Abstract
A convenient assay combining solution hybridization and enzyme immunoassay for DNA-RNA hybrids (polymerase chain reaction-enzyme immunoassay [PCR-EIA]) was developed to detect human immunodeficiency virus type 1 (HIV-1) provirus amplified by the PCR and was compared with oligomer hybridization with 32P-labeled SK19. In PCR-EIA, a fragment of the HIV-1 gag gene from peripheral blood mononuclear cells was first amplified with primer pair SK38/SK39 or O1/O2. PCR-amplified material was reacted in solution with a biotinylated RNA probe. Biotinylated hybrids were measured in a microtiter-plate EIA with antibiotin antibody and a beta-D-galactosidase-conjugated monoclonal antibody to DNA-RNA hybrids. Ten copies of HIV-1 DNA could be detected by PCR-EIA by using two different sets of primers. HIV-1 DNA was detected in 104 of 108 peripheral blood mononuclear cell samples by using SK38/39 and oligomer hybridization, in 104 of 108 samples by using SK38/SK39 and PCR-EIA, and in 104 of 108 samples by using O1/O2 and PCR-EIA. HIV-1 provirus was detected in 107 of 108 samples by using a combination of two sets of primers. One sample from a seropositive patient was negative in all three PCR assays, and six samples gave discordant results between primer pairs. Six of the latter samples scored negative in a PCR for beta-globin but became positive when the sample was diluted before amplification. When applied to clinical samples, PCR-EIA generated results similar to those of an isotopic assay for detection of amplified DNA.
Full text
PDF


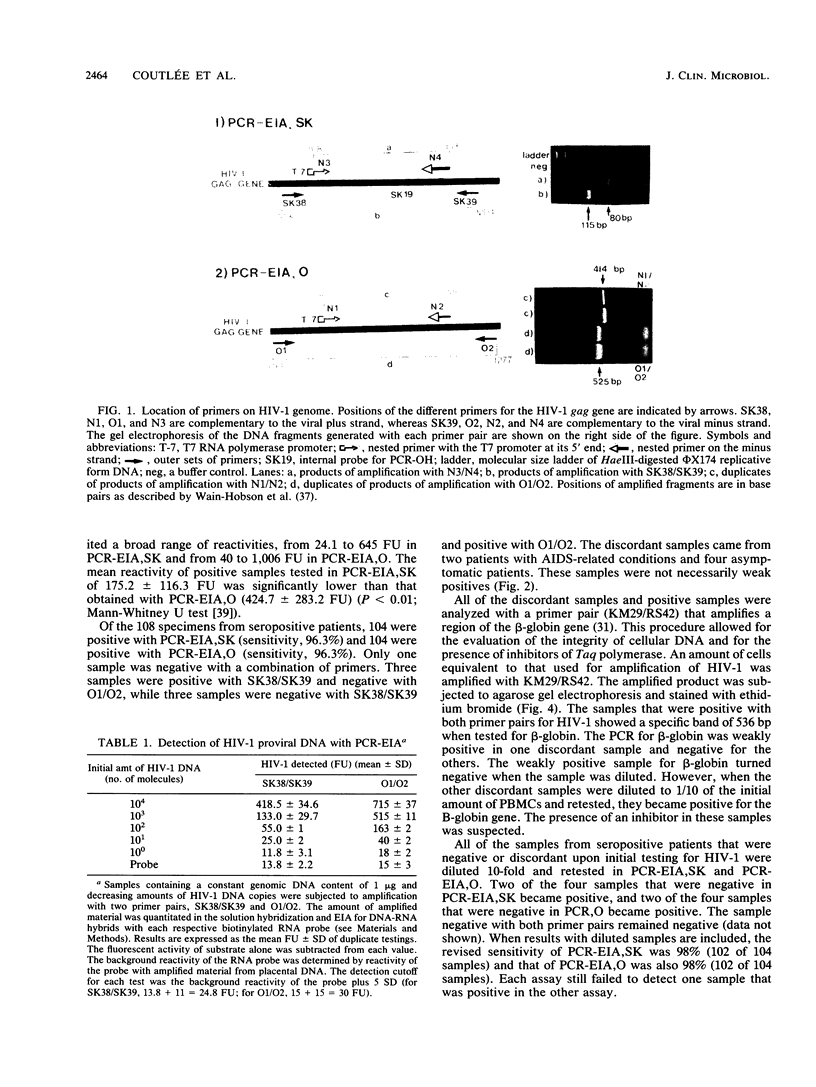
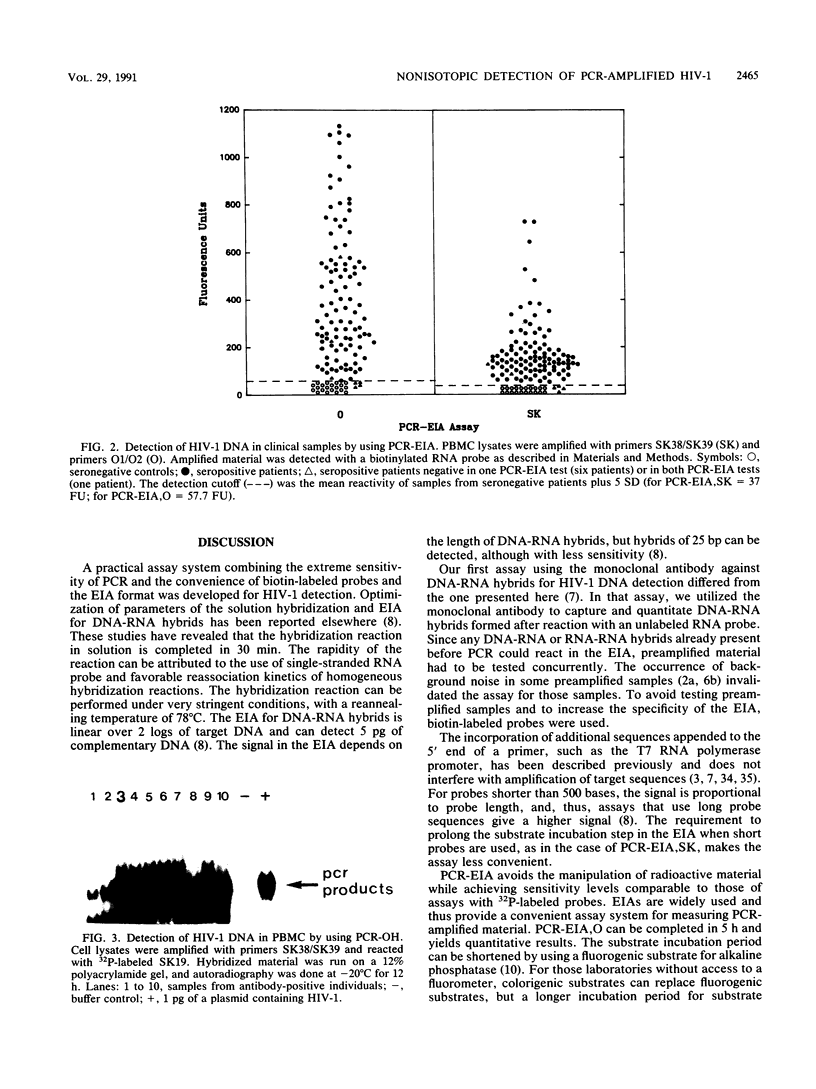


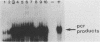
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott M. A., Poiesz B. J., Byrne B. C., Kwok S., Sninsky J. J., Ehrlich G. D. Enzymatic gene amplification: qualitative and quantitative methods for detecting proviral DNA amplified in vitro. J Infect Dis. 1988 Dec;158(6):1158–1169. doi: 10.1093/infdis/158.6.1158. [DOI] [PubMed] [Google Scholar]
- Albert J., Fenyö E. M. Simple, sensitive, and specific detection of human immunodeficiency virus type 1 in clinical specimens by polymerase chain reaction with nested primers. J Clin Microbiol. 1990 Jul;28(7):1560–1564. doi: 10.1128/jcm.28.7.1560-1564.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobo L., Coutlee F., Yolken R. H., Quinn T., Viscidi R. P. Diagnosis of Chlamydia trachomatis cervical infection by detection of amplified DNA with an enzyme immunoassay. J Clin Microbiol. 1990 Sep;28(9):1968–1973. doi: 10.1128/jcm.28.9.1968-1973.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boguslawski S. J., Smith D. E., Michalak M. A., Mickelson K. E., Yehle C. O., Patterson W. L., Carrico R. J. Characterization of monoclonal antibody to DNA.RNA and its application to immunodetection of hybrids. J Immunol Methods. 1986 May 1;89(1):123–130. doi: 10.1016/0022-1759(86)90040-2. [DOI] [PubMed] [Google Scholar]
