Abstract
The effects of Tat, an HIV-1 protein, on intravenous cocaine-induced locomotor activity were examined in ovariectomized rats. Animals were habituated to activity chambers, administered an IV baseline/saline injection, and 24 h later, received bilateral, intra-accumbal microinjections of Tat1-72 (15 μg/μl) or vehicle. Twenty four hours later, rats received the first of 14 daily IV cocaine injections (3.0 mg/kg/inj, 1/day) or saline. Locomotor activity was measured in automated chambers for 30 min following baseline and after the 1st and 14th cocaine injections. Observational time sampling following cocaine was also performed. Following acute cocaine/saline, Tat significantly increased cocaine-induced total activity over the 30-min session, with no significant effects for activity in the central compartment. Repeated cocaine injections produced behavioral sensitization with ~2-fold higher levels of total activity, ~3-fold higher levels of centrally directed activity, and increased locomotor scores via direct observations. Following repeated cocaine/saline, Tat altered the development of cocaine-induced behavioral sensitization for total activity with prior Tat exposure attenuating the development of cocaine-induced sensitization. Collectively, these data show that bilateral microinjection of Tat into the N Acc alters IV cocaine-induced behavior, suggesting that Tat may produce behavioral changes by disrupting the mesocorticolimbic pathway.
Keywords: Tat, HIV, Cocaine, behavioral sensitization, locomotor activity
1. Introduction
Patients with HIV, who also have a history of drug use, are more likely to develop neuropsychiatric disorders and HIV dementia (Bell et al., 1996; Davies et al., 1997; Koutsilieri et al., 2002). It has been suggested that chronic drug use potentiates the effects of HIV (Nath et al., 2001) perhaps by increasing HIV replication in the brain (Shapshak et al., 1996) and/or facilitating the onset of HIV encephalitis, such that neuropsychiatric disorders manifest at an earlier stage of AIDS for chronic drug users. Despite these reports, the mechanisms underlying the HIV/drug synergism is not well understood.
The unique feature of HIV-induced neurodegeneration is that neuronal cell loss occurs in the absence of neuronal infection. HIV-1 is harbored in the perivascular macrophage/microglia, rather than the neuron, and research suggests that viral proteins which are released into the extracellular space are integral in the neuropathology associated with HIV (Nath, 2002; Nath & Geiger, 1998). Thus, transactivator of transcription (Tat) protein is one particular protein implicated in HIV associated dementia (HAD; Nath, 2002). Tat is detected in serum (~1 ng/ml) and in the extracellular matrix of perivascular compartments in the brain of HIV patients (Westendorp et al., 1995). Elevated Tat mRNA levels (Wesselingh et al., 1993; Wiley et al., 1996) and Tat have been measured in the brain tissue of patients with HAD (Valle et al., 2000), and moreover, in the brains of HIV-1 infected primates (Hudson et al., 2000).
Previous research indicates that Tat protein may be integral to the HIV/drug synergism that produces neural dysfunction. First, Tat alone has been shown to produce neurotoxicity via oxidative modification of proteins in both cell culture studies (Aksenova et al., 2005; Nath et al., 2002; Wallace et al., 2006) and in experiments that microinjected Tat into the striatum of adult rats (Aksenov et al., 2001; Bansal et al., 2000). Second, in vitro studies demonstrate that the combination of Tat and cocaine produces neurotoxicity, and furthermore, that cocaine enhances Tat mediated oxidative cell damage (Kendall et al., 2005; Turchan et al., 2001). Together, these data suggest that the synergistic effects of Tat and cocaine can support a neural environment which produces neurotoxicity, and perhaps neurocognitive effects in HIV+ patients.
HIV aggregates in the caudate nucleus and N Acc, structures within the basal ganglia that are responsible for control of voluntary movement and the initiation of motivated behavior, respectively (Wiley et al., 1998). The N Acc in particular receives the highest concentration of HIV (Wiley et al., 1998), and interestingly, is also an integral structure in the addictive effects of psychostimulant drugs such as cocaine (Kalivas, 2004; Pierce & Kalivas, 1997; Robinson & Berridge, 1993). For example, the N Acc receives extensive DA input from the ventral tegmental area following cocaine injection. Repeated cocaine exposure produces a long-lasting neurobehavioral alteration termed behavioral sensitization, which refers to the augmentation of a behavioral response, e.g. locomotor activity, following repeated drug administration (Downs and Eddy, 1932; Emmett-Olglesby, 1995; Kalivas and Weber, 1988; Post, 1980; Post and Contel, 1983; Zahniser and Peris, 1992). Contemporary theories of drug abuse posit that the transition from casual drug taking to addiction is mediated via a behavioral sensitization process (Robinson & Berridge, 1993; Pierce & Kalivas, 1997; Kalivas, 2004; Kalivas & Volkow, 2005). Thus, the N Acc, which receives the highest concentration of HIV, is also important in the process of behavioral sensitization (Wise & Bozarth, 1985; Robbins et al., 1989; Koob, 1992; Robinson & Berridge, 1993; Wiley et al., 1998; Nestler, 2001; Kalivas, 2004; Li et al., 2004).
In the present experiment, Tat was microinjected into the N Acc and the effects of Tat on acute and repeated IV cocaine-induced locomotor behavior was examined in rats. Rats were microinjected with Tat or VEH, and the behavioral response to acute and repeated IV cocaine (or saline) was measured on days 1 and 14, respectively, using automated activity chambers and a behavioral observation method previously described (Booze et al., 1999a; Fray et al., 1980; Harrod et al., 2004; 2005a; 2005b; Wallace et al., 1996). The aim of this experiment was to examine the effect of Tat microinjection on the behavioral response to acute and repeated IV cocaine in order to determine if intra-accumbal Tat alters cocaine-mediated behavior. Based on the findings of previous research (Aksenov et al., 2003; Bansal et al., 2000; Joyce and Koob, 1981; Koob et al., 1981; Kubos et al., 1987) it was hypothesized that a Tat-induced dysfunction of the N Acc would attenuate IV cocaine-induced sensitized locomotor activity in rats.
2. Methods
2.1. Animals
Sixty-four adult ovariectomized female, Sprague–Dawley rats (70 days old) were obtained from Harlan Laboratories, Inc. (Indianapolis, IN). All rats were surgically implanted with an Intracath IV catheter (22 ga, Becton/Dickinson General Medical Corp., Grand Prarie, TX), which was used as a SC, dorsally implanted port for chronic IV injections. The subcutaneous implantable access port for rats was developed and described by Mactutus et al. (1994). Ovariectomized rats were used because estrogen is protective against the neurotoxic effects of Tat (Wallace et al., 2006). The use of male rats does not avoid this issue because testosterone is also neuroprotective against Tat-induced neurotoxicity via an estrogen receptor mediated process (Kendall et al., 2005). Serial ovariectomy and indwelling jugular catheter surgeries occurred for all rats during the same procedure at Harlan Laboratories. Upon arrival at the animal care facilities, rats were placed in quarantine for 7 days, and then transferred to the colony. Animals were pair housed throughout the experiment and the catheters were flushed daily with 0.2 ml of heparinized (2.5%) saline. Rodent food (Pro-Lab Rat, Mouse Hamster Chow #3000) and water were provided ad lib. The colony was maintained at 21 ± 2° C, 50 ± 10% relative humidity and a 12L:12D cycle with lights on at 0700 h (EST). The animals were maintained according to the National Institute of Health (NIH) guidelines in AAALAC-accredited facilities. The experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at the University of South Carolina, Columbia.
