Abstract
Female androgen receptor (AR) knockout mice (AR−/−) generated by an in-frame Ar exon 3 deletion are subfertile, but the mechanism is not clearly defined. To distinguish between extra- and intraovarian defects, reciprocal ovarian transplants were undertaken. Ovariectomized AR−/− hosts with wild-type (AR+/+) ovary transplants displayed abnormal estrus cycles, with longer cycles (50%, P < 0.05), and 66% were infertile (P < 0.05), whereas AR+/+ hosts with either AR−/− or surgical control AR+/+ ovary transplants displayed normal estrus cycles and fertility. These data imply a neuroendocrine defect, which is further supported by increased FSH (P <0.05) and estradiol (P <0.05), and greater LH suppressibility by estradiol in AR−/− females at estrus (P <0.05). Additional intraovarian defects were observed by the finding that both experimental transplant groups exhibited significantly reduced pups per litter (P < 0.05) and corpora lutea numbers (P < 0.05) compared with surgical controls. All groups exhibited normal uterine and lactation functions. AR−/− uteri were morphologically different from AR+/+ with an increase in horn length (P < 0.01) but a reduction in uterine diameter (P < 0.05), total uterine area (P < 0.05), endometrial area (P < 0.05), and myometrial area (P < 0.01) at diestrus, indicating a role for AR in uterine growth and development. Both experimental transplant groups displayed a significant reduction in uterine diameter (P < 0.01) compared with transplanted wild-type controls, indicating a role for both AR-mediated intraovarian and intrauterine influences on uterine physiology. In conclusion, these data provide direct evidence that extraovarian neuroendocrine, but not uterine effects, as well as local intraovarian AR-mediated actions are important in maintaining female fertility, and a disruption of AR signaling leads to altered uterine development.
AR−/− female mice exhibit decreased fertility and ovulation due to defects in neuroendocrine and intra-ovarian mechanisms as well as uterine development but normal uterine function.
Androgens classically mediate their genomic effects via the androgen receptor (AR), a protein encoded by an X chromosome gene and a member of the nuclear receptor superfamily (1). Although the biological effects of androgens in male physiology are well characterized, their physiological roles in the female other than as precursors for conversion to estrogens by aromatase (2) have only recently been recognized (3,4,5). The direct intraovarian actions of androgens via ARs is supported by the universality of AR expression in mammalian ovaries such as in rodents (6,7), domestic species (8,9), and primates (10,11). Furthermore, numerous in vitro and in vivo studies demonstrate direct pharmacological androgen effects on follicle growth (12,13,14,15). AR is also expressed in the hypothalamus and the pituitary (16,17,18,19,20,21) where it is regulated by testosterone (T) and estradiol (E2) (22). Hence, AR signaling has the potential to influence both intraovarian mechanisms and neuroendocrine pathways regulating the hypothalamic GnRH and pituitary LH and FSH release.
AR is also expressed in the uterus and similar patterns of expression are found in both rodents (23,24) and humans (25,26,27,28). Exogenous androgens, notably the nonaromatizable androgen dihydrotestosterone (29), promote growth and differentiation of the rodent uterus (30,31,32). Androstenedione, an aromatizable androgen, inhibits the growth of human endometrial epithelial cells in vitro, an effect blocked by an antiandrogen (cyproterone acetate) (28). Both of these findings support a direct role for AR-mediated actions. AR mRNA in the endometrium is highest in early compared with later pregnancy consistent with a role for AR in uterine function, especially implantation (33). In rodents, the endometrial stromal cells are transformed into decidual cells in response to implanting blastocysts. It has been hypothesized that androgens may play a role in maintaining the decidual cell reaction (34) and that abnormal concentrations of androgens may disturb periimplantation or lead to early pregnancy loss by disrupting uterine prostaglandin regulation (35).
