Abstract
Artificial copulomimetic cervical stimulation (CS) induces an immediate release of oxytocin (OT) and prolactin (PRL) followed by a daily PRL rhythm characterized by nocturnal and diurnal surges. Although we have shown that the initial release of PRL is induced by the immediate release of OT, we tested whether the PRL that is released in response to CS is responsible for the initiation and maintenance of the subsequent PRL surges. Thus, we injected OVX rats centrally or peripherally with ovine PRL (oPRL) at 2200 h. Central oPRL induced PRL surges in OVX rats that were similar in size and timing to those of CS rats, whereas peripheral oPRL induced surges that were of smaller amplitude and delayed. We then infused a PRL antagonist (S179D, 0.1 ng/h) centrally into OVX and OVX-CS rats and measured the release of endogenous PRL and the activity of neuroendocrine dopaminergic neurons. Central infusion of S179D did not influence basal PRL secretion in OVX rats but prevented the expression of the CS-induced PRL surges and the accompanying noontime increase of CS-induced dopaminergic activity when continued for 3 d. However, central infusion of S179D only on the day of CS did not prevent the daily rhythm of PRL surges. These results demonstrate that PRL acts centrally to induce the PRL rhythm and that PRL in the brain is essential for the maintenance but not for the initiation of the CS-induced rhythmic PRL surges.
Prolactin stimulates a prolactin secretory rhythm.
In rodents, the mating stimulus or stimulation of the uterine cervix (CS) induces a unique pattern of prolactin (PRL) secretion characterized by two daily increases that recur for 10–12 d: a nocturnal surge peaking at 0300 h and a diurnal surge peaking at 1700 h (1,2). These surges of PRL are necessary to rescue the corpus luteum of the estrous cycle and prolong its ability to secrete progesterone (3). The PRL rhythm, however, can be initiated by CS of ovariectomized (OVX) rats, indicating that ovarian steroids are not required for its occurrence (4), but instead a steroid-independent activation of several brain areas is necessary (5). There is evidence that the stimulus is stored as a “memory” in the brain, which allows the PRL surges to occur for several days without reinforcement of the stimulus. This memory would also be responsible for the reestablishment of the PRL rhythm after a transient inhibitory disturbance of the system (6). Once established, this memory of CS can be stored for several days without any change in PRL secretion before activating the surges, as it occurs in delayed pseudopregnancy (7).
PRL is predominantly secreted by lactotrophs in the anterior pituitary gland, and the major control of its secretion is inhibitory by hypothalamic dopamine (DA), secreted by neurons in the arcuate and periventricular nucleus. These neurons are anatomically and functionally divided into three different subpopulations: tuberoinfundibular DA (TIDA), tuberohypophyseal DA (THDA), and periventricular hypophyseal DA (PHDA) neurons, projecting through different pathways to the median eminence (ME) and to the neural and intermediate lobes of the hypophysis (NIL) (8). DA from the three subpopulations has been shown to tonically inhibit the lactotrophs (9), and a release from this inhibition is necessary for PRL secretion to occur. Indeed, there is a decrease in the DA concentrations in hypophyseal stalk plasma (10), along with decreased activity and DA content in DA neurons (11), corresponding to the initiation of PRL surges induced by CS. However, administration of concentrations of DA that mimic those of stalk blood only partially inhibits PRL levels (12), suggesting that other factors are involved in the control of PRL release.
Among several identified factors that have the capability to stimulate PRL secretion, oxytocin (OT) seems to play a pivotal role. OT has been shown to stimulate PRL secretion when administered peripherally or directly on pituitary cells in culture (13). Antagonism of OT prevents the PRL surge on proestrus (14). OT neurons of the paraventricular nucleus have a periodic activity that coincides with the expected PRL surges in the CS rats (15), and OT directly stimulates PRL-secreting lactotrophs from CS rats through a calcium-dependent mechanism (16), suggesting that OT is also involved in the control of the CS-induced PRL secretion. Activation of OT receptors in the ventromedial hypothalamus are required to establish mating-induced pseudopregnancy (17); however, peripheral infusion of an OT antagonist delayed, but did not prevent, the CS-induced PRL surges (18). This suggests that the memory of CS was triggered and maintained even though the rhythmic PRL surges were inhibited until the OT antagonist was cleared from the system, as was illustrated with a mathematical model. Thus, peripheral OT is a key element of the PRL rhythm but is not part of the memory mechanism for CS.
These experiments raise the question of whether PRL itself triggers the memory of CS. To address this, we first injected ovine PRL (oPRL) into OVX rats, both centrally and peripherally. Next, we infused a PRL antagonist (S179D) centrally into OVX-CS rats, to see whether this would block the CS-induced PRL rhythm. Evidence suggests that the rhythm is due to bidirectional feedback between lactotrophs and hypothalamic DA neurons (19). We therefore measured dopaminergic activity in the ME and NIL, indicative of the activity of TIDA and THDA/PHDA neurons, respectively. Mathematical modeling was used to help interpret the experimental results.
Materials and Methods
Animals
Adult female Sprague Dawley rats weighing 250–300 g (Charles River, Raleigh, NC) were kept in a Laboratory Animal Resources-care facility, housed in groups of three in plastic cages under a 12-h light, 12-h dark cycle (lights on at 0600 h) and controlled temperature (25 C). Food and water were provided ad libitum. All rats were OVX bilaterally through a single ventral midline incision under isoflurane anesthesia (Aerrane; Baxter, Deerfield, IL) and allowed to recover for at least 1 wk. Animal procedures used were approved by the Florida State University Animal Care and Use committee.
