Abstract
The GnRH receptor (GnRHR) responds to pulsatile GnRH signals to coordinate pituitary gonadotropin synthesis and secretion. Previously, a 1.2-kb fragment of the 5′-flanking region isolated from the mouse GnRHR gene was shown to target expression to pituitary gonadotropes in vivo. The 1.2-kb gene promoter fused to the simian virus 40 large T antigen (TAg) was used to generate transgenic mice that form gonadotrope-derived pituitary tumors at 4–5 months of age. Transgenic female mice have hypogonadotropic hypogonadism, infantile gonads, and are infertile throughout their life span, whereas males remain reproductively intact until their tumors become large. We hypothesized that the targeted TAg expression causes a sex-specific disruption of the reproductive axis at the level of the pituitary gland. To test this hypothesis, we characterized the pituitary gonadotropin β-subunit and TAg expression patterns, and measured plasma gonadotropin and gonadal steroid levels in female and male mice before and after pituitary tumor development. TAg expression was observed in transgenic females and males 15 d of age, before tumor development. Interestingly, and in contrast to the transgenic males, pituitary LHβ and FSHβ subunit protein levels, and plasma LH and FSH levels, were reduced in transgenic females. Reproductive organs in transgenic female mice remained underdeveloped but were normal in transgenic males. We conclude that the expression of the TAg transgene driven by the GnRHR gene promoter results in female-specific infertility due to disruption of gonadotropin production and secretion even before tumor development.
Transgenic targeting of SV40 T antigen in gonadotropes results in female-specific infertility due to disruption of gonadotropin production and secretion, even prior to tumor development.
The GnRH receptor (GnRHR) is expressed primarily in pituitary gonadotropes, and mediates GnRH-stimulated expression and secretion of the gonadotropins, LH and FSH (1,2,3). A 1.2-kb 5′-flanking region of the mouse GnRHR has been sufficient for both gonadotrope-specific and GnRH-stimulated GnRHR gene expression, and effectively directs reporter gene expression in the αT3-1 gonadotrope cell line, but not in heterologous cell lines (4,5,6). These findings have been further supported by transgenic mouse studies that used this region (7) or a larger 1.9-kb fragment that encompasses this region (8) to specifically target transgenes to the pituitary, and more specifically to the gonadotrope. Promoter-specific regulation of oncogenes, such as the simian virus 40 (SV40) large T antigen (TAg), has been used widely for tissue-specific targeting of transgene expression (9,10,11,12) and for the generation of immortalized cell lines from the resultant tumors (13,14,15).
GnRHR-TAg transgenic mice, created using this 1.2-kb 5′-flanking region of the mouse GnRHR gene fused upstream of the SV40 TAg coding sequence, develop debilitating pituitary tumors of apparent gonadotrope origin at 4–5 months of age. Histological evaluation demonstrated that the tumors expressed gonadotrope-specific genes, including α-glycoprotein subunit, LHβ, FSHβ, and GnRHR, as well as SV40 TAg (7). However, at younger ages, and before tumor formation, sex-specific differences in fertility were evident; all transgenic females were infertile throughout their life span, whereas the males were reproductively intact until their tumors became large.
The cause of female-specific infertility in these transgenic mice is unclear because these animals are unable to reproduce even before apparent pituitary tumor development. At 2 months of age, their ovarian histology shows only preovulatory follicles with no corpus luteum formation, suggesting an arrest of follicular development and anovulationb. Follicular maturation, ovulation, and corpus luteum formation were induced by superovulatory doses of human chorionic gonadotropin, suggesting an intact ovarian response to gonadotropin stimulation and implicating a defect in pituitary gonadotropin secretion in the transgenic females (7). In contrast, transgenic male mice are able to reproduce normally before pituitary tumor development, suggesting a sexual dimorphism in gonadotrope function. These observations have led us to investigate the hypothesis that the cause of the infertility in transgenic female mice may be impaired gonadotropin production and secretion due to SV40 TAg oncogene expression even before pituitary tumor development.
To address these questions, we measured plasma gonadotropin and gonadal steroid hormone levels in adult GnRHR-TAg transgenic mice both before and after pituitary tumor development, and further characterized the gross anatomical differences of internal reproductive organs in these mice. We also analyzed SV40 TAg and gonadotropin β-subunit expression in the pituitary before and after pituitary tumor development. The data presented here are consistent with the observed female-specific infertility in the GnRHR-TAg transgenic mice, and suggest that the presence of the transgene interrupts gonadotropin production and secretion earlier in females than males.
Materials and Methods
Animal husbandry
The GnRHR-TAg transgenic mouse line used in this study (M29) was described previously and bears the transgene in the heterozygous state (7). GnRHR-TAg transgenic and nontransgenic littermates were raised in an FVB background by breeding transgenic males with nontransgenic females in a 12-h light, 12-h dark cycle and fed ad libitum. Genotyping was performed by PCR for the GnRHR-TAg transgene (supplemental Table 1, which is published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). Daily vaginal cytology was monitored to analyze estrous cyclicity. All experiments were approved by the Harvard Medical Area Standing Committee on Animals in the Harvard Medical School Center for Animal Resources and Comparative Medicine.
Sample collection
Blood samples (300–400 μl) were collected without anesthesia by retro-orbital sinus phlebotomy (16) 4 h after lights-on. The same animals were subjected to sampling at both 2 and 5 months of age when available. Collected blood samples were centrifuged at 4 C, and plasma was stored at −80 C until analyses.
Pituitary glands from transgenic and nontransgenic littermates were collected at 2 or 5 months of age after cardiac perfusion and postfixation with 4% formaldehyde containing 0.2% picric acid in PBS. Reproductive organs from 2- and 5-month-old mice were collected for anatomical comparison.
Histology and immunohistochemistry
Paraffin-embedded pituitaries were sectioned (5 μm) and stained by hematoxylin-eosin (H&E) alone or by Wilder’s method for reticulin, followed by H&E. For pituitary immunohistochemistry, samples were serially cryosectioned (10 μm), treated with 0.3% H2O2 in PBS to remove endogenous peroxidase, and stained either with polyclonal rabbit anti-SV40 TAg antibody (no. SC-20800, 1:4,000 dilution; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), goat anti-LHβ antibody (for LHβ subunit, no. SC-7824, 1:4,000 dilution; Santa Cruz Biotechnology), or rabbit antirat FSHβ antibody (no. AFP7798-1289, 1:50,000 dilution; National Hormone and Peptide Program) in PBS containing 0.3% Triton X-100 with 1% normal serum overnight at 4 C. After incubation with the appropriate secondary antibody and Vectastain Elite ABC Kit (Vector Laboratories, Burlingame, CA), sections were visualized with ImmunoPure Metal Enhanced 3,3′-diaminobenzidine tetrahydrochloride (Pierce, Rockford, IL). Sections were dehydrated then mounted with Permount (Thermo Fisher Scientific Inc., Waltham, MA).