- Chien A., Edgar D. B., Trela J. M. Deoxyribonucleic acid polymerase from the extreme thermophile Thermus aquaticus. J Bacteriol. 1976 Sep;127(3):1550–1557. doi: 10.1128/jb.127.3.1550-1557.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewley J. P. The polymerase chain reaction, a review of the practical limitations for human immunodeficiency virus diagnosis. J Virol Methods. 1989 Aug;25(2):179–187. doi: 10.1016/0166-0934(89)90031-1. [DOI] [PubMed] [Google Scholar]
- Coutlee F., Viscidi R. P., Yolken R. H. Comparison of colorimetric, fluorescent, and enzymatic amplification substrate systems in an enzyme immunoassay for detection of DNA-RNA hybrids. J Clin Microbiol. 1989 May;27(5):1002–1007. doi: 10.1128/jcm.27.5.1002-1007.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutlee F., Yolken R. H., Viscidi R. P. Nonisotopic detection of RNA in an enzyme immunoassay using a monoclonal antibody against DNA-RNA hybrids. Anal Biochem. 1989 Aug 15;181(1):153–162. doi: 10.1016/0003-2697(89)90410-7. [DOI] [PubMed] [Google Scholar]
- Coutlée F., Bobo L., Mayur K., Yolken R. H., Viscidi R. P. Immunodetection of DNA with biotinylated RNA probes: a study of reactivity of a monoclonal antibody to DNA-RNA hybrids. Anal Biochem. 1989 Aug 15;181(1):96–105. doi: 10.1016/0003-2697(89)90399-0. [DOI] [PubMed] [Google Scholar]
- Coutlée F., Yang B. Z., Bobo L., Mayur K., Yolken R., Viscidi R. Enzyme immunoassay for detection of hybrids between PCR-amplified HIV-1 DNA and a RNA probe: PCR-EIA. AIDS Res Hum Retroviruses. 1990 Jun;6(6):775–784. doi: 10.1089/aid.1990.6.775. [DOI] [PubMed] [Google Scholar]
- Davis G. R., Blumeyer K., DiMichele L. J., Whitfield K. M., Chappelle H., Riggs N., Ghosh S. S., Kao P. M., Fahy E., Kwoh D. Y. Detection of human immunodeficiency virus type 1 in AIDS patients using amplification-mediated hybridization analyses: reproducibility and quantitative limitations. J Infect Dis. 1990 Jul;162(1):13–20. doi: 10.1093/infdis/162.1.13. [DOI] [PubMed] [Google Scholar]
- Eisenstein B. I. The polymerase chain reaction. A new method of using molecular genetics for medical diagnosis. N Engl J Med. 1990 Jan 18;322(3):178–183. doi: 10.1056/NEJM199001183220307. [DOI] [PubMed] [Google Scholar]