2.2. Experimental Design and Procedures
2.2.1. Behavioral Apparatus
The activity monitors were square (40 × 40 cm) locomotor activity chambers (Hamilton-Kinder Inc., Poway, CA) that detected free movement of animals by infrared photocell interruptions. This equipment used an infrared photocell grid (32 emitter/detector pairs) to measure locomotor activity. The chambers were converted into round (~ 40 cm diameter) compartments by adding clear Plexiglas inserts; photocell emitter/detector pairs were tuned by the manufacturer to handle the extra perspex width. Recent research from our laboratory suggests that circular chambers may facilitate detecting behavioral sensitization (Harrod et al., 2006). All activity monitors were located in an isolated room.
2.2.2. Locomotor activity: Habituation and saline baseline
Rats in the vehicle-saline (VEH-SAL), Tat-saline (TAT-SAL), vehicle-cocaine (VEH-COC), and Tat-cocaine (TAT-COC) groups were habituated to the locomotor activity chambers for two 60-min sessions, one/day. Twenty four hours after the second habituation session, all rats were injected with IV saline and placed into the activity chambers for 30-min to measure baseline activity.
2.2.3. Intra-accumbal Tat microinjection
All rats received intra-accumbal Tat or vehicle (VEH; physiological saline) injections approximately 24 hours after the saline baseline activity session. Previous work demonstrates that the development of cocaine-induced sensitization is associated with structural changes in the core, but not the shell region of the N Acc (Li et al., 2004). We therefore administered Tat to the core region of the N Acc to determine if the sensitization process was disrupted. Rats were administered intra-accumbal Tat or vehicle injections according to methods previously described (Aksenov et al., 2003; Bansal et al., 2000). Briefly, rats were anesthetized using a mixture of ketamine hydrochloride and xylazine by intraperatoneal (IP) injection (7.5 mg ketamine/100 g b.wt. 30 mg xylazine/100 g b.wt.). Bilateral injection of 15 μg/μl of Tat was administered, using a silanized Hamilton Syringe at the following coordinates: 1.8 mm anterior, ± 1.6 mm lateral, and 7.0 mm ventral from bregma on the skull surface, over an interval of one min, with a one min tissue recovery period before withdrawal.
Previous research shows that injection of a ketamine/xylazine cocktail prior to cocaine may attenuate cocaine-induced behavioral sensitization when cocaine is delivered via the IP route of administration (Torres et al., 1994). These interactions may relate to the temporal occurrence of the two drugs or to repeated daily injections of ketamine (Rofael and Abdel-Rahman, 2002). In the present experiment, the ketamine/xylazine cocktail was administered before surgery, 24-h prior to the first cocaine injection. To the best of our knowledge, there is no evidence to support the contention that a single ketamine/xylazine injection preceding cocaine injection by 24-h could affect the induction of behavioral sensitization.
2.2.4. IV cocaine-induced locomotor activity
IV injections of cocaine (3.0 mg/kg/injection) or saline were initiated 24 hours after Tat injection according to a 2 × 2, protein (VEH, TAT) × drug (SAL, COC), design. The behavioral testing began twenty-four hours after Tat injection because previous research demonstrated that maximum cell damage was observed twenty-four hours after Tat administration (Aksenov et al., 2003; Bansal et al., 2000). The animals were immediately placed into locomotor activity chambers following cocaine or saline injection. The animals’ locomotor activity response to cocaine was assessed for 30 min on only two occasions: immediately after the first (Day 1) and last (Day 14) cocaine injections; however, rats were administered daily IV cocaine or saline injections in the home cage on days 2 – 13. This latter procedure is important to preclude the repeated pairing of cocaine injection and the testing environment that otherwise confounds the neural expression of sensitization with learning via classical conditioning (Anagnostaras and Robinson, 1996; Li et al., 2004).
Two automated measures of activity – total activity and activity in center of compartment – were investigated. Total activity represents all detectable movements (photocell interruptions) that occur in the chamber. Activity in the center (in cm) in a circular region located in the center most portion of the compartment was the second dependent measure. Previous research has demonstrated that repeated IV cocaine injections produce an increase, or behavioral sensitization, of centrally directed activity in male and female rats (Booze et al., 1999b; Wallace et al., 1996). The increased centrally directed activity following acute and repeated IV injection is not likely due to an anxiolytic effect of the drug as cocaine has been shown to produce a dose-dependent increase of anxiogenic behaviors in an elevated plus maze (DeVries & Pert, 1998; Paine et al., 2002). Rather, rats exhibit thigmotaxic behavior and perform a number of locomotor and orofacial-related behaviors. The stimulant effects of drugs like cocaine increase activity in the center (Booze et al., 1999b; Harrod et al., 2004; Wallace et al., 1996), demonstrating cocaine-induced behavioral sensitization of locomotor activity. In order to determine the activity in center, Hamilton-Kinder, Inc. software was used to impose a circular region (~25% of total area) in the center of the compartment during the data reduction phase (i.e., following completion of the activity session) of the experiment, yielding the activity data in the center. Time course data for both automated measures of activity are presented in 10 min blocks of a 30-min session. Activity sessions were not video taped.
In addition to the automated monitoring, an observational time sampling procedure was employed. An observer, unaware of the treatment condition of the animal, observed and recorded the animal’s behavior, using a well-established protocol (Harrod et al., 2004; Harrod et al., 2005a; 2005b). Previous research by Booze et al. (1997) determined that a 3.0 mg/kg/injection of cocaine resulted in peak arterial plasma levels within one-min of IV administration. Thus, five of the six observational time points occurred within the first 30 min of the session to insure that the maximal amount of cocaine-induced locomotion would be observed. The current observational procedure represents an adaptation of Fray et al. (1980). Briefly, the present procedure excluded the sway and miscellaneous categories and added scan, headbob, yawn, and lying down. Another modification included observations at 1, 5, 10, 15, and 30 min, whereas Fray et al. (1980) observed behaviors at 10, 20, and 30 min. Testing occurred between 1500–1700 h under dim light conditions, in the absence of direct overhead lighting (< 10 lux). All animals were sacrificed according to procedures used in Harrod et al. (2004). Brains were collected, frozen on dry ice, and stored at 80°C. Cryostat-cut sections (20 μm) were collected through the nucleus accumbens and Nissl-stained to verify injection sites.
2.3. Drug Treatment
Recombinant Tat1-72 was produced as described in Ma & Nath (1997). Tat was dissolved in a sterile buffer [vehicle (VEH); 10 mM Tris HCl, 300 nM NaCl, pH ~7.58, sterile]. Previous research from our laboratory demonstrated that Tat1-72 dose-dependently (1, 5, 15, 50 μg) produced striatal toxicity in rats (Aksenov et al., 2001; Aksenov et al., 2003; Bansal et al., 2000). The highest dose of Tat (50 μg) induced sub-lethal histopathological changes in the striatum as there were no Tat-induced “clinical” outcomes such as convulsions, tremors, or morbidity (Aksenov et al., 2003). Rats in the present study were bilaterally injected with 15 μg of Tat. The 15 μg dose of Tat is within the range of doses (5, 15, and 50 μg Tat1-72) shown to produce significant increases in glial fibrillary acidic (GFAP) protein levels (astrocytosis) between 7 and 30 days after a single microinjection. The 15 μg concentration of Tat1-72 was also chosen because the increase in GFAP levels in response to this dose co-occurs with a time-course that would be expected to overlap with the initiation of cocaine-induced behavioral sensitization using our behavioral procedures (i.e., ~ 7 days; Harrod et al., 2005a).