Experimental design
Experiment 1: central or systemic oPRL administration
OVX rats were implanted with a guide cannula into the lateral cerebral ventricle for intracerebroventricular (icv) administration. After 1 wk, a catheter tubing was inserted into the jugular vein of all animals during the morning, and oPRL or vehicle was injected icv or systemically at 2200 h of the same day. Blood samples were withdrawn during the next 2 d to detect the occurrence of the diurnal and nocturnal surges of PRL.
Experiment 2: effect of central PRL antagonist infusion on PRL secretion
OVX rats were implanted with a guide cannula into the lateral cerebral ventricle for icv administration. After 1 wk recovery, rats were implanted into the jugular vein with catheter tubing. The icv infusion was performed by connecting an osmotic pump directly into the guide cannula, starting around 1300 h, and continued for 3 d. Blood samples were withdrawn every 2–4 h from d 1 until d 3 to detect the PRL levels.
Experiment 3: effect of central PRL antagonist infusion on CS-induced PRL secretory rhythm
OVX rats were treated according to experiment 2. The drug infusion started around 1200 h, 4 h before the first CS, and continued for 1 or 3 d. CS was performed at 1700 and 0900 h in the presence of the PRL antagonist. Blood samples were withdrawn according to experiment 2 times to detect the diurnal and nocturnal surges of PRL.
Experiment 4: effect of CS and PRL antagonist infusion on the activity of DA neurons
All animals from experiments 2 and 3 were decapitated at 1200 h of d 3, and the ME and NIL were quickly removed, frozen on dry ice, and stored at −80 C. Concentrations of DA and its metabolite, dihydroxyphenylacetic acid (DOPAC), were measured in the dissected areas.
The icv cannulation
Under ketamine (Ketaset; Fort Dodge Animal Health, Fort Dodge, IA; 49 mg/ml) and xylazine (Anased; Lloyd Laboratories, Shenandoah, IA; 1.8 mg/ml) anesthesia (100 μl/100 g body weight), animals were positioned in a stereotaxic apparatus with the incisor bar at −3.3 mm. A 22-gauge guide cannula (C313G; Plastics One, Inc., Roanoke, VA) was implanted in the right cerebral lateral ventricle (coordinates: 1.0 mm posterior to bregma, 1.6 mm lateral to the midline, and 3.2–3.7 mm below the outer surface of the skull). The correct vertical positioning of the cannula in the lateral ventricle was determined by displacement of the meniscus in a water manometer that detects pressure differences among compartments (20). The cannula, protected with a plastic-capped mandril (C313DC; Plastics One), was attached to the bone with stainless-steel screws and acrylic cement. After surgery, all rats were allowed to recover for 1 wk in individual cages.
oPRL injections
oPRL (National Institute of Diabetes and Digestive and Kidney Diseases Ovine Prolactin AFP#10677C; courtesy of Dr. Albert F. Parlow and the National Hormone and Pituitary Program) was dissolved in 0.03 m sodium bicarbonate and 0.15 m sodium chlorate (Sigma Chemical Co., St. Louis, MO) (pH 10.8). For icv administration, the drug (0.15 μg/2 μl) or its vehicle was administered under light isoflurane anesthesia via a stainless steel needle (0.2 mm diameter) connected by PE-10 polyethylene tubing to a Hamilton syringe (Hamilton, Reno, NV) controlled by an injection pump (KDS100; KD Scientific, Holliston, MA) set to dispense 2 μl solution/min. After each injection, the needle was left inside the cannula for an additional 60 sec to avoid solution reflux. The sc administration of oPRL (15 or 150 μg/ 200 μl) or vehicle was performed in freely moving animals.
S179D infusions
The PRL antagonist S179D PRL (21) was dissolved in sterile 0.9% NaCl, and 100 μl of the solutions was inserted in each osmotic pump (AP-1003D; Alzet, Durect Corp., Cupertino, CA). The pumps were connected to the guide cannula and implanted sc in the back of the animal. The drug was infused at a rate of 0.1 ng/ h for 1 or 3 d.
Cervical stimulation
The uterine cervix was stimulated with a Teflon Rod electrode (5 mm diameter), with two platinum wires protruding from the tip. The stimulation was performed twice on each rat at 1700 and 0900 h of the following day and consisted of two consecutive trains of electric current of 10 sec duration (rectangle pulses, 1 msec of 25 V at 200 Hz). This stimulation has been used in our laboratory to initiate pseudopregnancy, characterized by a daily PRL rhythm (11,18,22).
Jugular vein catheter implantation and blood samples
On the day before the experiment, rats were anesthetized with isoflurane and a catheter tube (Micro-Renathane; Braintree Scientific, Braintree, MA) filled with sterile saline (0.9% NaCl; Teknova, Hollister, CA) was inserted through the external jugular vein into the right atrium, fitted subcutaneously and exteriorized at the back of the animal, as previously described (23). All the stainless-steel surgical instruments were soaked in chlorhexidine disinfectant (Novalsan; Fort Dodge Animal Health) until surgery. After surgeries, the catheter tube was filled with gentamicin sulfate (Alexis, San Diego, CA) to prevent bacterial growth and to maintain catheter patency (24). In the morning of the experiment day, an extension of Micro-Renathane tubing (MRE-040, 0.040′ outer diameter ×0.025′ inner diameter; Braintree), filled with saline, was connected to the jugular catheter, and the rats were left undisturbed in their cages. Blood samples of 300 μl were withdrawn into plastic heparinized syringes, and the same volume of sterile 0.9% NaCl was injected through the catheter immediately after removal of each blood sample.
