Abstract
We describe a continuous peptide methylation assay using the Neurospora crassa Dim-5 histone H3 lysine 9 (H3K9) methyltransferase as a model system. The assay uses streptavidin FlashPlates coated with target peptide. Since no washing and pipeting steps were required after the addition of the enzyme/S-adenosyl-L-methionine (AdoMet) mixture to the microplate, a continuous readout of the reaction progress was possible. We show that this assay is highly reproducible (with errors in the order of ±3%). The continuous assay is well suited for the simultaneous analysis of up to 384 samples, thus allowing for a rapid screening of methylation rates of different substrates under different conditions or in the presence of inhibitors.
Posttranslational modification of histone tails including methylation, acetylation, or ubiquitination is an important epigenetic signal involved in gene and chromatin regulation (1,2). Histone lysine methyltransferases (HKMTs) are a large group of enzymes that specifically methylate histone tails at defined sites using S-adenosyl-L-methionine (AdoMet) as methyl group donor (3,4). Understanding the properties and mechanism of these enzymes is important, as many of them represent potential targets in cancer therapy (5). Different assays exist to determine the activity of HKMTs. Some use unlabeled AdoMet and detect the methylation of the peptide by mass spectrometry (6,7). Because of atomic mass resolution, mass spectrometric assays are very reliable and allow the distinction between mono-, di-, and trimethylated products (8). In addition, antibodies against different methylation states of lysines are used in the detection of histone methylation in fixed chromatin (9-12). Alternatively, the turnover of the coenzyme can be followed using a coupled fluorescent assay (13), in which the methyl donor product S-adenosy-L-homocysteine is enzymatically hydrolyzed to homocysteine and adenosine, and the homocysteine concentration is then determined by conjugation of its free sulfhydryl moiety to a fluorophore.
In an alternative approach, the transfer of a radioactively labeled methyl group from AdoMet to the peptide substrate can be detected. Radioactive assays need separation of modified peptide and unreacted AdoMet, which can be achieved by precipitation (14), gel electrophoresis (14-16), or using avidin/biotin technology if a biotinylated peptide is used as substrate (17). The biotin/ avidin microplate peptide methylation assay previously described by us is convenient, very accurate, reproducible, and inexpensive (17). Since it yields quantitative results, it can be used for a characterization of the enzymatic properties of HKMTs and other protein methyltransferases. Also, the assay is well suited for high-throughput applications. However, one general disadvantage of all assays mentioned above is that they are discontinuous in nature.
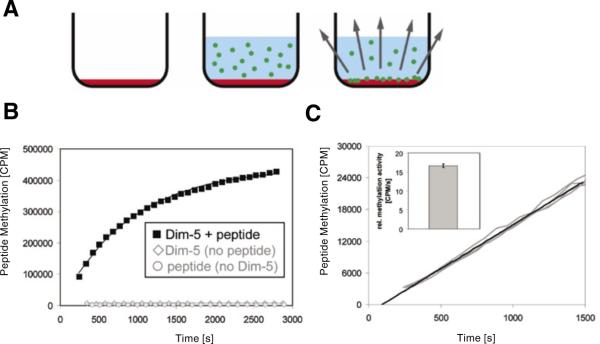
Here, we have modified the setup of the radioactive avidin/biotin microplate assay to overcome this limitation (Figure 1A). To this end, avidincoated FlashPlate streptavidin 96-well scintillant-coated microplates (Perkin Elmer, Waltham, MA, USA) were used (18). In these plates, the interior of each well is permanently coated with a thin layer of polystyrene-based scintillant followed by covalent binding of streptavidin molecules. Using a biotin tag, the peptide is immobilized at the surface of the plate. After washing off the unbound peptide, the enzyme is added in buffer containing AdoMet bearing a tritiated methyl group. Due to the short range of the β-particles emitted by tritium, this does not lead to a strong scintillation signal, because most β-particles are quenched by solvent before they reach the wall of the plate. After enzymatic transfer of the radioactive methyl groups to the peptide substrates, however, they closely approach the walls of the microplate, which leads to a strong scintillation signal (Figure 1A).
Figure 1. Experimental design.
