Abstract
The PTEN tumor suppressor gene modulates cell growth and survival known to be regulated by the activation of the transcription factor NF-κB, suggesting PTEN might affect the NF-κB activation pathway. We found that PTEN inhibited NF-κB activation induced by TNF. The suppression of NF-κB activation correlated with sequential inhibition of the tumor necrosis factor-induced expression of NF-κB-regulated anti-apoptotic (IAP1, IAP2, Bcl-2, Bcl-xL, cFLIP, Bfl-1/A1, and survivin) gene products. Downregulation of the antiapoptotic genes by PTEN increased TNF-induced apoptosis, as indicated by caspase activation, TUNEL, annexin staining, and estrase assay. We conclude that the ectopic expression of PTEN enhances TNF-induced apoptosis and downregulates the proliferation of glioma cells through the suppression of various molecules including NFκB, and various mediators of cellular survival and proliferation and that this targets might be essential for its central role in the growth and survival of glioma cancer cells.
Keywords: PTEN, TNF-α, NFκB, apoptosis
Introduction
Gliomas remain one of the human tumors most refractory to treatment, despite continuing advances in radiotherapy, chemotherapy, and surgical techniques. The most malignant subtype, glioblastoma multiforme, is particularly aggressive, with a median survival of less than 1 year seen in most series of patients, even for patients treated with aggressive surgery, radiotherapy, and chemotherapy regimens (1–3). Consequently, various alternate approaches to the treatment of gliomas, including molecular therapies are attracting much interest. Resistance of tumor cells to the induction of apoptosis is an important reason for the failure of anticancer treatments in patients with gliomas, and several factors working in concert have been implicated as sources of this treatment resistance. One factor that has frequently been implicated in the development of tumor cell resistance to apoptosis is nuclear factor kappa B (NFκB). NFκB regulates the expression of many genes that are essential for cell growth and differentiation, such as cytokines, growth factors and their receptors, and adhesion molecules (4–8). It also up regulates the expression of genes that actively participate in controlling cell survival (Bcl-2, Bcl-xL, survivin, and the inhibitor-of-apoptosis [IAP] family) (9–11), angiogenesis (interleukin (IL)-8, basic fibroblast growth factor, and vascular endothelial growth factor) (12,13); and metastasis (matrix metalloproteases [MMPs] 2, 7, and 9) (14). Consistent with its regulatory role, the constitutive activation of NFκB is frequently observed in different types of cancers (15) and has been correlated with cancer cell resistance to radiation- and chemotherapeutic agent-induced apoptosis (5). Conversely, the antagonism of NFκB is an important step in initiating the transcription of specific genes that facilitate apoptosis.
Natural resistance to tumor necrosis factor (TNF)-α̣ is another cause of treatment failure in patients with gliomas. TNF-α̣was first observed in the serum of endotoxin-treated mice,in which it was found to induce tumor necrosis in vivo and to selectively kill transformed and neoplastic cell lines in vitro (16). TNF-α signaling is transduced through its receptors to simultaneously elicit two opposing effects: the induction of apoptosis and the transcription of antiapoptotic genes, such as the genes that encode NFκB and activator protein 1 (AP-1) (17,18). Although in certain cell types and under certain conditions, TNF-α̣ can induce apoptosis, its clinical use has been limited because of the natural resistance of numerous tumor cells to TNF-induced apoptosis noted above (19–21).
PTEN is a tumor suppressor gene inactivated in many common malignancies, including glioblastoma, melanoma, and endometrial, lung, and prostate cancer (22–26). PTEN is believed to regulate cell survival signaling through the phosphatidylinositol 3-kinase (PI3K)/Akt pathway. In particular, PTEN dephosphorylates the D3 position of the key lipid second messenger phosphatidylinositol 3,4,5-triphosphate (PIP3) (27–28). PIP3, produced by PI3K once activated by receptor tyrosine kinases, activates Ras, or G proteins, and stimulates several downstream targets, including the serine/threonine protein kinase Akt (also known as protein kinase B) (22–26). Activated Akt protects cells from apoptotic death by phosphorylating substrates such as BAD, procaspase-9, and forkhead transcription family members (29–31). Finally, multiple laboratories have shown that the PI3K/Akt pathway provides cell survival signals, in part, through the activation of the NFκB transcription factor (32–35).
To better understand the role of PTEN in the resistance of glioma cells to apoptotic agents and to formulate potential therapies that alter PTEN expression, it is important to obtain greater insight into the effect of PTEN expression on cellular processes. The role of TNF-α̣ in activating NFκB has been well established. In this study, we tested our hypothesis that PTEN mediates its effects by modulating NFκB and enhanced TNF-mediated apoptosis in glioma cells, which confirmed our hypothesis.
Materials and Methods
Materials
Tissue culture reagents and Lipofectamine were purchased from Invitrogen Life Technologies Inc. (Carlsbad, CA). Anti-PTEN, anti-p50, anti-p65, anti-poly (ADP-ribose) polymerase (PARP), anti-inhibitor of apoptosis protein 1 (IAP1), anti-IAP2, anti-Bcl-2, anti-Bcl-xL, and anti-Bfl-1/A1 antibodies were obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Anti-β-actin antibody was purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO).
Cell Culture and Retroviral Gene Construction and Stable Transfections
U251 and U87 human glioblastoma cells (American Type Culture Collection, Manassas, VA), which have been shown previously to have a mutated PTEN gene (36), were maintained in culture medium (Dulbecco’s modified Eagle medium/F12, 5% fetal bovine serum) in a humidified atmosphere containing 5% CO2 at 37° C. The PTEN gene was stably expressed in U251 and U87 glioma cells as previously described (37).
