Abstract
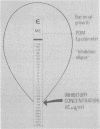
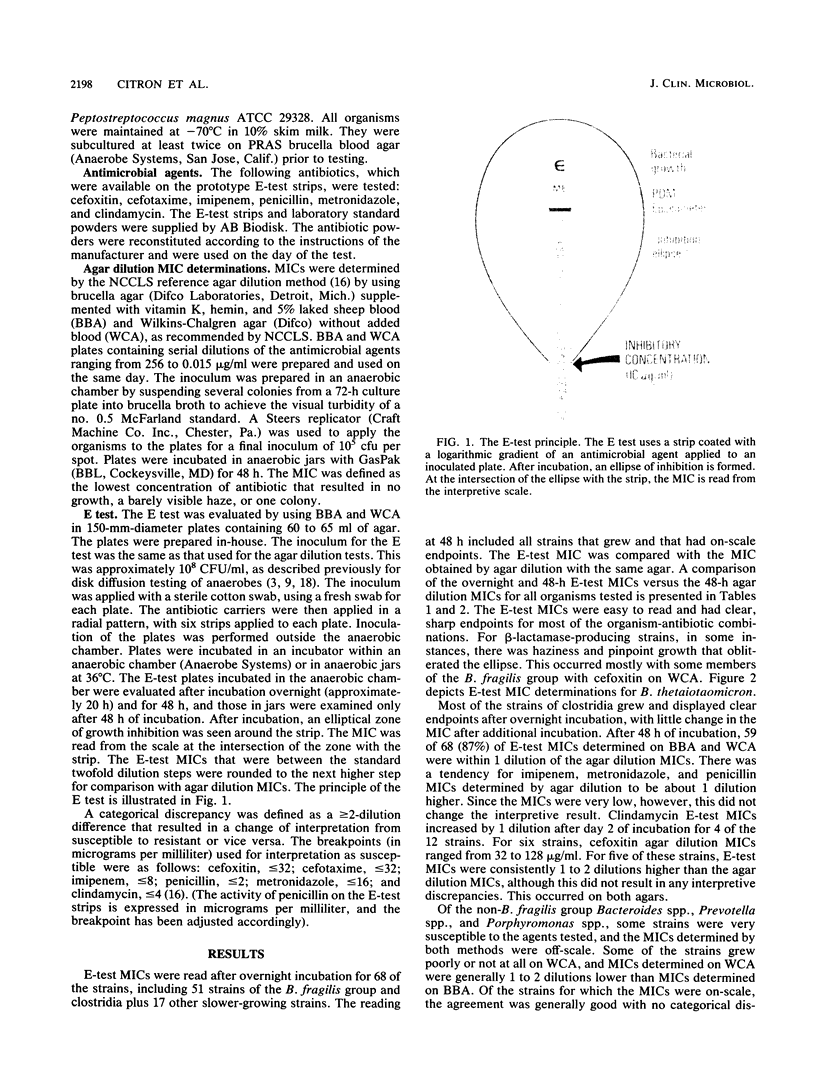
The susceptibilities of 105 clinical isolates of anaerobic bacteria were determined by a new method, the E test (AB Biodisk, Solna, Sweden), and were compared with the MICs for these organisms obtained by the reference agar dilution method by using supplemented brucella and Wilkins-Chalgren agars. The E test is a plastic strip with a predefined antibiotic gradient immobilized on one side and a MIC interpretive scale printed on the other side. Strips with cefoxitin, cefotaxime, imipenem, penicillin, metronidazole, and clindamycin were used in this study. A suspension of the test strain equal to the visual turbidity of a no. 0.5 McFarland standard was prepared and swabbed onto a 150-mm-diameter plate. The strips were applied in a radial fashion, and the plates were incubated under anaerobic conditions. After growth had occurred, an ellipse of inhibition was seen around each strip. At the point of intersection of the ellipse with the strip, the MIC was read from the interpretive scale. For most antibiotic-organism combinations, the ellipse was clear and the endpoint was sharp. The E-test MICs were interpreted after overnight and 48-h incubation for 58 of the strains. After overnight incubation, 87% of the E-test MICs were within 1 dilution of the agar dilution MICs, and 98% were within 2 dilutions. After 48 h of incubation, agreement was 86 and 97% respectively. E-test MICs obtained for the Bacteroides fragilis group after overnight incubation were more comparable than those obtained after 48 h of incubation to agar dilution MICs determined at 48 h for all drugs except clindamycin. On brucella agar, there was a 2% categorical discrepancy rate between the E-test MICs and agar dilution MICs, which occurred mostly with cefoxitin. The E test is easy to perform and read, is suitable for all anaerobes, can be used to test single patient isolates as needed, and offers the laboratory a reliable method for susceptibility testing of anaerobic bacteria.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldridge K. E., Sanders C. V. Antibiotic- and method-dependent variation in susceptibility testing results of Bacteroides fragilis group isolates. J Clin Microbiol. 1987 Dec;25(12):2317–2321. doi: 10.1128/jcm.25.12.2317-2321.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A. L., Fuchs P. C., Gerlach E. H., Allen S. D., Acar J. F., Aldridge K. E., Bourgault A. M., Grimm H., Hall G. S., Heizmann W. Multilaboratory evaluation of an agar diffusion disk susceptibility test for rapidly growing anaerobic bacteria. Rev Infect Dis. 1990 Jan-Feb;12 (Suppl 2):S210–S217. doi: 10.1093/clinids/12.supplement_2.s210. [DOI] [PubMed] [Google Scholar]
- Brazier J. S., Goldstein E. J., Citron D. M., Ostovari M. I. Fastidious anaerobe agar compared with Wilkins-Chalgren agar, brain heart infusion agar, and brucella agar for susceptibility testing of Fusobacterium species. Antimicrob Agents Chemother. 1990 Nov;34(11):2280–2282. doi: 10.1128/aac.34.11.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook I. In-vitro sensitivity tests and in-vivo efficacy of cephalosporins against Bacteroides fragilis. J Antimicrob Chemother. 1990 Jul;26(1):158–161. doi: 10.1093/jac/26.1.158. [DOI] [PubMed] [Google Scholar]