(A) Drawing of the principle of the continuous peptide methylation assay using FlashPlates with scintillator embedded into the walls of the microplate. In the first step, the well of a FlashPlate is coated with target peptide (red shading). Then, enzyme and radioactive coenzyme are added (radioactively labeled methyl groups are depicted by green circles). The transfer of the methyl groups to the target peptide leads to a close approximation of radioactive methyl group and scintillator, which results in a scintillation signal. (B) After adding radioactive S-adenosyl-L-methionine (AdoMet) and enzyme (3.5 μM) to the well of a FlashPlate coated with 320 pmol peptide, a strong scintillation signal appeared that reflects the progress of the methylation reaction. If enzyme is omitted or if the plates are not coated with peptide, no signal change was detected. (C) Reproducibility of the assay. Four independent experiments using 3.5 nM enzyme were carried out in different wells coated with 320 pmol peptide, and the results were overlaid without any data normalization. Initial methylation rates were determined by averaging data points over the first 1500 s. As shown in the insert, the initial methylation rates determined by the slope were identical within ±3% error.
The Dim-5 histone 3 lysine 9 (H3K9) methyltransferase was expressed and purified as described (14). A synthetic peptide containing a biotin at its N terminus was purchased from IRIS Biotech (Marktredwitz, Germany) in high-performance liquid chromatography (HPLC)-purified form and was dissolved in water. Purity of the peptide was greater than 95%, as confirmed by HPLC and mass spectrometric analysis. The length of the peptide was 20 residues, corresponding to the first 19 amino acids of the histone H3 tail plus a methionine (Bt-MARTKQTARKSTGGKAPRKQ), with the target lysine residue bolded.
Substrate binding to the streptavidin surface of the FlashPlate PLUS streptavidin 96-well microplate (Perkin Elmer) was performed by pipeting 320 pmol biotinylated histone H3 tail peptide in 40 μL binding buffer [50 mM Tris-HCl, pH 8.0, 20 mM KCl, 5 mM dithiothreitol (DTT), 1 mM EDTA] in each well of the streptavidin-coated plate. After 8 h incubation at 4°C, the wells were washed five times with PBST (500 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM K2HP04, 0.05% v/v Tween 50, pH 7.2). Afterwards, 60 μL reaction mixture [50 mM glycine, pH 9.8, 2 mM DTT, 25 μg/mL bovine serum albumin (BSA), 10% glycerol] containing enzyme concentration as indicated and 0.35 μM tritium-labeled AdoMet (specific activity: 2.03-3.15 TBq/mmol; Perkin Elmer) were added. For readout, a radioactive microplate reader (Topcount NXT; Perkin Elmer) was used. The radioactive signal was detected and averaged for 10 s, and after each reading the counts and absolute time was written in the report file. From that file, data were extracted and rearranged manually using Excel or using an in-house program. For calibration, the peptide methylation reaction was carried out using 35 nM Dim-5. The reaction progress curve was fitted to a monoexponential curve to determine the maximum signal that corresponds to the methylation of the 320 pmol peptide. Experiments carried out with larger amounts of peptide (up to 600 pmol/well) showed a linear response of total methylation and signal, indicating that the 320 pmol used for most of the experiments was below the peptide binding capacity of the well.
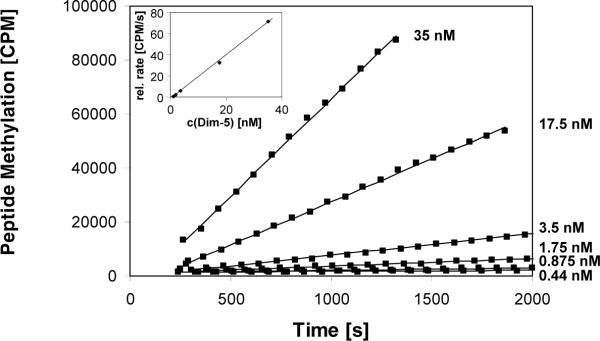
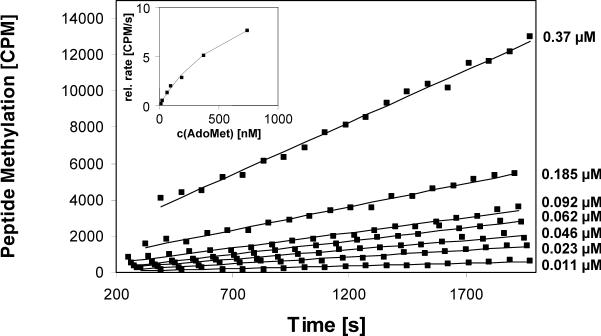
To demonstrate the principal feasibility of the assay, we used the Dim-5 histone lysine methyltransferase from Neurospora crassa, which trimethylates H3K9 in a processive reaction (8). After adding 3.5 μM enzyme, 0.35 μM radioactive cofactor and buffer into FlashPlates coated with peptide, a rapid and very strong increase in scintillation signal was observed (Figure 1B). No signal change was detectable in the absence of enzyme or peptide (Figure 1B). To investigate the reliability of the assay, four identical reactions containing 3.5 nM enzyme and 0.35 μM AdoMet were performed under multiple turnover conditions (using 1000 times less enzyme than before, to have a ratio of 320 pmol peptide and 0.2 pmol enzyme). During the initial phase, the methylation signal increased linearly with time (Figure 1C). The primary data of all four curves were readily superimposable. We determined the initial rate