Abstract
We reported a novel mutation of thyroid hormone receptor (TR)-β, F455S, in a patient with pituitary resistance to thyroid hormone (RTH), who showed impaired release of nuclear receptor corepressor and abnormal histone deacetylation. In the present study, we further analyzed the histone modifications and the dynamics of TR and RNA polymerase II on the TRH gene. The lysine residues 9 (H3K9) and 14 (K14) of the histone H3 were acetylated in the absence of thyroid hormone (TH), and addition of TH caused a temporary deacetylation of both residues. Although H3K4 was di- and trimethylated in the absence of T3, no methylation of H3K9 or K27 was detected. Long-term incubation with T3 decreased the level of trimethylated H3K4, the amount of TR, and the level of phosphorylated RNA polymerase II but not dimethylated H3K4. Treatment with an inhibitor for H3K4 methyltransferase, 5′-deoxy-5′-methylthioadenosine, decreased basal promoter activity but did not affect the repression by TH. Conversely, overexpression of MLL, an H3K4-specific methyltransferase, caused an increase in basal activity. In the presence of F455S, methylation of H3K4 and the dynamics of TR were intact, but both H3K9 and H3K14 were hyperacetylated, and T3-induced deacetylation was impaired, resulting in a high transcriptional level. These findings demonstrated that 1) negative regulation of the TRH gene by TH involves both the acetylation and methylation of specific residues of histone tails and changing the amount of TR, and 2) the major impairment to histone modifications in F455S was hyperacetylation of the specific histone tails.
Negative regulation of the gene by thyroid hormone involves both the acetylation and methylation of histone tails, and the major impairment in F455S was hyperacetylation.
Over 100 mutations of the thyroid hormone receptor (TR) β gene have been identified in patients with resistance to thyroid hormone (RTH) (1). In patients with RTH, impairment of the mechanism regulating the feedback of thyroid hormone to the hypothalamic TRH and pituitary TSH genes by mutated TRs causes inappropriate secretion of TSH from the pituitary, resulting in a high level of thyroid hormone in serum. In an earlier study, we found a novel mutant, F455S, in an 11-yr-old girl with a sporadic case of pituitary RTH who had high serum TSH and high thyroid hormone levels (2,3). To examine the molecular mechanism by which this mutant introduced phenotypes of pituitary RTH, we analyzed the mutant TR in vitro. The F455S mutant exhibited impaired transcriptional activity for genes negatively but not positively regulated by thyroid hormone. Glutathione S-transferase pull-down assays and EMSA demonstrated that the mutant TR exhibited a strong association with nuclear receptor corepressor (NCoR) and significant impairment of its release even after the addition of a large amount of T3 but showed a normal recruitment of steroid receptor coactivator-1. Because NCoR is known to recruit several histone deacetylases (HDACs), we expected histone modifications to occur in the presence of the F455S mutant. To examine histone modifications to genes, we had to establish a cell line stably expressing the target gene, which could form the chromatin structure. Therefore, we established cells stably expressing a human TRH gene and the F455S mutant or wild-type (WT) TR using a pituitary-derived cell line, GH4C1, because no hypothalamic cell line was available, and the TRH gene has been well characterized in this cell line. An important feature of established cell lines is that the amount of exogenous F455S mutant TR expressed is similar to that of the endogenous TR, which also functions appropriately for the TRH gene (shown in supplemental Fig. 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org) in the F455S cell line, reflecting the heterozygosity of the patient.
On the genes positively regulated by thyroid hormone, TR binds to target promoters and regulates promoter activity by recruiting specific coregulator protein complexes. In the unliganded state, TR assumes a conformation that stably interacts with corepressor molecules such as NCoR and silencing mediator of retinoic and thyroid hormone receptors (SMRT). Stimulation with T3 leads to the dissociation of corepressors and recruitment of coactivators including members of the p160/steroid receptor coactivator (SRC) family, TR-associated protein/vitamin D receptor-interacting protein (TRAP/DRIP) mediators, and finally RNA polymerase II (Pol II) to start its transcription (4).
With respect to histone modifications in thyroid hormone receptors, Li et al. (5) recently reported the importance of the methylation of H3K9 and the interaction of TR with SUV39H1 for repression by unliganded TRs, whereas Liu et al. (6) reported a ligand-dependent cyclic recruitment of TRs and multiple cofactors and the importance of acetylation status of the histones on several genes, including the GH, sarcoplasmic endoplasmic reticulum calcium-adenosine triphosphatase, and cholesterol 7α-hydroxylase genes. Interestingly, the profile of the dynamics of TR and cofactors differed among genes (5,6,7).
In contrast to our extensive knowledge of genes positively regulated by thyroid hormone, little is known about the mechanism of repression and the histone modifications in genes negatively regulated by thyroid hormone (8,9). Taking advantage of the above cell lines, we first characterized how coregulators were recruited to the TRH promoter after the addition of T3 (2). We found that TR and NCoR are recruited together on the TRH gene in the absence of T3, and the addition of T3 caused the release of NCoR and recruitment of HDAC2/3, inducing significant deacetylation of histone 3 within 30 min. Reacetylation then occurred gradually within 120 min on the TRH gene. The presence of the F455S mutant TR did not cause the complete release of NCoR or sufficient recruitment of HDAC2/3, resulting in insufficient deacetylation.