Studying the precise roles of androgens has been problematic due to the specificity and effectiveness of blockers used and the potential aromatization of some androgens, which allows for effects to be mediated indirectly via the estrogen receptors. These limitations hinder the interpretation of pharmacological studies aiming to identify the precise AR-mediated androgenic mechanism(s) influencing ovarian function and female fertility. Genetic methodology allowing the creation of AR knockout female mice (AR−/−) (3,4,5) has been an important advance in revealing a fundamental role for androgens acting via the AR in female reproductive physiology.
Various genetic approaches have converged to show that AR is important in maintaining female reproductive function (3,4,5), notably in optimizing follicle development, ovulation and fertility. However, the precise mechanism(s) causing the observed subfertility remains to be defined. We previously generated and characterized homozygous AR−/− female mice using Cre/LoxP recombination for an in-frame excision of exon 3 (3). The subfertility, with fewer litters and reduced pups per litter, was attributable to defective ovulation that could be overcome by gonadotrophin hyperstimulation, suggesting a predominant extraovarian defect in gonadotrophin regulation (3). Therefore, to test this hypothesis, we performed reciprocal ovarian transplants to allow us to assess the influence of intra- and extraovarian AR functions. We also assessed hormone levels at different stages of the estrus cycle, determined the role of AR-mediated actions influencing LH secretion after ovariectomy (OVX) and negative feedback by E2, and determined mRNA levels of LHβ at proestrus. Additionally, we defined the effect of AR-mediated androgen actions on uterine physiology and reproductive function.
Materials and Methods
Mice
Female homozygous androgen receptor knockout mice (AR−/−) were generated as previously described (3). Mice were housed under standard conditions (19–22 C, 12-h light, 12-h dark cycle) in cages with ad libitum access to water and food. Female mice were killed under anesthesia and organs dissected and weighed before fixation (4% paraformaldehyde at 4 C overnight) for histological processing. Serum was stored at −20 C. All procedures were approved by the Sydney South West Area Health Service Animal Welfare Committee within National Health Medical Research Council guidelines for animal experimentation.
Assessment of vaginal opening and estrus cycle
Weanling mice were inspected daily for vaginal opening and estrus cycling was determined in sexually mature females by light microscope analysis of vaginal epithelial cell smears (3). To define estrus cycle length, daily vaginal samples were collected for a minimum of two full estrus cycles or 14 consecutive days.
Fertility
Fertility was evaluated by a 13-wk mating trial with each female mated with one AR+/+ male of proven fertility. Cages were monitored daily and the presence of a vaginal plug (observed daily for 21 d after mating female with male), timing of litters, and number of pups per litter were recorded. After the birth of pups, the ability of the females to nurse offspring was assessed by survival of live pups (at 96 h postpartum) and weight of each pup from the first litter at 0, 1, 5, 10, 20, and 30 d of age.
Ovarian transplantation
Reciprocal paired ovarian transplants were performed between AR+/+ and AR−/− females as well as between AR+/+ and AR+/+ females as surgical controls for the procedure. Females served as both donors and recipients whenever possible. Experimental groups will be defined using the following abbreviations, where M is mouse and Ov is ovary. Surgical controls were ovariectomized AR+/+ mouse hosts bearing AR+/+ ovary transplants (designated as MAR+/+OvAR+/+), ovariectomized AR+/+ mouse hosts with AR−/− ovary transplants (designated as MAR+/+OvAR−/−), and ovariectomized AR−/− mouse hosts with AR+/+ ovary transplants (designated as MAR−/−OvAR+/+). The ovary transplantation procedure was a modification of a previous method (36). Five-week-old mice were anesthetized, and each ovary exposed via a flank incision. The surrounding bursa was incised and the ovary gently removed at the hilum. After excision from the donor, the ovaries were held in cold sterile saline until placed inside the ovarian bursa of the ovariectomized recipient. A single stitch of 8–0 silk suture (Johnson and Johnson Medical, North Ryde, New South Wales, Australia) was placed through the incision in the bursa. The reproductive tract was then returned to its normal anatomical position and the abdominal wall and skin closed using 6–0 silk sutures. Mice were housed individually and allowed to recover for 1 wk and then assessed for estrus cycles for 2 wk before being mated for a 13-wk mating trial period.