RIA
Blood samples were centrifuged at 1200 × g for 15 min at 4 C; the plasma was separated and frozen at −20 C until assayed. Plasma PRL was determined by double-antibody RIA using a kit provided by the National Institute of Diabetes and Digestive and Kidney Diseases (Bethesda, MD). The antiserum for PRL was antirat PRL-S9 and the reference preparation PRL-RP3. The lower limit of detection was 0.10 ng/ml, and the intra and interassay coefficients were less than 5 and 15%, respectively. oPRL does not interfere with this assay.
HPLC with electrochemical detection
The ME and NIL samples were thawed, homogenized, and sonicated in 0.2 m perchloric acid and 0.1 mm EDTA. The samples were centrifuged (20 min at 8000 × g). The supernatant was filtered through a 0.2-mm nylon microfiltration unit (Osmonics, Livermore, CA) and placed into autosampler vials, and the concentrations of DA and DOPAC were measured in the brain areas by HPLC with electrochemical detection, as previously described (18). Briefly, 20 μl of each sample was injected into the HPLC system by an autoinjector (model 542 autosampler; ESA, Inc., Chelmsford, MA). Separation was performed at 28 C on a 150- × 3-mm C18 column (MD-150, 3 μm; ESA). The mobile phase consisted of 75 mm sodium dihydrogen phosphate monohydrate (EM Science, Gibbstown, NJ), 1.7 mm 1-octane sulfonic acid (Fisher Scientific, Pittsburgh, PA), 25 μm EDTA (Fisher Scientific), and 6% acetonitrile (EM Science), titrated to pH 3.0 with phosphoric acid (Fisher Scientific). The pump (LC-20AD; Shimadzu, Kyoto, Japan) flow was 400 μl/min, and the detector (ESA Coulochem II) potential was −65 vs. −225 mV. The change in current on the second analytical electrode was measured by a coulometric detector and recorded using EZStart 7.3 SP1 (Shimadzu). DA and DOPAC were identified on the basis of their peak retention times. The amount of catecholamine in all sample peaks was estimated by comparison with the area under each peak for known amounts of each. The sensitivity of the assay was 15.6 pg DA and DOPAC. The intraassay coefficient of variation was less than 5% for DA and DOPAC.
Statistical analysis
Data are expressed as mean ± sem. Statistical differences were determined by two-way ANOVA followed by the Bonferroni post hoc test. Comparisons among times within the same experimental group were analyzed by one-way ANOVA followed by the Newman-Keuls post hoc test. P < 0.05 was considered statistically significant.
Results
oPRL administration stimulates the PRL secretory rhythm in OVX rats
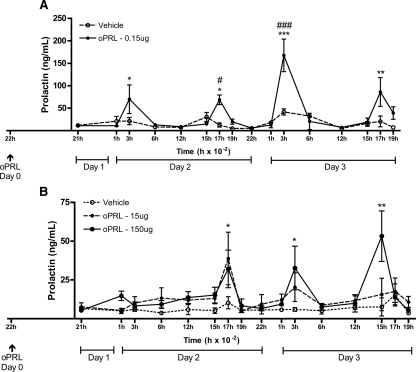
To determine whether PRL could trigger its own secretory rhythm, we injected oPRL systemically or centrally to OVX animals at 2200 h. This time point was chosen to mimic the PRL surge observed after a single OT injection in OVX animals, which induced the PRL secretory rhythm (25). Serum PRL levels were measured by a specific RIA that does not detect oPRL. Figure 1A shows that the icv injection of vehicle did not change basal levels of PRL in OVX rats. However, one single icv injection of oPRL at 2200 h of d 0 was able to induce a PRL secretory rhythm, increasing the plasma PRL concentrations at the times corresponding to the nocturnal (0300 h) and diurnal (1700 h) surges of CS rats on d 2 (P < 0.05) and d 3 (P < 0.001 and P < 0.01). When oPRL was injected systemically (Fig. 1B), only the higher dose used (150 μg) was able to induce a PRL secretory rhythm (P < 0.05 for 1700 h of d 2 and 0300 h of d 3, and P < 0.01 for 1500 h of d 3). The PRL surges induced systemically, however, were of smaller magnitude when compared with the central-induced PRL surges (around 50 vs. 200 ng/ml, respectively). They also began later than the centrally induced PRL surges (at 1700 h of d 2 vs. 0300 h of d 2).
Figure 1.
The icv or systemic oPRL administration initiates a daily PRL secretory rhythm. OVX rats were injected with oPRL or vehicle icv (0.15 μg) (A) or oPRL systemically (15 or 150 μg) (B) at 2200 h of d 0 (arrow). Blood samples were withdrawn during the next 2 d to determine the presence of the PRL diurnal and nocturnal surges (n = 5–11). Data are presented as mean ± sem. #, P < 0.05; ###, P < 0.001 vs. vehicle-injected group at the same time point. *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. basal PRL on 2100 h of d 1 in the same experimental group.
