Abstract
Anuran (frog) tadpoles and urodeles (newts and salamanders) are the only vertebrates capable of fully regenerating amputated limbs. During the early stages of regeneration these amphibians form a “blastema”, a group of mesenchymal progenitor cells that specifically directs the regrowth of the limb. We report that wnt-3a is expressed in the apical epithelium of regenerating Xenopus laevis limb buds, at the appropriate time and place to play a role during blastema formation. To test whether Wnt/β-catenin signaling is required for limb regeneration, we created transgenic Xenopus laevis tadpoles that express Dickkopf-1 (Dkk1), a specific inhibitor of Wnt/β-catenin signaling, under the control of a heat-shock promoter. Heat-shock immediately before limb amputation or during early blastema formation, blocked limb regeneration but did not affect the development of contralateral, unamputated limb buds. When the transgenic tadpoles were heat-shocked following the formation of a blastema, however, they retained the ability to regenerate partial hindlimb structures. Furthermore, heat-shock induced Dkk1 blocked fgf-8 but not fgf-10 expression in the blastema. We conclude that Wnt/β-catenin signaling has an essential role during the early stages of limb regeneration, but is not absolutely required after blastema formation.
Keywords: Xenopus laevis, limb, regeneration, Wnt/β-catenin signaling, transgenic, fgf-8, fgf-10
Introduction
All tetrapod limbs are thought to have evolved from paired fins of fish, to employ conserved mechanisms of development that originated with fins, and generally to have a common skeletal pattern (Tamura et al., 2001). Intriguingly, the regenerative responses of limbs after amputation are quite different between species. Animals such as mammals, birds, and lizards cannot restore lost limbs but instead merely undergo a wound healing response. In contrast, urodele amphibians such as newts and salamanders can regenerate their amputated limbs, while anuran amphibians are intermediate between urodele amphibians and other vertebrates in terms of their regenerative capacity. Xenopus laevis can completely regenerate developing hindlimb buds prior to the onset of metamorphosis, but the regenerative capacity declines gradually as metamorphosis proceeds (Dent, 1962; Muneoka et al., 1986).
In both urodele and anuran amphibians, limb regeneration progresses through a characteristic series of steps, beginning with wound healing, followed by formation of the blastema, and finally a redevelopment phase (Bryant et al., 2002; Gardiner et al., 2002; Han et al., 2005). Although the redevelopment stage of limb regeneration is thought to be equivalent to limb development, the early steps that result in the genesis of the blastema are critical in determining whether an amputated limb can successfully regenerate or whether it will undergo wound healing without regeneration. Considering the highly conserved mechanisms of limb development and conserved limb skeletal pattern among tetrapods, it is possible that elucidation of critical factor(s) important for blastema formation in regenerating amphibian limbs will contribute to development and improvement of tissue and organ replacement therapies (Stocum, 1997; Brockes and Kumar, 2005).
Based on the known roles for Wnt/β-catenin signaling during limb development (reviewed by Yang, 2003), we hypothesized that this signaling pathway might play an essential role in limb regeneration. Specifically, Wnt/β-catenin signaling is involved in the initiation of chick limb development and zebrafish pectoral fin formation, by inducing fgf-10 expression in the presumptive limb and fin region, respectively (Kawakami et al., 2001; Ng et al., 2002). In chick and mouse embryos, Wnt/ β-catenin signaling also has an essential role in the formation of a specialized ectodermal structure, an apical ectodermal ridge (AER) in the limb buds, through induction of fgf-8 expression (Kengaku et al., 1998; Barrow et al., 2003; Soshnikova et al., 2003). The feedback loop between FGF-10 and FGF-8 is well-known to be crucial for the outgrowth of the developing limb buds of chick (Ohuchi et al., 1997; Xu et al., 1998). Similarly, several recent studies indicate that both fgf-10 and fgf-8 are expressed in Xenopus and axolotl limb blastemas suggesting a crucial role in limb regeneration as well (Christen and Slack, 1997; Christensen et al., 2001, 2002; Endo et al., 2000; Han et al., 2001; Suzuki et al., 2005; Yokoyama et al., 2000, 2001).
Considering the essential roles of both pathways in the earliest regenerative steps, it is reasonable to hypothesize that Wnt/β-catenin signaling may serve to control in the initiation of limb regeneration by regulating downstream fgf-10 and/or fgf-8 expression. Furthermore, the Wnt/β-catenin pathway is implicated in the proliferation and maintenance of stem or progenitor cells of various adult tissues of mammals (reviewed by Beachy et al., 2004). Therefore, it is possible that Wnt/β-catenin signaling could be involved in either the initiation step of morphogenesis and/or the proliferation of stem or progenitor cells in regenerating limbs.
Functional analysis of genes and signaling pathways that might participate in regeneration has been hindered by the difficulty of manipulating gene function in postembryonic amphibians. However, the recent development of a transgenic system in Xenopus enables us to manipulate regeneration in anuran amphibians. To test the functional importance of Wnt signaling in regeneration we engineered Xenopus laevis that were transgenic for heat-shock inducible Dickkopf-1 (Dkk1), a secreted inhibitor of Wnt/β-catenin signaling (Glinka et al., 1998; Mao et al., 2001). By inducing this transgene at different time points during limb regeneration, we provide initial data establishing that Wnt/β-catenin signaling is required for limb regeneration.
Materials and Methods
Animal husbandry
Xenopus laevis were obtained from Nasco (Fort Atkinson, WI, USA). Tadpoles were kept in dechlorinated tap water containing 59g Instant Ocean Sea Salt (Aquarium System, INC., Mentor, OH, USA) / liter at 21-23°C, staged according to Nieuwkoop and Faber (1994), and fed with spirulina (Salt Creek, Inc.). At stage 58, the feeding was stopped until metamorphosis was completed.
DNA constructs and in situ hybridization
mmGFP5 (Siemering et al., 1996) was fused to the C-terminus of zebrafish Dkk-1 (Genbank accession no. AB023488). The Dkk1GFP5 fusion was then cloned downstream of the CMV promoter of the vector pCS2+ (CMV-Dkk1GFP5; Fig 1A). For the negative control, a plasmid in which only mmGFP5 is expressed under control of the CMV promoter was prepared (CMV-GFP5; Fig. 1A). For preparation of transgenic tadpoles, the Dkk1GFP5 was cloned downstream of the Xenopus hsp70 promoter (Wheeler et al., 2000; Hsp70-Dkk1GFP5; Fig. 2B).