Specimen collection
Pituitaries were collected and frozen at −80 C. Uteri and ovaries were weighed and fixed for histological analyses. One ovary from each mouse was randomly selected and processed through graded alcohols into glycol methacrylate resin (Technovit 7100; Heraeus Kulzer, Chatswood, Australia). Ovaries were serially sectioned at 20 μm, stained with periodic acid-Schiff, and counterstained with hematoxylin. Corpora lutea (CL) numbers per ovary were enumerated as described (3). Three tissue samples from each right uterine horn [one from each of the top (fimbrial end), middle, and bottom (cervical end)] were fixed, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin: these allowed analysis of differences in location along the length of the horn as well as genotype. Histomorphometric analysis of the uterine cross-sections, one cross-section from each of the three locations of the uterine horn/animal, was carried out using a light microscope calibrated using Stereo Investigator computer software (MicroBrightfield, Williston, VT).
Assessment of uterine function
AR+/+ and AR−/− female mice mated with an individual AR+/+ male of proven fertility were monitored daily to identify copulatory plugs, and pregnant females were left until gestation d 16 or the date of birth of pups. The number of CL in both ovaries were counted at gestation d 16. Uteri were stained as described (37) with the number of implantation sites, reabsorbing embryos, and fetuses in utero recorded. The percentage of pre- and postimplantation losses were calculated by the following formulas: preimplantation loss: 100 × (a-b)/a; postimplantation loss: 100 × (b-c)/b, where a is the number of CL, b is the total number of implantations, and c is the number of live fetuses. In pregnant females left until the date of birth of pups, total gestation length was determined and pups were weighed.
RNA extraction and RT-PCR
Deletion of AR exon 3 in the uterus was confirmed by RT-PCR using RNA extracted from uteri of 8-wk-old mice (n = 3/genotype). Reverse transcription and subsequent RT-PCR was performed as previously described (3). Primers identified intact (288 bp) and excised (171 bp) exon 3 AR (see Fig. 4A). Mouse β-actin was used as an internal control.
Figure 4.
Confirmation of the deletion of AR exon 3 in the uterus, and uterine weights, horn length, and diameter. AR+/+, black bars; AR−/−, white bars; MAR+/+OvAR+/+, black with white dots; MAR+/+OvAR−/−, black with white horizontal lines; MAR−/−OvAR+/+, white with black diagonal lines. A, Schematic diagram of intact and excised AR exon 3, showing location of primer pairs used for PCR genotype identification from RNA. B, Representation of RT-PCR analyses using cDNA from uteri of 8-wk-old mice, to confirm that uteri from AR−/− females carried the global exon 3 deleted transcripts. Intact AR exon 3 product is 288 bp and excised AR exon 3 product is 171 bp. Mouse β-actin, used as an internal control, had a product of 431 bp. C and G, Uterus weights. Data are the mean ± sem n = 5/genotype. D, Uteri (U) and ovaries (O) from 8-wk AR+/+ and AR−/− females at diestrus. E and H, Uterine horn length of AR+/+ and AR−/−females at diestrus (P < 0.01) and estrus (P < 0.01) and ovary transplant groups at diestrus (P < 0.01). Data are the mean ± sem (n = 3–5/genotype). F and I, Uterine diameter of AR+/+ and AR−/− females at diestrus (P < 0.01) and estrus (P < 0.05) and ovary transplant groups at diestrus (P < 0.01). Data are the mean ± sem n = 5/genotype. Different superscript letters denote statistically significant differences.
Quantitative real-time RT-PCR
Using real-time RT-PCR, mRNA expression for LHβ, FSHβ, and GnRH receptor was carried out on pituitaries collected from intact and ovary transplant females at diestrus. mRNA expression levels of LHβ were assessed in pituitaries collected from intact females at proestrous (collected at 1600 h on the afternoon proestrous was identified by vaginal epithelial cell smears). Total RNA was extracted from whole pituitaries using Tri reagent (Sigma, Castle Hill, Australia) according to the manufacturer’s protocol. Reverse transcription was performed as previously described (3). Quantitative RT-PCR analysis on resulting cDNA was performed in duplicate as previously described (3). Mouse ribosomal protein (Rpl19) was used as a housekeeping gene.