of the individual reactions by linear regression and found the standard error was smaller than ±3%, indicating excellent reproducibility. As expected for multiple turnover experiments, methylation rates increased linearly with enzyme concentration with very small error margins (Figure 2). We used the assay to determine the Michaelis constant (Km) value of the Dim-5 for AdoMet by varying the cofactor concentrations between 11 and 370 nM (Figure 3) using 3.5 nM enzyme. We measured a Km of 0.68 (-0.16, +0.25) μM and a maximal velocity (Vmax) of 14.7 (-2.1, +3.1) counts per minute (cpm)/s. The calibration curve shown in Figure 1B was used to calculate a turnover number (kcat) of 3.1/min, which is slightly higher than a value determined previously with the discontinuous assay, by varying the peptide concentration at a constant concentration of AdoMet (17). The error margins of the Km and Kcat values were determined by systematic simulations, in which the values of Km and Kcat were maximized and minimized by variation of the other parameter. The distributions of the squared deviations between experimental and theoretical data points were compared between the optimum fit and the simulation, and the values of Km and Kcat changed as long as the distribution of squared deviations in the simulation did not differ from the best fit to a statistically significant degree (P value > 0.05 as analyzed by Excel GTest). The assay cannot be used to determine the Km for the peptide.
Figure 2. Validation of the assay.
The continuous peptide methylation assay responds linearly with respect to enzyme concentration. Peptide methylation experiments were carried out at different enzyme concentrations ranging from 0.44 to 35 nΜ in wells coated with 320 pmol peptide. Initial slopes were determined and plotted against the enzyme concentration (insert). The standard error of the initial slopes of the linear regressions, as determined using Excel Analysis Functions at 95% confidence interval, was smaller than 10% in each case. The regression line of the secondary plot had an R value of 0.9993, and its slope has a standard error of ±2%.
Figure 3. Application of the continuous peptide methylation assay to determine the Michaelis constant (Km) value of Dim-5 for S-adenosyl-l-methionine (AdoMet).
Peptide methylation experiments were performed at different AdoMet concentrations ranging from 11 to 370 nM. The wells were coated with 320 pmol peptide, and the concentration of Dim-5 was 3.5 nM. Initial slopes were determined, plotted against the AdoMet concentration, and the data analyzed with respect to Km and maximal velocity (Vmax) values (insert).
In summary, the peptide methylation assay developed here is very accurate, since it yields continuous data that allow for stable averaging. The essential advantage of this assay is that, after addition of enzyme and AdoMet to the peptide-coated microplate, no washing or pipeting steps are required, allowing a continuous data collection. One inherent advantage of continuous assays is their high precision, due to the option of determining initial slopes from very many data points and due to the absence of noise introduced during downstream sample processing steps. The limited number of handling steps makes the assay easy to perform and suitable to collect accurate data for a large number of different substrates, under many different conditions, or in presence of different inhibitors. Using the counting parameters set here (counting each well for 10 s for each data point), counting one whole 96-well plate takes approximately 10 min, such that the assay is able to provide accurate, semi-continuous methylation data (with a 10-min interval between the data points) for up to 96 different samples. There are also 384-well FlashPlates available that enables the screening of 384 different samples at once. Therefore, this assay is ideal to characterize the enzymatic properties of different protein methyltransferase variants with different substrates or to perform mediumthroughput inhibitor screening.
ACKNOWLEDGMENTS
This work has been supported by the Federal Ministry of Education and Research (BMBF) BioFuture program and National Institutes of Health (NIH) grant no. GM068680. Thanks are due to Ms. M. Schwerdtfeger for technical assistance and Dr. R. Edler (Perkin Elmer) for technical advice.
Footnotes
COMPETING INTERESTS STATEMENT The authors declare no competing interests.