We now know more specific modifications of histone tails; for example, the acetylation of H3K9 and -K14, and methylation of H3K4, are associated with active genes, and in contrast, H3K9 and -K27 are frequently methylated in inactive and silenced genes (10,11,12). Furthermore, as mentioned above, the amount of TR has been reported to be cyclized on some genes. Therefore, in the present study, we analyzed histone modifications in the TRH gene in the presence of the WT TR or F455S mutant. We also examined the profile of change in the amount of TRβ, and RNA polymerase II, which is a parameter of transcriptional activity, and then examined how histone modifications and the dynamics of TR were involved in the dysregulation of the gene by the mutant TR F455S.
Materials and Methods
Materials
5′-Deoxy-5′-methylthioadenosine (MTA) was obtained from Sigma Chemical Co. (St. Louis, MO). The luciferase (Luc) reporter plasmid carrying the hTRH promoter (TRH-Luc), including the 790-bp promoter region and 54-bp exon 1, and plasmids expressing WT or mutant human TRβ1 were described previously (2). The plasmid expressing hMLL was provided by Prof. M. Seto, Aichi Cancer Institute, Japan, and Prof. J. L. Hess, University of Michigan Medical School.
Antibodies
Antibodies against the acetylated histone H3K9 (07-352), the acetylated histone H3K14 (07-353), the trimethyl histone H3K4 (07-473), the dimethyl histone H3K9 (07-441), the trimethyl histone H3K9 (07-442), H3 (06-755), and TRβ (06-539) were obtained from Upstate Biotechnology (Lake Placid, NY). Antibodies against the dimethyl histone H3K4 (ab7766), the trimethyl histone H3K27 (ab6002), and phospho-S5 RNA polymerase II (ab5131) were purchased from Abcam (Cambridge, MA). These antibodies were used in several previous reports for chromatin immunoprecipitation (ChIP) assays (13,14).
Mammalian cell culture and transfection
Cells stably expressing the TRH promoter and WT TR or the F455S mutant were established as reported and grown in DMEM supplemented with 10% fetal bovine serum (FBS) (2). Cells were split into six-well plates at subconfluency with DMEM supplemented with 10% FBS treated with AG1-X8 resin (Bio-Rad Laboratories, Hercules, CA) and activated charcoal (Sigma) to remove thyroid hormones. At a specific time after the addition of 100 nm T3 (Sigma), a ChIP assay was performed. In another experiment, 24 h after the addition of MTA, cells were harvested for a Luc assay.
For the transient transfection analysis, GH4C1 cells were grown in DMEM supplemented with 10% FBS. Cells were split into six-well plates at subconfluency 24 h before transfection. The transient transfection was performed using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA). The cells were transfected with 10 μg TRH-Luc, 0.5 μg WT TR expression vector, and 0–5 μg mixed-lineage leukemia (MLL) expression vector. The total amount of transfected plasmid was adjusted by adding an empty expression vector. After transfection (16 h), cells were further incubated in the absence or presence of 100 nm T3 for 24 h and then harvested for a Luc assay. In this system, as a negative control, the promoter activity of thymidine kinase was not affected by the expression of exogenous TR and/or by T3 (supplemental Fig. 2).
ChIP assay
ChIP analyses were performed according to the manufacturer’s instructions (Upstate Biotechnology) with some modifications. Briefly, after the addition of T3, cells (2 × 107) were treated with the cross-linking reagent formaldehyde (final concentration, 1%) for 10 min at 37 C. They were then rinsed twice with cold PBS containing 0.5 mm phenylmethylsulfonyl fluoride and 1 μg/ml aprotinin. Cells were collected by centrifugation for 4 min at 4 C and resuspended in 150 μl sodium dodecyl sulfate (SDS) lysis buffer (1% SDS; 10 mm EDTA; 50 mm Tris-HCl, pH 8.1) with proteinase inhibitors and incubated for 10 min on ice. Samples were sonicated on ice five times for 8 sec each (until the average length of the sheared genomic DNA was 0.2–1.0 kbp) and centrifuged for 10 min. One percent of the supernatant was used as input, and the remaining amount was subjected to the ChIP procedure. Next, 40 μl salmon sperm DNA/protein A agarose-50% slurry was added to reduce the nonspecific background and incubated for 30 min at 4 C with agitation. The solution was then incubated with 1–3 μg specific antibody or IgG as a negative control and rotated at 4 C overnight. Immunoprecipitated chromatin complexes were isolated by adding 50 μl salmon sperm DNA/protein A agarose-50% slurry and rotating the reactions for 1 h at 4 C. Immunoprecipitates were washed with low-salt immune complex wash buffer [20 mm Tris-HCl (pH 8), 1/2 mm EDTA, 0.1% SDS, 1% Triton X-100], followed by high-salt wash buffer [20 mm Tris-HCl (pH 8),1/2 mm EDTA, 0.1% SDS, 1% Triton X-100, 500 mm NaCl], LiCl immune complex wash buffer [10 mm Tris-HCl (pH 8.1), 0.25 m LiCl, 1% Nonidet P-40, 1% deoxycholate, 1 mm EDTA], and finally twice in 1× Tris-EDTA (pH 8.0). To elute the immunoprecipitated chromatin complexes from the resin, 100 μl elution buffer (1% SDS, 0.1 m NaHCO3) was added to the beads, and the tubes were vortexed and incubated at room temperature for 15 min with rotation. The supernatant was then collected, and the elution was repeated with a fresh 100 μl elution buffer. After the eluants were combined in one tube, the protein-DNA cross-linking was reversed by adding 5 m NaCl to a final concentration of 200 mm and heating at 65 C for 4 h. Inputs were diluted to 200 μl and subjected to the same procedure. Each sample was supplemented with 8 μl 1 m Tris-Cl (pH 6.5), 4 μl 0.5 m EDTA, and 5 μg proteinase K (Invitrogen) and subsequently incubated at 45 C for 1 h. Samples were then extracted with phenol/chloroform/isoamyl alcohol (25:24:1), and the DNA was precipitated with ethanol and subsequently resuspended in 50 μl H2O. Samples were analyzed by conventional PCR and real-time PCR. Conventional PCR was performed with 10 μl immunoprecipitate or input (see above), 0.5 μm of each primer, 1.5 mm MgCl2, 0.2 mm dNTP mixture, 1× thermophilic buffer, and 2.5 U AmpliTaq DNA polymerase (Applied Biosystems, Foster City, CA) in a total volume of 50 μl. The primers for the human TRH promoter were forward, 5′-ctgagcgctgcagactcctgacct-3′, and reverse, 5′-tgttcacctcgatatgtgcatctgt-3′. The primers for the Luc gene were forward, 5′-ggcgcgttatttatcggagtt-3′, and reverse, 5′-ttcatactgttgagcaattcacgtt-3′. Initially, PCR was performed with serial dilutions of input DNA to determine the linear range of the amplification for each gene. PCR was performed for 25 cycles, and all PCR signals were visualized by Southern blot analysis and quantified with a Molecular Imager FX (Bio-Rad). The values were corrected using the input values. Real-time PCR was performed with Sybr Green in an ABI Prism 7500 by using 2 μl immunoprecipitate or input according to the manufacturer’s specified parameters. The primers for the human TRH promoter were the same as above. The values were corrected using the negative control and the input values.