OVX and estrogen replacement
After observation of increased serum FSH in intact AR−/− females collected at estrus, negative feedback was evaluated by measurement of LH levels after OVX before and after E2 administration as described (38,39). On the day of surgery (d 0) intact 8-wk-old females were anesthetized between 0900 and 1100 h, OVX, and implanted with either a blank SILASTIC brand capsule (Dow Corning Corp., Midland, MI) (40) (OVX group) or a capsule containing about 10 mg 17β-estradiol (40) (OVX+E2 group). Mice were exposed to a persistent pharmacological dose of E2 to assess the inhibition of LH secretion by E2. On the afternoon of d 7 females were killed by cardiac exsanguination under anesthesia with serum stored at −20 C until assay.
Hormone assays
Mouse serum FSH and LH were determined using species-specific immunofluorometric assays (DELFIA; PerkinElmer, Turku, Finland) as described (3). E2 and progesterone (P4) were measured in solvent extracts (ethylacetate-hexane and diethyl ether, respectively) of serum by time-resolved immunofluorometric (DELFIA) with detection limits of 20 pm and 0.5 nm, respectively (3).
Statistical analysis
Statistical analysis was performed using NCSS 2007 software (NCSS Statistical Software, Kaysville, UT) for t tests or ANOVA with post hoc test using Fisher’s least significant differences multiple comparison test. Proportions were analyzed by Fisher’s exact test or its extension using StatXact software (Cytel Software, Cambridge MA). The time to complete the first estrus cycle was analyzed by survival (Kaplan-Meier) analysis using log rank test to compare groups. Mixed-model ANOVA was used to analyze differences between both genotype and uterine location as main effects. All parametric tests were confirmed by nonparametric equivalent tests. P < 0.05 was considered statistically significant.
Results
Vaginal opening and estrus cycle
Vaginal opening occurred at similar ages in AR+/+ (30 ± 0.6 d), AR+/− (31 ± 1.1 d), and AR−/− (30 ± 1.5 d) mice.
All AR+/+ (10 of 10) and AR+/− (10 of 10) female mice cycled, whereas 30% of AR−/− females (three of 10) (P = 0.08) failed to cycle (Fig. 1A). All control MAR+/+OvAR+/+ (nine of nine) and 89% of MAR+/+OvAR−/− (eight of nine) females exhibited normal estrus cycles, whereas 44% (four of nine) of MAR−/−OvAR+/+ females failed to cycle (P = 0.08) (Fig. 1B).
Figure 1.
Estrus cycling and fertility. A and E, AR+/+, black bars; AR+/−, gray bars; and AR−/−, white bars. B, F, G, and H, MAR+/+OvAR+/+, black with white dots; MAR+/+OvAR−/−, black with white horizontal lines; MAR−/−OvAR+/+, white with black diagonal lines. C, AR+/+, black square; AR+/−, gray diamond; and AR−/−, white circle. D and I, MAR+/+OvAR+/+, gray square; MAR+/+OvAR−/−, black triangle; MAR−/−OvAR+/+, white triangle. A and B, Percentage of females to cycle. C and D, Percentage of females to complete one cycle and median time in days for mice to complete one cycle (P < 0.05). E and F, Average number of cycles in 2 wk (P < 0.01). G, Percentage of females to undergo a successful pregnancy (P < 0.05). H, Average number of litters per female over a 3-month breeding period (P < 0.05). I, Average cumulative number of pups per month over a 3-month period of continual mating (P < 0.01). Data are the mean ± sem (n ≥ 8/genotype). Different superscript letters denote statistically significant differences.