Pituitary sections were processed for colocalization of FSHβ and LHβ with rabbit antirat FSHβ (no. SC-7797, 1:3000; Santa Cruz Biotechnology) and goat anti-LHβ (no. SC7824, 1:250 dilution; Santa Cruz Biotechnology) antibodies, respectively. Colocalization of SV40 TAg (no. SC-20800, 1:300; Santa Cruz Biotechnology) with LHβ (no. SC-7824, 1:250; Santa Cruz Biotechnology) or FSHβ (guinea pig antirat FSHβ, no. AFP28122491GP; 1:500; National Hormone and Peptide Program) was conducted similarly with simultaneous incubation of the primary antibodies diluted in PBS containing 0.3% Triton X-100 containing 5% normal serum. Red and green immunofluorescence was visualized by incubation with appropriate secondary antibodies conjugated with either Alexa Fluor 488 or 594 (nos. A11055, A21207, A21206, and A11076; Invitrogen Corp., Carlsbad, CA) at 1:500–1:200 dilutions, respectively. The slides were mounted and sealed with Vectashield (Vector Laboratories).
Plasma hormone analyses
Immunoreactive plasma hormone levels were analyzed by RIA or sandwich immunoradiometric assay (IRMA). The LH and FSH RIA used reagents from the National Hormone and Peptide Program (16): antirat LHβ antibody (NIDDK-anti-rLH-S-112; 1:750,000 dilution), and antimouse FSHβ antibody (anti-mFSH AFP1760191; 1:200,000 dilution). The LH IRMA was performed by University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core Laboratory (Charlottesville, VA). The FSH IRMA was performed using a rat FSH IRMA kit (Alpco Diagnostics, Windham, NH). The 17β-estradiol and testosterone levels were measured using commercial RIA kits (MP Biomedicals, Orangeburg, NY).
Quantitative RT-PCR (QRT-PCR)
Total RNA was extracted from individual pituitaries with the RNeasy Micro Plus kit (QIAGEN, Inc., Valencia, CA) and reverse transcribed with oligo deoxythymidine primers (Promega Corp., Madison, WI) and SuperScript III (Invitrogen). Real-time PCR, with 200 nm gene-specific primers (supplemental Table 1) and Platinum SYBR green SuperMix (Invitrogen), was performed using the ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA). To calculate fold change, the comparative threshold cycle (CT) method was used for mRNA quantification with β-actin as a loading control and the mean of 15-d nontransgenic female for each gene as a reference value.
Data analysis
Results were analyzed by ANOVA, followed by post hoc comparisons with Fisher’s protected least significant difference test or the Newman-Keuls test. When appropriate, results were analyzed by the unpaired t test for the comparison of mean differences between two different groups, and by the paired t test for the comparison of mean differences between different ages in a genotype. An analysis for an outlier observation in samples (17) was performed based on the random sampling theory to determine whether the FSH level in a transgenic male mouse at 5 months of age was an outlier. All data are presented as the mean ± sem.
Results
Characterization of the reproductive phenotypes of GnRHR-TAg transgenic female mice
Two to 6-month-old female GnRHR-TAg transgenic and nontransgenic littermates genotyped by PCR (supplemental Fig. 1A) were monitored for estrous cyclicity by vaginal cytology, which revealed that transgenic females did not cycle normally. Five out of nine mice showed constant nonestrus cell morphology, and the other four showed constant estrus cell morphology during 40 d observation. There was no correlation between cell morphology and age. In contrast, nontransgenic female mice had regular estrous cyclicity with average cycle duration of 6.3 ± 0.3 d. Attempts to mate a set of transgenic female mice with nontransgenic males beginning at 2 months of age were unsuccessful, with no evidence of vaginal plugs. In contrast, transgenic male and nontransgenic female mice mated successfully. Body weights and sizes of female transgenic mice were similar to age-matched littermates.
GnRHR-TAg transgenic female mice have underdeveloped internal reproductive organs
Transgenic female mice examined at 2 months of age had underdeveloped ovaries and uteri compared with littermates at the same age (supplemental Fig. 1B). Similarly, at 5 months of age, the ovaries in transgenic females remained smaller, and the uteri thinner, than in nontransgenic littermates, suggesting this abnormality develops early and persists throughout adulthood in GnRHR-TAg transgenic females. In contrast, there were no apparent differences in male reproductive organs between genotypes at 2 or 5 months of age.
Transgenic male and female pituitary tumors reveal histological differences
At 2 months, histological examination of H&E stained transgenic female pituitary tissue cytoarchitecture showed a similar appearance to that of nontransgenic female pituitary tissue, with normal sinusoidal morphology, with a cell population composed of eosinophilic, basophilic, and chromophobic cells, and devoid of visible focal anomalies or evidence of tumor formation (Fig. 1A). Therefore, reproductive deficits in these mice were not associated with changes in pituitary histology. However, at 5 months the transgenic female pituitary showed an atypical morphology with loss of normal sinusoidal architecture, and overgrowth of aggressive neoplastic cells with a scattered distribution of bizarre multinucleated cells with numerous mitotic figures and apoptosis were observed. These findings are those of an aggressive atypical pituitary adenoma, and morphologically most consistent with a very aggressive neoplasm, or pituitary carcinoma (Fig. 1A).
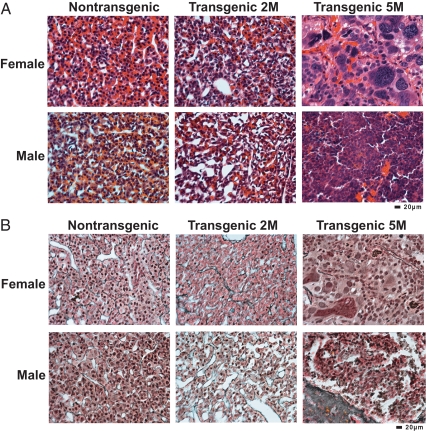
Figure 1.