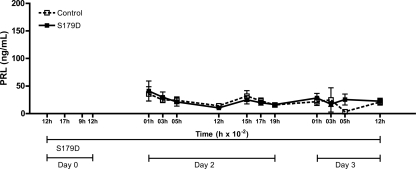
Central infusion of the PRL antagonist does not influence basal PRL secretion
To determine whether central infusion of a PRL antagonist interferes with basal PRL secretion, we infused S179D into the lateral ventricle of OVX rats without any other stimulus. Figure 2 shows basal levels of PRL secretion in control OVX rats and in rats infused with the PRL antagonist into the lateral ventricle for 3 d. The infusion of S179D does not interfere with the basal secretion of PRL.
Figure 2.
The icv infusion of a PRL antagonist has no effect on basal PRL levels. PRL antagonist (S179D, 0.1 ng/h) was infused into the lateral cerebral ventricle of OVX rats beginning approximately at 1200 h of dy 0. Blood samples were withdrawn for the next 2 d to monitor the basal PRL level (n = 5–11). Data are presented as mean ± sem.
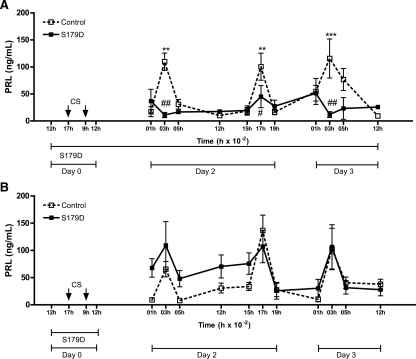
Continuous central infusion of the PRL antagonist prevented the expression of the CS-induced PRL secretory rhythm
The PRL antagonist was centrally infused in CS OVX rats, starting 5 h before the CS and continuing during the duration of the experiment (3 d) to determine whether the absence of central PRL action would interfere with the expression of the PRL secretory rhythm. In another set of animals, the drug was administered over a shorter time window (1 d), before and during CS on d 0 to investigate whether the absence of central PRL action would interfere with the establishment of the PRL secretory rhythm. In control OVX CS rats, PRL levels were elevated at 0300 h and 1700 h of d 2 (P < 0.01) and at 0300 h of d 3 (P < 0.001). These are time points at which the nocturnal and diurnal surges normally occur. The PRL antagonist infusion for 3 d prevented the expression of the CS-induced PRL surges (Fig. 3A). However, when the S179D was infused for only 1 d (at the time CS was performed), the drug did not block the PRL secretory rhythm induced by CS (Fig. 3B).
Figure 3.
Effect of icv PRL antagonist infusion on CS-induced PRL secretory rhythm. All animals were cervically stimulated at 1700 h of d 0 and 0900 h of d 1. OVX rats were infused with PRL antagonist (S179D, 0.1 ng/h) beginning around 1200 h of d 0. S179D infusion was maintained for 3 d (A) or 1 d (B). Blood samples were withdrawn during the next 2 d to observe the PRL diurnal and nocturnal surges (n = 5–11). Data are presented as mean ± sem. #, P < 0.05; ###, P < 0.001 vs. vehicle-injected group in the same time. *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. basal PRL on 2100 h of d 1 in the same experimental group.
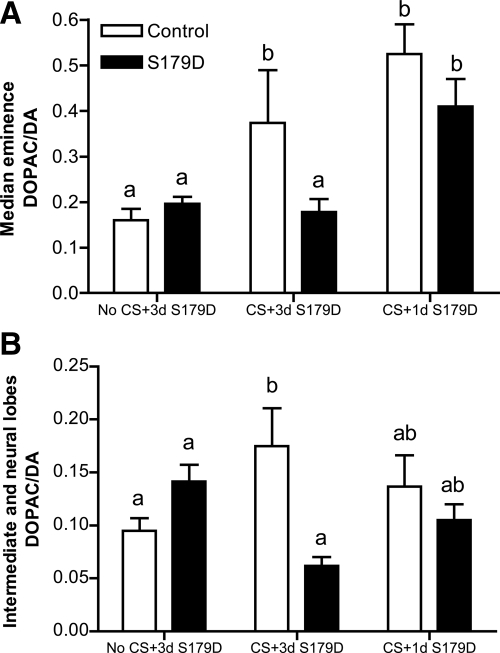
Continuous central infusion of the PRL antagonist prevented the CS-induced noontime increase of DA neuron activity
The activity of hypothalamic DA neurons was accessed at noon of d 3, motivated by previous results from our group that showed a decrease in DA neuronal activity at 0300 and 1700 h and an increase at 1200 h after CS in OVX rats (18). The DOPAC/DA ratio was measured in the ME and in the NIL as an index of DA activity of the TIDA, THDA, and PHDA neurons, respectively. As before (18), CS induced an increase in the DOPAC/DA ratio at 1200 h in the ME of both control CS rats (Fig. 4A). S179D infusion prevented this increase when applied for 3 d but not when applied for 1 d. The same effects of CS and S179D infusion were observed in the NIL (Fig. 4B), with a higher DOPAC/DA ratio in CS rats and prevention of this increase after S179D infusion for 3 d.
Figure 4.
Effect of icv PRL antagonist infusion on the activity of DA neurons. DOPAC/DA ratio in the ME (A) and in the NIL (B) of OVX animals without any manipulation infused with S179D for 3 d (No CS+3d S179D), cervically stimulated and infused with S179D for 3 d (CS+3d S179D) and cervically stimulated and infused with S179D for 1 d (CS+1d S179D) (n = 3–6). Data are presented as mean ± sem. Different letters represent statistical differences between groups (P < 0.05).