Fig. 1.
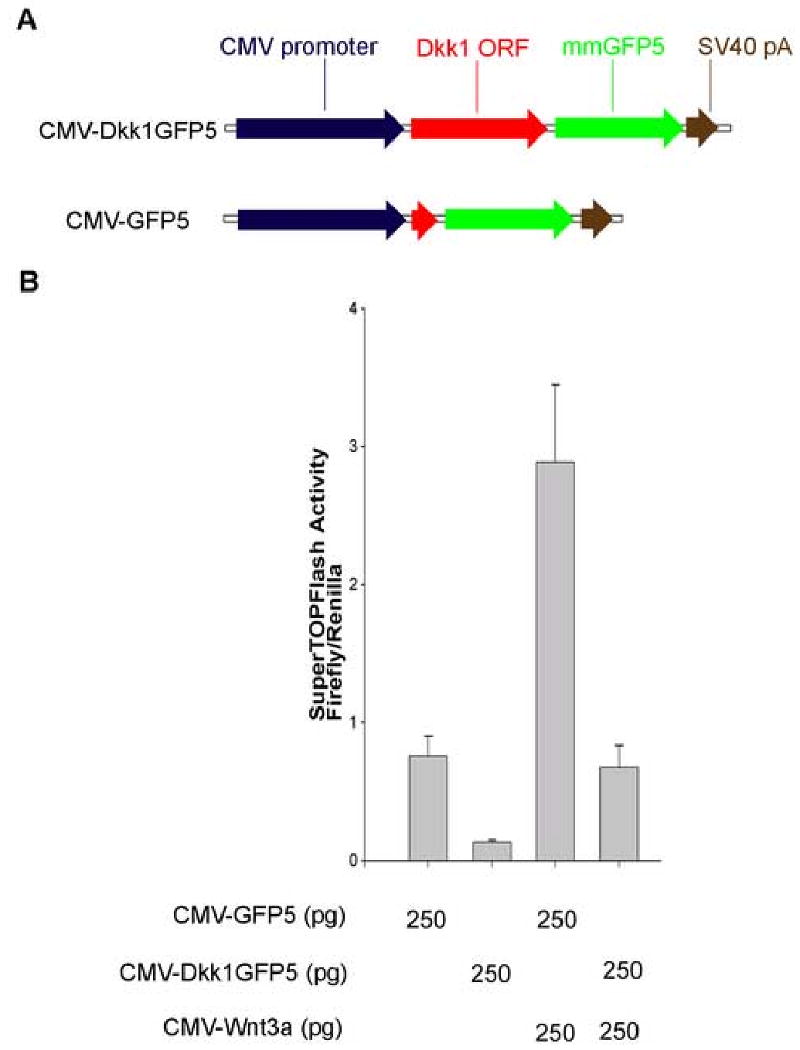
Dkk1GFP5 suppresses the activity of the β-catenin responsive reporter SuperTOPFlash in frog embryos. (A) Schematic representation of the injected constructs. Designs of constructs are described in Materials and Methods. (B) SuperTOPFlash reporter activation after injection of 250 pg CMV-GFP5 was compared to the activation occurring after injection of 250 pg CMV-Dkk1GFP5 with or without co-injection of 250 pg Xenopus CMV-Wnt3a DNA. Dkk1GFP5 suppressed both the endogenous activity of SuperTOPFlash (left two lanes) and the Wnt-3a-induced activation of SuperTOPFlash (right two lanes) in embryos. Firefly luciferase activity of the SuperTOPFlash reporter was normalized to renilla luciferase control activity. Error bars indicate the standard deviation from the mean (n=3).
Fig. 2.
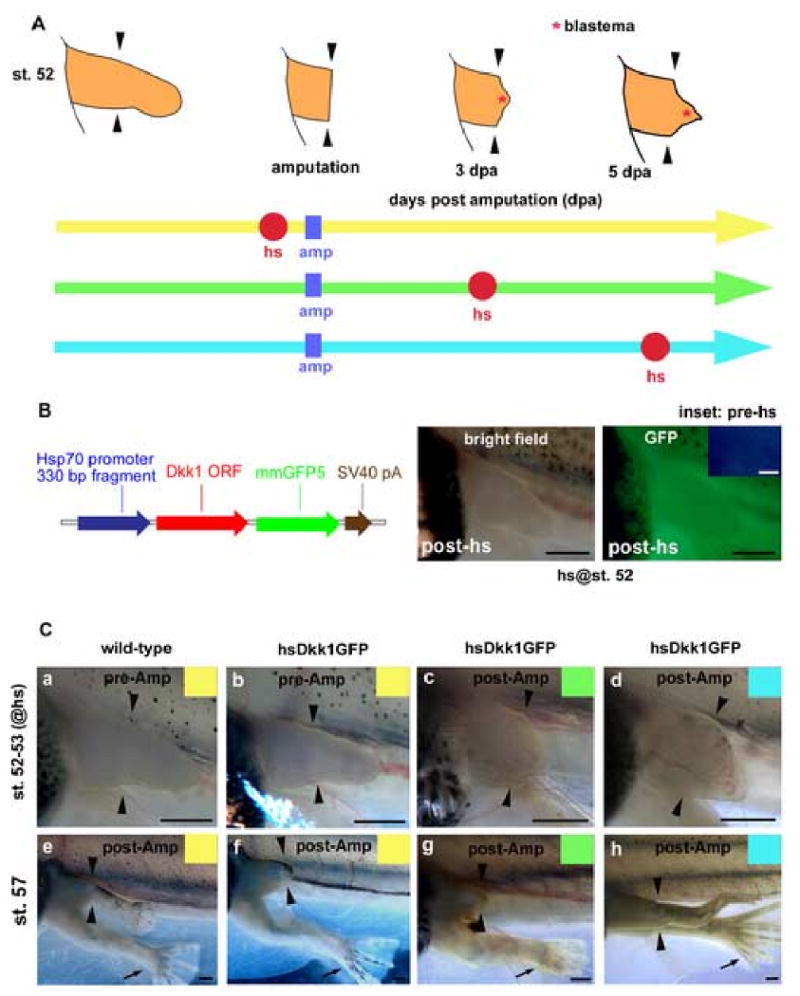
Wnt/β-catenin signaling is required for Xenopus limb regeneration. (A) Experimental scheme. Hindlimb buds of F0 tadpoles were amputated at the presumptive knee level (amp: represented as blue square). One heat-shock (hs: represented as red circle) was applied to tadpoles at 3 to 4 hours prior to amputation (yellow line), 3 dpa (days post amputation; green line) or 5 dpa (blue line). (B) Map of the heat-shock inducible Dkk1GFP transgene. Details are described in Materials and Methods. Expression of Dkk1GFP was induced in a transgenic tadpole carrying this transgene within 3 to 4 hours after heat-shock (left panel, bright field; right panel, GFP). No GFP expression was detected in the same tadpole before heat-shock (inset). (C) Live limb buds were photographed when tadpoles were heat-shocked (st. 52-53; a-d). The same amputated limb buds were photographed again when regenerated limbs became obvious in controls (st. 57; e-h). A wild-type tadpole heat-shocked prior to amputation regenerated the amputated limb bud completely (a and e). While the hsDkk1GFP tadpoles heat-shocked prior to amputation (b and f) or at 3 dpa (c and g) failed to regenerate, hsDkk1GFP tadpoles heat-shocked at 5 dpa regenerated incomplete hindlimbs (d and h). Note that un-amputated right limb buds developed normally (black arrows). Arrowheads show the presumptive knee level (amputation level). Scale bars, 500 μm.