Electrophoretic Mobility Shift Assay
U251 and U87 cells either expressing PTEN or vector alone were treated with 1 nM TNF for the indicated times (37) and incubated for 15 min at room temperature with radiolabeled NFκB-binding probe. For the supershift assays anti-p-50 and anti-p-65 antibodies were added to the incubation mixtures for 5 min before the radiolabeled probe was added. The protein-DNA complexes were then resolved on 5% nondenaturing polyacrylamide gels and visualized by autoradiography.
Immunoblotting
Cells were washed with ice-cold phosphate-buffered saline and lysed in ice-cold lysis buffer containing 1% Triton X-100, 50mM HEPES, pH 7.4, 150mM MgCl2, 1mM EGTA, 100mM NaF, 10mM Na-pyrophosphate, 1mM Na3VO4, 10% glycerol, 1mM phenylmethyl sulfonyl fluoride, and 10 ug/ml aprotinin. Proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), electroblotted to poly vinylidene difluoride membranes (Millipore, Billerica, MA), and then probed with various primary antibodies. After incubation with horseradish peroxidase–conjugated secondary antibodies, specific proteins were detected by chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ).
Live/Dead assay
To measure the effect of PTEN on TNF-induced apoptosis, we performed the Live/Dead assay (Molecular Probes), which determines intracellular esterase activity and plasma membrane integrity. This assay employs calcein, a polyanionic dye, which is retained within live cells and produces green fluorescence. It also employs the ethidium bromide homodimer dye (red fluorescence), which can enter the cells through damaged membranes and bind to nucleic acids but is excluded by the intact plasma membrane of live cells. Briefly, 1 x 105 cells were treated with 1 nM TNF for 16 h at 37 °C. Cells were stained with the Live/Dead reagent (5 μM ethidium bromide homodimer, 5 μM calcein-AM) and then incubated at 37° C for 30 min. Cells were analyzed under a fluorescence microscope (Labophot-2).
Annexin V Assay
An early indicator of apoptosis is the rapid translocation and accumulation of the membrane phospholipid phosphatidylserine from the cytoplasmic interface to the extracellular surface. This loss of membrane asymmetry can be detected by exploiting the binding properties of annexin V. In our quantification of cell apoptosis, we employed the annexin V antibody conjugated with FITC fluorescence dye. Briefly, 1 x 105 cells were treated with 1 nM TNF͂α for 16 h at 37 °C and then subjected to annexin V staining. Cells were washed in phosphate-buffered saline, resuspended in 100 μl of binding buffer containing FITC-conjugated anti-annexin V antibody (Santa Cruz Biotechnology), and then analyzed by flow cytometry (FACSCaliber; BD Bio-sciences, San Diego, CA).
TUNEL Assay
We also assayed cytotoxicity using the terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end-labeling (TUNEL) method, which examines DNA strand breaks during apoptosis, in which we used the Roche in situ cell death detection reagent (Roche Applied Science, Indianapolis, IN). Briefly, 1 x 105 cells were treated with 1nM TNF for 16 h at 37 °C. Thereafter, cells were washed with phosphate-buffered saline, air-dried, fixed with 4% paraformaldehyde, and then permeabilized with 0.1% Triton-X 100 in 0.1% sodium citrate. After being washed, cells were incubated with reaction mixture for 60 min at 37°C. Stained cells were mounted with mounting medium purchased from Sigma Chemical and analyzed under a fluorescence microscope (Labophot-2; Nikon, Tokyo, Japan). Pictures were captured using a Photometrics Coolsnap CF color camera (Nikon, Lewisville, TX) and MetaMorph version 4.6.5 software (Universal Imaging, Downingtown, PA).
Results
PTEN Inhibits the Induction of NFκB Activation by TNF
To determine the effect of PTEN on TNF-induced NF-B activation,we examined if TNF-α would activate NFκB in an electrophoretic mobility shift assay. Treatment of cells with 0.1nM TNF-α led to NFκB activation in a time-dependent manner in both cell lines (Fig. 1A and 1B). Further, the protein-DNA complex was supershifted by p50 and p65 antibody during NFκB activation (Fig. 1C). PTEN expression in U251 and U87 glioma cell lines significantly inhibited TNF’s ability to activate NFκB, revealing PTEN as a putative primary player in the regulation of the DNA-binding activity of NFκB.
Fig. 1. Effect of PTEN on TNF-induced NFκB activity.