The cocaine treatment was administered as a bolus injection delivered in a volume of 1 ml/kg body weight (15 s), and was followed by flushing (15 s) with 0.2 ml heparinized (2.5%) saline (i.e., the approximate volume of the catheter). The dose of cocaine hydrochloride (3.0 mg/kg/day; Sigma, St. Louis, MO) was calculated on the weight of the salt and dissolved in saline immediately prior to injection in a volume of 1 ml/kg. The injection rate used in the present experiment was used in the previous pharmacokinetic analysis (Booze et al., 1997) and is within the duration shown to produce moderate-to-robust behavioral sensitization to psychostimulant drugs (Booze et al., 1999a;Booze et al., 1999b; Harrod et al., 2004; 2005a; 2005b; Samaha et al., 2002; Wallace et al., 1996). The IV dosing regimen used in the present experiment has been shown to produce arterial drug concentrations and a pharmacokinetic profile in rats similar to levels demonstrated in human cocaine volunteers (Booze et al., 1997; Evans et al., 1996), and has been associated with producing reinforcing and euphoric effects in humans (Fischman and Schuster, 1982).
2.4. Data Analysis
The automated data were analyzed using analysis of variance (ANOVA) techniques (BMDP statistical Software, 1990; SPSS, 2003; Winer, 1971). A mixed factorial ANOVA, with protein (2 levels: VEH, Tat) and drug (2 levels: SAL, COC) as between-subjects factors, and day (2 levels: acute COC/SAL day 1, repeated COC/SAL day 14) and time (3 levels: 10, 20, and 30 min) as within-subjects factors, was conducted for total activity and activity in centermost portion of the compartment. Percent scores for cocaine-induced activity were calculated for acute and repeated data (Day 1 and Day 14, respectively) based on the 10-min time period. The individual data of the cocaine injected rats were divided by the mean of the saline controls and then multiplied by 100. A 3 (time) × 2 (protein), mixed model ANOVA was conducted on the percent scores of saline controls for both activity measures. For the within-subjects term (time), potential violations of sphericity were preferentially handled by the use of orthogonal contrasts or, if necessary, the Greenhouse–Geisser d.f. correction factor (Winer, 1971). Orthogonal contrasts were used to describe the linear and quadratic components of the temporal relationship between Tat and cocaine-mediated locomotor activity. An alpha level of p ≤ 0.05 was considered significant for all statistical tests used.
Chi square (χ2) tests were used to compare incidence scores between groups on the data derived from the observational time sampling procedure. Incidence refers to the occurrence or nonoccurrence, rather than the frequency, of a particular behavior. Incidence scores were derived by summing observations of a particular behavior, for example rearing, for a specific rat across six observations. Thus, the rearing incidence for each rat was summed across rats in a particular group. The incidence data are nominal and between subjects in nature and therefore were analyzed using the χ2 test. Scores below 5 are not reliably measured with the χ2 statistic (Siegel and Castellan, 1988). Therefore, composite scores were created to increase incidence scores, and therefore maintain statistical accuracy. Locomotor, head up/down, rearing, and scratching incidence scores were combined for a locomotor composite, and grooming, licking, and washing incidence were summed for the orofacial composite. A Yates correction procedure was used with the χ2 test. To further ensure statistical accuracy, a Bonferroni correction for multiple comparisons was used on the observed data. Thus, 12 comparisons were made for the locomotor and orofacial data separately, yielding a significance level of α ≤ 0.004 (i.e., 0.05/12). Fourteen rats were excluded from data analysis: 6 rats exhibited leaking or clogged IV catheters, whereas 8 animals received Tat or VEH injections outside of the nucleus accumbens region. These exclusions produced the following sample sizes: VEH-SAL n = 14; TAT-SAL n = 10; VEH-COC n = 13; and TAT-COC n =13.
3. Results
3.1. Histology
Analysis of Nissl-stained sections through the nucleus accumbens region confirmed injection into the core region of the nucleus accumbens; subjects with injection tracks outside of the nucleus accumbens were excluded from subsequent data analysis (n=8). No gross toxicity (e.g., no lesion) was present in the nucleus accumbens of Tat injected animals. However, increased numbers of reactive astrocytes, indicated by increased number of processes in staining density, were present in the area immediately adjacent to the injection track, similar to that previously reported following striatal Tat microinjections (Aksenov et al., 2003). Little or no reactivity was observed in VEH injected animals.
3.2. Body Weight
Following intra-accumbal Tat microinjection, the body weights were recorded across the 14-day cocaine injection period suggested no adverse effects of Tat or cocaine treatment. A 2 (day) × 2 (protein) × 2 (drug) ANOVA revealed a main effect of day [F (13, 598) = 620.8, p < 0.001; with a prominent linear component: F (1, 46) = 1261.3, p < 0.001, and a minor quadratic component: F (1, 46) = 4.8, p < 0.05] which indicated body weight increased over the 14-day experiment for all groups. However a significant protein × drug interaction [F (1, 46) = 5.5, p < 0.05] was found and localized to a difference in body weight on day 1 [protein × drug interaction, F(1, 46) = 6.25, p < 0.05], but not confirmed at later time points. Contrast analyses revealed that Tat-SAL and VEH-SAL groups initially differed from each other [F(1, 46) = 6.45, p < 0.05]. The specific group body weights on day 1 were (TAT-SAL: 239.1±5.3, VEH-SAL: 256.9±4.7, VEH-COC: 245.5±3.6, and TAT-COC: 251.9±5.4). No other main effects or interactions were statistically significant.
3.3. Automated Locomotor Activity
3.3.1. Total Activity
No significant differences in baseline total activity were detected; however, both the acute and repeated responses to cocaine were differentially affected by the prior intra-accumbal Tat injection.
Following 2 days of habituation in the activity chambers, total activity was recorded for all groups after a saline injection and is illustrated in figure 1. A 3 (time) × 2 (protein) × 2 (drug) ANOVA revealed a main effect of time [F (2, 92) = 143.0, pGG < 0.001] with prominent linear and quadratic components [F (1, 46) = 69.4, p < 0.001 and F (1, 46) = 167.7, p < 0.001, respectively]. However, as indicated in figure 1 all treatment groups displayed less activity over the 30-min session with no drug or protein effects or interactions.
FIG. 1.
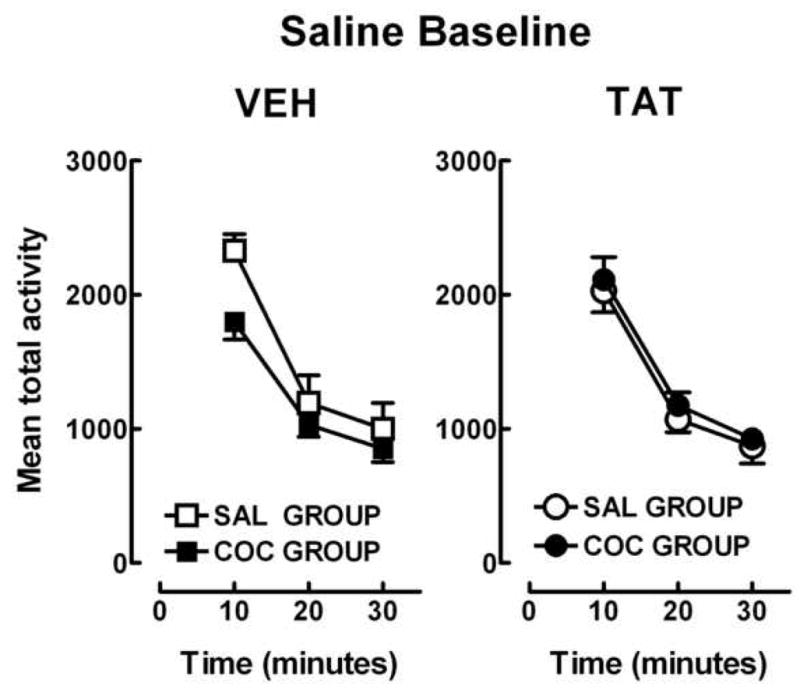
Mean total activity (± S.E.M.) for saline baseline day 1 during a 30-min session. All animals were habituated to the test environment for two sessions prior to baseline measurement. For clarity the two protein conditions, VEH and Tat, are presented in separate graphs. n = 10–14 rats/group.