Preparation of Dig-labeled wnt-3a (Wolda et al., 1993), fgf-8 (Yokoyama et al., 1998), fgf-10 (Yokoyama et al., 2000), Lmx-1 (Matsuda et al., 2001), Hoxa-13 (Endo et al., 2000) and msx-2 (unpublished) probes and in situ hybridization were performed as described previously (Endo et al., 1997). For making serial cryosections, specimens were fixed in MEMFA, dehydrated with 30% sucrose / PBS, embedded in OCT compound (Sakura), and serially sectioned at a 12μm thickness. Transcripts were detected by in situ hybridization on frozen sections using procedures described by Yoshida et al. (1996) with slight modifications.
Luciferase Assays
A total of 25 pg Super(8x)TOPFlash DNA (Veeman et al., 2003) together with 4 pg pRlu-N1 (h) DNA (Renilla reniform as the luciferase internal control; BioSignal Packard) was injected into two dorsal cells of four cell stage embryos. Three replicate samples each of four embryos were frozen for each group at late gastrula (st. 12.5) and luciferase assays were performed using the Promega luciferase assay system according to Tao el al. (2005) with slight modifications.
Transgenesis in Xenopus laevis
Transgenic Xenopus laevis embryos were generated by the REMI technique as previously described (Offield et al., 2000). To minimize potential leakiness of the transgene under the hsp70 promoter, embryos were reared at 16°C in 0.1X MMR (Wheeler et al., 2000) until tadpoles started swimming and feeding, then reared in 21-23°C.
For heat-shocking, tadpoles were placed in water warmed to 34°C for 30 min as described by Beck et al. (2003). Three to four hours after heat-shocking, tadpoles were examined under a fluorescent dissecting microscope and classified as GFP positive (hsDkk1GFP) or GFP negative (wild-type). Tadpoles with mosaic expression patterns of GFP, or that did not show GFP fluorescence 3 to 4 hours after heat-shocking but showed weak GFP the next day were excluded from the experiment.
Tadpole surgery
Tadpoles were anesthetized in 1:5000 ethyl-3-aminobenzoate (Sigma-Aldrich) dissolved in Holtfreter's solution. Left hindlimb buds were amputated at the presumptive knee level [according to the outside view and a fate map by Tschumi (1957)] with an ophthalmologic scalpel. After metamorphosis was completed, the cartilage pattern of amputated limbs was examined under a dissecting microscope to evaluate limb regeneration. If necessary, the limbs were stained with Alcian blue as described previously (Yokoyama et al., 2000). For in situ hybridization on sections of transgenic F0 tadpoles, both left and right hindlimb buds were amputated at the presumptive knee level.
Results and Discussion
Heat-shock inducible inhibition of Wnt/β-catenin signaling in Xenopus laevis
Our primary goal was to test the hypothesis that Wnt signaling is required for limb regeneration. To address this question we created transgenic Xenopus tadpoles that allowed us to inducibly inhibit endogenous Wnt/β-catenin signaling by overexpression of Dickkopf-1. Since a heat-shock inducible transgenic line for GFP-tagged Dickkopf-1 (hsDkk1GFP) can efficiently inhibit Wnt/β-catenin signaling in zebrafish (Stoick-Cooper et al., 2007), we used the same Dkk1GFP clone in Xenopus. After confirming that this fusion protein inhibits Wnt/β-catenin signaling in Xenopus embryos (Fig. 1), we cloned it downstream of the Xenopus hsp70 promoter (Fig. 2B). This Hsp70-Dkk1GFP (hsDkk1GFP) construct was then used to generate transgenic F0 animals.
As reported by Wheeler et al. (2000), no transgene expression under control by the hsp70 promoter was detected in transgenic animals during embryonic stages when embryos were kept at 16°C (data not shown), and under these conditions the embryos developed normally. Once embryos reached tadpole stages, leakiness of the transgene was not observed even at higher temperatures (21-23°C). Suggesting that basal expression of the transgene was very low prior to heat-shock, we observed no fluorescence of Dkk1GFP in these transgenic tadpoles at rearing temperatures (21-23°C; Fig. 2B inset). Establishing that the transgene was indeed induced by heat-shock, ubiquitous expression of Dkk1GFP was induced in F0 tadpoles 3 to 4 hours following a 30 min heat-shock at 34°C (Fig. 2B; 24% of total F0 tadpoles). Because of the random insertion of transgenes into Xenopus genomes by the REMI transgenic procedure (Kroll and Amaya, 1996), some F0 tadpoles did not express the transgene hence they were used as matched sibling negative controls (wild-type). The fluorescence of Dkk1GFP reaches a peak upon the next day of heat-shock and persists for several days in transgenic F0 tadpoles (data not shown). Ambiguous tadpoles that did not show GFP fluorescence 3 to 4 hours after heat-shock but showed weak GFP the following day were excluded from the experiment.