ChIP analyses were repeated a minimum of three times for statistical analyses as described below.
Luc assay
To determine the Luc activity, cell monolayers were rinsed twice with PBS and then lysed with 300 μl 25 mm glycylglycine (pH 7.8) containing 15 mm MgSO4, 4 mm EGTA, 1 mm dithiothreitol, and 1% vol/vol Triton X-100. Cells were scraped from the dishes and centrifuged at 12,000 × g for 5 min at 4 C. Assays for Luc activity were performed using 150-μl aliquots of cell lysate and 210 μl of 25 mm glycylglycine (pH 7.8) containing 15 mm MgSO4, 4 mm EGTA, 3.3 mm KPO4, 1 mm dithiothreitol, and 0.45 mm ATP. The reaction was initiated by the addition of 200 μl 0.2 mm d-luciferin, and light emission was measured for 10 sec using a luminometer. Luc activity was expressed as arbitrary light units per microgram of cellular protein.
RNA extraction and real-time PCR
Total RNA was extracted using Isogen (Nippongene, Toyama, Japan) according to the manufacturer’s instructions. Then cDNA was reverse transcribed from 160 ng total RNA (TaqMan reverse transcription reagents; Applied Biosystems, Foster City, CA), and 1 μl was subjected to real-time PCR. All reactions were performed in triplicate using Sybr Green and TaqMan probes in an ABI Prism 7500 according to the manufacturer’s specified parameters. The primers for the Luc gene were forward, 5′-ggcgcgttatttatcggagtt-3′, and reverse, 5′-ttcatactgttgagcaattcacgtt-3′. The TaqMan probe for GAPDH (Rn 99999916) was purchased from Applied Biosystems. The expression level of Luc mRNA relative to that of GAPDH mRNA was calculated using a standard curve, and the relative quantification was performed as described in ABI User Bulletin 2.
Statistical analysis
The statistical analysis was performed using ANOVA and Student’s t test, or the Wilcoxon/Kruskal-Wallis test with JMP (SAS Institute Inc., Cary, NC). The level of significance was set at P < 0.05 and P < 0.01.
Results
Deacetylation of specific lysine residues of histone 3 at the TRH promoter after thyroid hormone treatment
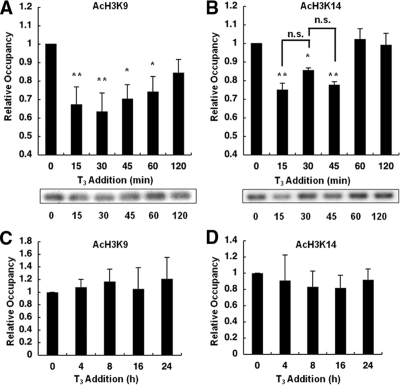
GH4C1 cells stably expressing the TRH promoter reporter gene and the WT TR were treated with 100 nm T3 for the indicated period. The time course of the TRH promoter’s occupancy by acetylated H3K9 and H3K14 was analyzed by ChIP assays. In our previous study, using an antibody recognizing the acetylation of both H3K9 and -K14, significant acetylation at the basal level and deacetylation after the treatment with T3 were observed. Therefore, we first examined which residues are responsible for this effect. As shown in Fig. 1A, lysine 9 at histone 3 was significantly acetylated at the basal level. Treatment with T3 led to significant deacetylation within 15 min to an approximately 40% reduction in the acetylation level and gradual reacetylation to approximately 85% of the basal level in 120 min. Similarly, deacetylation occurred at H3K14 within 15 min to approximately 70% of the control level (Fig. 1B). The difference between these two residues was that the H3K14 residue was completely reacetylated by 60 min. At 120 min, the status of H3K9 and H3K14 was restored to approximately 80 and 100% of the normal level, respectively. We also examined the acetylation status at 24 h but did not find any changes to either H3K9 or H3K14 at 4, 8, 16, or 24 h after the addition of T3 (Fig. 1, C and D). Furthermore, there was no significant change in the total amount of histone 3 (H3) on the TRH gene by T3 within 24 h (supplemental Fig. 3) and no significant acetylation of H3K9 or H3K14 in the coding region of the Luc gene (supplemental Fig. 4).
