Abstract
The aim of the present study was to understand the placental transfer of polychlorinated biphenyls (PCBs), specific hydroxylated PCB metabolites (OH-PCBs), and pentachlorophenol (PCP) in blood serum, in a birth cohort from eastern Slovakia. During the period 2002–2004, cord blood specimens were collected in parallel with maternal specimens from women delivering in the two eastern Slovak districts of Michalovce and Svidnik/Stropkov. A total of 92 pairs of mother-cord specimens at delivery were selected for this study. 4-OH-CB107, 3-OH-CB153, 4-OH-CB146, 3′-OH-CB138, 4-OH-CB187, and 4′-OH-CB172 were quantified. The median concentrations of Σ17PCBs, Σ6OH-PCBs, and PCP in cord serum were 0.92, 0.33, and 0.69 ng/g wet wt., respectively and highly correlated with the corresponding maternal serum levels (correlations were R2 = 0.61, 0.78, and 0.82, respectively). The median cord to mother ratios of the Σ17PCBs, Σ6OH-PCBs, and PCP were 0.18, 0.75, and 1.10, respectively. The median ratio of the Σ6OH-PCBs to the Σ17 PCBs in the cord serum was 0.38 from wet weight based concentrations, which was about four times higher than the ratio of these compounds in maternal serum (0.09). PCP was more abundant than any PCB or OH-PCB congener measured in cord serum. The higher cord to maternal ratios of OH-PCB metabolites as compared with the parent compounds suggests either a higher placental transfer rate or greater metabolism in the fetus as compared with the maternal compartment. These findings are consistent with their preferential binding to TTR that can cross the placenta. The cord to maternal ratio varies by congener (e.g., 4-OH-CB107 = 0.58, 4-OH-CB146=0.74, 3′-OH-CB138= 1.01).
Keywords: Cord blood, hydroxylated PCB metabolites, polychlorinated biphenyls, pentachlorophenol, Slovakia
1. Introduction
In the 1970s and 1980s, countries around the world including USA, Japan, Germany, and Slovakia took regulatory actions to restrict or ban the production and use of PCBs (polychlorinated biphenyls), and over the subsequent decades, a decline in PCB levels in the biota including human milk has been observed (Bignert et al., 1998; Norén and Meironyte, 2000). However, PCBs are still one of the world’s most widespread contaminants. PCBs were involved in several food poisoning incidents; i.e. Yusho (Masuda and Yoshimura, 1984), Yucheng (Hsu et al., 1985), and the Belgian chicken feed contamination (Bernard et al., 1999), as well as the contamination of neighborhoods surrounding production sites in Anniston, Alabama (now closed) (Hansen et al., 2003), and Michalovce, Slovak Republic (Hovander et al., 2006; Kocan et al., 2004; Pavuk et al., 2004).
PCBs are capable of crossing the placenta to reach the fetus (Ayotte et al., 2003; Covaci et al., 2002; Fukata et al., 2005). Animal experiments have demonstrated PCBs to be toxic to numerous systems, including alterations in the thyroid hormone system (Porterfield and Hendry, 1998), and adverse effects on cognitive and neurosensory development (Schantz et al., 1991; Goldey et al., 1995).
Studies in human also suggest adverse effects associated with in utero exposure to PCBs: lower birth weight (Hertz-Picciotto et al., 2005; Rylander et al., 1996), possible retardation of postnatal growth (Patandin et al., 1998), impaired immune response (Weisglas-Kuperus et al., 1995), and neurodevelopmental deficits (Stewart et al., 2004). Some studies specifically observed impairments in mental performance tests associated with cord blood PCBs (Rogan et al., 1986; Stewart et al., 2003). The apparent negative effects of PCBs on human health might have been the result of PCBs themselves, their metabolites such as OH-PCBs, or their contaminants, such as PCDFs. The metabolism of PCB congeners results in the formation of a large number of OH-PCB congeners (Bergman et al., 2006; Letcher et al., 2000). OH-PCBs have been shown to be transferred to the fetal compartment both in humans (Guvenius et al., 2003; Soechitram et al., 2004) and in animals (Brouwer et al., 1998; Sinjari and Darnerud, 1998; Meerts et al., 2002). Those OH-PCB metabolites with a para- or a meta-substituted hydroxyl group adjacent to chlorine atoms have a particularly high affinity for transthyretin (TTR) (Brouwer et al., 1998). Although TTR is not the primary transport protein for thyroid hormone in human blood, it still crosses the placenta and blood-brain barrier, resulting in the delivery of T4 and possibly OH-PCBs. In experimental animals, OH-PCB metabolites reduce thyroxine (T4) levels in the brain and blood of the fetus (Brouwer et al., 1998; Sinjari and Darnerud, 1998; Meerts et al., 2002) and therefore have the potential to affect behavioral development.
Unlike the parent PCB compounds, OH-PCBs are transferred in very low amounts via milk (Fängström et al., 2005). The studies of neurodevelopment in relation to postnatal exposure via breast milk have been inconsistent and often not significant (Jacobson and Jacobson, 2004). For this reason, although PCB exposure is higher through breastfeeding than in utero (Fukata et al., 2005; Guvenius et al., 2003), the prenatal exposure is believed to pose a greater threat to the infant than postnatal exposure. The analysis of umbilical cord blood is, therefore, central to the assessment of developmental effects, since it provides a direct measure of in utero exposure to PCBs and their metabolites.
Pentachlorophenol (PCP) is also of interest because it is present at high concentrations, higher than other halogenated phenolic compounds (HPCs) dominant in cord blood (Guvenius et al., 2003; Sandau et al., 2002), and is documented as an endocrine disruptor (Beard and Rawlings, 1999; Ishihara et al., 2003).
The present study of prenatal exposure to PCBs, PCP, and OH-PCBs was conducted in eastern Slovakia, in an area with PCB exposures among the highest in the world (Hovander et al., 2006; Kocan et al., 1994; Pavuk et al., 2004). We characterized and quantified the levels of 17 PCBs, 9 OH-PCB metabolites and PCP in cord blood sera specimens collected from mother-child pairs at delivery in two eastern Slovakian districts, Michalovce and Svidnik/Stropkov, to assess and compare the placental transfer and prenatal exposures to those compounds. Our ultimate goal is to improve the understanding of the relationship between prenatal exposure to halogenated endocrine disruptors and early childhood immune- and neuro-development.