Wnt/β-catenin is required for early stages of limb regeneration
We used stage 52 hindlimb buds as they consistently regenerate complete hindlimbs after amputation at the presumptive knee level (Dent, 1962; Yokoyama et al., 2000). We heat-shocked F0 tadpoles at stage 52 and then amputated their left hindlimb buds 3 to 4 hours after heat-shock (Fig. 2A; yellow line). While 69% of wild-type F0 tadpoles regenerated hindlimbs completely (Fig. 2C a, e and Fig. 3A), none of the hsDkk1GFP F0 tadpoles showed complete regeneration and only 18% showed partial regeneration (Fig. 2C b, f and Fig. 3B, see Fig. 3 for N values). Interestingly, un-amputated right limb buds of the hsDkk1GFP tadpoles developed normally after heat-shock (see black arrows in Fig. 2C). Therefore, Wnt/β-catenin signaling is required for limb regeneration but not for limb development at this stage. Furthermore, the normal development of the matched right limb bud controls excludes the possibility that the Dkk1GFP transgene has nonspecific inhibitory effects on limb outgrowth.
Fig. 3.
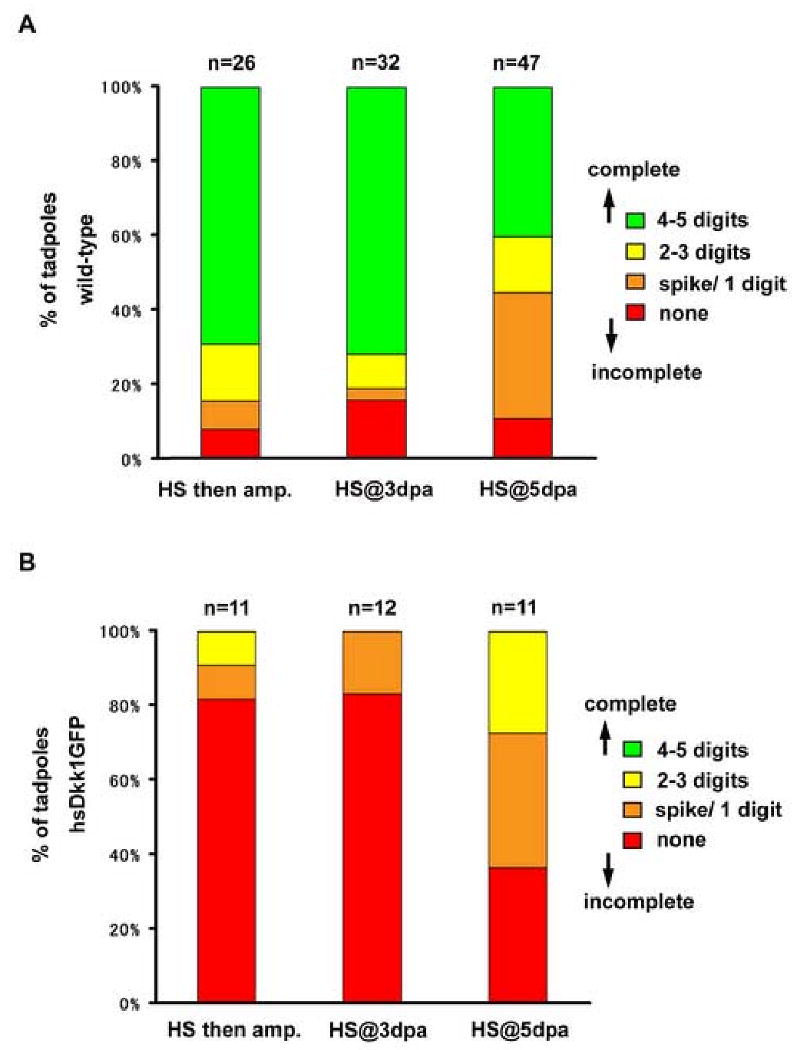
Percentage of wild-type and hsDkk1GFP tadpoles displaying varying degrees of regenerative responses after heat-shock as described in Fig. 1. (A) wild-type tadpoles. (B) hsDkk1GFP tadpoles. Regenerative capacity was evaluated by the number of regenerated digits.
To test for the requirement of Wnt/β-catenin signaling during subsequent phases of regeneration, left hindlimb buds of stage 52 F0 tadpoles were amputated at the presumptive knee level and heat-shocked following amputation, once at 3 dpa (days post amputation; Fig 2A; green line) or once at 5 dpa (Fig. 2A; blue line). At 3 dpa, the blastema is small, the reorganizing mesenchymal cells are in the process of accumulating and the overlying apical epithelium already appears thickened (see sections, Fig. 4C, F). When the F0 tadpoles were heat-shocked at 3 dpa, some regeneration response occurred in only 17% of the hsDkk1GFP tadpoles, compared with 84% in wild-type controls (Fig. 2C c, g and Fig. 3A, B). Heat shock induction of Dkk1GFP during apical epithelial thickening and early blastema formation reveals the requirement for Wnt signaling for regeneration at this stage.
Fig. 4.
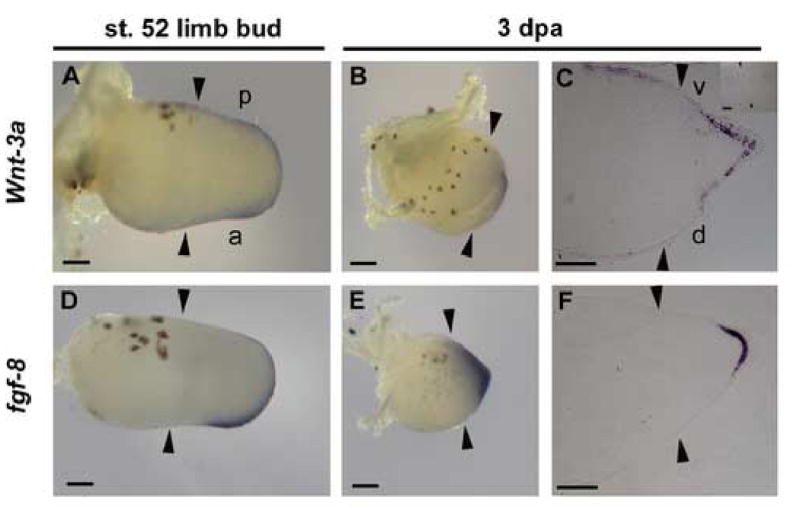
Expression of wnt-3a and fgf-8 in regenerating limb buds. (A and D) Stage 52 limb buds. (B, C, E and F) Regenerating blastemas at 3dpa. Right panels (C and F) show in situ hybridization on sectioned samples. Wnt-3a and fgf-8 are expressed in the inner layer of thickened apical epithelium of the blastema at 3 dpa. No specific hybridization signal was detected with an wnt-3a sense probe (C, inset). Arrowheads show amputation level (knee level). a, anterior; p, posterior; d, dorsal; v, ventral. Scale bars, 100 μm.