Figure 1.
Histone deacetylation at H3K9 and H3K14 on the TRH promoter. GH4C1 cells stably expressing the TRH promoter reporter gene and the WT TR were treated with 100 nm T3 for the period indicated. The short-term time course of the TRH promoter’s occupancy within 120 min and the long-term time course of occupancy by acetylated H3K9 (AcH3K9; A and C) and H3K14 (AcH3K14; B and D) were analyzed with ChIP assays. Both H3K9 and H3K14 were acetylated at the basal level, and treatment with 100 nm T3 led to transient deacetylation. No significant changes were observed in the acetylation status of H3K9 and H3K14 after an incubation longer than 2 h. Each level was compared with the basal level (0 min). Representative results of the ChIP assays are demonstrated. The data are presented as the mean ± se for four (A and B) or three (C and D) independent experiments. Asterisks indicate a significant difference vs. 0 min: *, P < 0.05; **, P < 0.01. n.s., Not significant.
Methylation status of specific lysine residues of histone 3 at the TRH gene promoter after thyroid hormone treatment
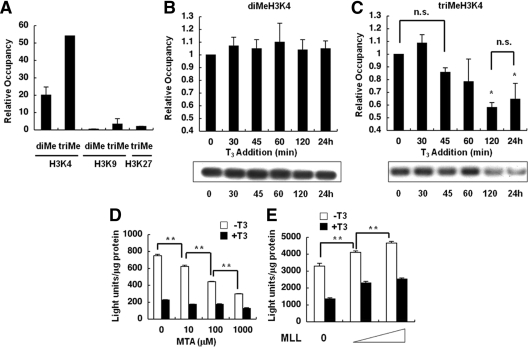
Because di- and trimethylation of H3K4 and -K9 and trimethylation of H3K27 have been reported to be important for gene expression, we examined the status of these modifications at the TRH promoter. As shown in Fig. 2A, compared with the control input, an extremely high level of trimethylation (approximately 50% of the input) at lysine 4 of histone 3 was observed at the TRH promoter in the absence of ligand T3. In addition, a lower level of dimethylation (approximately 20% of the input) was also observed at the same residue. In contrast, almost no di- or trimethylation of H3K9 and no trimethylation of H3K27 were observed, being less than 9% of the trimethylation level of the control.
Figure 2.
Histone methylation at H3K4, -K9, and -K27 on the TRH gene and effects of MTA and MLL. ChIP assays were performed with cells expressing the TRH promoter and the WT TR. A, ChIP assays revealed significant di- and trimethylation of H3K4 but no methylation of H3K9 and H3K27, at the basal level of the TRH gene. Values were expressed as a percentage of the input. B and C, Incubation with 100 nm T3 for longer than 2 h induced a significant reduction in the trimethylation of H3K4 (C) but no change in the level of dimethylation (B). Each level was compared with the basal level (0 min). Representative results of ChIP assays are demonstrated. D, Luc assays in GH4C1 cells stably expressing the TRH promoter and the WT TR in the absence or presence of 100 nm T3 demonstrated that treatment with 10 μm to 1.0 mm MTA inhibited the basal promoter activity in a dose-dependent manner. E, GH4C1 cells were transiently transfected with the TRH-Luc reporter gene, WT TR, and MLL. Overexpression of MLL caused a significant increase in the basal promoter activity of the TRH gene in a dose-dependent manner. The data are presented as the mean ± se for three (A–C) or six (D and E) independent experiments. *, P < 0.05 vs. 0 min (C); **, P < 0.01 (D and E). n.s., Not significant.
Although the addition of 100 nm T3 caused no significant change in the dimethylation of H3K4 on the TRH promoter (Fig. 2B), it led to a significant reduction in the trimethylation of H3K4 after 120 min, and the level remained low after 24 h (Fig. 2C). These findings suggest that prolonged administration of T3 caused a reduction in trimethylation at H3K4 of the gene.
Effect of the histone methyltransferase inhibitor MTA and overexpression of MLL on the promoter activity of the TRH gene
To confirm the importance of the methylation of H3K4 regarding the TRH promoter’s activity, we used a histone methyltransferase inhibitor, MTA, and overexpressed a histone methyltransferase of H3K4, MLL. As shown in Fig. 2D, incubation with MTA caused a significant decrease in the basal activity of the TRH promoter in a dose-dependent manner. In contrast, no significant changes were observed in the repression of the promoter activity by thyroid hormone. These findings further suggested that methylation of H3K4 was crucial for the basal activity of the TRH promoter but not for the repression of the gene by thyroid hormone.
This hypothesis was confirmed conversely by an experiment in which overexpression of MLL caused a significant increase in the basal activity of the TRH promoter in a dose-dependent manner but no change in the repression of the gene by thyroid hormone (Fig. 2E).