Pituitary histology of GnRHR-TAg transgenic mice reveals a sex-specific difference in tumor severity. A, H&E staining of female and male pituitary tissues from nontransgenic and GnRHR-TAg transgenic mice at 2 months of age (2M) revealed normal histology in both sexes. At 5 months of age (5M), both transgenic females and males displayed transformed cellular histology, but distinct in severity. B, Histological examination with reticulin staining of same tissue in A, demonstrating normal tissue organization in 2-month-old female and male nontransgenic and transgenic pituitaries by the presence of reticulin fibers (gray-black). Disruption of reticulin staining indicated tumor formation in transgenic 5-month-old female and male pituitaries with evidence of cellular transformation consistent with pituitary carcinoma in the female pituitary tissue and with that of adenoma in males. Magnification, ×400. Bars, 20 μm.
The male transgenic mice at 2 months of age revealed normal pituitary morphology, consistent with the normal reproductive phenotype (Fig. 1A). At 5 months, variable degrees of morphological changes were evident. Some transgenic males exhibited large pituitary tumors that retained normal acinar structure with small foci of densely packed cells, whereas others had pituitary tumors showing some degree of cytological atypia, including multinucleated cells and loss of sinusoidal architecture. Nonetheless, the highest degree of visible morphological changes in the transgenic males did not reveal the aggressive characteristics seen in the pituitary tumors of the females, and in contrast to the transgenic females, was consistent with benign pituitary adenoma rather than an atypical adenoma (Fig. 1A).
The lack of visible TAg-mediated transformation in both the 2-month transgenic females and males was confirmed by the presence of normal reticulin fiber staining, with the presence of collagen-rich reticulin membrane and normal sinusoidal organization (Fig. 1B). Therefore, at 2 months the female pituitary did not reveal morphological findings consistent with the formation of pituitary tumors as a cause of the hypogonadism and infertility already present at this age. In contrast, at 5 months, both GnRHR-TAg males and females showed disrupted reticulin staining and pituitary histology indicative of tumor formation (Fig. 1B).
Pituitary immunoreactivity of SV40 TAg is associated with reductions in LHβ and FSHβ immunoreactivity in GnRHR-TAg females
SV40 TAg expression was assessed and correlated with gonadotrope function by immunostaining alternating pituitary sections for SV40 TAg, LHβ, and FSHβ in transgenic and nontransgenic females (Fig. 2A) and males (Fig. 2B) at 2 and 5 months. Before any evidence of tumor development, transgenic female and male pituitaries were immunopositive for SV40 TAg at 2 months. Pituitary SV40 TAg immunoreactivity persisted in the transgenic mice at 5 months (Fig. 2). Nontransgenic mice were negative for SV40 TAg immunoreactivity.
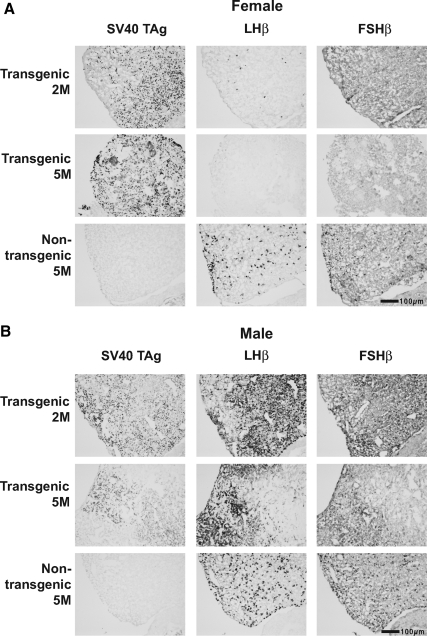
Figure 2.
GnRHR-TAg transgenic mice express SV40 TAg at 2 months of age (2M), and their pituitary LHβ and FSHβ levels are reduced earlier and to a greater extent in females than males. Representative images of immunohistochemistry of anterior pituitary serial sections immunostained for SV40 TAg, LHβ, and FSHβ from female (A) and male (B) mice at 2 or 5 months of age (5M). Magnification, ×100. Bars, 100 μm.
Interestingly, LHβ immunostaining was markedly reduced in transgenic female pituitaries at 2 months of age compared with nontransgenic controls, and was virtually undetectable by 5 months (Fig. 2A). Similarly, specific pituitary FSHβ immunostaining was reduced in transgenic females by 2 months and absent at 5 months. However, in contrast to the transgenic females, the immunostaining of transgenic male LHβ and FSHβ persisted at both 2 and 5 months, at levels equal to or greater than those in the 5-month-old nontransgenic control male pituitary (Fig. 2B). In nontransgenic pituitaries, it was noted that gonadotropin subunit immunoreactivity was greater in males, consistent with previous reports of greater FSHβ expression in males (18).