The acute response to cocaine (Day 1) is illustrated in figure 2A with the data expressed as percent of the saline control. A 3 (time) × 2 (protein) ANOVA revealed a significant main effect of time [F (2, 48) = 38.1, pGG < 0.001] with a prominent linear component [F(1, 24) = 54.8, p<0.001] and a significant time × protein interaction [F (2, 48) = 3.9, pGG ≤ 0.03; with a quadratic component: F(1, 24) = 4.9, p ≤ 0.04]], indicating that the groups significantly diverged across time. The time × protein interaction confirmed that acute cocaine-induced total activity is enhanced by Tat treatment across time.
FIG 2.
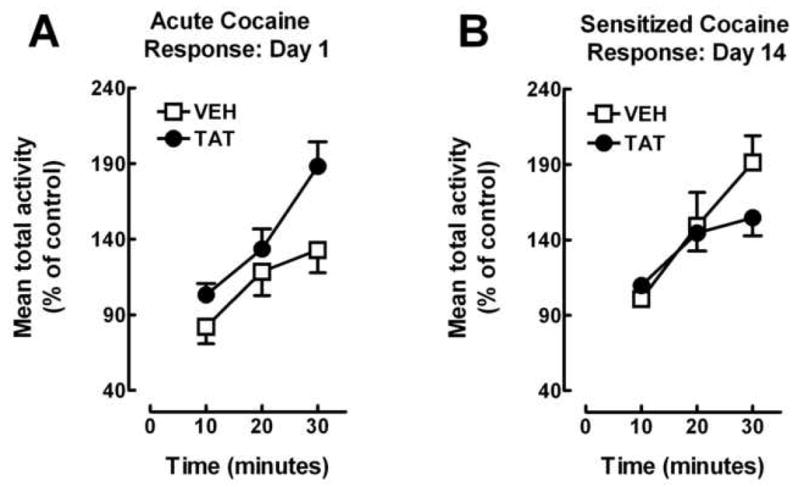
(A) Mean (± S.E.M.) total activity for acute cocaine response (Day 1) expressed as percent of saline control. n = 10–14 rats/group. The significant time × protein interaction indicates that Tat enhanced the locomotor effects of acute IV cocaine (3.0 mg/kg/injection (pGG ≤0.03). (B). Mean (± S.E.M.) total activity for sensitized cocaine response (Day 14) expressed in percent of control. n = 10–14 rats/group. The significant time × protein interaction indicates that ovariectomized females exhibit IV cocaine-induced sensitization following 14 daily injections (3.0 mg/kg/injection); Tat attenuated the cocaine-induced behavioral sensitization (pGG ≤0.02).
The sensitized response to cocaine (Day 14) is illustrated in figure 2B with the data expressed as percent of the saline control. A 3 (time) × 2 (protein) ANOVA revealed a significant main effect of time [F (2, 48) = 38.8, pGG < 0.001; with a prominent linear component: F (1, 24) = 83.2, p < 0.001], and a significant time × protein interaction [F(2, 48) = 4.6, pGG≤0.02; with a linear component: F (1, 24) = 9.5, p < 0.01]. The time × protein interaction confirmed that cocaine-induced sensitization is attenuated by prior Tat injection. The magnitude of the sensitized response was approximately 2-fold higher for the cocaine injected animals on day 14 (2301.9±139.9) compared to day 1 (1376.4±94.2).
3.3.2. Centrally directed activity
A significant difference in initial 10 min centrally directed activity between VEH-SAL and VEH-COC groups in response to saline was not interpretable. However, more importantly, there was no evidence for any differential response to acute or repeated cocaine as a function of the prior intra-accumbal Tat injection.
Analysis of baseline centrally directed activity data with a 3 (time) × 2 (protein) × 2 (drug) ANOVA revealed a main effect of time [F (2, 92) = 62.2, pGG < 0.001] with linear and quadratic components [F (1, 46) = 65.6, p < 0.001 and F (1, 46) = 61.6, p < 0.001]. All treatment groups displayed less activity over the 30-min session. A significant time × protein × drug effect was also noted (F(2, 92) = 3.86, pGG<0.05) with a significant quadratic component [F(1, 46) = 8.98, p<0.01]. The time × protein × drug interaction indicated a prominent difference in centrally directed activity between the animals assigned to the VEH-Sal and VEH-Coc groups in the initial 10 min interval. No other statistically significant effect was noted.
For the acute response to cocaine (Day 1) a 3 (time) × 2 (protein) ANOVA was conducted on percent of the saline control. A significant main effect of time [F (2, 48) = 9.5, pGG < 0.01] with a linear component [F (1, 24) = 10.8, p < 0.01] was noted, indicating increased activity in the center compartment over the 30-min session [10 min: 83.0±12.0; 20 min: 160.3±31.8; 30 min: 277.3±54.9]. No other statistically significant effect was noted.
For the sensitized response to cocaine (Day 14) a 3 (time) × 2 (protein) ANOVA conducted on percent of the saline controls revealed a significant main effect of time [F (2, 48) = 19.8, pGG < 0.001; with a prominent linear component: F (1, 24) = 21.3, p < 0.001; and a minor quadratic component: F (1, 24) = 5.4, p ≤ 0.03]. Again, increased activity in the center compartment was recorded over the 30-min session [10 min: 101.9±9.4; 20 min: 164.0±25.9; 30 min: 273.3±44.1]. No other statistically significant effect was noted. The magnitude of the sensitized response was approximately 3-fold higher for the cocaine injected animals on day 14 (1296.4±166.0) compared to day 1 (471.7±78.0).
3.4. Observational Data
A modified version of the observational time sampling method first described by Fray et al. (1980) was used in the present experiment to examine the incidence of specific behaviors that rats exhibited following acute and repeated IV cocaine administration.
3.4.1. Effects of Tat and IV cocaine on locomotor composite incidence scores
For acute cocaine (Day 1) chi square analyses of composite incidence revealed no significant differences between any protein and/or drug condition (data not shown).
Repeated (Day 14) cocaine injection induced significantly higher locomotor composite incidence scores in the VEH-COC rats compared to the VEH-SAL group [χ2 (1) = 25.4, p ≤ 0.004]. Further, VEH-COC, TAT-SAL and TAT-COC groups exhibited a significant increase in locomotor incidence between days 1 and 14 [χ2 (1) = 15.2, p < 0.001; χ2 (1) = 14.4, p < 0.001, respectively]. This increase in locomotor incidence across Days 1 – 14 suggests that IV cocaine induced behavioral sensitization of locomotor incidence in the cocaine treated groups (data not shown). No differential Tat effects were noted on Day 14.
3.4.2. Effects of Tat and IV cocaine on orofacial composite incidence scores
Chi square analyses indicate that there were no differences between groups following acute or repeated IV cocaine injection. Moreover, none of the orofacial composite incidence in any of the groups changed significantly from Day 1 to Day 14 (data not shown).