By 5 dpa, a cone-shaped blastema is formed. When heat-shocked at 5 dpa, 64% of the hsDkk1GFP tadpoles regenerated at least partially, compared with 89% in wild-type controls (The hsDkk1GFP tadpoles regenerated two digits at best; Fig 2C d, h and Fig. 3A, B). This result indicates that Wnt/β-catenin signaling is important, but not absolutely required for limb regeneration at this time-point. It is important to note that heat-shock itself at 5 dpa may have a slightly negative effect on regeneration. While about 70% of wild-type tadpoles heat-shocked before amputation or at 3 dpa regenerated completely (with 4 to 5 digits), only 40% of wild-type tadpoles heat-shocked at 5 dpa regenerated fully (Fig. 3A).
Wnt-3a is a candidate for regulating Wnt/β-catenin signaling in limb regeneration
Considering the inhibitory mechanism by which Dkk1 acts on Wnt/β-catenin signaling (Mao et al., 2001, 2002), a Wnt ligand that activates the β-catenin pathway should be expressed in regenerating limb buds during the period when heat-shock induced Dkk1GFP blocks regeneration. Among several Wnt ligands shown to activate β–catenin signaling (wnt-2b, wnt-3a, wnt-8, wnt-8b and wnt-10a), RT-PCR analysis showed that only wnt-3a was expressed in both regenerating limb buds during Dkk1GFP-sensitive regenerating window as well as in developing limb buds (data not shown). In chick embryo, wnt-3a is expressed in epithelial cell layers during the formation of the apical ectodermal ridge (AER), a specialized epithelial structure essential for the outgrowth and patterning of amniote limbs, and induces fgf-8 expression in β–catenin dependent manner (Kengaku et al., 1998). We examined the expression of wnt-3a and fgf-8 by in situ hybridization and found that both are expressed in the distal region of uncut stage 52 limb buds (Fig. 4A, D). Importantly, both genes were also expressed in the blastema of regenerating limbs (Fig. 4B, E). In situ hybridization on sectioned Xenopus regenerating limb buds further shows that wnt-3a and fgf-8 are specifically expressed in the apical epithelium of the blastema at 3 dpa (Fig. 4C, F).
These data suggest that wnt-3a is a candidate for mediating the function(s) of Wnt/β-catenin signaling during limb regeneration. In the initial process of amphibian limb regeneration, the amputated plane is rapidly covered with migrating epithelial cell layer that forms a specialized epithelial structure referred to as wound epithelium (Stocum, 1995; Han et al., 2005). As the regeneration process progresses, this epithelial cell layer thickens and forms an apical epithelial cap (AEC), a structure that is morphologically and functionally similar to the AER in amniote limb buds (Muneoka and Sassoon, 1992). The localization of transcript to the apical epithelium suggests that Wnt-3a and subsequent activation of Wnt/β-catenin signaling may function in the formation of the so-called “AEC” during limb regeneration.
To obtain more mechanistic insights into the roles of Wnt/β-catenin signaling in limb regeneration, we examined the expression of fgf-8 and fgf-10 following the induction of Dkk1GFP expression. F0 wild-type and hsDkk1GFP tadpoles were heat-shocked at 3 dpa or 5 dpa, and were fixed shortly (8 hours) after heat-shock to address the effect of Dkk1GFP on fgf-8 and fgf-10 expression (Fig. 5A). When tadpoles were heat-shocked at 5 dpa, fgf-8 expression was suppressed in the blastemas of hsDkk1GFP tadpoles (Fig. 5B b; n=5/6), while in all wild-type tadpoles the expression of fgf-8 remained unchanged, localized to the inner layer of the apical epithelium of the blastemas (Fig. 5B a; n=6/6). Similarly, fgf-8 expression was also suppressed in the hsDkk1GFP tadpoles heat-shocked at 3 dpa while all wild type tadpoles expressed fgf-8 (n=4/6; data not shown).
Fig. 5.
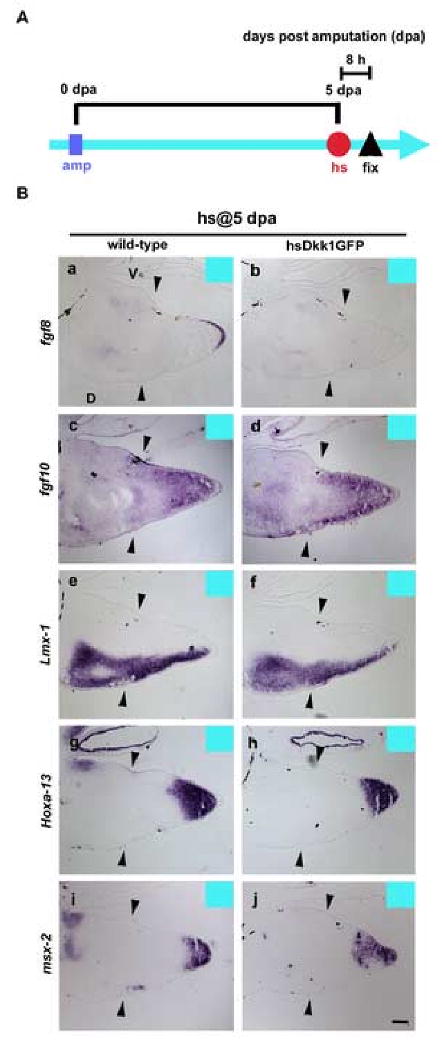
The Dkk1GFP represses fgf-8 expression in the regenerating blastemas, but not fgf-10 and other marker expressions. (A) Experimental scheme. One heat-shock was applied to tadpoles at 5 dpa (blue line). Wild-type and hsDkk1GFP tadpoles were fixed 8 hours after the heat-shock. (B) in situ hybridization on sectioned samples of blastemas. Sectioned samples were hybridized with fgf-8 (a and b), fgf-10 (c and d), Lmx-1 (e and f), Hoxa-13 (g and h) or msx-2 (i and j). To guarantee the correct comparisons of the gene expression level, wild-type (a, c, e, g and i) and hsDkk1GFP (b, d, f, h and j) tadpole sections were subjected to the completely same procedure of in situ hybridization together, respectively. Arrowheads show amputation level (knee level). D, dorsal; V, ventral. Scale bar, 100 μm.