2. Materials and methods
2.1. Cohorts
During the period 2002–2004, over 1100 mothers were enrolled into a birth cohort study in the Michalovce district, where the Chemko, Inc. chemical plant produced PCBs from 1959 to 1984, and in Svidnik/Stropkov district as a lower exposure area with similar population characteristics, about 70 km to the north. Hospital staff trained in the study protocols administered informed consent to all participants and collected the maternal and cord blood at delivery. The study participants gave written informed consent. A total of 762 and 341 women participated from Michalovce and Svidnik/Stropkov districts, respectively. We collected a total of 1087 pairs of both maternal and cord sera. The general characteristics of our study cohort were described in previously (Park et al., 2007). This study complied with all applicable U.S. and international requirements with regard to research on human subjects and was approved by the respective Institutional Review Boards at the University of California, Davis and the Slovak Medical University.
2.2. Samples
A Medican cannula was used, instead of standard needles, to collect umbilical cord blood samples just after the delivery. An adapter was used to connect the cannula to nine mL plastic vacutainer tube (S-Monovette, Sarstedt, Germany). An adequate volume of blood was aspirated into the S-Monovette tubes without adding anticoagulant. The blood samples were allowed to clot no more than two h at 5–10 °C. After clotting, blood was centrifuged at 3000 rpm for 15 min. Isolated serum was stored frozen at −18 °C in pre-cleaned glass tubes with polytetrafluoroethylene (PTFE) liner screw caps. All the samples were transported to the laboratory of the Slovak Medical University, Bratislava in thermo boxes to prevent thawing. The samples were then stored at −18 °C till the analysis. Ninety-two aliquots (0.5–5 mL) were transported to the University of California, Davis (CA, USA) and stored at −80 °C until analysis of halogenated phenolic compounds (PCP and OH-PCB metabolites).
2.3. Chemicals and standards
Diazomethane was synthesized in hexane by using N-nitroso-N-methylurea (Sigma-Aldrich, USA) as described elsewhere (Sandau, 2000). The following hydroxylated PCBs (OH-PCBs) were purchased from Wellington Laboratory (TerraChem Inc., USA) and used as authentic reference standards for the identification and quantification of the analytes: 2,3,3′,4′,5-pentachlorobiphenyl-4-ol (4-OH-CB107), 2,2′,3,4′,5,5′-hexachlorobiphenyl-4-ol (4-OH-CB146), 2,2′,3′,4,4′,5-hexachlorobiphenyl-3-ol (3′-OH-CB138), 2,2′,3,3′,4′,5-hexachlorobiphenyl-4-ol (4′-OH-CB130), 2,2′,3,4′,5,5′,6-heptachlorobiphenyl-4-ol (4-OH-CB187), 2,2′,3′,4,4′,5,5′-heptachlorobiphenyl-3-ol (3′-OH-CB180), 2,2′,3,3′,4′,5,5′-heptachlorobiphenyl-4-ol (4′-OH-CB172). 2,2′,4,4′,5,5′-hexachlorobiphenyl-3-ol (3-OH-CB153) and 2,3,3′,4′,5,5′,6-heptachlorobiphenyl-4-ol (4-OH-CB193) were synthesized (Bergman et al., 1995). The numbering of OH-PCBs is based on that specified by Bergman et al (2006).
Pentachlorophenol (PCP) was purchased from Ultra Scientific (USA). 2′,3,3′,4′,5,5′-hexaclorobiphenyl-4-ol (4′-OH-CB159) was purchased from AccuStandard (USA) and used as an internal standard.
2.4. Analysis and clean up
We analyzed cord blood serum specimens for 17 PCB congeners (CB-28, 52, 101, 123/149, 118, 114, 153, 105, 138, 167, 156/171, 157, 180, 170 and 189) at the Slovak Medical University as described previously (Kocan et al., 1994; Pavuk et al., 2004). The lipid content was enzymatically determined at the Slovak Medical University, using method described elsewhere (Akins et al., 1989). Ninety-two selected cord serum specimens were analyzed in 10 batches for nine OH-PCB congeners and pentachlorophenol (PCP) at the University of California, Davis. The extraction procedure used in this study was identical to that described in an earlier publication (Park et al. 2007).
2.5. Instruments
OH-PCBs and PCP were determined as methyl derivatives by gas chromatography (Agilent 6890N) with a mass spectrometer (Agilent 5973N). MS was operated in electron capture negative ionization mode as described previously (Park et al., 2007). For most OH-PCB congeners and PCP, the molecular ions were monitored as the base peak. However, the most abundant fragment ions [(M+2-HCl)−] were monitored for meta-substituted congeners (e.g., 3′-MeO-CB138 and 3-MeO-CB153) since their molecular ions were weak in intensity.
2.6. Quantification and QA/QC
All glassware was washed, dried by acetone and hexane, and baked at 550 °C for 8 h. One procedure blank (one% potassium chloride solution, five mL) and one spiked control serum sample were analyzed with each batch as QA/QC samples. The control samples were prepared as described in the previous study (Park et al., 2007). Five levels of calibration standards for the quantification were prepared in methanol, stored in brown ampoule and placed in a refrigerator. The external calibration range was from 0.1 to 50 pg/μL. We derivatized them simultaneously with the serum sample extracts for more accurate quantification. 4′-OH-CB159 (2.00 ng), which, to our knowledge, has not been detected in human blood to date, was added to all samples before extraction as a recovery internal standard. CB-209 (3.15 ng) was spiked to the samples before GC analysis. Serum concentrations of OH-PCBs were corrected based on the recoveries of the internal standards.