4. Discussion
Intra-accumbal Tat1-72 (15 μg/μl bilaterally) infusion acutely enhanced cocaine-induced total activity, but attenuated sensitized total activity after repeated IV cocaine administration. No Tat effect was noted on activity in the center, locomotor incidence, or the orofacial measure of activity. Similar to previous research, repeated IV cocaine resulted in robust behavioral sensitization as indexed by automated and observed measures of locomotor activity (Wallace et al., 1996; Booze et al., 1999b; Harrod et al., 2005a; 2005b); however, prior Tat exposure attenuated the development of cocaine-induced behavioral sensitization. We investigated the effects of Tat-induced damage to the N Acc to better understand how HIV related proteins alter the behavioral effects of IV COC. The present experiment used a behavioral sensitization procedure to determine the effects of Tat on cocaine-mediated changes in locomotor activity. The behavioral sensitization procedure was used because this process is believed to play an integral role in drug abuse behavior and has been investigated extensively (Robinson & Berridge, 1993; Pierce and Kalivas, 1997; Berridge, 2007; Kalivas & Volkow, 2005). Thus, contemporary theories of chronic addictive behavior suggest that the neurobiological changes that underlie behavioral sensitization may also increase the motivation for repeated drug use (Piazza et al., 1990; Horger et al., 1992; Robinson & Berridge, 1993; Vezina 2004).
The current behavioral findings demonstrate that intra-accumbal infusion of Tat enhances the acute effects of IV cocaine on total activity, but attenuates sensitized total activity after repeated cocaine administration. Previous research demonstrates that lesions of the nucleus accumbens alter DA mediated behaviors (Joyce and Koob, 1981; Koob et al., 1981; Kubos et al., 1987). For example, 6-hydroxydopamine (6-OHDA) lesions of the nucleus accumbens, which produces specific destruction of catecholamine releasing neurons, attenuates the locomotor activating effects of low doses of amphetamine in rats (Joyce and Koob, 1981; Koob et al., 1981). The mechanisms of Tat neurotoxicity may involve changes in cell oxidative status (Westendorp et al., 1995; Kruman et al., 1998; Shi et al., 1998) and oxidative damage to cell components (Aksenov et al., 2001; Aksenov et al., 2006), such as the DA transporter (DAT; Wallace et al., 2006). Regardless, the attenuating effects of sensitized locomotor activity by intra-accumbal Tat in the present experiment might indicate a general change in motivation for natural and drug related reinforcers and rewards in HIV-1-infected patients.
A number of studies now suggest that Tat-induced neurodegeneration and subsequent oxidative stress are the result of direct interaction between Tat1-72 and neurons (Aksenov et al., 2003; Aksenov et al., 2006). Although the exact mechanism by which Tat interacts with the neuronal membrane is not known, it is clear that a number of membrane bound proteins – and consequently their function and expression – are affected by Tat exposure. Thus, N-methyl-D-aspartate receptors (NMDAr), as well as NMDAr subtypes, have been implicated in Tat neurotoxicity (Prendergast et al., 2002; Self et al., 2003; Song et al., 2003), and moreover, Tat has been shown to decrease DAT number (Wang et al., 2004) and function (Wallace et al., 2006). It is well known that NMDAr are well expressed in the nucleus accumbens, and that ascending DA projections from the ventral tegmental area to the nucleus accumbens are replete with DAT. Thus, not only does Tat produce neurotoxicity, it alters membrane-bound proteins which regulate postsynaptic membrane function and the maintenance of synaptic levels of catecholamines which are integral for motivated behaviors, learning, affect, and memory. Therefore it will be important for future work to determine the extent to which Tat interacts with membrane bound proteins such as receptors and transporters given the role that these proteins play in the regulation of synaptic neurotransmitter levels, and ultimately behavior.
Previous research showed that ovariectomized rats did not express sensitization to repeated cocaine injection (Peris et al., 1991; van Haaren and Meyer, 1991; Sircar and Kim, 1999). The results of the present experiment are not consistent with these findings as repeated IV cocaine produced ~2-fold higher levels of total activity, ~3-fold higher levels of centrally directed activity, and increased locomotor scores via direct observations. Although previous research from our laboratory showed that ovariectomized rats exhibited less behavioral sensitization of total activity following repeated IV cocaine compared to intact female rats (same dose as present experiment; Harrod et al., 2005a), the current findings indicate that ovariectomized rats expressed IV cocaine-induced behavioral sensitization. The present study, as well as Harrod et al., (2005a), administered repeated cocaine via the IV route, whereas Peris et al., (1991), van Haaren and Meyer, (1991), and Sircar and Kim, (1999) administered intraperitoneal cocaine injections. One possible explanation for the discrepancy between studies which demonstrate cocaine-induced behavioral sensitization in ovariectomized rats and those that do not may be related to the brain levels of cocaine achieved. The IV route affords 100% bioavailability of drug, thus increasing the amount of cocaine in the brain relative to intraperitoneal injection. Thus, it is conceivable that ovariectomized animals require more cocaine to acquire behavioral sensitization. It is important to use ovariectomized rats in studies categorizing the neurotoxic effects of HIV viral proteins because estrogen is neuroprotective against Tat -induced damage (Lee et al., 2004; Wallace et al., 2006). Future experiments will be needed to determine the extent of Tat-mediated toxicity in gonadally intact female rats and furthermore, to understand if these viral proteins alter the development of cocaine-induced behavioral sensitization.
The development of behavioral sensitization is mediated by the ventral tegmental area, and that the nucleus accumbens is important for the expression of sensitization (Pierce & Kalivas, 1997). It should be noted that the present experiment did not test the expression of behavioral sensitization. In order to fully determine the expression of behavioral sensitization future experiments should use a design that administers a cocaine “challenge” following the drug-free period (Pierce and Kalivas, 1997).
HIV infected patients who also exhibit chronic drug abuse reveal increased incidence of HAD and other psychopathology, relative to HIV patients who do not abuse drugs (Bell et al., 1996; Davies et al., 1997). The increased expression of HAD in people who chronically self-administer drugs suggests a synergistic effect of HIV related viral proteins such as Tat and gp120, and addictive compounds such as crack cocaine and methamphetamine (Turchan et al., 2001; Kendall et al., 2005). A central question for basic science researchers investigating the relationship between HIV and neural dysfunction is how do viral proteins like Tat1-72 and cocaine interact to produce neurotoxicity, and ultimately HAD? Although the exact mechanisms of Tat/COC toxicity are currently unknown, findings from basic science research demonstrate that the combination of Tat and cocaine result in enhanced neurotoxicity, even though the dose of cocaine used in those experiments was not, by itself, toxic (Kendall et al., 2005). Interestingly, these experiments further show that the cocaine-enhanced neurotoxicity is, at least in part, mediated through DA receptors as blockade of D1 receptors with SCH 23390, a D1 receptor antagonist, attenuated cocaine-enhanced toxicity when Tat and cocaine were administered in combination to hippocampal cell cultures (Aksenov et al., 2006). Notably, HIV produces continuous levels of viral protein which are present during repeated cocaine use. However, a single exposure to Tat has been shown to initiate a cascade of molecular events with a long temporal course (e.g., Aksenov et al., 2006) and is capable of inducing long-term neurotoxicity in the hippocampus when injected neonatally (Fitting et al., 2008). The results of the present experiment follow with this protocol of using a single intermediate dose of Tat. The present findings demonstrate Tat mediated toxicity when combined with cocaine, and further suggest that understanding the synergistic effects of repeated Tat administration and cocaine will be important to effectively treat patients who exhibit co-morbidity of HIV and drug dependence.