- Jackson J. B., Kwok S. Y., Sninsky J. J., Hopsicker J. S., Sannerud K. J., Rhame F. S., Henry K., Simpson M., Balfour H. H., Jr Human immunodeficiency virus type 1 detected in all seropositive symptomatic and asymptomatic individuals. J Clin Microbiol. 1990 Jan;28(1):16–19. doi: 10.1128/jcm.28.1.16-19.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jason J., Ou C. Y., Moore J. L., Lawrence D. N., Schochetman G., Evatt B. L. Prevalence of human immunodeficiency virus type 1 DNA in hemophilic men and their sex partners. Hemophilia-AIDS Collaborative Study Group. J Infect Dis. 1989 Nov;160(5):789–794. doi: 10.1093/infdis/160.5.789. [DOI] [PubMed] [Google Scholar]
- Keller G. H., Huang D. P., Manak M. M. A sensitive nonisotopic hybridization assay for HIV-1 DNA. Anal Biochem. 1989 Feb 15;177(1):27–32. doi: 10.1016/0003-2697(89)90007-9. [DOI] [PubMed] [Google Scholar]
- Kemp D. J., Smith D. B., Foote S. J., Samaras N., Peterson M. G. Colorimetric detection of specific DNA segments amplified by polymerase chain reactions. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2423–2427. doi: 10.1073/pnas.86.7.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoth K., Roberds S., Poteet C., Tamkun M. Highly degenerate, inosine-containing primers specifically amplify rare cDNA using the polymerase chain reaction. Nucleic Acids Res. 1988 Nov 25;16(22):10932–10932. doi: 10.1093/nar/16.22.10932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Goedert J. J., Hughes S. H. A method for the rapid screening of human blood samples for the presence of HIV-1 sequences: the probe-shift assay. AIDS Res Hum Retroviruses. 1989 Jun;5(3):345–354. doi: 10.1089/aid.1989.5.345. [DOI] [PubMed] [Google Scholar]
- Kwok S., Higuchi R. Avoiding false positives with PCR. Nature. 1989 May 18;339(6221):237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- Kwok S., Kellogg D. E., McKinney N., Spasic D., Goda L., Levenson C., Sninsky J. J. Effects of primer-template mismatches on the polymerase chain reaction: human immunodeficiency virus type 1 model studies. Nucleic Acids Res. 1990 Feb 25;18(4):999–1005. doi: 10.1093/nar/18.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifson A. R., Stanley M., Pane J., O'Malley P. M., Wilber J. C., Stanley A., Jeffery B., Rutherford G. W., Sohmer P. R. Detection of human immunodeficiency virus DNA using the polymerase chain reaction in a well-characterized group of homosexual and bisexual men. J Infect Dis. 1990 Mar;161(3):436–439. doi: 10.1093/infdis/161.3.436. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou C. Y., Kwok S., Mitchell S. W., Mack D. H., Sninsky J. J., Krebs J. W., Feorino P., Warfield D., Schochetman G. DNA amplification for direct detection of HIV-1 in DNA of peripheral blood mononuclear cells. Science. 1988 Jan 15;239(4837):295–297. doi: 10.1126/science.3336784. [DOI] [PubMed] [Google Scholar]
