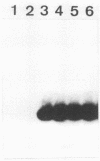
Abstract
A simple and reliable method using a polymerase chain reaction (PCR) was devised to identify methicillin-resistant staphylococci. By using lysates of the strain to be tested as templates and 22-mer oligonucleotides as primers, a 533-bp region of mecA, the structural gene of a low-affinity penicillin-binding protein (PBP 2'), was amplified by PCR and detected by agarose gel electrophoresis. Results obtained by this method were compared with those obtained by broth microdilution MIC determination for 210 and 100 clinical isolates of Staphylococcus aureus and coagulase-negative staphylococci, respectively. Of 99 mecA-negative S. aureus isolates, 100% of the strains were methicillin susceptible and 98% of the strains were oxacillin susceptible. Three strains (3%) of 111 mecA-positive S. aureus isolates exhibited almost the same susceptibility to beta-lactams as the mecA-negative ones and did not produce detectable amounts of PBP 2' despite the presence of the mecA gene. One of them yielded typically methicillin-resistant variants at a low frequency with concomitant recovery of PBP 2' production. The mecA gene was also found in coagulase-negative Staphylococcus epidermidis, Staphylococcus haemolyticus, Staphylococcus sciuri, Staphylococcus saprophyticus, and Staphylococcus caprae and conferred resistance on most of the bacteria.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer G. L., Pennell E. Detection of methicillin resistance in staphylococci by using a DNA probe. Antimicrob Agents Chemother. 1990 Sep;34(9):1720–1724. doi: 10.1128/aac.34.9.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A. L., Jones R. N. Reliability of high-content disks and modified broth dilution tests for detecting staphylococcal resistance to the penicillinase-resistant penicillins. J Clin Microbiol. 1987 Oct;25(10):1897–1901. doi: 10.1128/jcm.25.10.1897-1901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger-Bächi B. Insertional inactivation of staphylococcal methicillin resistance by Tn551. J Bacteriol. 1983 Apr;154(1):479–487. doi: 10.1128/jb.154.1.479-487.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers H. F. Coagulase-negative staphylococci resistant to beta-lactam antibiotics in vivo produce penicillin-binding protein 2a. Antimicrob Agents Chemother. 1987 Dec;31(12):1919–1924. doi: 10.1128/aac.31.12.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb W. B., Annear D. I. Spontaneous loss of methicillin resistance in Staphylococcus aureus at room-temperature. Lancet. 1972 Dec 9;2(7789):1257–1257. doi: 10.1016/s0140-6736(72)92315-x. [DOI] [PubMed] [Google Scholar]
- Hartman B. J., Tomasz A. Expression of methicillin resistance in heterogeneous strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1986 Jan;29(1):85–92. doi: 10.1128/aac.29.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman B. J., Tomasz A. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J Bacteriol. 1984 May;158(2):513–516. doi: 10.1128/jb.158.2.513-516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu K., Suzuki E., Takayama H., Katayama Y., Yokota T. Role of penicillinase plasmids in the stability of the mecA gene in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1990 Apr;34(4):600–604. doi: 10.1128/aac.34.4.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNOX R., SMITH J. T. The nature of penicillin resistance in staphylococci. Lancet. 1961 Sep 2;2(7201):520–522. doi: 10.1016/s0140-6736(61)92958-0. [DOI] [PubMed] [Google Scholar]
- Kornblum J., Hartman B. J., Novick R. P., Tomasz A. Conversion of a homogeneously methicillin-resistant strain of Staphylococcus aureus to heterogeneous resistance by Tn551-mediated insertional inactivation. Eur J Clin Microbiol. 1986 Dec;5(6):714–718. doi: 10.1007/BF02013311. [DOI] [PubMed] [Google Scholar]
- Murakami K., Nomura K., Doi M., Yoshida T. Production of low-affinity penicillin-binding protein by low- and high-resistance groups of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1987 Sep;31(9):1307–1311. doi: 10.1128/aac.31.9.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre J., Williamson R., Bornet M., Gutmann L. Presence of an additional penicillin-binding protein in methicillin-resistant Staphylococcus epidermidis, Staphylococcus haemolyticus, Staphylococcus hominis, and Staphylococcus simulans with a low affinity for methicillin, cephalothin, and cefamandole. Antimicrob Agents Chemother. 1990 Sep;34(9):1691–1694. doi: 10.1128/aac.34.9.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabath L. D. Mechanisms of resistance to beta-lactam antibiotics in strains of Staphylococcus aureus. Ann Intern Med. 1982 Sep;97(3):339–344. doi: 10.7326/0003-4819-97-3-339. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Song M. D., Wachi M., Doi M., Ishino F., Matsuhashi M. Evolution of an inducible penicillin-target protein in methicillin-resistant Staphylococcus aureus by gene fusion. FEBS Lett. 1987 Aug 31;221(1):167–171. doi: 10.1016/0014-5793(87)80373-3. [DOI] [PubMed] [Google Scholar]
- Stratton C. W., Gelfand M. S., Gerberding J. L., Chambers H. F. Characterization of mechanisms of resistance to beta-lactam antibiotics in methicillin-resistant strains of Staphylococcus saprophyticus. Antimicrob Agents Chemother. 1990 Sep;34(9):1780–1782. doi: 10.1128/aac.34.9.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesch W., Strässle A., Berger-Bächi B., O'Hara D., Reynolds P., Kayser F. H. Cloning and expression of methicillin resistance from Staphylococcus epidermidis in Staphylococcus carnosus. Antimicrob Agents Chemother. 1988 Oct;32(10):1494–1499. doi: 10.1128/aac.32.10.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornsberry C., McDougal L. K. Successful use of broth microdilution in susceptibility tests for methicillin-resistant (heteroresistant) staphylococci. J Clin Microbiol. 1983 Nov;18(5):1084–1091. doi: 10.1128/jcm.18.5.1084-1091.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubukata K., Nonoguchi R., Song M. D., Matsuhashi M., Konno M. Homology of mecA gene in methicillin-resistant Staphylococcus haemolyticus and Staphylococcus simulans to that of Staphylococcus aureus. Antimicrob Agents Chemother. 1990 Jan;34(1):170–172. doi: 10.1128/aac.34.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubukata K., Yamashita N., Konno M. Occurrence of a beta-lactam-inducible penicillin-binding protein in methicillin-resistant staphylococci. Antimicrob Agents Chemother. 1985 May;27(5):851–857. doi: 10.1128/aac.27.5.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsui Y., Yokota T. Role of an altered penicillin-binding protein in methicillin- and cephem-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1985 Sep;28(3):397–403. doi: 10.1128/aac.28.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Salihy S. M., James A. M. Loss of methicillin-resistance from resistant strains of Staphylococcus aureus. Lancet. 1972 Aug 12;2(7772):331–332. doi: 10.1016/s0140-6736(72)92937-6. [DOI] [PubMed] [Google Scholar]