As the interval between the heat-shock and fixation was short (8 hours), no significant morphological difference was observed among wild-type and hsDkk1GFP tadpoles. Our data show, then, that fgf-8 expression is dependent upon Wnt/β-catenin signaling during limb regeneration. In contrast to fgf-10 being thought to be regulated by Wnt/β-catenin signaling in limb bud and fin formation (Kawakami et al., 2001; Ng et al., 2002; Agarwal et al., 2003), we observed that expression of fgf-10 was not directly affected by the Dkk1GFP (Fig. 5B c, d,;n=6/6). However, it is still possible that Dkk1GFP may indirectly inhibit fgf10 expression through the suppression of fgf-8 in blastemas later than 8 hours after heat-shock since FGF-10 and FGF-8 constitute a positive feedback loop essential for limb outgrowth in amniote embryo (Ohuchi et al., 1997; Xu et al., 1998). One possible explanation for the difference of regeneration response among hsDkk1 tadpoles heat-shocked at 3 dpa and heat-shocked at 5 dpa is that the feedback loop between FGF-10 and FGF8 may be easily truncated by the Dkk1GFP through the suppression of fgf-8 during the blastema formation as expression levels of fgf-10 and fgf-8 are still low. However, once a cone-shape blastema is formed and once the strong expression of fgf-10 and fgf-8 is established, the feedback loop may be maintained with overcoming the temporal suppression of fgf-8 by the Dkk1GFP and result in partial limb regeneration.
Several reports strongly suggest that Wnt/β-catenin signaling controls the expression of fgf-8 in the developing limb buds of chick and mouse (Kengaku et al., 1998; Barrow et al., 2003; Soshnikova et al., 2003). Furthermore, in transgenic mice carrying a Wnt/β-catenin responsive reporter, the mice show reporter activity in the AER, in the fgf-8 expressing domain of limb buds. Moreover, defects in Wnt/β -catenin signaling caused the reduction of reporter activity as well as the absence of fgf-8 expression in the apical epithelium (Maretto et al., 2003). Based on these results, fgf-8 expression in the apical epithelium can be taken as an index of Wnt/β-catenin activity in limbs during morphogenesis. To exclude the possibility that the Dkk1GFP transgene suppressed not only Wnt/β-catenin signaling but non-specifically repressed other genes, in the present study we examined expression of Hoxa-13 (Fig. 5B g, h; n=6) and msx-2 (Fig. 5B i, j; n=6) and found that neither was altered by Dkk1GFP in blastemas. Based on these resuls, we concluded that the Dkk1GFP specifically blocked canonical Wnt/β -catenin signaling in blasetema of tadpoles and resulted in the suppression of fgf-8 gene expression in the hsDkk1GFP tadpoles.
Although we still cannot exclude the possibility that there may be other Wnt ligands expressed that mediate Wnt/β-catenin signaling during limb regeneration, the wnt-3a expression domain clearly overlaps with that of fgf-8 in the blastema and furthermore, wnt-3a is known to induce fgf-8 expression during the AER formation process of limb bud in chick embryo (Kengaku et al., 1998). Therefore, it is likely that wnt-3a plays a role in the initiation of limb regeneration by inducing fgf-8 expression in a β-catenin dependent manner.
Conclusions
Based on the critical roles of Wnt/β-catenin signaling in limb bud initiation during limb development and in stem cell renewal in amniotes, we hypothesized that Wnt/β-catenin signaling plays an essential role in initiation of limb regeneration. To test this hypothesis, we created transgenic Xenopus laevis tadpoles that express a Wnt/β-catenin antagonist, Dkk1, under the control of a heat-shock promoter and we used heat shock at various time-points during limb regeneration to express Dkk1 and thus to inhibit endogenous Wnt/β-catenin signaling. A single heat-shock, just prior to limb amputation or during early blastema formation, blocked limb regeneration with high efficiency. However, induction of Dkk1 by heat-shock after blastema formation allowed tadpoles to escape complete block of regeneration resulting in the production of incomplete limbs. Dkk1 inhibition of Wnt/β-catenin signaling during regeneration repressed fgf-8 but not fgf-10 in the regenerating blastema. These findings help to position Wnt signaling in the hierarchy of signaling events important to early stages of limb regeneration. In conclusion, we demonstrate that Wnt/β-catenin signaling plays an essential role during the early phases of limb regeneration and is important, but not absolutely required, during the subsequent phases of limb regeneration in Xenopus.