REFERENCES
- 1.Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- 2.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Martin C, Zhang Y. The diverse functions of histone lysine methylation. Nat. Rev. Mol. Cell Biol. 2005;6:838–849. doi: 10.1038/nrm1761. [DOI] [PubMed] [Google Scholar]
- 4.Cheng X, Collins RE, Zhang X. Structural and sequence motifs of protein (histone) methylation enzymes. Annu. Rev. Biophys. Biomol. Struct. 2005;34:267–294. doi: 10.1146/annurev.biophys.34.040204.144452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kubicek S, O'Sullivan RJ, August EM, Hickey ER, Zhang Q, Teodoro ML, Rea S, Mechtler K, et al. Reversal of H3K9me2 by a small-molecule inhibitor for the G9a histone methyltransferase. Mol. Cell. 2007;25:473–481. doi: 10.1016/j.molcel.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Bonaldi T, Regula JT, Imhof A. The use of mass spectrometry for the analysis of histone modifications. Methods Enzymol. 2004;377:111–130. doi: 10.1016/S0076-6879(03)77006-2. [DOI] [PubMed] [Google Scholar]
- 7.Bonaldi T, Imhof A, Regula JT. A combination of different mass spectroscopic techniques for the analysis of dynamic changes of histone modifications. Proteomics. 2004;4:1382–1396. doi: 10.1002/pmic.200300743. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Yang Z, Khan SI, Horton JR, Tamaru H, Selker EU, Cheng X. Structural basis for the product specificity of histone lysine methyltransferases. Mol. Cell. 2003;12:177–185. doi: 10.1016/s1097-2765(03)00224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters AH, Kubicek S, Mechtler K, O'Sullivan RJ, Derijck AA, Perez-Burgos L, Kohlmaier A, Opravil S, et al. Partitioning and plasticity of repressive histone methylation states in mammalian chromatin. Mol. Cell. 2003;12:1577–1589. doi: 10.1016/s1097-2765(03)00477-5. [DOI] [PubMed] [Google Scholar]
- 10.Sarma K, Nishioka K, Reinberg D. Tips in analyzing antibodies directed against specific histone tail modifications. Methods Enzymol. 2004;376:255–269. doi: 10.1016/S0076-6879(03)76017-0. [DOI] [PubMed] [Google Scholar]
- 11.Perez-Burgos L, Peters AH, Opravil S, Kauer M, Mechtler K, Jenuwein T. Generation and characterization of methyl-lysine histone antibodies. Methods Enzymol. 2004;376:234–254. doi: 10.1016/S0076-6879(03)76016-9. [DOI] [PubMed] [Google Scholar]
- 12.Schotta G, Lachner M, Sarma K, Ebert A, Sengupta R, Reuter G, Reinberg D, Jenuwein T. A silencing pathway to induce H3-K9 and H4-K20 trimethylation at constitutive heterochromatin. Genes Dev. 2004;18:1251–1262. doi: 10.1101/gad.300704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collazo E, Couture JF, Bulfer S, Trievel RC. A coupled fluorescent assay for histone methyltransferases. Anal. Biochem. 2005;342:86–92. doi: 10.1016/j.ab.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Tamaru H, Khan SI, Horton JR, Keefe LJ, Selker EU, Cheng X. Structure of the Neurospora SET domain protein DIM-5, a histone H3 lysine methyltransferase. Cell. 2002;111:117–127. doi: 10.1016/s0092-8674(02)00999-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strahl BD, Ohba R, Cook RG, Allis CD. Methylation of histone H3 at lysine 4 is highly conserved and correlates with transcriptionally active nuclei in Tetrahymena. Proc. Natl. Acad. Sci. USA. 1999;96:14967–14972. doi: 10.1073/pnas.96.26.14967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Couture JF, Collazo E, Hauk G, Trievel RC. Structural basis for the methylation site specificity of SET7/9. Nat. Struct. Mol. Biol. 2006;13:140–146. doi: 10.1038/nsmb1045. [DOI] [PubMed] [Google Scholar]
- 17.Gowher H, Zhang X, Cheng X, Jeltsch A. Avidin plate assay system for enzymatic characterization of a histone lysine methyltransferase. Anal. Biochem. 2005;342:287–291. doi: 10.1016/j.ab.2005.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown BA, Cain M, Broadbent J, Tompkins S, Henrich G, Joseph R, Casto S, Harney H, et al. FlashPlate technology. In: Devlin JP, editor. High Throughput Screening. Dekker; New York: 1997. pp. 317–328. [Google Scholar]