- Finegold S. M. Susceptibility testing of anaerobic bacteria. J Clin Microbiol. 1988 Jul;26(7):1253–1256. doi: 10.1128/jcm.26.7.1253-1256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold S. M., Wexler H. M. Therapeutic implications of bacteriologic findings in mixed aerobic-anaerobic infections. Antimicrob Agents Chemother. 1988 May;32(5):611–616. doi: 10.1128/aac.32.5.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Bourgault A. M., Lamothe F. Disk diffusion susceptibility testing of the Bacteroides fragilis group. Antimicrob Agents Chemother. 1987 Oct;31(10):1596–1599. doi: 10.1128/aac.31.10.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C. C., Wexler H. M., Becker S., Garcia M., Finegold S. M. Cell-wall-defective variants of Fusobacterium. Antimicrob Agents Chemother. 1989 Mar;33(3):369–372. doi: 10.1128/aac.33.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen J. H., Howell A. W., Maher L. A. Quantitative antimicrobial susceptibility testing of Haemophilus influenzae and Streptococcus pneumoniae by using the E-test. J Clin Microbiol. 1991 Jan;29(1):109–114. doi: 10.1128/jcm.29.1.109-114.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen J. H., Redding J. S., Howell A. W. Evaluation of broth disk elution methods for susceptibility testing of anaerobic bacteria with the newer beta-lactam antibiotics. J Clin Microbiol. 1986 Mar;23(3):545–550. doi: 10.1128/jcm.23.3.545-550.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzynski T. A., Yrios J. W., Helstad A. G., Field C. R. Aerobically incubated thioglycolate broth disk method for antibiotic susceptibility testing of anaerobes. Antimicrob Agents Chemother. 1976 Oct;10(4):727–732. doi: 10.1128/aac.10.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorian V. In vitro simulation of in vivo conditions: physical state of the culture medium. J Clin Microbiol. 1989 Nov;27(11):2403–2406. doi: 10.1128/jcm.27.11.2403-2406.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter V. L., Kwok Y., Finegold S. M. Susceptibility of Bacteroides fragilis to six antibiotics determined by standardized antimicrobial disc susceptibility testing. Antimicrob Agents Chemother. 1973 Feb;3(2):188–193. doi: 10.1128/aac.3.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tally F. P., Cuchural G. J., Jr, Malamy M. H. Mechanisms of resistance and resistance transfer in anaerobic bacteria: factors influencing antimicrobial therapy. Rev Infect Dis. 1984 Mar-Apr;6 (Suppl 1):S260–S269. doi: 10.1093/clinids/6.supplement_1.s260. [DOI] [PubMed] [Google Scholar]
- Wexler H. M., Reeves D., Finegold S. M. Effect of inoculum size and medium on activity of seven antimicrobial agents against Bacteroides fragilis strains. Clin Ther. 1989 Nov-Dec;11(6):828–833. [PubMed] [Google Scholar]
- Zabransky R. J., Birk R. J., Kurzynski T. A., Toohey K. L. Predicting the susceptibility of anaerobes to cefoperazone, cefotaxime, and cefoxitin with the thioglycolate broth disk procedure. J Clin Microbiol. 1986 Aug;24(2):181–185. doi: 10.1128/jcm.24.2.181-185.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]