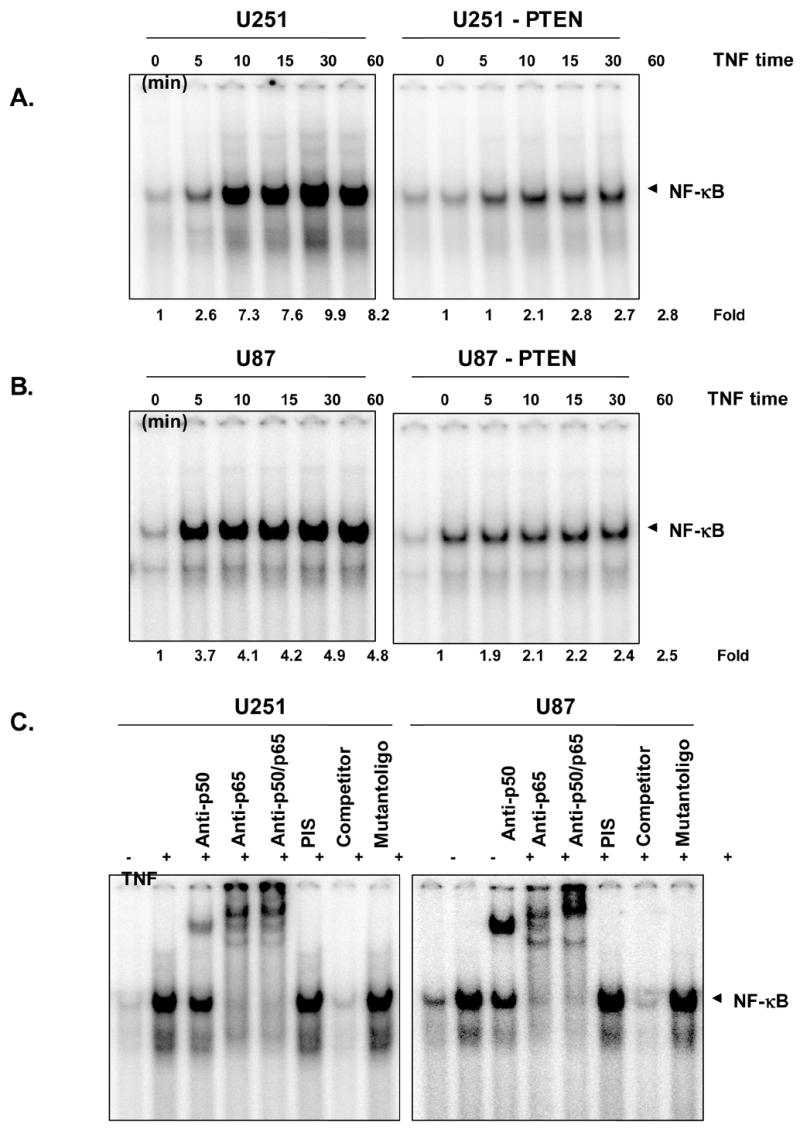
A, U251/U251-PTEN and B, U87/U87-PTEN cells were treated with TNF for various times before harvesting. Nuclear extracts were prepared and assayed for NFκB activation by an electrophoretic mobility shift assay, as described in Materials and Methods. C, for the supershift assays, antibodies was added to the incubation mixtures before the probe. These data are representative of three independent experiments.
PTEN Potentiates TNF-induced Apoptosis
Activation of NFκB by TNF and other chemotherapeutic agents leads to resistance to apoptosis (39–41) so we first determined the potential of PTEN to enhance apoptosis induced by TNF using the live and dead assay, 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide. The Live and Dead assay (which measures intracellular esterase activity and plasma membrane integrity) showed that PTEN up-regulated TNF-α induced cytotoxicity, in that there was an increase in the proportion of cells showing cytotoxicity from 1% to 9% for U251 cells and from 1% to 11% for U87 cells. When PTEN and TNF-α were used together, the proportion of U251 cells showing cytotoxicity increased from 2% to 26% and the proportion of U87 cells showing cytotoxicity increased from 1% to 34% (Fig. 2A). Whether this increased cytotoxicity was due to apoptosis was investigated by Annexin V staining technique. Annexin V staining indicated that PTEN up-regulated TNF-induced early apoptosis ((Fig. 2B ). TNF-α induced apoptosis is affected when PTEN suppresses NFκB. That is, stimulation with TNF-α alone did not induce a greater proportion of annexin V–positive U251 and U87 cells, but PTEN expression dramatically enhanced the proportion of apoptotic U251 cells, from 3.1% to 28.6%, and of U87 cells, from 4.8% to 30.4% (Fig. 2B). PTEN by itself also had little effect on the induction of annexin V–positive cells.
Fig. 2. PTEN sensitizes cells to TNF-induced apoptosis.
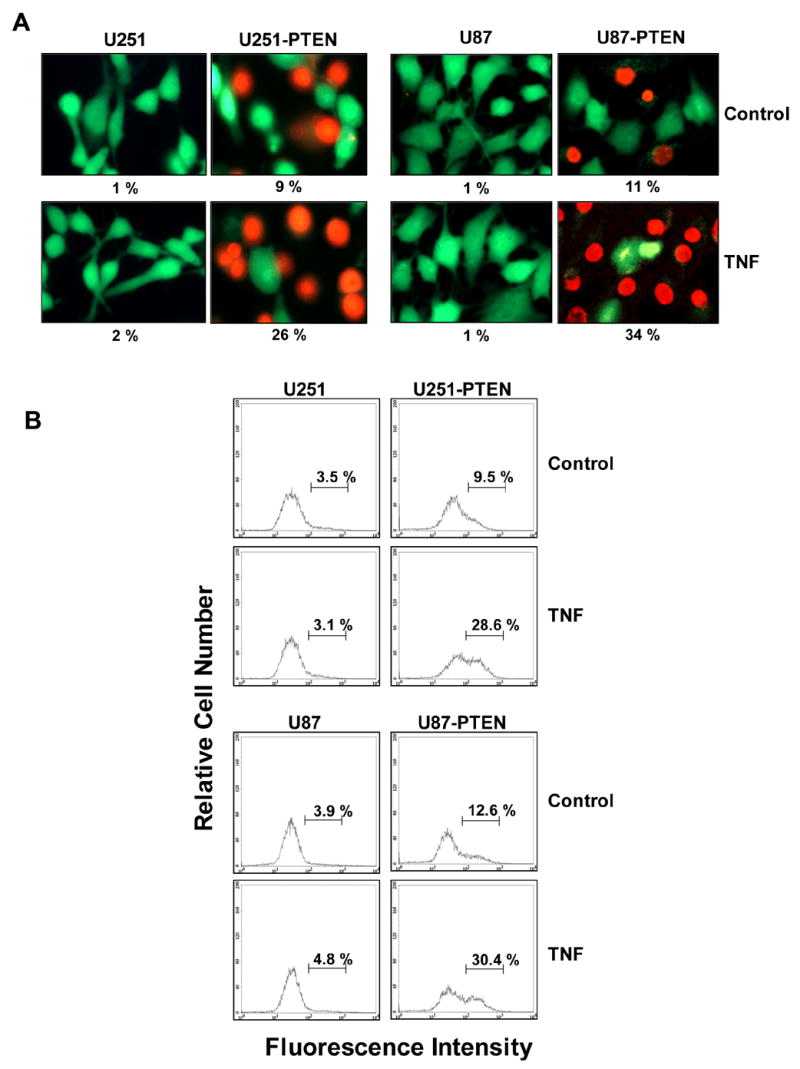
