang B, Hooks WB, Carson J, Weston B. Bioorg Med Chem Lett. 2002;12:2175–2177. doi: 10.1016/s0960-894x(02)00339-6. [DOI] [PubMed] [Google Scholar]
- 16.Yang W, Fan H, Gao S, Gao X, Ni W, Karnati V, Hooks WB, Carson J, Weston B, Wang B. Chem Biol. 2004;11:439–448. doi: 10.1016/j.chembiol.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 17.Springsteen G, Wang B. Tetrahedron. 2002;58:5291–5300. [Google Scholar]
- 18.Yan J, Springsteen G, Deeter S, Wang B. Tetrahedron. 2004;60:11205–11209. [Google Scholar]
- 19.Lorand JP, Edwards JO. J Org Chem. 1959;24:769–774. [Google Scholar]
- 20.Sugihara JM, Bowman CM. J Am Chem Soc. 1958;80:2443. [Google Scholar]
- 21.Shinkai S, Takeuchi M. Trends Anal Chem. 1996;15:188–193. [Google Scholar]
- 22.Yoon J, Czarnik AW. J Am Chem Soc. 1992;114:5874–5875. [Google Scholar]
- 23.Wang W, Gao X, Wang B. Curr Org Chem. 2002;6:1285–1317. [Google Scholar]
- 24.Cao HS, Heagy MD. J Fluor. 2004;14:569–584. doi: 10.1023/b:jofl.0000039344.34642.4c. [DOI] [PubMed] [Google Scholar]
- 25.James TD, Linnane P, Shinkai S. Chem Commun. 1996:281–288. [Google Scholar]
- 26.Wiskur SL, Lavigne JJ, Metzger A, Tobey SL, Lynch V, Anslyn EV. Chem Eur J. 2004;10:3792–3804. doi: 10.1002/chem.200305737. [DOI] [PubMed] [Google Scholar]
- 27.Jiang S, Rusin O, Escobedo JO, Kim KK, Yang Y, Fakode S, Warner IM, Strongin RM. J Am Chem Soc. 2006;128:12221–12228. doi: 10.1021/ja063651p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alptürk O, Rusin O, Fakayode SO, Wang W, Escobedo JO, Warner IM, Král V, Strongin RM. Proc Natl Acad Sci USA. 2006;103:9756–9760. doi: 10.1073/pnas.0603758103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asher SA, Alexeev VL, Goponenko AV, Sharma AC, Lednev IK, Wilcox CS, Finegold J Am Chem Soc. 2003;125:3322–3329. doi: 10.1021/ja021037h. [DOI] [PubMed] [Google Scholar]
- 30.Jeff T, Suri JT, Cordes DB, Cappuccio FE, Wessling RA, Singaram B. Angew Chem, Int Ed. 2003;42:5857–5859. doi: 10.1002/anie.200352405. [DOI] [PubMed] [Google Scholar]
- 31.Badugu R, Lakowicz JR, Geddes CD. Bioorg Med Chem. 2005;13:113–119. doi: 10.1016/j.bmc.2004.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stones D, Manku S, Lu X, Hall DG. Chem Eur J. 2004;10:92–100. doi: 10.1002/chem.200305400. [DOI] [PubMed] [Google Scholar]
- 33.Arimori S, Phillips MD, James TD. Tetrahedron Lett. 2004;45:1539–1542. [Google Scholar]
- 34.Riggs JA, Hossler KA, Smith BD, Karpa MJ, Griffin G, Duggan PJ. Tetrahedron Lett. 1996;37:6303–6306. [Google Scholar]
- 35.Draffin SP, Duggan PJ, Duggan SAM. Org Lett. 2001;3:917–920. doi: 10.1021/ol015560x. [DOI] [PubMed] [Google Scholar]
- 36.Gardiner SJ, Smith BD, Duggan PJ, Karpa MJ, Griffin GJ. Tetrahedron. 1999;55:2857–2864. [Google Scholar]
- 37.Shino D, Kataoka K, Koyama Y, Yokoyama M, Okano T, Sakutai Y. J Intelligent Mater System Structure. 1994;5:311–314. [Google Scholar]
- 38.Shiino D, Murata Y, Kubo A, Kim YJ, Kataoka K, Koyama Y, Kikuchi A, Yokoyama M, Sakurai Y, Okano T. J Controlled Release. 1995;37:269–276. [Google Scholar]