- Ou C. Y., McDonough S. H., Cabanas D., Ryder T. B., Harper M., Moore J., Schochetman G. Rapid and quantitative detection of enzymatically amplified HIV-1 DNA using chemiluminescent oligonucleotide probes. AIDS Res Hum Retroviruses. 1990 Nov;6(11):1323–1329. doi: 10.1089/aid.1990.6.1323. [DOI] [PubMed] [Google Scholar]
- Paterlini P., Lallemant-Le Coeur S., Lallemant M., M'Pelé P., Dazza M. C., Terre S., Moncany M., Jourdain G., Courgnaud V., N'Zingoula S. Polymerase chain reaction for studies of mother to child transmission of HIV1 in Africa. J Med Virol. 1990 Jan;30(1):53–57. doi: 10.1002/jmv.1890300112. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Schowalter D. B., Sommer S. S. The generation of radiolabeled DNA and RNA probes with polymerase chain reaction. Anal Biochem. 1989 Feb 15;177(1):90–94. doi: 10.1016/0003-2697(89)90019-5. [DOI] [PubMed] [Google Scholar]
- Stoflet E. S., Koeberl D. D., Sarkar G., Sommer S. S. Genomic amplification with transcript sequencing. Science. 1988 Jan 29;239(4839):491–494. doi: 10.1126/science.3340835. [DOI] [PubMed] [Google Scholar]
- Viscidi R. P., O'Meara C., Farzadegan H., Yolken R. Monoclonal antibody solution hybridization assay for detection of human immunodeficiency virus nucleic acids. J Clin Microbiol. 1989 Jan;27(1):120–125. doi: 10.1128/jcm.27.1.120-125.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wain-Hobson S., Sonigo P., Danos O., Cole S., Alizon M. Nucleotide sequence of the AIDS virus, LAV. Cell. 1985 Jan;40(1):9–17. doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]
- Wang A. M., Doyle M. V., Mark D. F. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehle C. O., Patterson W. L., Boguslawski S. J., Albarella J. P., Yip K. F., Carrico R. J. A solution hybridization assay for ribosomal RNA from bacteria using biotinylated DNA probes and enzyme-labeled antibody to DNA:RNA. Mol Cell Probes. 1987 Jun;1(2):177–193. doi: 10.1016/0890-8508(87)90026-0. [DOI] [PubMed] [Google Scholar]
- de Franchis R., Cross N. C., Foulkes N. S., Cox T. M. A potent inhibitor of Taq polymerase copurifies with human genomic DNA. Nucleic Acids Res. 1988 Nov 11;16(21):10355–10355. doi: 10.1093/nar/16.21.10355. [DOI] [PMC free article] [PubMed] [Google Scholar]