Heterogeneous SV40 TAg immunoreactivity and overlap with LHβ and FSHβ-positive cell populations
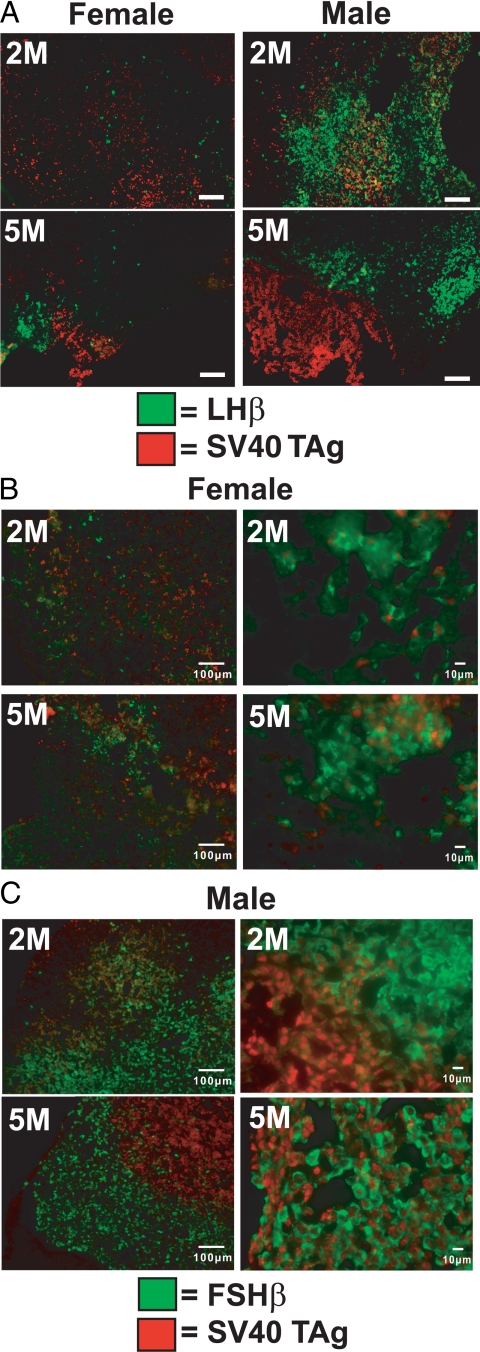
Changes in gonadotropin subunit expression in transgenic mice were further characterized by double immunofluorescence for LHβ or FSHβ with SV40 TAg in pituitary sections from the GnRHR-TAg mice. In this analysis, intracellular colocalization of gonadotropin subunit and TAg proteins would not be expected because their distributions are restricted to cytoplasm and nucleus, respectively. Nonetheless, double immunofluorescence can reveal areas of overlap in staining patterns, suggesting expression of both proteins in individual cells. In transgenic female mice, although LHβ staining was low, there was no observable overlap with SV40 TAg and LHβ staining at 2 or 5 months (Fig. 3A). In contrast, transgenic males at 2 months had areas of fluorescence overlap in which both proteins were present. However, by 5 months of age, heterogeneity was noted, with sites of immunoreactive LHβ distinct from those of SV40 TAg (Fig. 3A). These results suggest that the presence of SV40 TAg in the tumor may suppress LHβ expression by the time tumor formation occurs.
Figure 3.
Heterogeneous patterns of SV40 TAg and gonadotropin subunit detection in GnRHR-TAg transgenic pituitaries. A, Dual immunostaining of LHβ (green) and SV40 TAg (red) in pituitary sections of female and male GnRHR-TAg transgenic mice at 2 months (2M) and 5 months (5M). Magnification, ×100. Bars, 100 μm. B and C, Dual immunostaining of FSHβ (green) and SV40 TAg (red) in pituitary sections of female (B) and male (C) GnRHR-TAg transgenic mice at 2 months (2M) and 5 months (5M). Lower magnification images (B and C, left column; magnification, ×100; bars, 100 μm) illustrate differences in FSHβ (green) and SV40 TAg (red) staining patterns between males and females at each age. Higher magnification images (B and C, right column, magnification, ×400; bars, 10 μm) of a different field of view from the same tissues in the left column were shown to emphasize varying degrees of FSHβ and SV40 TAg overlap.
In pituitaries of transgenic females (Fig. 3B), some overlap of TAg and FSHβ was present at both 2 and 5 months, although in small amounts given the low levels of FSHβ. In transgenic males (Fig. 3C), these patterns were more heterogeneous, with overlap in some areas and with other areas of predominantly FSHβ or SV40 immunoreactivity (Fig. 3C, right column).
GnRHR-TAg transgenic mice express LHβ and FSHβ in distinct pituitary cell populations
To examine potential sex differences in LHβ and FSHβ expression patterns, colocalization studies were performed by double-immunofluorescent staining. As expected, nontransgenic females and males (supplemental Fig. 2) showed colocalization (yellow color in merged images) of the LHβ (green) and FSHβ (red) subunits in the majority of the stained cells at 2 and 5 months. As noted by immunohistochemistry, GnRHR-TAg transgenic female mice had markedly reduced LHβ and FSHβ levels at both 2 and 5 months of age. Colocalization of these subunits was not observed in the female anterior pituitary gland at either 2 or 5 months of age; however, this may merely reflect the low levels of detectable gonadotropins (supplemental Fig. 2A). In contrast, GnRHR-TAg transgenic males showed clear evidence of colocalization of the β-subunits at 2 months of age, similar to that observed in nontransgenic males. This colocalization was reduced, but still evident, at 5 months of age (supplemental Fig. 2B).
GnRHR-TAg transgenic female mice have reduced plasma LH and FSH levels
To determine the effects of the GnRHR-TAg transgene on gonadotropin secretion, immunoreactive serum LH and FSH from transgenic mice and control littermates were measured by RIA at 2 and 5 months. In nontransgenic males and females, there were no changes in circulating LH and FSH levels with age. However, in transgenic females, LH was reduced at both ages compared with the wild-type controls (Fig. 4A), a finding in agreement with the results of our immunohistochemical analyses. In contrast, LH levels in transgenic male mice did not differ from their nontransgenic male counterparts (Fig. 4B).
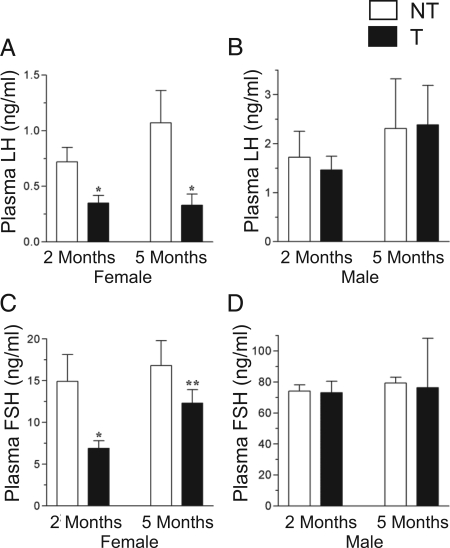
Figure 4.
Plasma gonadotropin levels in GnRHR-TAg transgenic (T) (black bars) mice measured by RIA. Circulating LH levels in 2- and 5-month female (A) and male (B) mice, and FSH levels in female (C) and male (D) mice at 2 and 5 months, measured by RIA. Data are presented as mean ± sem. *, P < 0.05 vs. nontransgenic (NT) (white bars) littermate controls at the same age; **, P < 0.05 vs. transgenic female mice at 2 months of age.
In parallel to the reduction in circulating plasma LH levels, transgenic female mice had significantly reduced plasma FSH levels at 2 months of age (Fig. 4C). This reduction is again consistent with FSHβ immunohistochemical analyses. Interestingly, at 5 months, plasma FSH levels were higher than at 2 months in the transgenic females, and were no longer different from nontransgenic females.