2.7. Statistical Analysis
We selected a total of 92 pairs of maternal-cord blood sera specimens for OH-PCB assays; to enhance statistical power, we sampled more heavily from those pairs in which the maternal PCB levels were above the 75th percentile. Thus, our sample was comparised of 50% below the 75% percentile, 15% from 75th to 85th percentile, 22% from 85th to 95th percentile and 13% above 95th percentile. We report mean, standard deviation, and the cutpoints for the highest and lowest deciles for the distributions of PCB and OH-PCB concentrations in maternal and cord sera from our cohort of 1103 using weights proportional to the inverse of the sampling fractions. We report the measured concentrations of PCP treated the samples as randomly selected, since PCP was not associated with PCBs. We conducted parametric tests using log-transformed PCB and OH-PCB values to determine the differences and relationships. We calculated R-squared values from a weighted Pearson correlation analysis to determine the relationships between individual PCBs and their presumed metabolites in maternal and cord sera. We conducted Student t-tests of differences in the concentrations between maternal and cord sera, and between PCBs and their metabolites. We used the non-parametric Mann-Whitney test for PCP data to examine the difference between maternal and cord serum concentrations. We report the concentration of phenolic compounds, OH-PCBs and PCP on a wet weight basis since they are not accumulated in lipids, but rather have high affinity to blood proteins (Bergman et al. 1994; Letcher et al. 2000).
3. Results
Nine OH-PCB congeners and PCP were identified. The average recoveries of the OH-PCB congeners ranged from 78±10% to 97±12% where the recoveries between two matrices were within the analytical error. The average recovery of PCP was 97±19%. Recoveries of the internal standard (4′-OH-CB159) were on average 83±7%. In cord blood serum, major PCB precursors (CB-153, 138, 180, 170), PCP, and major OH-PCB metabolites (4-OH-CB107, 3-OH-CB153, 4-OH-CB146, 3′-OH-CB138, 4-OH-CB187, and 4′-OH-CB172) were detected in >98% of all cord sera specimens.
The cord and maternal blood concentrations of potential PCB precursors for the OH-PCB metabolites, the sum of 17 PCB congeners (Σ17PCBs), six major OH-PCB metabolites, their sum (Σ6OH-PCBs), PCP, and lipid weights are presented in Table 1. The mothers’ age ranged from 18 to 44 years. We observed little association between mother’s age and the concentrations of mother and cord PCBs, PCP and OH-PCBs, and the mother to cord ratios of these compounds. Concentrations of both PCBs and OH-PCB metabolites in cord serums were about two times higher in Michalovce subjects than in those from Svidnik/Stropkov (data not shown). However, the ratios of PCBs and OH-PCBs in cord to maternal blood between the two areas were not significantly different (Student t-test, p=0.98 (PCBs) and p=0.38 (OH-PCBs)). Based on this result, the data from the two districts were combined. The concentrations of Σ17PCBs, Σ6OH-PCBs, and PCP in cord serum were highly correlated with maternal serum levels (Pearson R2 = 0.62 and 0.81 for Σ17PCBs and Σ6OH-PCBs, respectively, and Spearman R2 = 0.82 for PCP) (not shown here).
Table 1.
Distribution* of PCBs, OH-PCBs, and PCP, measured in Slovak maternal and cord blood serums collected from mothers at time of delivery
| Maternal Serum | Cord Serum | Median Ratio of Cord/Mother | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10th percentile | Median | 90th percentile | Mean | SD | 10th percentile | Median | 90th percentile | Mean | SD | ||
| mg/mL | mg/mL | Ratio | |||||||||
| Lipid | 8.49 | 10.6 | 13.1 | 10.6 | 2.07 | 1.87 | 2.55 | 3.14 | 2.58 | 0.57 | 0.25 |
| PCBs | ng/mL wet wt. | ng/mL wet wt. | Ratio | ||||||||
| CB-118 | 0.03 | 0.12 | 0.30 | 0.17 | 0.23 | 0.01 | 0.01 | 0.06 | 0.03 | 0.05 | 0.12 |
| CB-153 | 0.73 | 1.54 | 3.87 | 1.97 | 1.68 | 0.10 | 0.30 | 0.61 | 0.38 | 0.33 | 0.19 |
| CB-105 | 0.01 | 0.02 | 0.08 | 0.04 | 0.06 | 0.002 | 0.004 | 0.005 | 0.004 | 0.007 | 0.16 |
| CB-138 | 0.42 | 0.95 | 2.43 | 1.29 | 1.20 | 0.07 | 0.22 | 0.47 | 0.28 | 0.27 | 0.21 |
| CB-180 | 0.57 | 1.27 | 3.51 | 1.85 | 1.64 | 0.07 | 0.25 | 0.61 | 0.33 | 0.28 | 0.18 |
| CB-170 | 0.26 | 0.55 | 1.60 | 0.81 | 0.73 | 0.02 | 0.10 | 0.25 | 0.13 | 0.12 | 0.16 |
| aΣPCBs | 2.13 | 4.50 | 11.8 | 6.13 | 5.37 | 0.30 | 0.92 | 1.99 | 1.21 | 1.05 | 0.18 |
| OH-PCBs | ng/g wet wt. | ng/g wet wt. | Ratio | ||||||||
| 4-OH-CB107 | 0.01 | 0.03 | 0.08 | 0.04 | 0.05 | 0.01 | 0.01 | 0.05 | 0.03 | 0.04 | 0.57 |
| 3-OH-CB153 | 0.02 | 0.06 | 0.18 | 0.08 | 0.08 | 0.02 | 0.04 | 0.10 | 0.05 | 0.05 | 0.68 |
| 4-OH-CB146 | 0.04 | 0.09 | 0.28 | 0.14 | 0.18 | 0.03 | 0.07 | 0.18 | 0.11 | 0.16 | 0.78 |
| 3′-OH-CB138 | 0.02 | 0.06 | 0.13 | 0.07 | 0.07 | 0.02 | 0.06 | 0.13 | 0.07 | 0.07 | 1.01 |
| 4-OH-CB187 | 0.08 | 0.17 | 0.57 | 0.26 | 0.29 | 0.06 | 0.11 | 0.26 | 0.16 | 0.20 | 0.68 |
| 4′-OH-CB172 | 0.01 | 0.03 | 0.10 | 0.05 | 0.07 | 0.02 | 0.03 | 0.09 | 0.05 | 0.08 | 1.03 |
| bΣOH-PCBs | 0.21 | 0.44 | 1.23 | 0.65 | 0.70 | 0.15 | 0.33 | 0.90 | 0.48 | 0.56 | 0.75 |
| PCP | 0.33 | 0.65 | 1.40 | 1.02 | 1.51 | 0.37 | 0.69 | 1.64 | 1.12 | 1.64 | 1.10 |
| Ratio | Ratio | ||||||||||
| OH-PCB/PCB | 0.05 | 0.09 | 0.15 | 0.10 | 0.04 | 0.20 | 0.38 | 0.74 | 0.41 | 0.21 | |
These distributions represent the full cohort of 1087 pairs of mothers and cords were obtained using weights inversely proportional to the sampling probabilities.