Interpretation of data through mathematical modeling
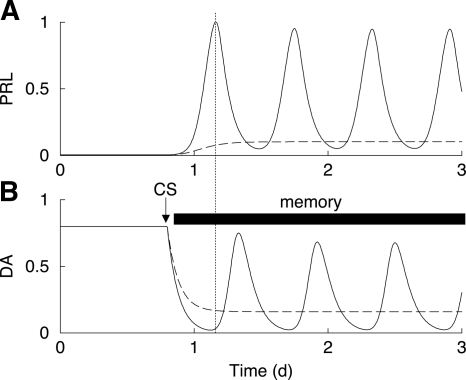
The inhibitory action of DA on lactotrophs (8) and the stimulatory action of PRL on DA neurons (26) form a feedback cycle. Because the stimulatory feedback of PRL onto DA neurons is delayed for up to several hours (27,28), this cycle has the potential for oscillations. We have developed a mathematical model based on the hypothesis that this feedback cycle is the engine for the CS-induced PRL rhythm (18,19). In this model, the PRL-DA cycle alone produces two PRL surges per day after CS (Fig. 5). Other elements of the full model, such as the effects of OT and vasoactive intestinal polypeptide, are not included in Fig. 5 so as to focus on PRL-DA interactions.
Figure 5.
Model simulation of the PRL (A) and DA (B) circuit after CS. The circuit is oscillatory while the memory is maintained (solid curves). The PRL antagonist administration disrupts this oscillatory pattern in PRL and DA (dashed curves).
Before CS, the model system is at a steady state. The CS triggers a long-lasting memory response that results in partial inhibition of DA neuron activity. That is, the constant stimulatory drive to the model DA neurons (the Td parameter) is reduced after simulated CS. The PRL-DA circuit is oscillatory as long as this memory is maintained (Fig. 5, solid curves). The morning PRL surge causes a delayed surge of DA neuron activity at about noon, as shown experimentally in Fig. 4. This elevated DA inhibits PRL secretion, but a surge of PRL secretion occurs later in the afternoon when the DA neuron activity returns to a low level. Thus, the surges in PRL and the surges in DA activity are dependent upon, and out of phase with, each other.
A PRL receptor antagonist will have one effect, and possibly two, on the system. First, the antagonist will sever the stimulatory pathway from PRL to the DA neurons. Figure 5 (dashed curves) shows a model simulation where this feedback is severed. The CS causes a reduction in DA neuron activity, so the PRL level is elevated. However, the elevated PRL has no influence on the DA neurons due to the antagonist blockade of PRL receptors on DA neurons, so no rhythm is produced. The DA level is not elevated at noon, because this elevation is an integral part of the PRL rhythm. These model results are consistent with the data in Figs. 3A and 4.
If the trigger for the CS memory requires a direct action of PRL, then a second effect of the antagonist will be the elimination of the memory. Thus, no PRL rhythm will occur. The fact that a PRL antagonist applied only for 1 d at the time of cervical stimulation did not preclude induction of a PRL rhythm (Fig. 3B) argues against a direct role for PRL as a trigger for the memory.
Discussion
The present experiments provide additional information about the mechanism and factors involved in the rhythmic PRL secretion induced by CS. We showed that a single PRL injection is able to induce a PRL secretory rhythm in OVX rats. A much smaller dose of PRL was required to induce the PRL surges when administered centrally than when administered peripherally, suggesting that endogenous PRL acts primarily on the brain to affect its own secretory rhythm. Central infusion of a PRL antagonist demonstrated that the presence of PRL in the brain is essential for the maintenance of the CS-induced PRL rhythm but not for its initiation. The fact that the blockade of PRL receptors in the brain did not prevent the initiation of the CS-induced PRL surges suggests that an elevated PRL level in the brain is sufficient, but not necessary, to trigger the PRL rhythm. Thus, although central PRL infusion is sufficient to start the rhythm, the rhythm can also be started by CS with the effects of central PRL antagonized.
It is well known that peripheral PRL is the major luteotropic stimulus that transforms the corpus luteum of the estrous cycle into the corpus luteum of pseudopregnancy, prolonging its ability to secrete progesterone and support implantation and pregnancy (29). A central PRL action has previously been suggested to be involved with the initiation, maintenance, and termination of pseudopregnancy. We point out that these are separate processes, and central PRL may be important for some, but not necessarily all. Earlier experiments demonstrated that pseudopregnancy can be induced by injecting PRL peripherally in cycling rats, but several systemic PRL injections were necessary to successfully induce pseudopregnancy (30,31). Peripheral OT injection induces a PRL release followed by a PRL rhythm, similar to that induced by CS (25). Subcutaneous injections of PRL failed to block the nocturnal surge of pseudopregnancy, but it was totally blocked after PRL injection into the lateral ventricle (32). Moreover, implantation of PRL into the ME has been shown to suppress PRL release and terminate pseudopregnancy (33) and pregnancy (34). Thus, although in our data the PRL injection disrupts the steady-state of the PRL-DA system to initiate the PRL secretory rhythm, the implantation of PRL after CS may perturb the oscillatory PRL-DA circuit created by CS and in this case terminate the surges.