In male mice, plasma FSH levels were similar between genotypes at 2 and 5 months (Fig. 4D). Notable variation in transgenic male FSH measurements was found at the 5-month time point (mean value 76.3 ± 31.8 ng/ml, n = 4) and could be attributed to a high FSH level in a single transgenic male mouse. The FSH level in this mouse was 171 ng/ml (3.8-fold above the mean; 44.7 ± 4.7 ng/ml, n = 3). This measurement was repeated 2 wk later in the same mouse and had increased even further (408 ng/ml); therefore, this mouse was excluded from Fig. 4D as an outlier (17). In contrast, LH levels in this mouse declined during the same 2-wk period (from 1.6–0.6 ng/ml).
To explore these results further, an FSH IRMA was performed in a larger sample size at the 5-month age. Again, circulating FSH levels in transgenic female mice did not show any significant difference compared with nontransgenic females (supplemental Fig. 3A). However, transgenic male mice had variably increased plasma FSH levels in this assay (412.1 ± 122.8 ng/ml, range 22.7–2074.1, n = 18), compared with nontransgenic males (33.8 ± 2.8 ng/ml). Circulating LH levels were also repeated using a sandwich IRMA, with greater sensitivity at lower concentrations of LH, in this additional set of mice (supplemental Fig. 3B). Similar to the RIA results, the plasma LH levels in transgenic females were significantly lower than those in nontransgenic female littermates. In male mice, LH IRMA results confirmed that there is no difference between levels in nontransgenic and transgenic males.
GnRHR-TAg transgenic female mice have reduced plasma gonadal steroid levels
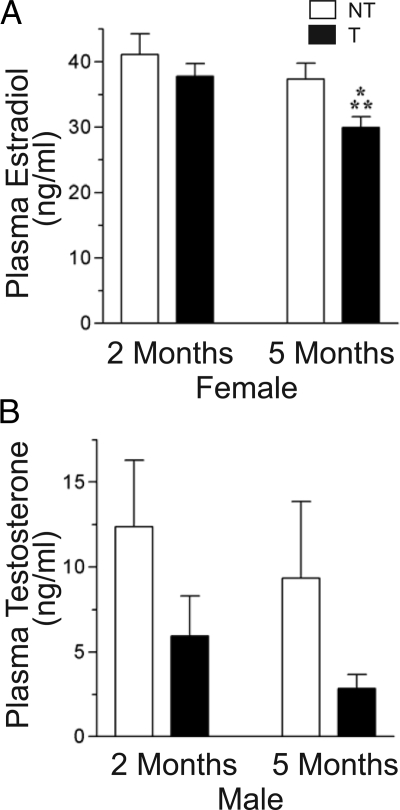
To determine gonadal steroidogenic activity in GnRHR-TAg transgenic mice, plasma estradiol levels in females and testosterone levels in males were measured. Nontransgenic female mice had no change in estradiol levels over the period of measurement (Fig. 5A). In transgenic female mice, estradiol levels were not significantly different from those in nontransgenic littermates at 2 months of age. However, at 5 months their estradiol levels were significantly reduced.
Figure 5.
Plasma gonadal steroid levels in GnRHR-TAg transgenic (T) mice. Steroid hormone levels were analyzed by RIA for 17β-estradiol in female mice (A) and testosterone in male mice (B). *, P < 0.05 vs. nontransgenic (NT) littermate controls at 5 months of age; **, P < 0.05 vs. transgenic female mice at 2 months of age.
In nontransgenic male mice, average testosterone levels were not different at the two time points examined (Fig. 5B). In transgenic male mice, plasma testosterone levels showed a trend toward a decrease at both 2 and 5 months of age, although these reductions did not reach statistical significance.
GnRHR-TAg transgenic mice have reduced LHβ and FSHβ gene expression during development
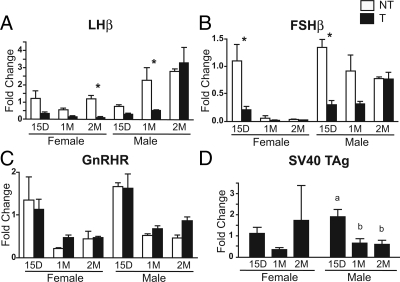
We used QRT-PCR to assay pituitary LHβ, FSHβ, GnRHR, and SV40 TAg mRNA levels during postnatal development to correlate with the changes in protein levels observed at 2 months of age (Fig. 6). Gonadotrope-specific and SV40 TAg gene expression in transgenic and nontransgenic mice at 15 d, 1 month, and 2 months of age revealed reductions in LHβ and FSHβ expression during development in the GnRHR-TAg mice. LHβ, FSHβ, and GnRHR expression in nontransgenic mice paralleled patterns previously observed in developing rats, including the female-specific decrease in FSHβ expression between 15 d and 1 month (Fig. 6, A–C) (19,20). At 15 d there were no differences in LHβ and FSHβ mRNA levels between males and females in either the wild-type or transgenic mice, although both were clearly decreased in the transgenic mice at this age (Fig. 6, A and B). By 2 months, sex-specific differences in the patterns of LHβ and FSHβ expression in the transgenic mice became apparent. LHβ mRNA levels remained persistently decreased in the transgenic females, whereas levels increased in the transgenic males to levels comparable to those in the nontransgenic male mice. A similar pattern for FSHβ expression was also observed. Interestingly, the developmentally high level of FSHβ transcript at d 15 in nontransgenic mice was absent in the transgenic females and males. GnRHR expression levels were also highest at d 15 and decreased by 1 month for both sexes and genotypes (Fig. 6C). Levels of SV40 TAg transgene expression were not different between transgenic males and females, and roughly matched the developmental changes observed for GnRHR mRNA levels (Fig. 6D).
Figure 6.
Gonadotrope-specific and SV40 TAg gene expression in GnRHR-TAg transgenic and nontransgenic mice at 15 d (15D), 1 month (1M), and 2 months (2M) of age by QRT-PCR. Relative changes in pituitary LHβ (A), FSHβ (B), GnRHR (C), and SV40 TAg gene (D) expression in nontransgenic (NT) (white bars) and transgenic (T) (black bars) females and males. Fold change, comparative CT method was used for mRNA quantification with β-actin as a loading control and the mean of 15-d nontransgenic female for each gene as a reference value. Sample sizes, n = 4. A–C, Asterisks (*) indicate significant difference between genotypes (post hoc, P < 0.05; after significant ANOVA comparison within sex). D, Dissimilar letters indicate significant difference between ages (post hoc, P < 0.05, after significant ANOVA comparison within sex).