Acknowledgments
We thank Dr. Stefan Hoppler for providing the Xenopus hsp70 promoter and for advice on how to control this promoter, and Dr. Gilbert Weidinger for the zebrafish Dkk1 clone and for discussions. We thank Travis Biechele for technical help with luciferase assays, Jeanot Muster and Jerry Ament for excellent frog care, and Dr. Charlotte Hubbert for reading the manuscript. We thank Drs. Hiroyuki Ide and Koji Tamura for the gift of Xenopus fgf-10, fgf-8, Lmx-1, Hoxa-13 and msx-2 clones. HY was supported by JSPS Research Fellowships for Young Scientists and JSPS Postdoctoral Fellowships for Research Abroad. C. S. C. is a recipient of a NIH-funded Cardiovascular Pathology Training Grant. This work was supported by National Institutes of Health award RO1 GM073887-01. RTM is an investigator of the HHMI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agarwal P, Wylie JN, Galceran J, Arkhitko O, Li C, Deng C, Grosschedl R, Bruneau BG. Tbx5 is essential for forelimb bud initiation following patterning of the limb field in the mouse embryo. Development. 2003;130:623–633. doi: 10.1242/dev.00191. [DOI] [PubMed] [Google Scholar]
- Barrow JR, Thomas KR, Boussadia-Zahui O, Moore R, Kemler R, Capecchi MR, McMahon AP. Ectodermal Wnt3/β-catenin signaling is required for the establishment and maintenance of the apical ectodermal ridge. Genes Dev. 2003;17:394–409. doi: 10.1101/gad.1044903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324–331. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- Beck CW, Christen B, Slack JMW. Molecular pathways needed for regeneration of spinal cord and muscle in a vertebrate. Dev Cell. 2003;5:429–439. doi: 10.1016/s1534-5807(03)00233-8. [DOI] [PubMed] [Google Scholar]
- Brockes JP, Kumar A. Appendage regeneration in adult vertebrates and implications for regenerative medicine. Science. 2005;310:1919–1923. doi: 10.1126/science.1115200. [DOI] [PubMed] [Google Scholar]
- Bryant SV, Endo T, Gardiner DM. Vertebrate limb regeneration and the origin of limb stem cells. Int J Dev Biol. 2002;46:887–896. [PubMed] [Google Scholar]
- Christen B, Slack JMW. FGF-8 is associated with anteroposterior patterning and limb regeneration in Xenopus. Dev Biol. 1997;192:455–466. doi: 10.1006/dbio.1997.8732. [DOI] [PubMed] [Google Scholar]
- Christensen RN, Weinstein M, Tassava RA. Fibroblast growth factors in regenerating limbs of Ambystoma: cloning and semi-quantitative RT-PCR expression studies. J Exp Zool. 2001;290:529–540. doi: 10.1002/jez.1097. [DOI] [PubMed] [Google Scholar]
- Christensen RN, Weinstein M, Tassava RA. Expression of fibroblast growth factors 4, 8, and 10 in limbs, flanks and blastemas of Ambystoma. Dev Dyn. 2002;223:193–203. doi: 10.1002/dvdy.10049. [DOI] [PubMed] [Google Scholar]
- Coelho CND, Sumoy L, Rodgers BJ, Davidson DR, Hill R, Upholt WB, Kosher RA. Expression of the chicken homeobox-containing gene GHox-8 during embryonic chick development. Mech Dev. 1991;34:143–154. doi: 10.1016/0925-4773(91)90051-7. [DOI] [PubMed] [Google Scholar]
- Dent JN. Limb regeneration in larvae and metamorphosing individuals of the South African clawed toad. J Morphol. 1962;110:61–77. doi: 10.1002/jmor.1051100105. [DOI] [PubMed] [Google Scholar]
- Endo T, Yokoyama H, Tamura K, Ide H. Shh expression in developing and regenerating limb buds of Xenopus laevis. Dev Dyn. 1997;209:227–232. doi: 10.1002/(SICI)1097-0177(199706)209:2<227::AID-AJA8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Endo T, Tamura K, Ide H. Analysis of gene expressions during Xenopus forelimb regeneration. Dev Biol. 2000;220:296–306. doi: 10.1006/dbio.2000.9641. [DOI] [PubMed] [Google Scholar]
- Gardiner DM, Endo T, Bryant SV. The molecular basis of amphibian limb regeneration: integrating the old with the new. Semin Cell Dev Biol. 2002;13:345–352. doi: 10.1016/s1084952102000903. [DOI] [PubMed] [Google Scholar]
- Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- Han MJ, An JY, Kim WS. Expression patterns of Fgf-8 during development and limb regeneration of the axolotl. Dev Dyn. 2001;220:40–48. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1085>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Han M, Yang X, Taylor G, Burdsal CA, Anderson RA, Muneoka K. Limb regeneration in higher vertebrates: developing a roadmap. Anat Rec B New Anat. 2005;287:14–24. doi: 10.1002/ar.b.20082. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Capdevila J, Büscher D, Itoh T, Rodríguez Esteban C, Izpisúa Belmonte JC. WNT signals control FGF-dependent limb initiation and AER induction in the chick embryo. Cell. 2001;104:891–900. doi: 10.1016/s0092-8674(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Kengaku M, Capdevilla J, Rodriguez-Esteban C, De La Pena J, Johnson RL, Izpisúa Belmonte JC, Tabin CJ. Distinct WNT pathways regulating AER formation and dorsoventral polarity in the chick limb bud. Science. 1998;280:1274–1277. doi: 10.1126/science.280.5367.1274. [DOI] [PubMed] [Google Scholar]
- Kroll KL, Amaya E. Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development. 1996;122:3173–3183. doi: 10.1242/dev.122.10.3173. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, Delius H, Hoppe D, Stannek P, Walter C, Glinka A, Niehrs C. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002;417:664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/β-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Yokoyama H, Endo T, Tamura K, Ide H. Epidermal signal regulates Lmx-1 expression and dorsal-ventral pattern during Xenopus limb regeneration. Dev Biol. 2001;229:351–362. doi: 10.1006/dbio.2000.9973. [DOI] [PubMed] [Google Scholar]
- Muneoka K, Holler-Dinsmore G, Bryant SV. Intrinsic control of regenerative loss in Xenopus laevis limbs. J Exp Zool. 1986;240:47–54. doi: 10.1002/jez.1402400107. [DOI] [PubMed] [Google Scholar]
- Muneoka K, Sassoon D. Molecular aspects of regeneration in developing vertebrate limbs. Dev Biol. 1992;152:37–49. doi: 10.1016/0012-1606(92)90154-9. [DOI] [PubMed] [Google Scholar]