The past experiments in which injections of PRL were able to initiate pseudopregnancy in cycling animals suggested that PRL induced an increase of progesterone secretion, and this increase required PRL treatment to be continued during the first 4 d after estrus (31). A single progesterone injection can also induce pseudopregnancy (35), but we bypassed the influence of ovarian steroids by using OVX animals and were able to induce the PRL secretory rhythm with a single PRL injection. The fact that a much higher concentration of PRL was required when injected peripherally suggests that a small fraction of the oPRL injected sc was transported to the brain to initiate the rhythm in OVX rats. This hypothesis is supported by other experiments that demonstrate that oPRL is functionally effective in the brain, after icv injections (36,37,38,39). PRL is actively transported from the blood into the cerebrospinal fluid by PRL receptors in the choroid plexus (40,41,42) so that PRL concentrations in cerebrospinal fluid reflect changes in plasma PRL levels (43). Another possibility for the origin of the PRL in the brain would be a local production, because there is evidence that PRL is produced by neurons and may act as a neurohormone to modulate hypothalamic function (44,45,46).
To inhibit the central effects of PRL, we used the PRL antagonist S179D, a mutant mimic of the phosphorylated PRL (21). Although S179D has been described to act as an agonist at higher concentrations (47), the dose used in this study (0.1 ng/h) has been experimentally shown to act as an antagonist (48).
The central infusion of S179D into OVX rats did not influence basal PRL secretion. The absence of a change in PRL after antagonist administration in control animals suggests that the antagonist was not able to block the tonic stimulatory action of PRL in DA neurons. However, S179D infusion for 3 d in CS rats totally blocked the expression of the PRL secretory rhythm. This suggests different regulation of basal and CS PRL rhythms. The ability of S179D to block the CS rhythm demonstrates that the presence of PRL in the brain is fundamental for the maintenance of this rhythm. This supports the hypothesis that the DA-PRL feedback loop is the mechanism for the CS-induced PRL rhythm (Fig. 5). The administration of the PRL antagonist probably prevented the PRL feedback onto DA neurons, inhibiting the expression of the rhythm. Activation of the TIDA neurons by PRL requires activation of the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway (28,49), and this pathway has been shown to be severely impaired after S179D treatment (50). We have previously shown that DA neuronal activity is elevated at 1200 h in the TIDA and THDA neurons of OVX-CS rats, in antiphase with PRL surges (18). In our present data, CS also increased DA activity in TIDA and THDA neurons at the same time, but the 3-d infusion of the PRL antagonist prevented this increase. These results confirm that the presence of PRL in the brain is required for the maintenance of the PRL secretory rhythm in OVX rats, and mathematical modeling suggests that its primary target is hypothalamic DA neurons.
To test whether PRL release in the brain was necessary for the initiation of the PRL surges, we infused S179D into CS-OVX rats for 1 d on the day of CS, and blood samples were collected after the clearance of the drug. We found that CS was able to initiate the PRL secretory rhythm even in the presence of S179D, suggesting that the PRL antagonist did not interfere with the triggering of the memory of CS. In accordance with this assumption, the infusion of S179D for 1 d did not prevent the noontime increase in DA activity in the TIDA and THDA neurons observed in the CS rats, demonstrating that the DA-PRL feedback loop was not affected in these animals. Thus, a direct action of PRL in the brain is not necessary for the initiation of the CS-induced PRL secretory rhythm. Understanding the exact mechanism that triggers the CS-induced PRL secretory rhythm requires further investigation. One possibility would be an interaction between PRL and other neurohormones that influence PRL secretion, such as OT.
Footnotes
This work was supported by the National Institutes of Health Grants DK43200 (to M.E.F.) and DA19356 (to R.B. and M.E.F.). A.M.W. was supported by California Breast Cancer Research Program, 10PB-0127.
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 12, 2009
Abbreviations: CS, Cervical stimulation; DA, dopamine; DOPAC, dihydroxyphenylacetic acid; icv, intracerebroventricular; ME, median eminence; NIL, neural and intermediate lobes of the hypophysis; oPRL, ovine PRL; OT, oxytocin; OVX, ovariectomized; PHDA, periventricular hypophyseal DA; PRL, prolactin; THDA, tuberohypophyseal DA; TIDA, tuberoinfundibular DA.