Alteration of the amount of TRβ on the TRH gene on prolonged incubation with T3
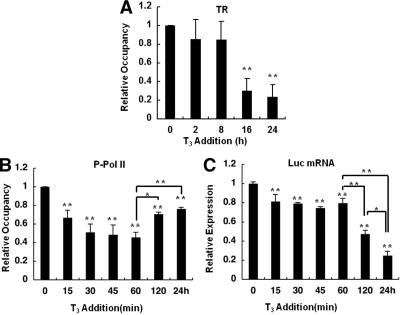
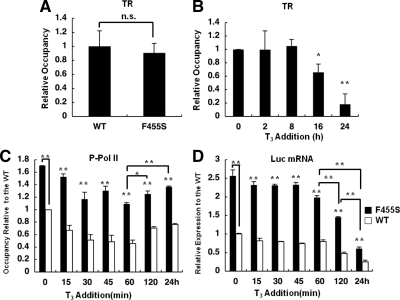
We previously reported that there was no significant change in the amount of TR at the TRH gene promoter within 120 min after the addition of T3 (2). We examined the status of TRβ at 24 h and found that incubation with T3 caused a significant reduction in the amount of TR after 16 and 24 h, to approximately 20 and 16% of the value in the absence of T3, respectively (Fig. 3A). These findings suggest that the prolonged administration of T3 caused a decrease in the amount of TR on the TRH gene. We confirmed that the amount of TR was not altered significantly by T3 using Western blotting (supplemental Fig. 5).
Figure 3.
Profiles of TRs and transcriptional activity of the TRH gene after a long incubation with T3. ChIP assays were performed with cells expressing the WT TR using antibodies against TRβ and Pol II phosphorylated at serine-5 (P-Pol II). A, ChIP assays showed that prolonged treatment with 100 nm T3 caused a significant reduction in the amount of TR on the TRH promoter. B, The amount of P-Pol II on the TRH promoter was reduced at 15 and 60 min after the addition of T3 but had gradually recovered by 120 min and 24 h. Each level was compared with the basal level (0 min). C, Real-time PCR for Luc mRNA was performed using GH4C1 cells stably expressing the TRH promoter reporter gene and the WT TR. Addition of 100 nm T3 decreased Luc mRNA expression at 15 min, and that level was maintained until 60 min. A further reduction was observed at 120 min and at 24 h. Each level was compared with the basal level (0 min). The data are presented as the mean ± se for three independent experiments. Asterisks indicate a significant difference vs. 0 min: *, P < 0.05; **, P < 0.01.
Real-time transcriptional activity of the TRH promoter after the addition of T3
Because the amount of phosphorylated Pol II has been reported to reflect transcriptional activity in some genes, to examine how the above histone modifications are involved in transcription of the gene, we performed a ChIP assay using an antibody specific for Pol II phosphorylated at serine 5. As shown in Fig. 3B, addition of T3 caused a significant reduction in the amount of Pol II to 66.7 ± 8.1% of the control level (P < 0.01) within 15 min, and this reduction persisted for 60 min. However, no further reduction was observed at 120 min (70.5 ± 2.1%) and 24 h (76.3 ± 1.6%). We therefore analyzed the transcriptional activity by measuring the TRH Luc mRNA level. As shown in Fig. 3C, addition of T3 caused a significant reduction in the Luc mRNA level to 81.5 ± 3.2% of the control level (P < 0.01) within 15 min. We confirmed that this reduction was not due to reduced stability of the mRNA using actinomycin D (supplemental Fig. 6). Furthermore, this reduction persisted for 60 min, and further reductions were observed at 120 min (47.3 ± 3.9% of the control) and 24 h (24.8 ± 4.5%), suggesting expression of the TRH gene to require other factors such as trimethylation of H3K4 in addition to the recruitment of Pol II.
Hyperacetylation and aberrant histone deacetylation of a specific residue of histone 3 with the F455S mutant TR
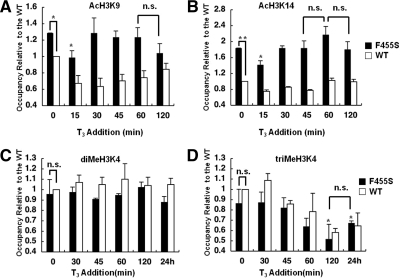
We next examined the effect of the F455S mutant on the acetylation status of H3K9 and -K14. In the presence of the F455S TR, the acetylation of H3K9 showed an approximately 1.3-fold increase in the absence of T3 compared with that with the WT TR (Fig. 4A). On the other hand, the level of acetylated H3K14 was more significantly increased at approximately 180% that of the control WT (Fig. 4B). Addition of T3 caused a similar profile of deacetylation within 15 min both at H3K9 and at H3K14 compared with the WT, as shown in Fig. 4, A and B. However, H3K9 and H3K14 were hyperacetylated in the presence or absence of T3, and the reacetylation was completed in 30 min at both sites, suggesting that the presence of the mutant TR induced hyperacetylation, thereby causing insufficient deacetylation of the histone tail on the TRH promoter.
Figure 4.
Acetylation and methylation status in the presence of the F455S mutant TR. ChIP assays were performed with cells expressing the WT TR (white bars) or F455S mutant TR (black bars) using antibodies against acetylated H3K9 (AcH3K9) (A), acetylated H3K14 (AcH3K14) (B), diMeH3K4 (C), and triMeH3K4 (D). The values are expressed relative to the WT cells (white bars), and the value for the WT cells at 0 min was set as 1.0. A and B, Residues H3K9 and -K14 were hyperacetylated, and reacetylation started earlier in F455S cells. C and D, No significant changes in diMeH3K4 or triMeH3K4 were observed at the basal level of the TRH gene compared with that of the WT TR. After the addition of 100 nm T3, the profile of diMeH3K4 and triMeH3K4 in F455S cells was similar to that in WT cells. The data are presented as the mean ± se for three independent experiments. Asterisks indicate a significant difference vs. 0 min: *, P < 0.05; **, P < 0.01. n.s., Not significant.