Discussion
Together, these studies indicate that SV40 TAg expression may be sufficient to disrupt gonadotropin production, and that tumor development itself may not be the sole cause of hypogonadism and infertility. A sex-specific reduction in female gonadotropin levels corresponded to impaired internal reproductive organ development (smaller ovaries and thinner uteri), indicative of estrogen deficiency, and infertility. Although we have not yet identified the specific mechanisms by which SV40 TAg disrupts gonadotropin production, the repression of cellular specification of gonadotropes is unlikely because GnRHR expression levels, a marker specific for gonadotropes in the pituitary, were normal. However, we can speculate that SV40 TAg may interfere, either directly or indirectly, with LHβ and FSHβ subunit gene expression, and, therefore, protein biosynthesis. Alternatively, hypogonadism and infertility may be a consequence of disruption of the periodicity of LH and FSH release in females, with a resulting failure to generate an LH surge.
Developmentally, GnRHR binding sites are first detected in the pituitary in rodents on embryonic d 12 (21). It has been reported previously in Wistar rats that GnRHR mRNA levels in females are maximal at postnatal d 20 but then decrease to their neonatal levels by d 25, whereas in male rats, GnRHR levels reach their maximum at postnatal d 35 (20,22). Our measurements of GnRHR expression (and SV40 TAg expression) in mice showed the highest levels at d 15 in both males and females, with little difference between sexes. Therefore, if differences in SV40 TAg expression levels contributed to an alteration of gonadotropin expression or transformation, the difference likely had occurred earlier than d 15. Alternatively, pulsatile GnRH is known to regulate its own receptor in the pituitary of both males and females, contributing to basal GnRHR expression (23,24). Preovulatory hypersecretion of GnRH in females transiently increases GnRHR expression and would be expected to have corresponding effects on SV40 TAg expression, which would not occur in transgenic males (23,25), and thereby contribute to sex-specific differences.
Here, we have shown that there are sex-specific effects of transgene expression on LH and FSH production, and on tumor development in GnRHR-TAg transgenic mice. In transgenic female mice, LH levels were decreased at 2 months of age and remained low at 5 months. In contrast, FSH levels were significantly reduced compared with nontransgenic controls at 2 months of age but then increased by 5 months of age, coincident with tumor development. The differential effects on LH and FSH in transgenic females at 5 months of age may reflect different sensitivities to TAg, or alternatively may reflect low levels of FSH production by the tumors in the females. Furthermore, a subset of 5-month-old transgenic male mice with pituitary tumors had increases in plasma FSH levels as well, with 50% of them having FSH levels greater than 200 ng/ml. These data are consistent with the possibility that the gonadotrope-derived pituitary tumors developed by these males may secrete high levels of immunologically active FSH in some cases.
Our colocalization studies suggest that in male transgenic mice, the expression of SV40 TAg appears to be distinct from that of LHβ, whereas FSHβ expression is detected overlapping with TAg positive cells, indicating the existence of heterogeneity in gonadotrope response to SV40 TAg expression. It is possible that tumors may develop preferentially from FSH-secreting gonadotropes, or alternatively that SV40 TAg expression selectively suppresses LH production, possibly by cellular dedifferentiation (18), but the same levels are insufficient to suppress FSHβ expression completely. These possibilities are supported by the lack of overlap between pituitary LHβ and SV40 TAg expression, the presence of mixed levels of colocalization of TAg with FSHβ positive cell in the tumors at 5 months of age, and the identification of individual transgenic males with high levels of circulating FSH.
The analysis of gonadotropin β-subunit expression from 15-d to 2-month aged transgenic males and females demonstrated sexually dimorphic effects consistent with the female-specific infertility at 2 months of age. From the youngest age studied by mRNA analysis or measurements of circulating gonadotropins, LH expression or plasma levels decrease significantly only in the transgenic female mice. This implicates an increased sensitivity to SV40-mediated effects in the female, though a specific mechanism is not yet clear. The loss of estrous cyclicity in transgenic females may be the result of pulsatile GnRH hypersecretion during pubertal onset, leading to increased transformation of gonadotropes by the transgene. This is supported by the identical patterns of LHβ and FSHβ expression in males and females only before puberty. In transgenic males, GnRHR and, therefore, SV40 TAg expression are predicted to be more stable at basal levels. Less GnRHR-directed oncogene expression may account for the larger prevalence of FSH-secreting tumors in males that appear more consistent with functional adenomas. These observations are parallel to sex-specific differences previously reported in the gonadotrope-targeted SV40 TAg driven by the FSHβ promoter that resulted in FSH-secreting tumors forming only in males and the absence of tumors in females that express lower levels of FSHβ and, therefore, express less SV40 TAg (18).
Plasma estradiol levels were only reduced in transgenic female mice at 5 months of age, even though at 2 months, there were low gonadotropin levels with reductions in ovary size and uterine thickness, both reflective of low estrogen activity. Although the failure to detect a reduction in plasma estradiol levels at 2 months may be due to insufficient sensitivity of the estradiol assay to reliably distinguish normal from low estradiol levels, the reduction at 5 months of age coincides with an increase in FSH levels in transgenic female mice. This suggests that a decrease in estradiol feedback inhibition at this age may contribute to the increase in FSH secretion in these mice. It is also possible that in early stages of development, gonadotropin secretion, although reduced, is sufficient to support some ovarian estradiol synthesis. This is consistent with our observation that a subset of transgenic females displayed vaginal cell morphology typical of constant estrus. Such low levels of gonadotropin production may be sufficient to stimulate some estradiol synthesis, but the disruption of estrous cyclicity in these mice illustrates the lack of a proestrus surge of gonadotropins, which would be expected to result in infertility. Supporting this hypothesis is a previous report, in which treatment of a patient with hypogonadotropic hypogonadism with increasing doses of pulsatile GnRH restored estradiol levels at lower doses, whereas induction of ovulatory cycles required higher doses (26).
Transgenic male mice, in contrast to the females, remain fertile and have no decrease in gonadotropin levels compared with nontransgenic littermates. Although plasma testosterone levels appeared to be modestly reduced in transgenic male mice, these decreases did not reach statistical significance. This testosterone production may be sufficient to support ongoing reproductive capacity in the transgenic male mice. Fertility in male mice is also less dependent upon the precise pattern of LH and FSH secretion than in female mice, as evidenced by the presence of ongoing spermatogenesis and fertility in FSHβ subunit-deficient (27) and FSH receptor-deficient (28,29,30) male mice, whereas their female counterparts are infertile (27,31).