References
- Butcher RL, Fugo NW, Collins WE 1972 Semicircadian rhythm in plasma levels of prolactin during early gestation in the rat. Endocrinology 90:1125–1127 [DOI] [PubMed] [Google Scholar]
- Freeman ME, Smith MS, Nazian SJ, Neill JD 1974 Ovarian and hypothalamic control of the daily surges of prolactin secretion during pseudopregnancy in the rat. Endocrinology 94:875–882 [DOI] [PubMed] [Google Scholar]
- Morishige WK, Rothchild I 1974 Temporal aspects of the regulation of corpus luteum function by luteinizing hormone, prolactin and placental luteotrophin during the first half of pregnancy in the rat. Endocrinology 95:260–274 [DOI] [PubMed] [Google Scholar]
- Freeman ME, Neill JD 1972 The pattern of prolactin secretion during pseudopregnancy in the rat: a daily nocturnal surge. Endocrinology 90:1292–1294 [DOI] [PubMed] [Google Scholar]
- Freeman ME, Banks JA 1980 Hypothalamic sites which control the surges of prolactin secretion induced by cervical stimulation. Endocrinology 106:668–673 [DOI] [PubMed] [Google Scholar]
- Voogt JL 1980 Regulation of nocturnal prolactin surges during pregnancy in the rat. Endocrinology 106:1670–1676 [DOI] [PubMed] [Google Scholar]
- De Greef WJ, Zeilmaker GH 1976 Prolactin and delayed pseudopregnancy in the rat. Endocrinology 98:305–310 [DOI] [PubMed] [Google Scholar]
- Ben-Jonathan N, Hnasko R 2001 Dopamine as a prolactin (PRL) inhibitor. Endocr Rev 22:724–763 [DOI] [PubMed] [Google Scholar]
- DeMaria JE, Livingstone JD, Freeman ME 1998 Characterization of the dopaminergic input to the pituitary gland throughout the estrous cycle of the rat. Neuroendocrinology 67:377–383 [DOI] [PubMed] [Google Scholar]
- De Greef WJ, Neill JD 1979 Dopamine levels in hypophysial stalk plasma of the rat during surges of prolactin secretion induced by cervical stimulation. Endocrinology 105:1093–1099 [DOI] [PubMed] [Google Scholar]
- Lerant A, Herman ME, Freeman ME 1996 Dopaminergic neurons of periventricular and arcuate nuclei of pseudopregnant rats: semicircadian rhythm in Fos-related antigens immunoreactivities and in dopamine concentration. Endocrinology 137:3621–3628 [DOI] [PubMed] [Google Scholar]
- Gibbs DM, Neill JD 1978 Dopamine levels in hypophysial stalk blood in the rat are sufficient to inhibit prolactin secretion in vivo. Endocrinology 102:1895–1900 [DOI] [PubMed] [Google Scholar]
- Lumpkin MD, Samson WK, McCann SM 1983 Hypothalamic and pituitary sites of action of oxytocin to alter prolactin secretion in the rat. Endocrinology 112:1711–1717 [DOI] [PubMed] [Google Scholar]
- Johnston CA, Negro-Vilar A 1988 Role of oxytocin on prolactin secretion during proestrus and in different physiological or pharmacological paradigms. Endocrinology 122:341–350 [DOI] [PubMed] [Google Scholar]
- Arey BJ, Freeman ME 1992 Activity of oxytocinergic neurons in the paraventricular nucleus mirrors the periodicity of the endogenous stimulatory rhythm regulating prolactin secretion. Endocrinology 130:126–132 [DOI] [PubMed] [Google Scholar]
- Egli M, Bertram R, Sellix MT, Freeman ME 2004 Rhythmic secretion of prolactin in rats: action of oxytocin coordinated by vasoactive intestinal polypeptide of suprachiasmatic nucleus origin. Endocrinology 145:3386–3394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northrop LE, Erskine MS 2008 Selective oxytocin receptor activation in the ventrolateral portion of the ventromedial hypothalamus is required for mating-induced pseudopregnancy in the female rat. Endocrinology 149:836–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee DT, Poletini MO, Bertram R, Freeman ME 2007 Oxytocin action at the lactotroph is required for prolactin surges in cervically stimulated ovariectomized rats. Endocrinology 148:4649–4657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram R, Egli M, Toporikova N, Freeman ME 2006 A mathematical model for the mating-induced prolactin rhythm of female rats. Am J Physiol Endocrinol Metab 290:E573–E582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner AA, Branco LG 2001 Carbon monoxide is the heme oxygenase product with a pyretic action: evidence for a cGMP signaling pathway. Am J Physiol Regul Integr Comp Physiol 280:R448–R457 [DOI] [PubMed] [Google Scholar]
- Chen TJ, Kuo CB, Tsai KF, Liu JW, Chen DY, Walker AM 1998 Development of recombinant human prolactin receptor antagonists by molecular mimicry of the phosphorylated hormone. Endocrinology 139:609–616 [DOI] [PubMed] [Google Scholar]
- Gorospe WC, Freeman ME 1981 An ovarian role in prolonging and terminating the two surges of prolactin in pseudopregnant rats. Endocrinology 108:1293–1298 [DOI] [PubMed] [Google Scholar]
- Harms PG, Ojeda SR 1974 A rapid and simple procedure for chronic cannulation of the rat jugular vein. J Appl Physiol 36:391–392 [DOI] [PubMed] [Google Scholar]
- Thrivikraman KV, Huot RL, Plotsky PM 2002 Jugular vein catheterization for repeated blood sampling in the unrestrained conscious rat. Brain Res Brain Res Protoc 10:84–94 [DOI] [PubMed] [Google Scholar]
- Egli M, Bertram R, Toporikova N, Sellix MT, Blanco W, Freeman ME 2006 Prolactin secretory rhythm of mated rats induced by a single injection of oxytocin. Am J Physiol Endocrinol Metab 290:E566–E572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMaria JE, Lerant AA, Freeman ME 1999 Prolactin activates all three populations of hypothalamic neuroendocrine dopaminergic neurons in ovariectomized rats. Brain Res 837:236–241 [DOI] [PubMed] [Google Scholar]