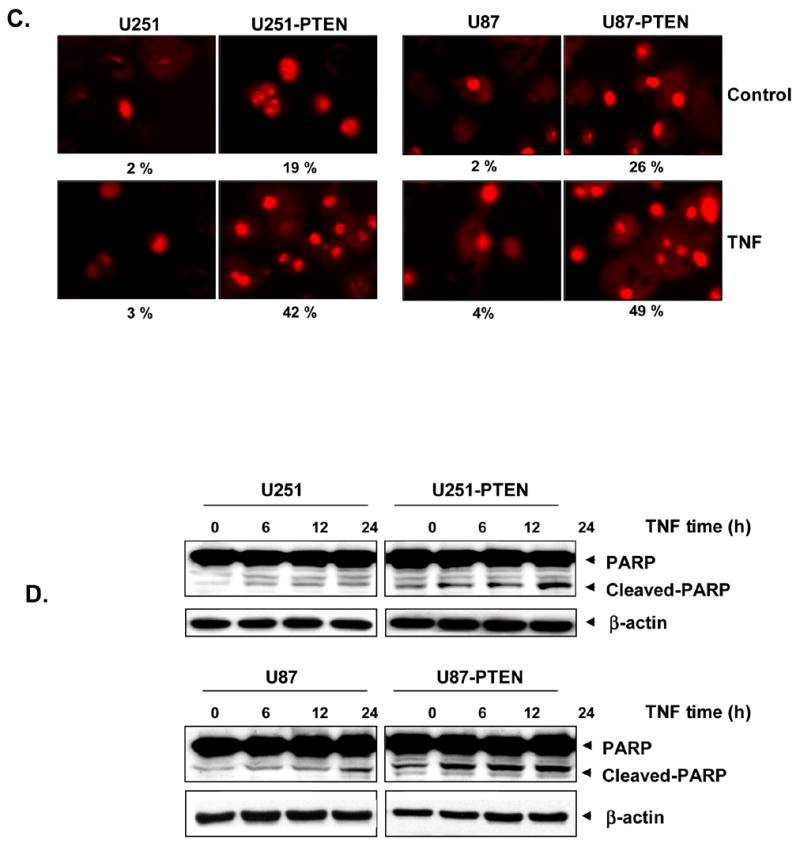
A, U251/U251-PTEN and U87/U87-PTEN cells (1 x 105 cells/ml) were treated with 1 nM TNF for 16 h. Cells were stained with the Live/Dead reagent for 30 min. Cells were analyzed under a fluorescence microscope. B, U251/U251-PTEN and U87/U87-PTEN cells were treated with 1 nM TNF for 16 h and then underwent annexin V staining. Cells were washed, incubated with FITC-conjugated anti-annexin V antibody, and then analyzed by flow cytometry. C, U251/U251-PTEN and, U87/U87-PTEN cells (1 x 105 cells/well) were treated with 1 nM TNF for 16 h. Cells were washed with phosphate-buffered saline, air-dried, fixed, permeabilized, and then stained with TUNEL assay reagent, after which they were analyzed under a fluorescence microscope. D, U251/U251-PTEN and, U87/U87-PTEN cells (5 x 105 cells/well) were treated with 1 nM TNF for the times indicated, and whole-cell lysates were subjected to SDS-PAGE. Western blot analysis was performed using anti-PARP antibody. β-actin was blotted as a loading control.
TUNEL staining confirmed that TNF-induced apoptosis was enhanced by PTEN expression (Fig. 2C). This assay showed that PTEN upregulated TNF-α induced apoptosis such that the proportion of apoptotic U251 cells increased from 2% to 20% and the proportion of apoptotic U87 cells increased from 1% to 34% When PTEN and TNF-α were used together, the proportions of apoptotic U25l and U87 cells increased from 3% to 42% and from 4% to 49%, respectively (Fig. 2C).
Effect on apoptotic proteins
Immunoblot analysis of the extracts from cells treated with TNF clearly showed activation of the downstream caspases leading to cleavage of the 118 kDa PARP protein into an 87 kDa fragment in PTEN expressing cells, another hallmark of cells undergoing apoptosis (Figure 2D). Where as control cells did not show any PARP cleavage in response to TNF treatment. The combined results from this study therefore showed that PTEN enhances TNF-α induced apoptotic effects.