ΣPCBs: 28, 52, 101, 123/149, 118, 114, 153, 105, 138, 167, 156/171, 157, 180, 170, and 189.
ΣOH-PCBs: 4-OH-CB107, 3-OH-CB153, 4-OH-CB146, 3′-OH-CB138, 4-OH-CB187, 4′-OH-CB172.
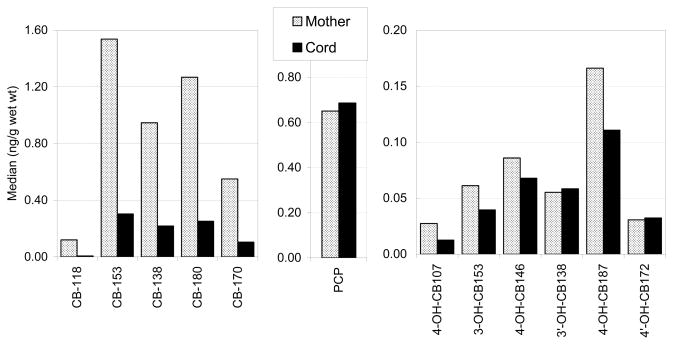
The median concentrations of Σ17PCBs, Σ6OH-PCBs, and PCP in cord serum were 0.92, 0.33, and 0.69 ng/g wet wt., respectively. Concentrations of OH-PCBs in cord serum were slightly lower than those in maternal serum, using the Student t-test on log-transformed weighted data (p<0.05). The distribution of PCP showed similar median concentrations of 0.65 and 0.69 ng/g wet wt. in maternal and cord serum, respectively (Mann-Whitney test; p=0.33). The medians of ΣPCBs were 0.92 and 4.50 ng/g wet wt. in cord and maternal serums, respectively (p<0.001).
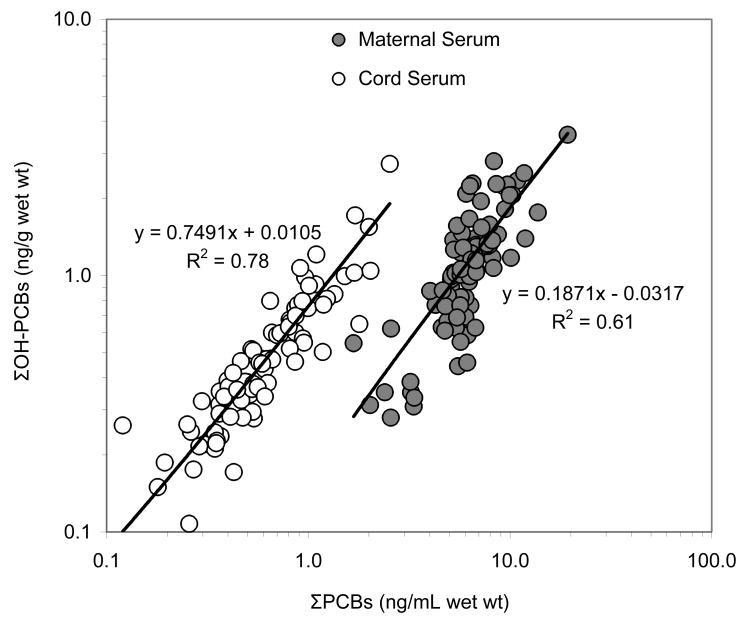
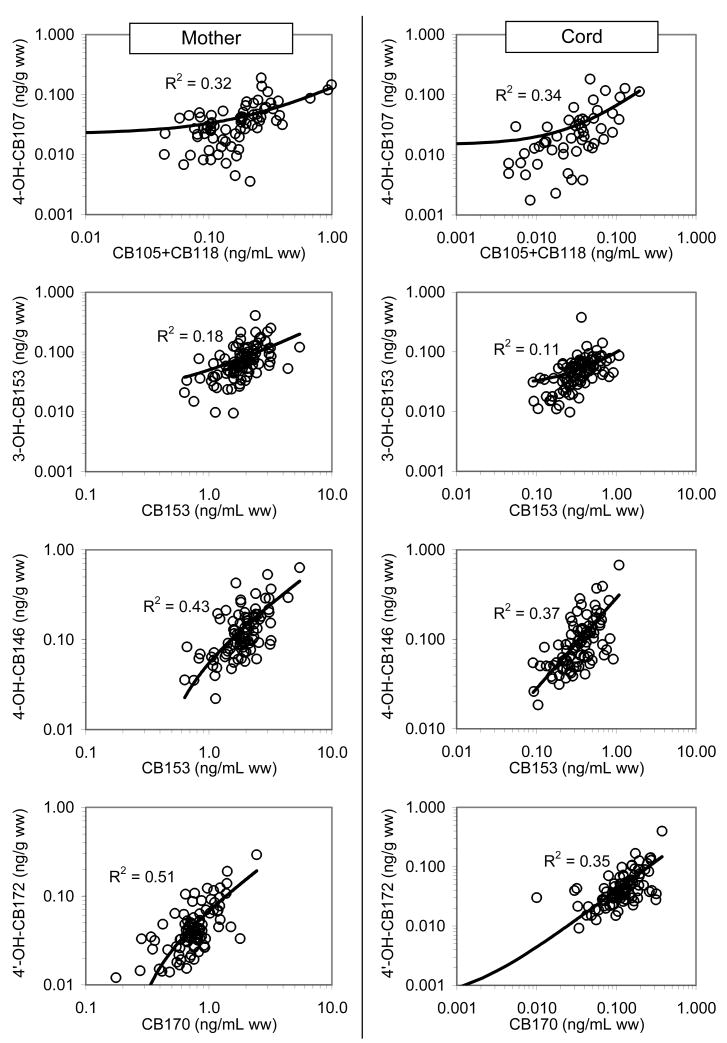
The median of selected PCBs, OH-PCB metabolites, and PCP in the maternal/cord serums are shown in Figure 1. The median mother to cord ratios of ΣPCBs, ΣOH-PCBs, and PCP were 0.18, 0.75, and 1.10, respectively. The ΣOH-PCB metabolites significantly correlated with the ΣPCBs in cord serum (R2=0.78, p<0.001) and in maternal serum (R2=0.61, p<0.001) (Figure 2). Using log transformed data, the presumed individual PCB precursors and their metabolites for both maternal and cord serum were related as shown in Figure 3, with correlations ranging from R2=0.11 to 0.51.