- 39.Yang W, Gao X, Wang B. Med Res Rev. 2003;23:346–368. doi: 10.1002/med.10043. [DOI] [PubMed] [Google Scholar]
- 40.Yang W, Gao S, Wang B. In: Organoboronic Acids. Hall D, editor. John Wiley and Sons; New York: 2005. pp. 481–512. [Google Scholar]
- 41.Gao X, Zhang Y, Wang B. Org Lett. 2003;5:4615–4618. doi: 10.1021/ol035783i. [DOI] [PubMed] [Google Scholar]
- 42.Yang W, Lin L, Wang B. Heterocycl Commun. 2004;10:383–388. [Google Scholar]
- 43.Wang J, Jin S, Wang B. Tetrahedron Lett. 2005;46:7003–7006. [Google Scholar]
- 44.Yang W, Lin L, Wang B. Tetrahedron Lett. 2005;46:7981–7984. [Google Scholar]
- 45.Wang J, Lin N, Jin S, Wang B. Chem Biol Drug Design. 2006;67:137–144. doi: 10.1111/j.1747-0285.2005.00338.x. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, Gao X, Hardcastle K, Wang B. Chem-Eur J. 2006;12:1377–1384. doi: 10.1002/chem.200500982. [DOI] [PubMed] [Google Scholar]
- 47.Gao X, Zhang Y, Wang B. Tetrahedron. 2005;61:9111–9117. [Google Scholar]
- 48.Biswas M, Nguyen P, Marder TB, Khundkar LR. J Phys Chem A. 1997;101:1689–1695. [Google Scholar]
- 49.Elangovan A, Yang SW, Lin JH, Kao KM, Ho TI. Org Biomol Chem. 2004;2:1597–1602. doi: 10.1039/b403775h. [DOI] [PubMed] [Google Scholar]
- 50.Leroy-Lhez S, Fages F. Eur J Org Chem. 2005:2684–2688. [Google Scholar]
- 51.Metivier R, Amengual R, Leray I, Michelet V, Genet JP. Org Lett. 2004;6:739–742. doi: 10.1021/ol036399o. [DOI] [PubMed] [Google Scholar]
- 52.Odom SA, Parkin SR, Anthony JE. Org Lett. 2003;5:4245–4248. doi: 10.1021/ol035415e. [DOI] [PubMed] [Google Scholar]
- 53.Schiedel MS, Briehn CA, Bauerle P. Angew Chem, Int Ed. 2001;40:4677–4680. doi: 10.1002/1521-3773(20011217)40:24<4677::aid-anie4677>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 54.Yamaguchi S, Shirasaka T, Tamao K. Org Lett. 2000;2:4129–4132. doi: 10.1021/ol006660q. [DOI] [PubMed] [Google Scholar]
- 55.Zheng SL, Reid S, Lin N, Wang B. Tetrahedron Lett. 2006;47:2331–2335. [Google Scholar]
- 56.Gao X, Zhang Y, Wang B. New J Chem. 2005;29:579–586. [Google Scholar]
- 57.Norrild JC. J Chem Soc Perkin Trans. 2001;2:719–726. [Google Scholar]
- 58.Ori A, Shinkai S. J Chem Soc, Chem Commun. 1995:1771–1772. [Google Scholar]
- 59.Goldman M, Wehry EL. Anal Chem. 1970;42:1186–1188. [Google Scholar]
- 60.Yang W, Springsteen G, Yan J, Deeter S, Wang B. Bioorg Med Chem Lett. 2003;13:1019–1022. doi: 10.1016/s0960-894x(03)00086-6. [DOI] [PubMed] [Google Scholar]
- 61.Boduroglu S, Khoury El, Reddy JM, Rinaldi VD, Hu PL. J Bioorg Med Chem Lett. 2005;15:3974–3977. doi: 10.1016/j.bmcl.2005.05.075. [DOI] [PubMed] [Google Scholar]
- 62.Canete A, Melendrez MX, Saitz C, Zanocco AL. Synth Commun. 2001;31:2143–2148. [Google Scholar]
- 63.de Silva AP, Gunaratne HQN, Jayasekera KR, O'Callaghan S, Samankumara KRA, Sandanayake S. Chem lett. 1995:123–124. [Google Scholar]
- 64.Erdelyi M, Gogoll A. J Org Chem. 2001;66:4165–4169. doi: 10.1021/jo0057250. [DOI] [PubMed] [Google Scholar]