PTEN Down-modulated TNF-induced NF-κB-dependent Antiapoptotic Gene Expression
Because NF-κB regulates the expression of the anti-apoptotic proteins such as IAP1/2 (42, 43), Bcl-2 (44), Bcl-xL (45), cFLIP (46), Bfl-1/A1 (47), and survivin (48), we examined whether PTEN can modulate the expression of these anti-apoptotic gene products induced by TNF in U251 and U87 cells. The results of western blot analysis showed that TNF induced the expression of most of the anti-apoptotic genes studied in glioma cells and the expression of PTEN in these cells suppressed the expression of these anti-apoptotic proteins (Fig. 3A and 3B).
Fig. 3. PTEN inhibits the TNF-induced expression of NF-κB -dependent genes.
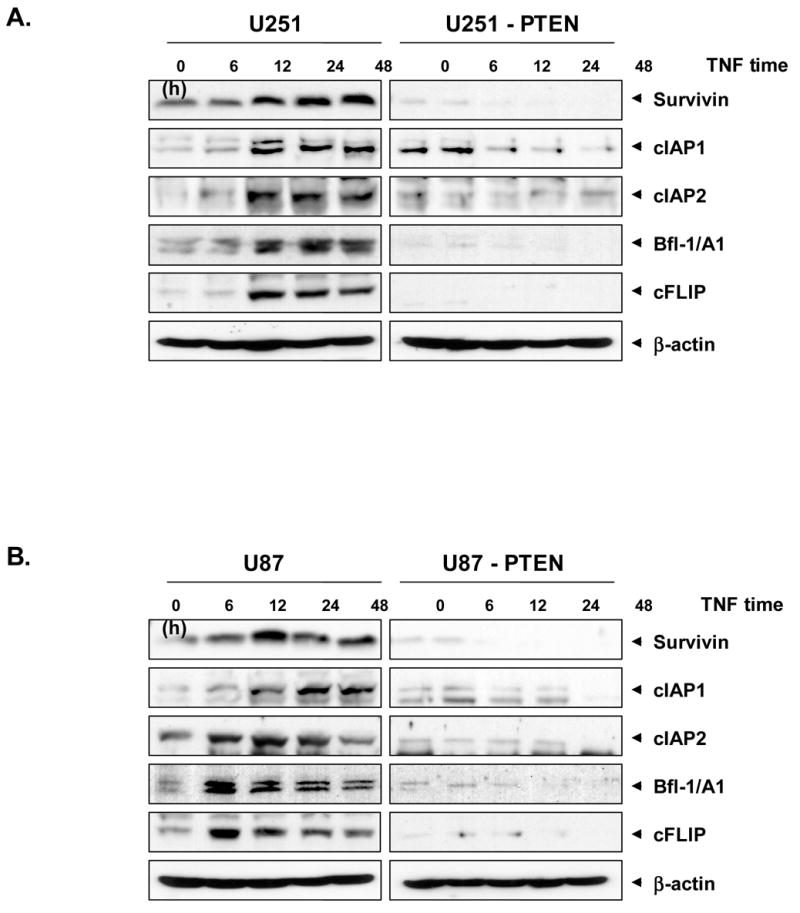
U251/U251 –PTEN (A) and U87/U87-PTEN) (B) cells were treated with 1 nM TNF for the times indicated. Whole-cell extracts were prepared and analyzed in Western blots using antibodies against IAP1/2, cFLIP, Bfl-1/A1 and survivin. As shown, PTEN inhibited the NFκB-dependent gene expression induced by TNF in U251 and U87 cells.
Discussion
The present study showed that PTEN targets the antiapoptotic gene NFκB induced by TNF-α by suppressing several TNF-induced antiapoptotic gene products, thereby confirming our hypothesis that PTEN mediates its effects by modulating NFκB and identifying a function of PTEN in its role as a tumor suppressor. Because NFκB is a transcriptional activator and its antiapoptotic function is mediated by the induction of several genes (49), PTEN’s suppression of NFκB in order to induce apoptosis by TNF-α is a unique finding that suggests a broader regulatory role for PTEN than has previously been anticipated.
In human glioma cell lines, NFκB is constitutively activated and confers resistance to TNF-induced apoptosis. (50–51). Conversely, inhibition of NFκB activation prevents cell cycle progression and inhibits the growth of U251 glioma cells treated with TNF in a PI3K/Akt pathway–dependent manner (52–54). PTEN is a crucial phosphatase involved in the regulation of Akt phosphorylation, in that the presence of an active PTEN protein blocks Akt phosphorylation by the dephosphorylation of the PI3K product PIP3 (25). In an effort to make glioma cells sensitive to TNF-induced apoptosis and block NFκB activation as well as to inhibit proliferation, we therefore used PTEN as a negative regulator of the PI3K/Akt/NFκB pathway. In this way, we showed that PTEN could regulate the DNA-binding activity of NFκB, as evidenced by TNF’s failure to activate NFκB in PTEN-expressing U251 and U87 glioma cell lines.