Figure 1.
Comparative presentation of the PCB, OH-PCB, and PCP concentrations in maternal (dotted bars) and cord (black bars) serums.
Figure 2.
Correlation of Σ17 PCBs and Σ6 OH-PCBs in maternal and cord serums, presented as white and grey rings, respectively.
Figure 3.
Comparison of correlations of individual PCB precursors vs. their OH-PCB metabolites between maternal and cord serums
4. Discussion
Although there are many reports on the PCB analysis of human cord, the analysis of halogenated phenolic compounds for this matrix is less common. To our knowledge, there are only three recent publications on cord blood analysis of halogenated phenolic compounds (HPCs) (Guvenius et al., 2003; Sandau et al., 2000; Soechitram et al., 2004). Only Guvenius et al. and Soechitram et al. reported the HPCs’ data from maternal-cord blood pairs. It is readily seen that the ratio of cord to maternal serum concentration is much higher for OH-PCB metabolites and PCP than it is for PCBs (Table 1 and Figure 1). In cord serum, PCP was the most abundant HPC and even higher than any PCB congeners, whereas, in maternal serum, it was lower than the concentrations of CB-153, 138, 170, and 180. The cord median ratio of the ΣOH-PCBs to the ΣPCBs in wet wt. was 0.38 which was slightly higher than that reported from other areas; ~0.2 in coastal populations in Quebec (Sandau et al., 2002), ~0.31 in Dutch mothers (Soechitram et al., 2004), and ~0.33 in Swedish mothers (Guvenius et al., 2003). The pattern of individual PCBs and OH-PCB metabolites observed in this study showed a slight discrepancy from other studies (Guvenius et al., 2003; Sandau et al., 2002; Soechitram et al., 2004), possibly due to the differences in the exposure of mothers to various PCBs, and variation in the formation and retention of OH-PCB metabolites in mothers.
PCB congeners are transferred on a 1:1 basis between the lipid compartment in maternal blood and that in cord blood. Since the lipid level is much lower in cord than in maternal blood, the volume of PCBs being transferred is, relatively speaking, low, and hence, the concentration on a fresh weight basis is lower in cord than in maternal serum (or plasma). OH-PCBs, in contrast, are not bound to lipids (or dissolved in the lipids) but instead bind to serum or plasma proteins. The concentrations of OH-PCBs in cord were almost the same as in the maternal serum, which would be highly coincidental if maternal OH-PCBs were not being transferred easily across the placenta. While it is interesting to speculate about a higher rate of PCB hydroxylation in the fetal compartment, we are not aware of evidence supporting such activity. Since OH-PCBs are reversibly bound to transthyretin, the transplacental transfer is dependent on this molecule. Empirically, we see that the regression line of OH-PCBs vs. PCBs measured from cord serum was tighter and steeper than the one from maternal serums (Figure 2). This tighter regression may indicate that PCBs and OH-PCB metabolites observed in the placenta were transferred mainly from the mothers. Nevertheless, a possible contribution of metabolism in the placental/fetal compartment cannot be excluded. Perhaps the strongest argument in favor of more efficient transfer of the metabolites as compared with the parent compounds is the higher binding affinity to TTR. The role of thyroid binding globulin, however, remains unknown, and this is the primary binding protein in humans.
PCBs and OH-PCBs showed significant correlations between mother and cord serum, supporting that these compounds cross the placenta. The median cord to maternal ratio for the ΣOH-PCBs (0.75) was about 4 times higher than that from PCBs (0.18) from wet wt. based concentrations. The median ratio of cord vs. mother for OH-PCBs in this study was similar to Swedish subjects of 0.71 (Guvenius et al., 2003) and slightly higher than Dutch subjects of 0.53 (Soechitram et al., 2004). The ratios for PCBs from other studies was 0.23 at Dutch mothers (Soechitram et al., 2004), 0.18 at Swedish mothers (Guvenius et al., 2003), 0.30 at Japanese mothers (Fukata et al., 2005). This again confirms the previous observations that OH-PCBs preferentially cross the placenta over PCBs, which makes them more important in the health risk assessment of fetus. Among halogenated phenolic compounds, PCP, 3′-OH-CB138, and 4′-OH-CB172 showed the highest ratios, proving they have the best selectivity to cross the placenta. PCB congeners showed consistently low ratios. This result might be related to the observation for the population living in Nunavik and Lower North Shore of Quebec that free T4 concentrations in cord blood plasma were negatively correlated with the ΣHPCs (PCP plus OH-PCBs) while they were not correlated with any PCB congeners or the ΣPCBs (Sandau et al., 2002).
Pairs of OH-PCB metabolites and potential or known PCB precursors have been described elsewhere (Letcher et al., 2000). In summary, the 1,2 shift mechanism involves the biotransformations of CB-105 and CB-118 to 4-OH-CB107, CB-138 and CB-153 to 4-OH-CB146, and 170/180 to 4′-OH-CB172, while an oxygen (hydroxyl group) can be directly inserted to transform CB-153 and 138 to 3-OH-CB153 and 3′-OH-CB138, respectively. The simple regression analysis does not provide information on which PCB congeners contribute to the formation of specific metabolites since correlations among PCB congeners are moderately high, leading to correlations with unrelated metabolites: e.g., 4-OH-CB146 vs. CB-118 (R2=0.49); 4-OH-CB172 vs. CB-153 and CB-138 (R2=0.30 and 0.39, respectively).
As for OH-PCB metabolites, 4-OH-CB187 was the predominant congener in almost all cord specimens and comprised 31±5% of ΣOH-PCBs. It was followed by 4-OH-CB146 which was detected at a higher concentration than 4-OH-CB187 in only four out of 92 cord serums. These results were in accordance with other studies (Guvenius et al., 2003; Sandau et al., 2002; Soechitram et al., 2004). These two combined with four other metabolites (3-OH-CB153, 3′-OH-CB138, 4′-OH-CB172, and 4-OH-CB107) together constituted 89±5% of the OH-PCB congeners we measured. 3′-OH-CB180 and 4-OH-CB193 were only detected at trace levels. It may be concluded that analysis of the six OH-PCBs listed in Table 1 covers all major OH-PCB metabolites well enough to be suggested for future analysis.