The current behavioral findings are based on a single concentration of Tat, and therefore, further dose-response studies are needed to determine the concentration of intra-accumbal Tat that disrupts the expression of IV cocaine-induced behavioral sensitization. Moreover, one Tat exposure was administered in the current experiment. Given that HAD is mediated by the continuous expression of Tat, it will be important for future experiments to utilize Tat transgenic mice in studies investigating the acute and repeated effects of cocaine (Kim et al., 2003). Clearly, Tat is not the only HIV viral protein which contributes to brain damage in crack cocaine dependent individuals. Recent research shows that the combination of Tat and gp120 produces a synergistic neurotoxic effect in the rat brain (Bansal et al., 2000; Nath et al., 2002). Therefore, future studies will be necessary to examine the behavioral effects of Tat and gp120 in the nucleus accumbens, and the subsequent behavioral response to repeated IV COC. It will also be necessary for future research to investigate the effects of intra-accumbal Tat directly on motivated behaviors, such as food and drug-maintained responding, to better understand how cocaine use and HIV related viral proteins may alter the motivation to engage in behaviors necessary to be healthy and to maintain positive social interactions. Together, this research will begin to elucidate the effects of HIV viral proteins on the mesocorticolimbic DA system and will guide the development of treatment strategies to further prevent behavioral problems in individuals who acquire HIV and exhibit co-morbid drug taking behavior.
Acknowledgments
This research and the investigators were supported by grants DA09160, HD043680, DA11337, DA13137, and DA21287. The authors would like to thank Cantey Land, Ph.D. for her technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aksenov MY, Aksenova MV, Nath A, Ray PD, Mactutus CF, Booze RM. Cocaine-mediated enhancement of Tat toxicity in rat hippocampal cell cultures: The role of oxidative stress and D1 dopamine receptor. Neurotoxicology. 2006;27(2):217–28. doi: 10.1016/j.neuro.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Aksenov MY, Hasselrot U, Bansal AK, Wu G, Nath A, Anderson C, Mactutus CF, Booze RM. Oxidative damage induced by the injection of HIV-1 Tat protein in the rat striatum. Neurosci Lett. 2001;305:5–8. doi: 10.1016/s0304-3940(01)01786-4. [DOI] [PubMed] [Google Scholar]
- Aksenov MY, Hasselrot U, Wu G, Nath A, Anderson C, Mactutus CF, Booze RM. Temporal relationships between HIV-1 Tat-induced neuronal degeneration, OX-42 immunoreactivity, reactive astrocytosis, and protein oxidation in the rat striatum. Brain Res. 2003;987:1–9. doi: 10.1016/s0006-8993(03)03194-9. [DOI] [PubMed] [Google Scholar]
- Aksenova MV, Aksenov MY, Mactutus CF, Booze RM. Cell culture models of oxidative stress and injury in the central nervous system. Current Neurovasc Res. 2005;2(1):73–89. doi: 10.2174/1567202052773463. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Robinson TE. Sensitization to the psychomotor stimulant effects of amphetamine: modulation by associative learning. Behav Neurosci. 1996;110(6):1397–1414. doi: 10.1037//0735-7044.110.6.1397. [DOI] [PubMed] [Google Scholar]
- Bansal AK, Mactutus CF, Nath A, Maragos W, Hauser KF, Booze RM. Neurotoxicity of HIV-1 proteins gp120 and Tat in the rat striatum. Brain Res. 2000;879:42–49. doi: 10.1016/s0006-8993(00)02725-6. [DOI] [PubMed] [Google Scholar]
- Becker JB, Molenda M, Hummer DL. Gender differences in the behavioral responses to cocaine and amphetamine: Implications for mechanisms mediating gender differences in drug abuse. Ann N Y Acad Sci. 2001;937:172–187. doi: 10.1111/j.1749-6632.2001.tb03564.x. [DOI] [PubMed] [Google Scholar]
- Bell JE, Donaldson YK, Lowrie S, McKenzie CA, Elton RA, Chiswick A, Brettle RP, Ironside JW, Simmonds P. Influence of risk group and zidovudine therapy on the development of HIV encephalitis and cognitive impairment in AIDS patients. AIDS. 1996;10(5):493–499. doi: 10.1097/00002030-199605000-00007. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology. 2007;191(3):391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- BMDP Statistical Software. University of California Press; Berkeley, CA: 1990. [Google Scholar]
- Booze RM, Lehner AF, Wallace DR, Welch MA, Mactutus CF. Dose-response cocaine pharmacokinetics and metabolite profile following intravenous administration and arterial sampling in unanesthetized, freely moving male rats. Neurotoxicol Teratol. 1997;19(1):7–15. doi: 10.1016/s0892-0362(96)00180-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booze RM, Welch MA, Wood ML, Billings KA, Apple SR, Mactutus CF. Behavioral sensitization following repeated intravenous nicotine administration: gender differences and gonadal hormones. Pharmacol Biochem Behav. 1999a;64(4):827–839. doi: 10.1016/s0091-3057(99)00169-0. [DOI] [PubMed] [Google Scholar]
- Booze RM, Wood ML, Welch MA, Berry S, Mactutus CF. Estrous cyclicity and behavioral sensitization in female rats following repeated intravenous cocaine administration. Pharmacol Biochem Behav. 1999b;64(3):605–610. doi: 10.1016/s0091-3057(99)00154-9. [DOI] [PubMed] [Google Scholar]
- Davies J, Everall IP, Weich S, McLaughlin J, Scaravilli F, Lantos PL. HIV-associated brain pathology in the United Kingdom: an epidemiological study. AIDS. 1997;11:1145–1150. doi: 10.1097/00002030-199709000-00010. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Pert A. Conditioned increases in anxiogenic-like behavior following exposure to contextual stimuli associated with cocaine are mediated by corticotropin-releasing factor. Psychopharmacology (Berl) 1998;137(4):333–40. doi: 10.1007/s002130050627. [DOI] [PubMed] [Google Scholar]
- Downs AW, Eddy NB. The effect of repeated doses of cocaine on the rat. J Pharmacol Exp Ther. 1932;46:199–200. [Google Scholar]
- Emmett-Olglesby MW. Sensitization and tolerance to motivational and subjective effects of psychostimulants. In: Hammer RP Jr, editor. The neurobiology of cocaine: Cellular and molecular mechanisms. Boca Raton: CRC Press; 1995. pp. 31–47. [Google Scholar]
- Evans SM, Cone EJ, Henningfield JE. Arterial and venous cocaine plasma concentrations in humans: relationship to route of administration, cardiovascular effects and subjective effects. J Pharmacol Exp Ther. 1996;279(3):1345–1356. [PubMed] [Google Scholar]
- Fischman MW, Schuster CR. Cocaine self-administration in humans. Fed Proc. 1982;41:241–246. [PubMed] [Google Scholar]
- Fitting S, Booze RM, Hasselrot U, Mactutus CF. Differential long-term neurotoxicity of HIV-1 proteins in the rat hippocampal formation: a design-based stereological study. Hippocampus. 2008;18(2):135–147. doi: 10.1002/hipo.20376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fray PJ, Sahakian BJ, Robbins TW, Koob GF, Iverson SD. An observational method for quantifying the behavioral effects of dopamine agonists: Contrasting effects of d-amphetamine and apomorphine. Psychopharmacology. 1980;69:253–259. doi: 10.1007/BF00433091. [DOI] [PubMed] [Google Scholar]