Intact histone methylation on the TRH gene with the F455S mutant
We next examined the methylation status of H3K4 of the TRH promoter in the presence of F455S. As shown in Fig. 4, C and D, no significant difference was observed in either the di- or trimethylation status of H3K4 between the WT and F455S TRs in the absence or presence of T3. Similar to the findings with the WT TR, addition of T3 caused no significant change in the dimethylation of H3K4 but led to a significant decrease in the trimethylation of H3K4 to 60 and 78% of the basal level within 120 min and 24 h, respectively.
Intact profile of TR on the TRH gene in the presence of F455S TR
The total amount of TR in GH4C1 cells expressing both exogenous F455S TR and the endogenous WT TRs in the absence of T3 was similar to that in the control cells (WT TR) expressing both exogenous and endogenous WT TRs (Fig. 5A). We found a significant reduction in the amount of TR at 16 and 24 h, in the presence of the F455S TR (Fig. 5B), suggesting the alteration in the total amount of TR not to be a major cause of the impaired regulation of the TRH gene by the mutant TRs.
Figure 5.
Profile of TRs and transcriptional activity of the TRH promoter in the presence of F455S TR. ChIP assays were performed with cells expressing the WT TR or F455S mutant TR using antibodies against TR. A, The total amount of TR in GH4C1 cells expressing F455S in the absence of T3 was similar to that in the cells expressing WT TR. B, Prolonged treatment with 100 nm T3 caused a significant reduction in the amount of TR on the TRH promoter in cells expressing the F455S mutant. C, In the absence of T3, approximately 170% of the control level of Pol II phosphorylated at serine-5 (P-Pol II) was recruited to the TRH promoter in F455S cells (black bars). After the addition of T3, the level of P-Pol II remained high. The value is expressed relative to that of WT cells, and the level for WT cells at 0 min was set as 1.0. The white bars indicate the data for WT cells. D, Luc mRNA was measured in WT cells (white bars) or F455S TR cells (black bars). Approximately 250% of the Luc mRNA level in WT cells was expressed in F455S TR cells in the absence of T3. After the addition of T3, the level of mRNA remained high. The value is expressed relative to that of WT cells, and the level of WT cells at 0 min was set as 1.0. The data are presented as the mean ± se for at least three independent experiments. Asterisks indicate a significant difference vs. 0 min: *, P < 0.05; **, P < 0.01. n.s., Not significant.
Strong transcriptional activity of the TRH gene in the presence of F455S TR
We further examined transcriptional levels in the presence of F455S TR. In the absence of T3, reflecting the hyperacetylation of H3K9 and -K14, the amount of phosphorylated Pol II on the TRH promoter was significantly elevated to approximately 170% of the WT level (Fig. 5C). The profile of phosphorylated Pol II on the TRH promoter after the addition of T3 was similar to that of the WT shown in Fig. 3B, but the level remained high. Furthermore, the reduction of Pol II after the addition of T3 was significantly impaired at 15–45 min compared with that of the WT TR (10.4 ± 2.6 vs. 33.2 ± 8.1% at 15 min, P < 0.05; 23.5 ± 4.3 vs. 51.4 ± 10.3% at 45 min, P < 0.01; Fig. 5C).
We further analyzed the transcriptional activity by measuring the TRH Luc mRNA level. The mRNA level in the absence of T3 was significantly elevated to approximately 250% of the WT level (Fig. 5D). The profile of TRH-Luc mRNA expression after the addition of T3 was similar to that of the WT shown in Fig. 3C; however, the repression by T3 in 15–45 min was not sufficient as shown in Fig. 3C (9.6 ± 3.4 vs. 18.5 ± 3.2% at 15 min, P < 0.05; 9.4 ± 2.3 vs. 25.4 ± 1.4% at 45 min, P < 0.01; Fig. 5D). These findings suggested that a high expression level of the TRH promoter was maintained even after the addition of T3 and showed impairment of repression of the gene probably by the dominant-negative activity of the F455S mutant TR against the WT TR.
Discussion
In the present study, we demonstrated that H3K9 and -K14, reported to be critical histone modifications for gene expression, on the TRH gene showed a similar transient deacetylation induced by thyroid hormone. We previously reported the acute recruitment of HDAC2 and HDAC3 to the TRH gene within 15 min after the addition of T3. Although the mechanism by which each residue, H3K9 and -K14, was deacetylated remains unclear, whether or not the same HDAC worked on both residues, the acetylation status of certain residues of the histone tail affects the modifications of other residues; i.e. acetylation of H3K9 induced acetylation of H3K14 through phosphorylation of H3S10 (15). Therefore, it is possible that the acetylation status of H3K9 affected that of K14, and vice versa, on the TRH gene.
There are several reports regarding the involvement of histone methylation in transcriptional regulation by nuclear receptors (16,17,18). Although Li et al. (5) reported the importance of the methylation of H3K9 and the interaction of TR with SUV39H1 for repression by unliganded TRs, this is the first report regarding that of genes negatively regulated by thyroid hormone. In the case of the TRH gene, no methylation was observed at H3K9 and H3K27, whereas significant methylation was observed at H3K4. Because it is generally accepted that the methylation of H3K9 and -K27 is closely linked to transcriptional repression, and that of H3K4 to active euchromatin regions, the methylation status of the TRH gene may reflect the strong gene activity.