With slight variation, the rank order of abundance of individual PCBs and OH-PCB metabolites observed in the cord blood was as follows: CB-153 > CB-180 > CB-138 > 4-OH-CB187 > CB-170 > 4-OH-CB146 > 3′-OH-CB138 > 3-OH-CB153 > 4′-OH-CB172 > 4-OH-CB107 = CB-118 > CB-105. This pattern was similar to maternal blood although the ratio between congeners was not identical, possibly due to the different selectivity for transfer across the placenta or to metabolism by the fetus. Certain chemical structure is required to bind to TTR, which involves a hydroxyl group in either the para- or meta-position of a biphenyl ring, adjacent to chlorine atoms on both sides (Lans et al., 1993; Letcher et al., 2000).
PCP was the most dominant halogenated compound in cord serum but not in maternal serum, in accordance with other reports (Guvenius et al., 2003; Sandau et al., 2002). Cord PCP showed even higher median concentration than that of the ΣOH-PCBs by a factor of 1.8. The median concentration of PCP was higher than CB-153, the most abundant PCB congener by a factor of two and higher than 4-OH-CB187, the most abundant OH-PCB congener in the serum, by a factor of 5.3. In other words, in this population, the fetus is exposed to a greater amount of PCP than any PCB or OH-PCB congeners. PCP was among the halogenated phenolic compounds showing the highest placental transfer rate, supporting high affinity (> 95%) to blood protein in both animal (Braun et al., 1977) and human (Uhl et al., 1986). It has been reported that PCP interferes with thyroid hormone in the blood (Beard and Rawlings, 1999; Ishihara et al., 2003; van den Berg, 1990). PCP itself was negatively associated with T3, free T4, and TBG concentrations in cord bloods from the coastal population of Quebec (Sandau et al., 2002). Other possible adverse health effects of PCP observed from in vitro and animal studies include abnormalities in reproduction (Beard and Rawlings, 1999) and immunotoxicity (Daniel et al., 1995). PCP was not associated with PCBs and OH-PCBs, indicating a different source of PCP than of PCB compounds. PCP is extensively used as a fungicide for wood preservation (Wild et al., 1992) and it is rather volatile leading to risks for uptake via inhalation in the Slovak subjects. A minor PCP contribution may come from hexachlorobenzene (HCB) metabolism, since the HCB levels are significant in the population (Renner, 1988).
5. Conclusions
This study demonstrates that mothers in eastern Slovakia area carry substantial body burdens of halogenated phenolic compounds such as PCP and OH-PCB metabolites. In cord serum, PCP is present in the highest concentration among all compounds analyzed in this study. The amount of PCBs, OH-PCBs, and PCP transferred via placenta may pose a risk for disruption of the thyroid hormone system and adverse effects on neurodevelopment. Generally, halogenated phenolic compounds (OH-PCB metabolites and PCP) showed the higher ratios of cord to maternal serum than the neutral PCBs, suggesting that the HPCs are more easily transferred to the fetal compartment and must be included in the risk assessment of fetal exposure.
Acknowledgments
This research was supported by the U.S. National Institutes of Health, National Cancer Institute, #R01-CA96525, and National Institute of Environmental Health Service, #1P01-ES01129. The authors would like to thank the many people who assisted with recruitment and collection of the specimens, with special appreciation for Dr. Jaroslav Jasovsky, Dr. Frantisek Krul, Katka Revicka and Peter Suchanek. We especially would like to thank Dr. Courtney Sandau in Center for Disease Control, Georgia, Atlanta for helping with the inter-laboratory comparison study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akins JR, Waldrep K, Bernert JT. The estimation of total serum lipids by a completely enzymatic summation method. Clin Chim Acta. 1989;184(3):219–226. doi: 10.1016/0009-8981(89)90054-5. [DOI] [PubMed] [Google Scholar]
- Ayotte P, Muckle G, Jacobson JL, Jacobson SW, Dewailly E. Assessment of pre- and post-natal exposure to polychlorinated biphenyls: Lessons from the Inuit cohort study. Environ Health Perspect. 2003;111(9):1253–1258. doi: 10.1289/ehp.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard AP, Rawlings NC. Thyroid function and effects on reproduction in ewes exposed to the organochlorine pesticides lindane or pentachlorophenol (PCP) from conception. J Toxicol Environ Health-Part A. 1999;58(8):509–530. doi: 10.1080/009841099157124. [DOI] [PubMed] [Google Scholar]
- Bergman Ǻ, Hovander L, Malmberg T, Ludewig G, Robertson LW. Metabolism and metabolites of polychlorinated biphenyls (PCBs) Crit Rev Toxicol. 2006 doi: 10.3109/10408444.2014.999365. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman Å, Klasson-Wehler E, Kuroki H, Nilsson A. Synthesis and mass spectrometry of some methoxylated PCB. Chemosphere. 1995;30:1921–1938. [Google Scholar]
- Bernard A, Hermans C, Broeckaert F, De Poorter G, De Cock A, Houins G. Food contamination by PCBs and dioxins (vol 401, pg 231, 1999) Nature. 1999;401(6752):446–446. doi: 10.1038/45717. [DOI] [PubMed] [Google Scholar]
- Bignert A, Olsson M, Persson W, Jensen S, Zakrisson S, Litzen K, Eriksson U, Haggberg L, Alsberg T. Temporal trends of organochlorines in northern Europe, 1967–1995. Relation to global fractionation, leakage from sediments and international measures. Environ Pollut. 1998;99:177–198. doi: 10.1016/s0269-7491(97)00191-7. [DOI] [PubMed] [Google Scholar]