Numerous studies have shown that the activation of NFκB causes cell death pathways to be blocked (55). Further, NFκB must be activated to protect cells from the apoptotic cascade induced by TNF̃α and other stimuli (56–60). In particular, TNF̃α binding to the TNF receptor (TNFR) can initiate apoptosis and activate the NFκB transcription factor, which in turn suppresses apoptosis by an unknown mechanism. One clue to this was the finding that NFκB activation blocks the activation of caspase-8, a cell death executing protein. In addition, the stimulation of cells with TNF̃α, a potent inflammatory cytokine, generates two types of signals: one that initiates programmed cell death (61) and one that leads to activation of the NFκB transcription factor (62), which subsequently activates the inflammatory response. How specific cell types fare in the face of this differential activity depends upon the balance between the two signals. For example, the direct inhibition of NFκB or one of the upstream signaling moieties during TNF̃α activation results in apoptosis in various cell types that are naturally resistant to TNF-induced apoptosis. Similarly, fibroblasts and macrophages from NFκB subunit p65–deficient mice are more sensitive to TNF-induced apoptosis. Logically it follows from this that activating NFκB induces the expression of genes that counteract apoptotic signals and prevent cell death. Other antiapoptotic genes have been shown to be activated by NFκB, including the Bcl-2 homologues A1/Bfl-1, Bcl-xL, IEX-1, and XIAP.
In this study we used PTEN to counteract the NFκB activation by TNF-α via the PI3K/Akt pathway, as reported earlier by our group (63), to subsequently inhibit the expression of several antiapoptotic gene products. The expression of PTEN inhibited the expression of these antiapoptotic genes and sensitized the glioma cells to TNF-induced apoptosis. Therefore, the antagonism of NFκB by PTEN in TNF-resistant cells sensitized cells to TNF-induced apoptosis, which is important to increasing the efficacy of the chemotherapy against malignant gliomas. In summary, we have provided evidence that PTEN expression targets the transcription factors NFκ-B and that this target might be essential for its central role in the growth and survival of glioma cancer cells. In view of the relative inability of standard therapies to successfully eradicate malignant gliomas, the hope for cure ultimately rests totally or partially on identifying such targets for therapy.
Acknowledgments
This work was supported by grants from the University Cancer Foundation at The University of Texas M. D. Anderson Cancer Center to D.K. grant R01 CA–056041 from the National Institute of Health to WKA Yung and a grant from the Gilland Foundation to W. K. A. Yung. We thank Betty P. Notzon (Department of Scientific publications, M. D. Anderson) for editorial assistance, Dr. John F. de Groot (Department of Neuro-Oncology, M. D. Anderson) for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Walker MD, Alexander E, Hunt WE. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas: A cooperative clinical trial. J Neurosurg. 1978;49:333–343. doi: 10.3171/jns.1978.49.3.0333. [DOI] [PubMed] [Google Scholar]
- 2.Walker MD, Green SB, Byar DP. TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. N Engl J Med. 1980;303:1323–1329. [Google Scholar]
- 3.Davis FG, McCarthy BJ, Freels S. The conditional probability of survival of patients with primary malignant brain tumors: surveillance, epidemiology, and end results (SEER) data. Cancer. 1999;85:85–491. [PubMed] [Google Scholar]
- 4.Huang S, DeGuzman A, Bucana CD, Fidler IJ. Nuclear factor-kappaB activity correlates with growth, angiogenesis, and metastasis of human melanoma cells in nude mice. Clin Cancer Res. 2000;6:2573–2581. [PubMed] [Google Scholar]
- 5.Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 6.Gilmore TD. The Re1/NF-kappa B/I kappa B signal transduction pathway and cancer. Cancer Treat Res. 2003;115:241–265. [PubMed] [Google Scholar]
- 7.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 8.Boone DL, Lee EG, Libby S, Gibson PJ, Chien M, Chan F, Madonia M, Burkett PR, Ma A. Recent advances in understanding NF-kappaB regulation. Inflamm Bowel Dis. 2002;8:201–212. doi: 10.1097/00054725-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Chu ZL, McKinsey TA, Liu L, Gentry JJ, Malim MH, Ballard DW. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-kappaB control. Proc Natl Acad Sci U S A. 1997;94:10057–10062. doi: 10.1073/pnas.94.19.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.You M, Ku PT, Hrdlickova R, Bose HR., Jr ch-IAP1, a member of the inhibitor-of-apoptosis protein family, is a mediator of the antiapoptotic activity of the v-Rel oncoprotein. Mol Cell Biol. 1997;17:7328–7341. doi: 10.1128/mcb.17.12.7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stehlik C, de Martin R, Kumabashiri I, Schmid JA, Binder BR, Lipp J. Nuclear factor (NF)-kappaB-regulated X-chromosome-linked iap gene expression protects endothelial cells from tumor necrosis factor alpha-induced apoptosis. J Exp Med. 1998;188:211–216. doi: 10.1084/jem.188.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang S, Robinson JB, Deguzman A, Bucana CD, Fidler IJ. Blockade of nuclear factor-kappaB signaling inhibits angiogenesis and tumorigenicity of human ovarian cancer cells by suppressing expression of vascular endothelial growth factor and interleukin 8. Cancer Res. 2000;60:5334–5339. [PubMed] [Google Scholar]