It is of interest that the addition of T3 for longer than 2 h caused a significant reduction in trimethylated H3K4 (triMeH3K4) but no change in dimethylated H3K4 (diMeH3K4). Although the biological significance of the difference between di- and trimethylation of H3K4 remains unclear, it has been reported that trimethylation strongly correlates with transcription start sites, whereas dimethylation was observed elsewhere in the vicinity of the active gene (19). Therefore, alteration of the degree of trimethylation of H3K4 in the promoter region may be involved in the activation of the TRH gene. In fact, the present study using MTA and overexpression of MLL demonstrated that methylation was crucial for the activity of the gene. These observations suggest the reduction in triMeH3K4 to be important for the repression of the gene on long-term administration of thyroid hormone. Previously, we reported that treatment with a HDAC inhibitor, trichostatin A (TSA), completely abolished repression of the promoter activity by T3 (2). On the basis of these findings, it is suggested that 1) histone trimethylation of H3K4 was crucial for the basal activity of the TRH promoter, 2) deacetylation plays a predominant role in the repression of the gene on short-term incubation with thyroid hormone (∼2 h), and 3) long-term incubation with T3 reduced the promoter activity of the TRH gene probably by decreasing the methylation of H3K4.
Furthermore, the present study demonstrated that the real-time transcription of the TRH promoter had a similar profile to the sum of the deacetylation of H3K9/K14 and demethylation of triMeH3K4. Therefore, combined changes in the status of acetylation and methylation may be involved in the negative regulation of the TRH gene by thyroid hormone. Furthermore, the profile of Pol II phosphorylated at serine 5 on the TRH promoter was similar to that of real-time transcription, except at 120 min and 24 h, suggesting that in addition to phosphorylated Pol II, other factors such as triMeH3K4 may be important for expression of the TRH gene. In fact, it has been reported that RNA Pol II phosphorylated at serine 5 is involved with initiation of the transcription and is also required for the trimethylation of H3K4 (20,21).
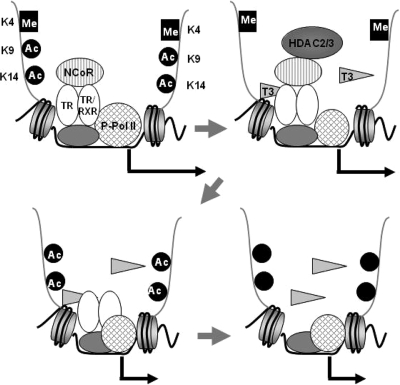
Another novel finding of the present study was that prolonged administration of T3 caused the release of TR from the TRH promoter. The reduction in the amount of TR on the TRH gene may be involved in the demethylation of triMeH3K4, because the two showed a similar profile on the TRH gene. Although cyclic recruitment of TR to the targeted gene positively regulated by thyroid hormone has been reported (6), the present and previous studies demonstrated no such cyclization of TR in the TRH promoter region within 24 h (2). Therefore, the dynamics of TR on targeted genes may differ between the genes positively and negatively regulated by thyroid hormone. Based on the present observations together with those of the previous study, a schematic model was developed for the TRH gene, as shown in Fig. 6.
Figure 6.
A model of the dynamics of coregulators, the histone modifications, and TR on the TRH gene in response to T3. In the absence of T3, TR, NCoR and phosphorylated Pol II are recruited to the TRH gene, H3K4 is methylated, and H3K9 and -K14 are acetylated. The addition of T3 causes the recruitment of HDACs 2 and 3 to deacetylate H3K9 and -K14, and the amount of phosphorylated Pol II was decreased to suppress the gene. After the release of NCoR and HDACs, H3K9 and -K14 start to be reacetylated. Prolonged administration of T3 causes demethylation of H3K4 and subsequently the release of TR from the gene to suppress the gene.
The presence of the mutant F455S induced aberrant acetylation but caused no change in methylation or the amount of TR on the TRH gene. We also found a significant enhancement of the basal promoter activity of the TRH gene and impaired suppression by thyroid hormone. These findings might be due to the impaired release of the corepressor NCoR and recruitment of HDAC in the presence of F455S TR as previously reported (2). Considering that NCoR may act as a coactivator for genes negatively regulated by thyroid hormone at least in vitro (22), these abnormalities may cause hyperacetylation and insufficient deacetylation on the TRH gene, resulting in the recruitment of excess Pol II and strong expression of the TRH gene, which may reflect the dominant-negative activity of the F455S mutant on the WT TR.
In conclusion, we demonstrated here the role of histone modifications, the amount of TR, and the status of Pol II in the regulation of the TRH gene by thyroid hormone and abnormalities in the mutant TR F455S. Because the regulation of the TRH gene by thyroid hormone was observed in a specific region, particularly in the hypothalamic paraventricular nucleus, further study is required to confirm that the model, including histone modifications, proposed in the present study works in a site-specific manner in vivo (23). However, this is the first model to show how a gene is negatively regulated by thyroid hormone and the involvement of specific histone modifications with RTH.
Supplementary Material
Footnotes
Disclosure Summary: The authors have nothing to declare.
First Published Online March 19, 2009
Abbreviations: ChIP, Chromatin immunoprecipitation; diMeH3K4, dimethylated H3K4; FBS, fetal bovine serum; HDAC, histone deacetylase; Luc, luciferase; MLL, mixed-lineage leukemia; MTA, 5′-deoxy-5′-methylthioadenosine; NCoR, nuclear receptor corepressor; Pol II, RNA polymerase II; RTH, resistance to thyroid hormone; SDS, sodium dodecyl sulfate; TR, thyroid hormone receptor; triMeH3K4, trimethylated H3K4; WT, wild type.