- Harrod SB, Booze RM, Welch M, Browning CE, Mactutus CF. Acute and repeated intravenous cocaine-induced locomotor activity is altered as a function of sex and gonadectomy in rats. Pharmacol Biochem Behav. 2005a;82(1):170–181. doi: 10.1016/j.pbb.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Harrod SB, Fitting S, Jarrell HM, Booze RM, Mactutus CF. Poster. Society for Research on Nicotine & Tobacco (SRNT); Florida, Orlando, USA: 2006. Parametric analysis of chamber shape on nicotine-induced sex differences in the expression of behavioral sensitization. [Google Scholar]
- Harrod SB, Mactutus CF, Bennett K, Hasselrot U, Wu G, Welch M, Booze RM. Sex differences and repeated intravenous nicotine: behavioral sensitization and dopamine receptors. Pharmacol Biochem Behav. 2004;78(3):581–592. doi: 10.1016/j.pbb.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Harrod SB, Mactutus CF, Browning CE, Welch M, Booze RM. Homecage observations following acute and repeated IV cocaine in intact and gonadectomized rats. Neurotoxicol Teratol. 2005b;27(6):891–896. doi: 10.1016/j.ntt.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Horger BA, Giles MK, Schenk S. Preexposure to amphetamine and nicotine predisposes rats to self-administer a low dose of cocaine. Psychopharmacology. 1992;107(2–3):271–6. doi: 10.1007/BF02245147. [DOI] [PubMed] [Google Scholar]
- Hudson L, Liu J, Nath A, Jones M, Raghavan R, Narayan O, Male D, Everall I. Detection of the human immunodeficiency virus regulatory protein tat in CNS tissues. J Neurovirol. 2000;6:145–155. doi: 10.3109/13550280009013158. [DOI] [PubMed] [Google Scholar]
- Joyce EM, Koob GF. Amphetamine-, scopolamine- and caffeine-induced locomotor activity following 6-hydroxydopamine lesions of the mesolimbic dopamine system. Psychopharmacology. 1981;73(4):311–313. doi: 10.1007/BF00426456. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Recent understanding in the mechanisms of addiction. Curr Psychiatry Rep. 2004;6(5):347–351. doi: 10.1007/s11920-004-0021-0. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162(8):1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Weber B. Amphetamine injection into the ventral mesencephalon sensitizes rats to peripheral amphetamine and cocaine. J Pharmacol Exp Ther. 1988;245(3):1095–1102. [PubMed] [Google Scholar]
- Kendall SL, Anderson CF, Nath A, Turchan-Cholewo J, Land CL, Mactutus CF, Booze RM. Gonadal steroids differentially modulate neurotoxicity of HIV and cocaine: Testosterone and ICI 182,780 sensitive mechanisms. BMC Neuroscience. 2005;8;6(1):40. doi: 10.1186/1471-2202-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BO, Liu Y, Ruan Y, Xu ZC, Schantz L, He JJ. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am J Pathol. 2003;162(5):1693–707. doi: 10.1016/S0002-9440(10)64304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neural mechanisms of drug reinforcement. Ann N Y Acad Sci. 1992;654:171–191. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Stinus L, Le Moal M. Hyperactivity and hypoactivity produced by lesions to the mesolimbic dopamine system. Behav Brain Res. 1981;3(3):341–359. doi: 10.1016/0166-4328(81)90004-8. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ. Gender differences in cocaine use and treatment response. J Subst Abuse Treat. 1993;10(1):63–66. doi: 10.1016/0740-5472(93)90100-g. [DOI] [PubMed] [Google Scholar]
- Koutsilieri E, Sopper S, Scheller C, ter Meulen V, Riederer P. Involvement of dopamine in the progression of AIDS Dementia Complex. J Neural Transm. 2002;109(3):399–410. doi: 10.1007/s007020200032. [DOI] [PubMed] [Google Scholar]
- Kubos KL, Moran TH, Robinson RG. Differential and asymmetrical behavioral effects of electrolytic or 6-hydroxydopamine lesions in the nucleus accumbens. Brain Res. 1987;13;401(1):147–151. doi: 10.1016/0006-8993(87)91174-7. [DOI] [PubMed] [Google Scholar]
- Kruman II, Nath A, Mattson MP. HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp Neurol. 1998;54:276–288. doi: 10.1006/exnr.1998.6958. [DOI] [PubMed] [Google Scholar]
- Lee YW, Eum SY, Nath A, Toborek M. Estrogen-mediated protection against HIV Tat protein-induced inflammatory pathways in human vascular endothelial cells. Cardiovasc Res. 2004;63(1):139–48. doi: 10.1016/j.cardiores.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Li Y, Acerbo MJ, Robinson TE. The induction of behavioural sensitization is associated with cocaine-induced structural plasticity in the core (but not shell) of the nucleus accumbens. Eur J Neurosci. 2004;20(6):1647–54. doi: 10.1111/j.1460-9568.2004.03612.x. [DOI] [PubMed] [Google Scholar]
- Ma M, Nath A. Molecular determinants for cellular uptake of Tat protein of human immunodeficiency virus type 1 in brain cells. J Virol. 1997;71(3):2495–2499. doi: 10.1128/jvi.71.3.2495-2499.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mactutus CF, Herman AS, Booze RM. Chronic intravenous model for studies of drug (ab)use in the pregnant and/or group-housed rat: An initial study with cocaine. Neurotoxicol Teratol. 1994;16:183–191. doi: 10.1016/0892-0362(94)90116-3. [DOI] [PubMed] [Google Scholar]
- Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis. 2002;1;186(Suppl 2):S193–8. doi: 10.1086/344528. [DOI] [PubMed] [Google Scholar]
- Nath A, Geiger J. Neurobiological aspects of human immunodeficiency virus infection: neurotoxic mechanisms. Prog Neurobiol. 1998;54(1):19–33. doi: 10.1016/s0301-0082(97)00053-1. [DOI] [PubMed] [Google Scholar]
- Nath A, Hauser KF, Wojna V, Booze RM, Maragos W, Prendergast M, Cass W, Turchan JT. Molecular basis for interactions of HIV and drugs of abuse. J Acquir Immune Defic Syndr. 2002;1;31(Suppl 2):S62–9. doi: 10.1097/00126334-200210012-00006. [DOI] [PubMed] [Google Scholar]
- Nath A, Maragos WF, Avison MJ, Schmitt FA, Berger JR. Acceleration of HIV dementia with methamphetamine and cocaine. J Neurovirol. 2001;7:66–71. doi: 10.1080/135502801300069737. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Neurobiology. Total recall-the memory of addiction. Science. 2001;292:2266–2267. doi: 10.1126/science.1063024. [DOI] [PubMed] [Google Scholar]
- Paine TA, Jackman SL, Olmstead MC. Cocaine-induced anxiety: alleviation by diazepam, but not buspirone, dimenhydrinate or diphenhydramine. Behav Pharmacol. 2002;13(7):511–23. doi: 10.1097/00008877-200211000-00001. [DOI] [PubMed] [Google Scholar]