One explanation for transgenic female infertility may include a developmental role of gonadotropins, particularly FSH, before puberty. A juvenile increase and decrease of FSHβ expression maturation is present in the nontransgenic mice, a pattern characteristic in female rats approaching puberty (19). However, in the transgenic mice, FSHβ mRNA levels are 5-fold less at d 15 than nontransgenics; thus, the transgenic females are never exposed to the prepubertal increase in FSH. Female FSHβ mRNA levels then decline to the lower levels similar to those in nontransgenic females. Although this absence of a prepubertal increase in FSHβ is also true for transgenic males, both FSH and the FSH receptor have both been dispensable for male but not female fertility (27,31).
Targeted tumorigenesis in transgenic mice by SV40 TAg expression under the regulation of genes expressed at each specific stage of pituitary development has been a powerful tool for the study of gonadotrope physiology and differential LH and FSH expression (13,18,32,33,34,35). We describe that the expression of SV40 TAg directed by a 1.2-kb region of the GnRHR promoter demonstrated female-specific hypogonadotropic hypogonadism. Both LH and FSH levels are reduced in transgenic female mice, but not in male mice. In addition, transgenic female mice have decreased estradiol levels at 5 months of age, and have smaller ovaries and thinner uteri than nontransgenic females, and are infertile throughout their life span, even before tumor development. In contrast, transgenic male mice have no detectable changes in gonadotropin levels except for elevated FSH levels at 5 months of age, no significant reduction in testosterone levels, normal internal reproductive organs, and remain fertile. Therefore, the differential effects on gonadotropin expression are sex specific and likely reflect the direct actions of GnRHR-TAg expression, or alternatively result from the transformation process itself.
Supplementary Material
Acknowledgments
We thank William W. Chin and Constance T. Albarracin for critical discussions, Joel Lawitts and Beth Israel Deaconess Medical Center Transgenic Core Facility for the revival of frozen embryos, Noriyuki Koibuchi for assistance with the establishment of initial mouse colonies, and Shuyun Xu, Uche I. Nwankpa, and Ipek M. Kutlu for mouse maintenance and genotyping.
Footnotes
This work was supported by National Institutes of Health Grants R21 HD050412 (to U.B.K.), R01 HD19938 (to U.B.K.), R01 HD33001 (to U.B.K.), and T32 DK07529-21 (to J.C.G.), and a Lalor Foundation Research Fellowship Grant (to K.-H.J.). The plasma LH immunoradiometric assay was done by the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core Laboratory under National Institutes of Health/National Institute of Child Health and Human Development (Specialized Cooperative Centers Program in Reproduction and Infertility Research) Grant U54-HD28934.
Present address for K.-H.J.: Department of Advanced Biological Research, R&D Center, Mazence, Suwon, Kyeongki 443-813, Korea.
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 12, 2009
Abbreviations: CT, Threshold Cycle; GnRHR, GnRH receptor; H&E, hematoxylin-eosin; IRMA, immunoradiometric assay; QRT-PCR, quantitative RT-PCR; SV40, simian virus 40; TAg, large T antigen.
The authors have verified that the cross-sections of transgenic and littermate nontransgenic female mouse ovaries in Fig. 2 of the previously published report (7) were unintentionally mislabeled (W. W. Chin, personal communication).
References
- Gharib SD, Wierman ME, Shupnik MA, Chin WW 1990 Molecular biology of the pituitary gonadotropins. Endocr Rev 11:177–199 [DOI] [PubMed] [Google Scholar]
- Kaiser UB, Conn PM, Chin WW 1997 Studies of gonadotropin-releasing hormone (GnRH) action using GnRH receptor-expressing pituitary cell lines. Endocr Rev 18:46–70 [DOI] [PubMed] [Google Scholar]
- Sealfon SC, Weinstein H, Millar RP 1997 Molecular mechanisms of ligand interaction with the gonadotropin-releasing hormone receptor. Endocr Rev 18:180–205 [DOI] [PubMed] [Google Scholar]
- Albarracin CT, Kaiser UB, Chin WW 1994 Isolation and characterization of the 5′-flanking region of the mouse gonadotropin-releasing hormone receptor gene. Endocrinology 135:2300–2306 [DOI] [PubMed] [Google Scholar]
- Clay CM, Nelson SE, DiGregorio GB, Campion CE, Wiedemann AL, Nett RJ 1995 Cell-specific expression of the mouse gonadotropin-releasing hormone (GnRH) receptor gene is conferred by elements residing within 500 bp of proximal 5′ flanking region. Endocrine 3:615–622 [DOI] [PubMed] [Google Scholar]
- Duval DL, Nelson SE, Clay CM 1997 The tripartite basal enhancer of the gonadotropin-releasing hormone (GnRH) receptor gene promoter regulates cell-specific expression through a novel GnRH receptor activating sequence. Mol Endocrinol 11:1814–1821 [DOI] [PubMed] [Google Scholar]
- Albarracin CT, Frosch MP, Chin WW 1999 The gonadotropin-releasing hormone receptor gene promoter directs pituitary-specific oncogene expression in transgenic mice. Endocrinology 140:2415–2421 [DOI] [PubMed] [Google Scholar]
- McCue JM, Quirk CC, Nelson SE, Bowen RA, Clay CM 1997 Expression of a murine gonadotropin-releasing hormone receptor-luciferase fusion gene in transgenic mice is diminished by immunoneutralization of gonadotropin-releasing hormone. Endocrinology 138:3154–3160 [DOI] [PubMed] [Google Scholar]
- Hanahan D 1985 Heritable formation of pancreatic β-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature 315:115–122 [DOI] [PubMed] [Google Scholar]