- 13.Shibata A, Nagaya T, Imai T, Funahashi H, Nakao A, Seo H. Inhibition of NF-kappaB activity decreases the VEGF mRNA expression in MDA-MB-231 breast cancer cells. Breast Cancer Res Treat. 2002;73:237–24352. doi: 10.1023/a:1015872531675. [DOI] [PubMed] [Google Scholar]
- 14.Rabbani SA, Harakidas P, Guo Y, Steinman D, Davidsen SK, Morgan DW. Synthetic inhibitor of matrix metalloproteases decreases tumor growth and metastases in a syngeneic model of rat prostate cancer in vivo. Int J Cancer. 2000;87:276–282. doi: 10.1002/1097-0215(20000715)87:2<276::aid-ijc20>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal BB. Apoptosis and nuclear factor-kappa B: a tale of association and dissociation. Biochem Pharmacol. 2000;60:1033–1039. doi: 10.1016/s0006-2952(00)00393-2. [DOI] [PubMed] [Google Scholar]
- 16.Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci. 1975;72:3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu H, Xiong J, Goeddel DV. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell. 1999;81:495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 18.Hsu H, Shu HB, Pan MG, Goeddel DV. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 19.Sugarman BJ, Aggarwal BB, Hass PE, Figari IS, Palladino MA, Jr, Shepard HM. Recombinant human tumor necrosis factor-alpha: effects on proliferation of normal and transformed cells in vitro. Science. 1985;230:943–945. doi: 10.1126/science.3933111. [DOI] [PubMed] [Google Scholar]
- 20.Tsujimoto M, Yip YK, Vilcek J. Tumor necrosis factor: specific binding and internalization in sensitive and resistant cells. Proc Natl Acad Sci USA. 1985;827:626–7630. doi: 10.1073/pnas.82.22.7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Del Maestro RF, LopezTorres M, McDonald WB, Stroude EC, Vaithilingam IS. The effect of tumor necrosis factor-alpha on human malignant glial cells. J Neurosurg. 1992;76:652–659. doi: 10.3171/jns.1992.76.4.0652. [DOI] [PubMed] [Google Scholar]
- 22.Vazquez F, Sellers WR. The PTEN tumor suppressor protein: an antagonist of phosphoinositide 3-kinase signaling. Biochim Biophys Acta. 2000;1470:M21–M35. doi: 10.1016/s0304-419x(99)00032-3. [DOI] [PubMed] [Google Scholar]
- 23.Di Cristofano A, Pandolfi PP. The multiple roles of PTEN in tumor suppression. Cell. 2000;100:387–390. doi: 10.1016/s0092-8674(00)80674-1. [DOI] [PubMed] [Google Scholar]
- 24.Leevers SJ, Vanhaesebroeck B, Waterfield MD. Signalling through phosphoinositide 3-kinases: the lipids take centre stage. Curr Opin Cell Biol. 1999;11:219–225. doi: 10.1016/s0955-0674(99)80029-5. [DOI] [PubMed] [Google Scholar]
- 25.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. Trends Cell Biol. 1998;9:125–128. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 26.Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci U S A. 1999;96:4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 28.Myers MP, Pass I, Batty IH, Van der Kaay J, Stolarov JP, Hemmings BA, Wigler MH, Downes CP, Tonks NK. The lipid phosphatase activity of PTEN is critical for its tumor supressor function. Proc Natl Acad Sci U S A. 1998;95:13513–13518. doi: 10.1073/pnas.95.23.13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 30.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 31.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 32.Romashkova JA, Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signaling. Nature. 1999;401:86–89. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 33.Madrid L, Wang CY, Guttridge DC, Schottelius AJG, Baldwin AS, Jr, Mayo MW. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-kappaB. Mol Cell Biol. 2000;20:1626–1638. doi: 10.1128/mcb.20.5.1626-1638.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reddy SAG, Huang JH, Liao WL. Phosphatidylinositol 3-kinase as a mediator of TNF-induced NF-kappa B activation. J Immunol. 2000;164:1355–1363. doi: 10.4049/jimmunol.164.3.1355. [DOI] [PubMed] [Google Scholar]
- 35.Yang CH, Murti A, Pfeffer SR, Kim JG, Donner DB, Pfeffer LM. Interferon alpha /beta promotes cell survival by activating nuclear factor kappa B through phosphatidylinositol 3-kinase and Akt. J Biol Chem. 2001;276:13756–13761. doi: 10.1074/jbc.M011006200. [DOI] [PubMed] [Google Scholar]
- 36.Steck PA, Pershouse M, Jasser SA, Yung WKA, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T, Frye C, Hu R, Swedlund B, Teng DHF, Tavtigian SV. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 37.Koul D, Jasser SA, Lu Y, Davies MA, Shen R, Shi Y, Mills GB, Yung WK. Motif analysis of the tumor suppressor gene MMAC/PTEN identifies tyrosines critical for tumor suppression and lipid phosphatase activity. Oncogene. 2002;15:2357–64. doi: 10.1038/sj.onc.1205296. [DOI] [PubMed] [Google Scholar]
- 38.Reddy SAG, Huang JH, Liao WSL. Phosphatidylinositol 3-kinase in interleukin 1 signaling. Physical interaction with the interleukin 1 receptor and requirement in NFkappaB and AP-1 activation. J Biol Chem. 1997;272:29167–29172. doi: 10.1074/jbc.272.46.29167. [DOI] [PubMed] [Google Scholar]