- Freeman ME, Kanyicska B, Lerant A, Nagy G 2000 Prolactin: structure, function, and regulation of secretion. Physiol Rev 80:1523–1631 [DOI] [PubMed] [Google Scholar]
- Ma FY, Anderson GM, Gunn TD, Goffin V, Grattan DR, Bunn SJ 2005 Prolactin specifically activates signal transducer and activator of transcription 5b in neuroendocrine dopaminergic neurons. Endocrinology 146:5112–5119 [DOI] [PubMed] [Google Scholar]
- Smith MS, McLean BK, Neill JD 1976 Prolactin: the initial luteotropic stimulus of pseudopregnancy in the rat. Endocrinology 98:1370–1377 [DOI] [PubMed] [Google Scholar]
- Croft BT, Bartke A 1975 Pseudopregnancy in mice treated with ovine prolactin. J Reprod Fertil 45:513–514 [DOI] [PubMed] [Google Scholar]
- Alloiteau JJ, Vignal A 1958 Pseudopregnancy after short-term treatment of rats with prolactin. C R Hebd Seances Acad Sci 247:2485–2487 [PubMed] [Google Scholar]
- Kishi K, Kobayashi F 1984 Role of prolactin in controlling prolactin surges in pseudopregnant rats. Biol Reprod 30:879–885 [DOI] [PubMed] [Google Scholar]
- Dang BT, Voogt JL 1977 Termination of pseudopregnancy following hypothalamic implantation of prolactin. Endocrinology 100:873–880 [DOI] [PubMed] [Google Scholar]
- Vidal G, Mathiasen JR, Voogt JL 1991 Prolactin implants in the hypothalamus inhibit prolactin surges during pregnancy and alter prolactin-release in response to dopamine receptor blockade. J Neuroendocrinol 3:249–252 [DOI] [PubMed] [Google Scholar]
- Murakami N, Takahashi M, Suzuki Y 1980 Induction of pseudopregnancy and prolactin surges by a single injection of progesterone. Biol Reprod 22:253–258 [DOI] [PubMed] [Google Scholar]
- Augustine RA, Grattan DR 2008 Induction of central leptin resistance in hyperphagic pseudopregnant rats by chronic prolactin infusion. Endocrinology 149:1049–1055 [DOI] [PubMed] [Google Scholar]
- Toyoda F, Hasunuma I, Yamamoto K, Yamashita M, Kikuyama S 2005 Prolactin acts centrally to enhance newt courtship behavior. Gen Comp Endocrinol 141:172–177 [DOI] [PubMed] [Google Scholar]
- Fujikawa T, Soya H, Tamashiro KL, Sakai RR, McEwen BS, Nakai N, Ogata M, Suzuki I, Nakashima K 2004 Prolactin prevents acute stress-induced hypocalcemia and ulcerogenesis by acting in the brain of rat. Endocrinology 145:2006–2013 [DOI] [PubMed] [Google Scholar]
- Bridges RS, Numan M, Ronsheim PM, Mann PE, Lupini CE 1990 Central prolactin infusions stimulate maternal behavior in steroid-treated, nulliparous female rats. Proc Natl Acad Sci USA 87:8003–8007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangurian LP, Walsh RJ, Posner BI 1992 Prolactin enhancement of its own uptake at the choroid plexus. Endocrinology 131:698–702 [DOI] [PubMed] [Google Scholar]
- Walsh RJ, Slaby FJ, Posner BI 1987 A receptor-mediated mechanism for the transport of prolactin from blood to cerebrospinal fluid. Endocrinology 120:1846–1850 [DOI] [PubMed] [Google Scholar]
- Lerant A, Freeman ME 1998 Ovarian steroids differentially regulate the expression of PRL-R in neuroendocrine dopaminergic neuron populations: a double label confocal microscopic study. Brain Res 802:141–154 [DOI] [PubMed] [Google Scholar]
- Login IS, MacLeod RM 1977 Prolactin in human and rat serum and cerebrospinal fluid. Brain Res 132:477–483 [DOI] [PubMed] [Google Scholar]
- Clapp C, Torner L, Gutiérrez-Ospina G, Alcántara E, López-Gómez FJ, Nagano M, Kelly PA, Mejía S, Morales MA, Martínez de la Escalera G 1994 The prolactin gene is expressed in the hypothalamic-neurohypophyseal system and the protein is processed into a 14-kDa fragment with activity like 16-kDa prolactin. Proc Natl Acad Sci USA 91:10384–10388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Hökfelt T, Eneroth P, Gustafsson JA, Skett P 1977 Prolactin-like immunoreactivity: localization in nerve terminals of rat hypothalamus. Science 196:899–900 [DOI] [PubMed] [Google Scholar]
- Paut-Pagano L, Roky R, Valatx JL, Kitahama K, Jouvet M 1993 Anatomical distribution of prolactin-like immunoreactivity in the rat brain. Neuroendocrinology 58:682–695 [DOI] [PubMed] [Google Scholar]
- Bernichtein S, Kinet S, Jeay S, Llovera M, Madern D, Martial JA, Kelly PA, Goffin V 2001 S179D-human PRL, a pseudophosphorylated human PRL analog, is an agonist and not an antagonist. Endocrinology 142:3950–3963 [DOI] [PubMed] [Google Scholar]
- Bridges R, Rigero B, Byrnes E, Yang L, Walker A 2001 Central infusions of the recombinant human prolactin receptor antagonist, S179D-PRL, delay the onset of maternal behavior in steroid-primed, nulliparous female rats. Endocrinology 142:730–739 [DOI] [PubMed] [Google Scholar]
- Grattan DR, Xu J, McLachlan MJ, Kokay IC, Bunn SJ, Hovey RC, Davey HW 2001 Feedback regulation of PRL secretion is mediated by the transcription factor, signal transducer, and activator of transcription 5b. Endocrinology 142:3935–3940 [DOI] [PubMed] [Google Scholar]
- Wu W, Coss D, Lorenson MY, Kuo CB, Xu X, Walker AM 2003 Different biological effects of unmodified prolactin and a molecular mimic of phosphorylated prolactin involve different signaling pathways. Biochemistry 42:7561–7570 [DOI] [PubMed] [Google Scholar]