- Braun WH, Young JD, Blau GE, Gehring PJ. Pharmacokinetics and metabolism of pentachlorophenol in rats. Toxicol Appl Pharmacol. 1977;41(2):395–406. doi: 10.1016/0041-008x(77)90041-2. [DOI] [PubMed] [Google Scholar]
- Brouwer A, Morse DC, Lans MC, Schuur AG, Murk AJ, Klasson-Wehler E, Bergman Ǻ, Visser TJ. Interactions of persistent environmental organohalogens with the thyroid hormone system: Mechanisms and possible consequences for animal and human health. Toxicol Ind Health. 1998;14(1–2):59–84. doi: 10.1177/074823379801400107. [DOI] [PubMed] [Google Scholar]
- Covaci A, Jorens P, Jacquemyn Y, Schepens R. Distribution of PCBs and organochlorine pesticides in umbilical cord and maternal serum. Sci Total Environ. 2002;298(1–3):45–53. doi: 10.1016/s0048-9697(02)00167-5. [DOI] [PubMed] [Google Scholar]
- Daniel V, Huber W, Bauer K, Opelz G. Impaired in-vitro lymphocyte-responses in patients with elevated pentachlorophenol (PCP) blood levels. Arch Environ Health. 1995;50(4):287–292. doi: 10.1080/00039896.1995.9935956. [DOI] [PubMed] [Google Scholar]
- Fukata H, Omori M, Osada H, Todaka E, Mori C. Necessity to measure PCBs and organochlorine pesticide concentrations in human umbilical cords for fetal exposure assessment. Environ Health Perspect. 2005;113(3):297–303. doi: 10.1289/ehp.7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fängström B, Strid A, Grandjean P, Weihe Pál, Bergman Å. A retrospective study of PBDEs and PCBs in human milk from the Faroe Islands. Environ Health. 2005;4/12 doi: 10.1186/1476-069X-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldey ES, Kehn LS, Lau C, Rehnberg GL, Crofton KM. Developmental exposure to polychlorinated biphenyls (Aroclor 1254) reduces circulating thyroid hormone concentrations and causes hearing deficits in rats. Toxicol Appl Pharmacol. 1995;135:77–88. doi: 10.1006/taap.1995.1210. [DOI] [PubMed] [Google Scholar]
- Guvenius DM, Aronsson A, Ekman-Ordeberg G, Bergman Ǻ, Norén K. Human prenatal and postnatal exposure to polybrominated diphenyl ethers, polychlorinated biphenyls, polychlorobiphenylols, and pentachlorophenol. Environ Health Perspect. 2003;111(9):1235–1241. doi: 10.1289/ehp.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen LG, DeCaprio AP, Nisbet ICT. PCB congener comparisons reveal exposure histories for residents of Anniston, Alabama, USA. Fresenius Environ Bull. 2003;12(2):181–190. [Google Scholar]
- Harada M. Intrauterine poisoning: Clinical and epidemiological studies and significance of the problem. Bull Instit Constit Med. 1976;25:1–59. [Google Scholar]
- Hertz-Picciotto I, Charles MJ, James RA, Keller JA, Willman E, Teplin S. In utero polychlorinated biphenyl exposures in relation to fetal and early childhood growth. Epidemiology. 2005;16(5):648–656. doi: 10.1097/01.ede.0000173043.85834.f3. [DOI] [PubMed] [Google Scholar]
- Hovander L, Linderholm L, Athanasiadou M, Athanassiadis I, Bignert A, Fängström B, Kocan A, Petrik J, Trnovec T, Bergman Ǻ. Levels of PCBs and their metabolites in the serum of residents of a highly contaminated area in eastern Slovakia. Environ Sci Technol. 2004;40:3696–3703. doi: 10.1021/es0525657. [DOI] [PubMed] [Google Scholar]
- Hsu ST, Ma CI, Hsu SKH, Wu SS, Hsu NHM, Yeh CC, Wu SB. Discovery and epidemiology of PCB poisoning in Taiwan - A 4-year follow-up. Environ Health Perspect. 1985;59:5–10. doi: 10.1289/ehp.59-1568088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara A, Sawatsubashi S, Yamauchi K. Endocrine disrupting chemicals: interference of thyroid hormone binding to transthyretins and to thyroid hormone receptors. Mol Cell Endocrinol. 2003;199(1–2):105–117. doi: 10.1016/s0303-7207(02)00302-7. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW. Prospective studies of exposure to an environmental contaminant: The challenge of hypothesis testing in a multivariate correlational context. Psychol Sch. 2004;41(6):625–637. [Google Scholar]
- Kocan A, Drobna B, Petrik J, Jursa S, Chovancova J, Conka K, Balla B, Sovcikova E, Trnovec T. Human exposure to PCBs and some other persistent organochlorines in eastern Slovakia as a consequence of former PCB production. Organohalogen Compounds. 2004;66:3539–3546. [Google Scholar]
- Kocan A, Petrik J, Drobna B, Chovancova J. Levels of PCBs and some organochlorine pesticides in the human population of selected areas of the Slovak Republic. 1. Blood Chemosphere. 1994;29(9–11):2315–2325. doi: 10.1016/0045-6535(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Lans MC, Klassonwehler E, Willemsen M, Meussen E, Safe S, Brouwer A. Structure-dependent, competitive interaction of hydroxy-polychlorobiphenyls, hydroxy-dibenzo-p-dioxins and hydroxy-dibenzofurans with human transthyretin. Chem-Biol Interact. 1993;88(1):7–21. doi: 10.1016/0009-2797(93)90081-9. [DOI] [PubMed] [Google Scholar]
- Letcher RJ, Klasson WE, Bergman Ǻ. Methylsulfon and hydroxylated metabolites of polychlorinated biphenyls. In: Paasivirta J, editor. Handbook of Environmental Chemistry. New Types of Persistent Halogenated Compounds. Part K. Vol. 3. Berlin: Springer-Verlag; 2000. [Google Scholar]
- Lonky E, Reihman J, Darvill T, Mather J, Daly H. Neonatal behavioral assessment scale performance in humans influenced by maternal consumption of environmentally contaminated Lake Ontario fish. J Great Lakes Res. 1996;22(2):198–212. [Google Scholar]
- Masuda Y, Yoshimura H. Polychlorinated biphenyls and dibenzofurans in patients with Yusho and their toxicological significance - a review. Am J Ind Med. 1984;5(1–2):31–44. [PubMed] [Google Scholar]