- 39.Mayo MW, Wang CY, Cogswell PC, Rogers-Graham KS, Lowe SW, Der CJ, Baldwin AS., Jr Requirement of NF-kappa B Activation to Suppress p53-Independent Apoptosis Induced by Oncogenic Ras. Science. 1997;278:1812–1815. doi: 10.1126/science.278.5344.1812. [DOI] [PubMed] [Google Scholar]
- 40.Giri DK, Aggarwal BB. Constitutive Activation of NF-kappa B Causes Resistance to Apoptosis in Human Cutaneous T Cell Lymphoma HuT-78 Cells. J Biol Chem. 1998;273:14008–14014. doi: 10.1074/jbc.273.22.14008. [DOI] [PubMed] [Google Scholar]
- 41.Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NF-kappa B Antiapoptosis: Induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to Suppress Caspase-8 Activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 42.You M, Ku PT, Hrdlickova R, Bose HR., Jr ch-IAP1, a member of the inhibitor-of-apoptosis protein family is a mediator of the antiapoptotic activity of the v-Rel oncoprotein. Mol Cell Biol. 1997;17:7328–7341. doi: 10.1128/mcb.17.12.7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chu ZL, McKinsey TA, Liu L, Gentry JJ, Malim MH, Ballard DW. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-kappa B control. Proc Natl Acad Sci U S A. 1997;94:10057–10062. doi: 10.1073/pnas.94.19.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Catz SD, Johnson JL. Transcriptional regulation of bcl-2 by nuclear factor kappa B and its significance in prostate cancer. Oncogene. 2001;20:7342–7351. doi: 10.1038/sj.onc.1204926. [DOI] [PubMed] [Google Scholar]
- 45.Tamatani M, Che YH, Matsuzaki H, Ogawa S, Okado H, Miyake S, Mizuno T, Tohyama M. Tumor Necrosis Factor Induces Bcl-2 and Bcl-x Expression through NFkappa B Activation in Primary Hippocampal Neurons. J Biol Chem. 1999;274:8531–8538. doi: 10.1074/jbc.274.13.8531. [DOI] [PubMed] [Google Scholar]
- 46.Kreuz S. NF-kappaB inducers upregulate cFLIP, a cycloheximide-sensitive inhibitor of death receptor signaling. Mol Cell Biol. 2001;21:3964–3973. doi: 10.1128/MCB.21.12.3964-3973.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zong WX, Edelstein LC, Chen C, Bash J, Gelina C. The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-kappaB that blocks TNFalpha-induced apoptosis. Genes Dev. 1999;13:382–387. doi: 10.1101/gad.13.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu L, Fukuda S, Cordis G, Das DK, Maulik N. Anti-apoptotic protein survivin plays a significant role in tubular morphogenesis of human coronary arteriolar endothelial cells by hypoxic preconditioning. FEBS Lett. 2001;508:369–374. doi: 10.1016/s0014-5793(01)03084-8. [DOI] [PubMed] [Google Scholar]
- 49.Wang CY, Mayo MW, Baldwin AS., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 50.Otsuka G, Nagaya T, Saito K, Mizuno M, Yoshida J, Seo H. Inhibition of nuclear factor-kappaB activation confers sensitivity to tumor necrosis factor-alpha by impairment of cell cycle progression in human glioma cells. Cancer Res. 1999;59:4446–4452. [PubMed] [Google Scholar]
- 51.Nagai S, Washiyama K, Kurimoto M, Takaku A, Endo S, Kumanishi T. Aberrant nuclear factor-kappaB activity and its participation in the growth of human malignant astrocytoma. J Neurosurg. 2002;96:909–917. doi: 10.3171/jns.2002.96.5.0909. [DOI] [PubMed] [Google Scholar]
- 52.Sakurai H, Chiba H, Miyoshi H, Sugita T, Toriumi W. IkappaB kinases phosphorylate NF-kappaB p65 subunit on serine 536 in the transactivation domain. J Biol Chem. 1999;274:30353–30356. doi: 10.1074/jbc.274.43.30353. [DOI] [PubMed] [Google Scholar]
- 53.Mayo MW, Madrid LV, Westerheide SD, Jones DR, Yuan XJ, Baldwin AS, Whang YE. PTEN blocks tumor necrosis factor-induced NF-kappa B-dependent transcription by inhibiting the transactivation potential of the p65 subunit. J Biol Chem. 2002;277:11116–11125. doi: 10.1074/jbc.M108670200. [DOI] [PubMed] [Google Scholar]
- 54.Sizemore N, Lerner N, Dombrowski N, Sakurai H, Stark GR. Distinct roles of the Ikappa B kinase alpha and beta subunits in liberating nuclear factor kappa B (NF-kappa B) from Ikappa B and in phosphorylating the p65 subunit of NF-kappa B. J Biol Chem. 2002;277:3863–3869. doi: 10.1074/jbc.M110572200. [DOI] [PubMed] [Google Scholar]
- 55.Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J Clin Invest. 2001;107:241–246. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 57.Liu ZG, Hsu H, Goeddel DV, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 58.Van Antwerp D, Martin JS, Kafri T, Green DR, Verma IM. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 59.Wang CY, Mayo M, Baldwin AS. TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 60.Wu M, Lee H, Ballas R, Schauer S, Arsura M, Katz D, FitzGerald M, Rothestein T, Sherr D, Sonenshein GE. Inhibition of NF-kappaB/Rel induces apoptosis of murine B cells. EMBO J. 1996;15:4682–4690. [PMC free article] [PubMed] [Google Scholar]
- 61.Hale AJ, Smith CA, Sutherland LC, Stoneman VEA, Longthorne VL, Culhane AC, Williams GT. Apoptosis: molecular regulation of cell death. Eur J Biochem. 1996;236:1–26. doi: 10.1111/j.1432-1033.1996.00001.x. [DOI] [PubMed] [Google Scholar]
- 62.Barinaga M. Forging a path to cell death. Science. 1996;273:735–737. doi: 10.1126/science.273.5276.735. [DOI] [PubMed] [Google Scholar]
- 63.Koul D, Yao Y, Abbruzzese JL, Yung WKA, Reddy SAG. MMAC/PTEN inhibits Cytokine-induced NFkB activation without interfering with the IkB degradation pathway. J Biol Chem. 2001;276:11402–8. doi: 10.1074/jbc.M007806200. [DOI] [PubMed] [Google Scholar]