- Ng JK, Kawakami Y, Büscher D, Raya A, Itoh T, Koth CM, Rodríguez Esteban C, Rodríguez-León J, Garrity DM, Fishman MC, Izpisúa Belmonte JC. The limb identity gene Tbx5 promotes limb initiation by interacting with Wnt2b and Fgf10. Development. 2002;129:5161–5170. doi: 10.1242/dev.129.22.5161. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin) Garland Publishing; New York and London: 1994. [Google Scholar]
- Nohno T, Noji S, Koyama E, Nishikawa K, Myokai F, Saito T, Taniguchi S. Differential expression of msh-related homeobox genes CHox-7 and CHox-8 during chick limb development. Biochem Biophys Rec Comm. 1992;182:12–128. doi: 10.1016/s0006-291x(05)80120-2. [DOI] [PubMed] [Google Scholar]
- Offield MF, Hirsch N, Grainger RM. The development of Xenopus tropicalis transgenic lines and their use in studying lens developmental timing in living embryos. Development. 2000;127:1789–1797. doi: 10.1242/dev.127.9.1789. [DOI] [PubMed] [Google Scholar]
- Ohuchi H, Nakagawa T, Yamamoto A, Araga A, Ohta T, Ishimaru Y, Yoshioka H, Kuwana T, Nohno T, Yamasaki M, Itoh N, Noji S. The mesenchymal factor, FGF10, initiates and maintains the outgrowth of the chick limb bud through interaction with FGF8, an apical ectodermal factor. Development. 1997;124:2235–2244. doi: 10.1242/dev.124.11.2235. [DOI] [PubMed] [Google Scholar]
- Riddle RD, Ensini M, Nelson C, Tsuchida T, Jessel TM, Tabin C. Induction of the LIM homeobox gene Lmx-1 by WNT7a establishes dorsoventral pattern in the vertebrate limb. Cell. 1995;83:631–640. doi: 10.1016/0092-8674(95)90103-5. [DOI] [PubMed] [Google Scholar]
- Robert B, Lyons G, Simandl BK, Kuroiwa A, Buckingham M. The apical ectodermal ridge regulates Hox-7 and Hox-8 gene expression in developing chick limb buds. Gene Dev. 1991;5:2363–2374. doi: 10.1101/gad.5.12b.2363. [DOI] [PubMed] [Google Scholar]
- Siemering KR, Golbik R, Sever R, Haseloff J. Mutations that suppress the thermosensitivity of green fluorescent protein. Curr Biol. 1996;6:1653–1663. doi: 10.1016/s0960-9822(02)70789-6. [DOI] [PubMed] [Google Scholar]
- Soshnikova N, Zechner D, Huelsken J, Mshina Y, Behringer RR, Taketo MM, Crenshaw EB, III, Birchmeier W. Genetic interaction between Wnt/β-catenin and BMP receptor signaling during formation of the AER and the dorsal-ventral axis in the limb. Gene Dev. 2003;17:1963–1968. doi: 10.1101/gad.263003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocum DL. Wound Repair, Regeneration and Artificial Tissues. R. G. Landes; Austin: 1995. [Google Scholar]
- Stocum DL. New tissues from old. Science. 1997;276:15. doi: 10.1126/science.276.5309.15. [DOI] [PubMed] [Google Scholar]
- Stoick-Cooper C, Weidinger G, Riehle KJ, Hubbert C, Major MB, Fausto N, Moon RT. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development. 2007;134:479–489. doi: 10.1242/dev.001123. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Satoh A, Ide H, Tamura K. Nerve-dependent and –independent events in blastema formation during Xenopus froglet limb regeneration. Dev Biol. 2005;286:361–375. doi: 10.1016/j.ydbio.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Tamura K, Kuraishi R, Saito D, Masaki H, Ide H, Yonei-Tamura S. Evolutionary aspects of positioning and identification of vertebrate limbs. J Anat. 2001;199:195–204. doi: 10.1046/j.1469-7580.2001.19910195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Q, Yokota C, Puck H, Kofron M, Birsoy B, Yan D, Asashima M, Wylie CC, Lin X, Heasman J. Maternal Wnt11 activates the canonical Wnt signaling pathway required for the axis formation in Xenopus embryos. Cell. 2005;120:857–871. doi: 10.1016/j.cell.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Tschumi PA. The growth of hind limb bud of Xenopus laevis and its dependence on the epidermis. J Anat. 1957;9:149–173. [PMC free article] [PubMed] [Google Scholar]
- Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol. 2003;13:680–685. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- Vogel A, Rodriguez C, Warnken W, Izpisúa Belmonte JC. Dorsal cell fate specified by chick Lmx-1 during vertebrate limb development. Nature. 1995;378:716–720. doi: 10.1038/378716a0. [DOI] [PubMed] [Google Scholar]
- Wheeler GN, Hamilton FS, Hoppler S. Inducible gene expression in transgenic Xenopus embryos. Curr Biol. 2000;10:849–852. doi: 10.1016/s0960-9822(00)00596-0. [DOI] [PubMed] [Google Scholar]
- Wolda SL, Moody CJ, Moon RT. Overlapping expression of Xwnt-3A and Xwnt-1 in neural tissue of Xenopus laevis embryos. Dev Biol. 1993;155:46–57. doi: 10.1006/dbio.1993.1005. [DOI] [PubMed] [Google Scholar]
- Xu X, Weinstein M, Li C, Naski M, Cohen RI, Omitz DM, Leder P, Deng C. Fibroblast growth factor receptor 2 (FGFR2)-mediated reciprocal regulation loop between FGF8 and FGF10 is essential for limb induction. Development. 1998;125:753–765. doi: 10.1242/dev.125.4.753. [DOI] [PubMed] [Google Scholar]
- Yang Y. Wnts and wing: Wnt signaling in vertebrate limb development and musculoskeletal morphogenesis. Birth Defects Res (Part C) 2003;69:305–317. doi: 10.1002/bdrc.10026. [DOI] [PubMed] [Google Scholar]
- Yokouchi Y, Sasaki H, Kuroiwa A. Homeobox gene expression correlated with the bifurcation process of limb cartilage development. Nature. 1991;353:443–445. doi: 10.1038/353443a0. [DOI] [PubMed] [Google Scholar]
- Yokoyama H, Endo T, Tamura K, Yajima H, Ide H. Multiple digit formation in Xenopus limb bud recombinants. Dev Biol. 1998;196:1–10. doi: 10.1006/dbio.1998.8856. [DOI] [PubMed] [Google Scholar]
- Yokoyama H, Yonei-Tamura S, Endo T, Izpisúa Belmonte JC, Tamura K, Ide H. Mesenchyme with fgf-10 expression is responsible for regenerative capacity in Xenopus limb buds. Dev Biol. 2000;219:18–29. doi: 10.1006/dbio.1999.9587. [DOI] [PubMed] [Google Scholar]
- Yokoyama H, Ide H, Tamura K. FGF-10 stimulates limb regeneration ability in Xenopus laevis. Dev Biol. 2001;233:72–79. doi: 10.1006/dbio.2001.0180. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Urase K, Takahashi J, Ishii Y, Yasugi S. Muscus-associated antigen in epithelial cells of the chicken digestive tract: developmental change in expression and implications for morphogenesis-function relationships. Dev Growth Differ. 1996;38:185–192. doi: 10.1046/j.1440-169X.1996.t01-1-00008.x. [DOI] [PubMed] [Google Scholar]