- Peris J, Decambre N, Coleman-Hardee ML, Simpkins JW. Estradiol enhances behavioral sensitization to cocaine and amphetamine-stimulated striatal [3H]dopamine release. Brain Res. 1991;566(1–2):255–64. doi: 10.1016/0006-8993(91)91706-7. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, le Moal M, Simon H. Stress- and pharmacologically-induced behavioral sensitization increases vulnerability to acquisition of amphetamine self-administration. Brain Res. 1990;23;514(1):22–6. doi: 10.1016/0006-8993(90)90431-a. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997;25(2):192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Post RM. Intermittent versus continuous stimulation: Effect of time interval on the development of sensitization or tolerance. Life Sci. 1980;26:1275–1282. doi: 10.1016/0024-3205(80)90085-5. [DOI] [PubMed] [Google Scholar]
- Post RM, Contel NR. Human and animal studies of cocaine: Implications for the development of behavioral pathology. In: Creese I, editor. Stimulants: Neurochemical, behavioral and clinical perspectives. New York: Raven Press; 1983. pp. 169–203. [Google Scholar]
- Prendergast MA, Rogers DT, Mulholland PJ, Littleton JM, Wilkins LH, Jr, Self RL, Nath A. Neurotoxic effects of the human immunodeficiency virus type-1 transcription factor Tat require function of a polyamine sensitive-site on the N-methyl-D-aspartate receptor. Brain Res. 2002;8;954(2):300–307. doi: 10.1016/s0006-8993(02)03360-7. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Cador M, Taylor JR, Everitt BJ. Limbic-striatal interactions in reward-related processes. Neurosci Biobehav Rev. 1989;13:155–162. doi: 10.1016/s0149-7634(89)80025-9. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rofael HZ, Abdel-Rahman MS. The role of ketamine on plasma cocaine pharmacokinetics in rat. Toxicol Lett. 2002;129(1–2):167–76. doi: 10.1016/s0378-4274(02)00008-5. [DOI] [PubMed] [Google Scholar]
- Samaha AN, Li Y, Robinson TE. The rate of intravenous cocaine administration determines susceptibility to sensitization. J Neurosci. 2002;22(8):3244–3250. doi: 10.1523/JNEUROSCI.22-08-03244.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapshak P, Crandall KA, Xin KQ, Goodkin K, Fujimura RK, Bradley W, McCoy CB, Nagano I, Yoshioka M, Petito C, Sun NC, Srivastava AK, Weatherby N, Stewart R, Delgado S, Matthews A, Douyon R, Okuda K, Yang J, Zhangl BT, Cao XR, Shatkovsky S, Fernandez JB, Shah SM, Perper J. HIV-1 neuropathogenesis and abused drugs: current reviews, problems, and solutions. Adv Exp Med Biol. 1996;402:171–186. doi: 10.1007/978-1-4613-0407-4_23. [DOI] [PubMed] [Google Scholar]
- Shi B, Raina J, Lorenzo A, Busciglio J, Gabuzda D. Neuronal apoptosis induced by HIV-1 Tat protein and TNF-alpha: potentiation of neurotoxicity mediated by oxidative stress and implications for HIV-1 dementia. J Neurovirol. 1998;4(3):281–290. doi: 10.3109/13550289809114529. [DOI] [PubMed] [Google Scholar]
- Self RL, Mulholland PJ, Nath A, Harris BR, Prendergast MA. The human immunodeficiency virus type-1 transcription factor Tat produces elevations in intracellular Ca2+ that require function of an N-methyl-D-aspartate receptor polyamine-sensitive site. Brain Res. 2004;2;995(1):39–45. doi: 10.1016/j.brainres.2003.09.052. [DOI] [PubMed] [Google Scholar]
- Siegel S, Castellan NJ., Jr . Nonparametric Statistics for the Behavioral Sciences. 2. New York, NY: McGraw-Hill, Inc; 1988. [Google Scholar]
- Sircar R, Kim D. Female gonadal hormones differentially modulate cocaine-induced behavioral sensitization in Fischer, Lewis, and Sprague-Dawley rats. J Pharmacol Exp Ther. 1999;289(1):54–65. [PubMed] [Google Scholar]
- Song L, Nath A, Geiger JD, Moore A, Hochman S. Human immunodeficiency virus type 1 Tat protein directly activates neuronal N-methyl-D-aspartate receptors at an allosteric zinc-sensitive site. J Neurovirol. 2003;9(3):399–403. doi: 10.1080/13550280390201704. [DOI] [PubMed] [Google Scholar]
- SPSS 12.0 for Windows (1989–2003). SPSS Inc.
- Torres G, Rivier C, Weiss F. A ketamine mixture anesthetic inhibits neuroendocrine and behavioral consequences of cocaine administration. Brain Res. 1994;656(1):33–42. doi: 10.1016/0006-8993(94)91363-3. [DOI] [PubMed] [Google Scholar]
- Turchan J, Anderson C, Hauser KF, Sun Q, Zhang J, Liu Y, Wise PM, Kruman I, Maragos W, Mattson MP, Booze R, Nath A. Estrogen protects against the synergistic toxicity by HIV proteins, methamphetamine and cocaine. BMC Neurosci. 2001;2:3. doi: 10.1186/1471-2202-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle LD, Croul S, Morgello S, Amini S, Rappaport J, Khalili K. Detection of HIV-1 Tat and JCV capsid protein, VP1, in AIDS brain with progressive multifocal leukoencephalopathy. J Neurovirol. 2000;6:221–228. doi: 10.3109/13550280009015824. [DOI] [PubMed] [Google Scholar]
- van Haaren F, Meyer ME. Sex differences in locomotor activity after acute and chronic cocaine administration. Pharmacol Biochem Behav. 1991;39(4):923–927. doi: 10.1016/0091-3057(91)90054-6. [DOI] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Chang L, Volkow ND, Telang F, Logan J, Ernst T, Fowler JS. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain. 2004;127:2452–2458. doi: 10.1093/brain/awh269. [DOI] [PubMed] [Google Scholar]
- Wallace DR, Dodson S, Nath A, Booze RM. Estrogen attenuation of gp120- and tat-induced oxidative stress: role of the dopamine transporter. Synapse. 2006;59(1):51–60. doi: 10.1002/syn.20214. [DOI] [PubMed] [Google Scholar]
- Wallace DR, Mactutus CF, Booze RM. Repeated intravenous cocaine administration: locomotor activity and dopamine D2/D3 receptors. Synapse. 1996;23(3):152–163. doi: 10.1002/(SICI)1098-2396(199607)23:3<152::AID-SYN4>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Wesselingh SL, Power C, Glass JD, Tyor WR, McArthur JC, Farber JM, Griffin JW, Griffin DE. Intracerebral cytokine messenger RNA expression in acquired immunodeficiency syndrome dementia. Ann Neurol. 1993;33(6):576–582. doi: 10.1002/ana.410330604. [DOI] [PubMed] [Google Scholar]
- Westendorp MO, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin KM, Krammer PH. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature. 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Baldwin M, Achim CL. Expression of HIV regulatory and structural mRNA in the central nervous system. AIDS. 1996;10(8):843–847. doi: 10.1097/00002030-199607000-00007. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Soontornniyomkij V, Radhakrishnan L, Masliah E, Mellors J, Hermann SA, Dailey P, Achim CL. Distribution of brain HIV load in AIDS. Brain Pathol. 1998;8(2):277–84. doi: 10.1111/j.1750-3639.1998.tb00153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer BJ. Statistical Principles in Experimental Design. New York: McGraw-Hill; 1971. [Google Scholar]
- Wise RA, Bozarth MA. Brain mechanisms of drug reward and euphoria. Psychiatr Med. 1985;3:445–460. [PubMed] [Google Scholar]
- Zahniser NR, Peris J. Neurochemical mechanisms of cocaine-induced sensitization. In: Lakoski JM, Galloway MP, White FJ, editors. Cocaine: Pharmacology, physiology, and clinical strategies. Boca Raton, FL: CRC Press; 1992. pp. 229–260. [Google Scholar]