References
- Refetoff S, Dumitrescu AM 2007 Syndromes of reduced sensitivity to thyroid hormone: genetic defects in hormone receptors, cell transporters and deiodination. Best Pract Res Clin Endocrinol Metab 21:277–305 [DOI] [PubMed] [Google Scholar]
- Ishii S, Yamada M, Satoh T, Monden T, Hashimoto K, Shibusawa N, Onigata K, Morikawa A, Mori M 2004 Aberrant dynamics of histone deacetylation at the thyrotropin-releasing hormone gene in resistance to thyroid hormone. Mol Endocrinol 18:1708–1720 [DOI] [PubMed] [Google Scholar]
- Hattori S, Deguchi K, Onigata K, Yamada S, Nagashima T, Morikawa A 2000 A novel mutation (F455S) in thyroid hormone receptor β gene in a sporodic case of resistance to thyroid hormone. Endocr J Suppl 47:236 [Google Scholar]
- Oetting A, Yen PM 2007 New insights into thyroid hormone action. Best Pract Res Clin Endocrinol Metab 21:193–208 [DOI] [PubMed] [Google Scholar]
- Li J, Lin Q, Yoon HG, Huang ZQ, Strahl BD, Allis CD, Wong J 2002 Involvement of histone methylation and phosphorylation in regulation of transcription by thyroid hormone receptor. Mol Cell Biol 22:5688–5697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Xia X, Fondell JD, Yen PM 2006 Thyroid hormone-regulated target genes have distinct patterns of coactivator recruitment and histone acetylation. Mol Endocrinol 20:483–490 [DOI] [PubMed] [Google Scholar]
- Sharma D, Fondell JD 2002 Ordered recruitment of histone acetyltransferases and the TRAP/mediator complex to thyroid hormone-responsive promoters in vivo. Proc Natl Acad Sci USA 99:7934–7939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg AN, Monden T, Flynn TR, Boers ME, Cohen O, Wondisford FE 1995 The human thyrotropin-releasing hormone gene is regulated by thyroid hormone through two distinct classes of negative thyroid hormone response elements. Mol Endocrinol 9:540–550 [DOI] [PubMed] [Google Scholar]
- Satoh T, Monden T, Ishizuka T, Mitsuhashi T, Yamada M, Mori M 1999 DNA binding and interaction with the nuclear receptor corepressor of thyroid hormone receptor are required for ligand-independent stimulation of the mouse preprothyrotropin-releasing hormone gene. Mol Cell Endocrinol 154:137–149 [DOI] [PubMed] [Google Scholar]
- Kouzarides T 2007 Chromatin modifications and their function. Cell 128:693–705 [DOI] [PubMed] [Google Scholar]
- Li B, Carey M, Workman JL 2007 The role of chromatin during transcription. Cell 128:707–719 [DOI] [PubMed] [Google Scholar]
- Bernstein BE, Meissner A, Lander ES 2007 The mammalian epigenome. Cell 128:669–681 [DOI] [PubMed] [Google Scholar]
- Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA, Shi Y 2004 Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 119:941–953 [DOI] [PubMed] [Google Scholar]
- Dou Y, Milne TA, Tackett AJ, Smith ER, Fukuda A, Wysocka J, Allis CD, Chait BT, Hess JL, Roeder RG 2005 Physical association and coordinate function of the H3 K4 methyltransferase MLL1 and the H4 K16 acetyltransferase MOF. Cell 121:873–885 [DOI] [PubMed] [Google Scholar]
- Agalioti T, Chen G, Thanos D 2002 Deciphering the transcriptional histone acetylation code for a human gene. Cell 111:381–392 [DOI] [PubMed] [Google Scholar]
- Metzger E, Wissmann M, Yin N, Müller JM, Schneider R, Peters AH, Günther T, Buettner R, Schüle R 2005 LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature 437:436–439 [DOI] [PubMed] [Google Scholar]
- Dreijerink KM, Mulder KW, Winkler GS, Höppener JW, Lips CJ, Timmers HT 2006 Menin links estrogen receptor activation to histone H3K4 trimethylation. Cancer Res 66:4929–4935 [DOI] [PubMed] [Google Scholar]
- Cloos PA, Christensen J, Agger K, Helin K 2008 Erasing the methyl mark: histone demethylases at the center of cellular differentiation and disease. Genes Dev 22:1115–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BE, Kamal M, Lindblad-Toh K, Bekiranov S, Bailey DK, Huebert DJ, McMahon S, Karlsson EK, Kulbokas 3rd EJ, Gingeras TR, Schreiber SL, Lander ES 2005 Genomic maps and comparative analysis of histone modifications in human and mouse. Cell 120:169–181 [DOI] [PubMed] [Google Scholar]
- Hirose Y, Ohkuma Y 2007 Phosphorylation of the C-terminal domain of RNA polymerase II plays central roles in the integrated events of eucaryotic gene expression. J Biochem 141:601–608 [DOI] [PubMed] [Google Scholar]
- Hampsey M, Reinberg D 2003 Tails of intrigue: phosphorylation of RNA polymerase II mediates histone methylation. Cell 113:429–432 [DOI] [PubMed] [Google Scholar]
- Tagami T, Madison LD, Nagaya T, Jameson JL 1997 Nuclear receptor corepressors activate rather than suppress basal transcription of genes that are negatively regulated by thyroid hormone. Mol Cell Biol 17:2642–2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerson TP, Kauer J, Wolfe HC, Mobtaker H, Wu P, Jackson IM, Lechan RM 1987 Thyroid hormone regulates TRH biosynthesis in the paraventricular nucleus of the rat hypothalamus. Science 238:78–80 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.