- Meerts I, Assink Y, Cenijn PH, van den Berg JHJ, Weijers BM, Bergman Ǻ, Koeman JH, Brouwer A. Placental transfer of a hydroxylated polychlorinated biphenyl and effects on fetal and maternal thyroid hormone homeostasis in the rat. Toxicol Sci. 2002;68(2):361–371. doi: 10.1093/toxsci/68.2.361. [DOI] [PubMed] [Google Scholar]
- Norén K, Meironyte D. Certain organochlorine and organobromine contaminants in Swedish human milk in perspective of past 20–30 years. Chemosphere. 2000;40(9–11):1111–1123. doi: 10.1016/s0045-6535(99)00360-4. [DOI] [PubMed] [Google Scholar]
- Patandin S, Koopman-Esseboom C, De Ridder MAJ, Weisglas-Kuperus N, Sauer PJJ. Effects of environmental exposure to polychlorinated biphenyls and dioxins on birth size and growth in Dutch children. Pediat Res. 1998;44(4):538–545. doi: 10.1203/00006450-199810000-00012. [DOI] [PubMed] [Google Scholar]
- Park JS, Linderholm L, Charles MJ, Athanasiadou M, Petrik J, Kocan A, Drobna B, Trnovec T, Bergman3 Å, Hertz-Picciotto I. Polychlorinated biphenyls and their hydroxylated metabolites (OH-PCBs) in pregnant women from eastern Slovakia. Environ Health Perspect. 2007;115(1):20–27. doi: 10.1289/ehp.8913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuk M, Cerhan JR, Lynch CF, Schecter A, Petrik J, Chovancova J, Kocan A. Environmental exposure to PCBs and cancer incidence in eastern Slovakia. Chemosphere. 2004;54(10):1509–1520. doi: 10.1016/j.chemosphere.2003.10.038. [DOI] [PubMed] [Google Scholar]
- Porterfield SP, Hendry LB. Impact of PCBs on thyroid hormone directed brain development. Toxicol Ind Health. 1998;14(1–2):103–120. doi: 10.1177/074823379801400109. [DOI] [PubMed] [Google Scholar]
- Renner G. Hexachlorobenzene and its metabolism. Toxicol Environ Chem. 1988;18(1):51–78. [Google Scholar]
- Rice DC, Hayward S. Effects of postnatal exposure to a PCB mixture in monkeys on nonspatial discrimination reversal and delayed alternation performance. Neurotoxicology. 1997;18:479–494. [PubMed] [Google Scholar]
- Rogan WJ, Gladen BC, McKinney JD, Carreras N, Hardy P, Thullen J, Tinglestad J, Tully M. Neonatal effects of trans-placental exposure to PCBs and DDE. J Pediat. 1986;109(2):335–341. doi: 10.1016/s0022-3476(86)80397-3. [DOI] [PubMed] [Google Scholar]
- Rylander L, Stromberg U, Hagmar L. Dietary intake of fish contaminated with persistent organochlorine compounds in relation to low birth weight. Scand J Work Environ Health. 1996;22(4):260–266. doi: 10.5271/sjweh.140. [DOI] [PubMed] [Google Scholar]
- Sandau CD. Analytical Chemistry of Hydroxylated Metabolites of PCBs and Other Halogenated Phenolic Compounds in Blood and Their Relationship to Thyroid Hormone and Retinol Homeostasis in Humans and Polar Bears. Carleton University; Ottawa, Ontario: 2000. pp. 39–44. Ph.D. Dissertation. [Google Scholar]
- Sandau CD, Ayotte P, Dewailly E, Duffe J, Norstrom RJ. Pentachlorophenol and hydroxylated polychlorinated biphenyl metabolites in umbilical cord plasma of neonates from coastal populations in Quebec. Environ Health Perspect. 2002;110(4):411–417. doi: 10.1289/ehp.02110411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandau CD, Meerts I, Letcher RJ, McAlees AJ, Chittim B, Brouwer A, Norstrom RJ. Identification of 4-hydroxyheptachlorostyrene in polar bear plasma and its binding affinity to transthyretin: A metabolite of octachlorostyrene? Environ Sci Technol. 2000;34(18):3871–3877. [Google Scholar]
- Schantz SL, Levin ED, Bowman RE. Long-term neurobehavioral effects of perinatal polychlorinated biphenyl (PCB) exposure in monkeys. Environ Toxicol Chem. 1991;10:747–756. [Google Scholar]
- Sinjari T, Darnerud PO. Hydroxylated polychlorinated biphenyls: placental transfer and effects on thyroxin in the fetal mouse. Xenobiotica. 1998;28(1):21–30. doi: 10.1080/004982598239722. [DOI] [PubMed] [Google Scholar]
- Soechitram SD, Athanasiadou M, Hovander L, Bergman A, Sauer PJJ. Fetal exposure to PCBs and their hydroxylated metabolites in a Dutch cohort. Environ Health Perspect. 2004;112(11):1208–1212. doi: 10.1289/ehp.6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart P, Reihman J, Lonky E, Darvill T, Pagano J. Prenatal PCB exposure and neurobehavioral development in infants and children: Can the Oswego study inform the current debate? Psychol Sch. 2004;41(6):639–653. [Google Scholar]
- Stewart PW, Reihman J, Lonky EI, Darvill TJ, Pagano J. Cognitive development in preschool children prenatally exposed to PCBs and MeHg. Neurotoxicol Teratol. 2003;25(1):11–22. doi: 10.1016/s0892-0362(02)00320-3. [DOI] [PubMed] [Google Scholar]
- Uhl S, Schmid P, Schlatter C. Pharmacokinetics of pentachlorophenol in man. Arch Toxicol. 1986;58(3):182–186. doi: 10.1007/BF00340979. [DOI] [PubMed] [Google Scholar]
- van den Berg KJ. Interaction of chlorinated phenols with thyroxine binding sites of human transthryretin, albumin and thyroid binding globulin. Chem-Biol Interact. 1990;76:63–75. doi: 10.1016/0009-2797(90)90034-k. [DOI] [PubMed] [Google Scholar]
- Weisglas-Kuperus N, Sas TCJ, Koopmanesseboom C, Vanderzwan CW, Deridder MAJ, Beishuizen A, Hooijkaas H, Sauer PJJ. Immunological effects of background prenatal and postnatal exposure to dioxins and polychlorinated biphenyls in Dutch infants. Pediat Res. 1995;38(3):404–410. doi: 10.1203/00006450-199509000-00022. [DOI] [PubMed] [Google Scholar]
- Wild SR, Harrad SJ, Jones KC. Pentachlorophenol in the UK environment. 1 A budget and source inventory. Chemosphere. 1992;24(7):833–845. [Google Scholar]