- Suda Y, Aizawa S, Hirai S, Inoue T, Furuta Y, Suzuki M, Hirohashi S, Ikawa Y 1987 Driven by the same Ig enhancer and SV40 T promoter ras induced lung adenomatous tumors, myc induced pre-B cell lymphomas and SV40 large T gene a variety of tumors in transgenic mice. EMBO J 6:4055–4065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakso M, Sauer B, Mosinger Jr B, Lee EJ, Manning RW, Yu SH, Mulder KL, Westphal H 1992 Targeted oncogene activation by site-specific recombination in transgenic mice. Proc Natl Acad Sci USA 89:6232–6236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds HS, McCarthy SA, Chen J, Pipas JM, Van Dyke T 1993 Use of transgenic mice reveals cell-specific transformation by a simian virus 40 T-antigen amino-terminal mutant. Mol Cell Biol 13:3255–3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarid ET, Windle JJ, Whyte DB, Mellon PL 1996 Immortalization of pituitary cells at discrete stages of development by directed oncogenesis in transgenic mice. Development 122:3319–3329 [DOI] [PubMed] [Google Scholar]
- Turgeon JL, Kimura Y, Waring DW, Mellon PL 1996 Steroid and pulsatile gonadotropin-releasing hormone (GnRH) regulation of luteinizing hormone and GnRH receptor in a novel gonadotrope cell line. Mol Endocrinol 10:439–450 [DOI] [PubMed] [Google Scholar]
- Yusta B, Alarid ET, Gordon DF, Ridgway EC, Mellon PL 1998 The thyrotropin β-subunit gene is repressed by thyroid hormone in a novel thyrotrope cell line, mouse T αT1 cells. Endocrinology 139:4476–4482 [DOI] [PubMed] [Google Scholar]
- Jeong KH, Jacobson L, Widmaier EP, Majzoub JA 1999 Normal suppression of the reproductive axis following stress in corticotropin-releasing hormone-deficient mice. Endocrinology 140:1702–1708 [DOI] [PubMed] [Google Scholar]
- Grubbs FE 1969 Procedures for detecting outlying observations in samples. Technometrics 11:1–21 [Google Scholar]
- Kumar TR, Graham KE, Asa SL, Low MJ 1998 Simian virus 40 T antigen-induced gonadotroph adenomas: a model of human null cell adenomas. Endocrinology 139:3342–3351 [DOI] [PubMed] [Google Scholar]
- Wilson ME, Handa RJ 1997 Ontogeny of gene expression in the gonadotroph of the developing female rat. Biol Reprod 56:563–568 [DOI] [PubMed] [Google Scholar]
- Zapatero-Caballero H, Sanchez-Franco F, Fernandez-Mendez C, García-San Frutos M, Botella-Cubells LM, Fernandez-Vazquez G 2004 Gonadotropin-releasing hormone receptor gene expression during pubertal development of female rats. Biol Reprod 70:348–355 [DOI] [PubMed] [Google Scholar]
- Aubert ML, Begeot M, Winiger BP, Morel G, Sizonenko PC, Dubois PM 1985 Ontogeny of hypothalamic luteinizing hormone-releasing hormone (GnRH) and pituitary GnRH receptors in fetal and neonatal rats. Endocrinology 116:1565–1576 [DOI] [PubMed] [Google Scholar]
- Zapatero-Caballero H, Sanchez-Franco F, Guerra-Perez N, Fernandez-Mendez C, Fernandez-Vazquez G 2003 Gonadotropin-releasing hormone receptor gene expression during pubertal development of male rats. Biol Reprod 68:1764–1770 [DOI] [PubMed] [Google Scholar]
- Bauer-Dantoin AC, Weiss J, Jameson JL 1995 Roles of estrogen, progesterone, and gonadotropin-releasing hormone (GnRH) in the control of pituitary GnRH receptor gene expression at the time of the preovulatory gonadotropin surges. Endocrinology 136:1014–1019 [DOI] [PubMed] [Google Scholar]
- Kaiser UB, Jakubowiak A, Steinberger A, Chin WW 1993 Regulation of rat pituitary gonadotropin-releasing hormone receptor mRNA levels in vivo and in vitro. Endocrinology 133:931–934 [DOI] [PubMed] [Google Scholar]
- Schirman-Hildesheim TD, Ben-Aroya N, Koch Y 2006 Daily GnRH and GnRH-receptor mRNA expression in the ovariectomized and intact rat. Mol Cell Endocrinol 252:120–125 [DOI] [PubMed] [Google Scholar]
- Seminara SB, Beranova M, Oliveira LM, Martin KA, Crowley Jr WF, Hall JE 2000 Successful use of pulsatile gonadotropin-releasing hormone (GnRH) for ovulation induction and pregnancy in a patient with GnRH receptor mutations. J Clin Endocrinol Metab 85:556–562 [DOI] [PubMed] [Google Scholar]
- Kumar TR, Wang Y, Lu N, Matzuk MM 1997 Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet 15:201–204 [DOI] [PubMed] [Google Scholar]
- Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, LeMeur M, Sassone-Corsi P 1998 Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci USA 95:13612–13617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy H, Babu PS, Morales CR, Sairam MR 2001 Delay in sexual maturity of the follicle-stimulating hormone receptor knockout male mouse. Biol Reprod 65:522–531 [DOI] [PubMed] [Google Scholar]
- Abel MH, Wootton AN, Wilkins V, Huhtaniemi I, Knight PG, Charlton HM 2000 The effect of a null mutation in the follicle-stimulating hormone receptor gene on mouse reproduction. Endocrinology 141:1795–1803 [DOI] [PubMed] [Google Scholar]
- Danilovich N, Roy I, Sairam MR 2001 Ovarian pathology and high incidence of sex cord tumors in follitropin receptor knockout (FORKO) mice. Endocrinology 142:3673–3684 [DOI] [PubMed] [Google Scholar]
- Windle JJ, Weiner RI, Mellon PL 1990 Cell lines of the pituitary gonadotrope lineage derived by targeted oncogenesis in transgenic mice. Mol Endocrinol 4:597–603 [DOI] [PubMed] [Google Scholar]
- Camper SA 1987 Research applications of transgenic mice. Biotechniques 5:638–650 [Google Scholar]
- Weck J, Anderson AC, Jenkins S, Fallest PC, Shupnik MA 2000 Divergent and composite gonadotropin-releasing hormone-responsive elements in the rat luteinizing hormone subunit genes. Mol Endocrinol 14:472–485 [DOI] [PubMed] [Google Scholar]
- Pernasetti F, Spady TJ, Hall SB, Rosenberg SB, Givens ML, Anderson S, Paulus M, Miller WL, Mellon PL 2003 Pituitary tumorigenesis targeted by the ovine follicle-stimulating hormone β-subunit gene regulatory region in transgenic mice. Mol Cell Endocrinol 203:169–183 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.