AR−/− females took significantly longer (median 6 d) to complete the first estrus cycle than AR+/+ (median 4 d) and AR+/− (median 4 d) mice (P < 0.05) (Fig. 1C). MAR−/−OvAR+/+ mice also took significantly longer (median 9 d) (P < 0.05) to complete one estrus cycle than surgical control (MAR+/+OvAR+/+) (median 5 d) and MAR+/+OvAR−/− (median 5 d) mice (Fig. 1D).
Some AR−/− mice had irregular estrus cycles and overall completed significantly fewer estrus cycles in 2 wk (1.1 ± 0.3) compared with AR+/+ (2 ± 0.2) and AR+/− (2.2 ± 0.2) female mice (P < 0.01) (Fig. 1E). MAR−/−OvAR+/+ mice also completed significantly (P < 0.01) fewer estrus cycles in 2 wk (0.7 ± 0.2) than both MAR+/+OvAR+/+ (1.7 ± 0.2) and MAR+/+OvAR−/− (1.3 ± 0.2) mice (Fig. 1F).
Fertility
All control (MAR+/+OvAR+/+) (10 of 10) and MAR+/+OvAR−/− (eight of eight) and most (six of eight) MAR−/−OvAR+/+ females mated as indicated by the appearance of a copulatory plug.
A significantly reduced percentage of MAR−/−OvAR+/+ mice underwent a successful pregnancy with only three of eight (37.5%) giving birth to offspring compared with control (MAR+/+OvAR+/+) (10 of 10) and MAR+/+OvAR−/− (eight of nine) females (P < 0.05) (Fig. 1G).
During the 13-wk mating trial, MAR−/−OvAR+/+ mice had significantly fewer litters (1.1 ± 0.6) compared with control (MAR+/+OvAR+/+) (3.0 ± 0.3) and MAR+/+OvAR−/− (2.3 ± 0.4) (P < 0.05) females (Fig. 1H). Litter size was significantly smaller in both the experimental transplant groups, MAR−/−OvAR+/+ (2.1 ± 1.2 pups/litter) and the MAR+/+OvAR−/− (3.5 ± 0.8 pups/litter), compared with the litter size of control (MAR+/+OvAR+/+) female mice (7.3 ± 0.6 pups/litter) (P < 0.01). Additionally, the cumulative number of pups produced over the 3-month breeding trial period was significantly smaller in MAR−/−OvAR+/+ (6 ± 3.9 pups) (P < 0.05) and MAR+/+OvAR−/− (10 ± 2.8 pups) (P < 0.05) females compared with controls (MAR+/+OvAR+/+) (22 ± 2.4 pups) (Fig. 1I).
Body and ovary weights
Body weights were comparable at time of ovarian transplantation (MAR+/+OvAR+/+, 18.0 ± 0.4 g; MAR+/+OvAR−/−, 18.2 ± 0.4 g; and MAR−/−OvAR+/+, 18.1 ± 0.7 g) and at end of experiments (MAR+/+OvAR+/+, 28.5 ± 0.6 g; MAR+/+OvAR−/−, 28.0 ± 0.9 g; and MAR−/−OvAR+/+, 28.8 ± 1.6 g).
Ovary weight was significantly decreased in both the MAR−/−OvAR+/+ (2.4 ± 0.8 mg) and MAR+/+OvAR−/− (2.4 ± 0.5 mg) mice compared with control (MAR+/+OvAR+/+) females (5.3 ± 0.6 mg) (P < 0.05) (Fig. 2A).
Figure 2.
Ovary weights and average number of CL per ovary. MAR+/+OvAR+/+, black with white dots; MAR+/+OvAR−/−, black with white horizontal lines; MAR−/−OvAR+/+, white with black diagonal lines. A, Ovary weights (P < 0.01). B, Average number of CL per ovary at the diestrus stage (P < 0.05). All data are expressed as the mean ± sem (n ≥ 6/genotype). Different superscript letters denote statistically significant differences. C, Histological ovarian sections from ovary transplant groups. CL are indicated by an asterisk.
Ovarian histology
CL numbers in transplanted ovaries at diestrus were significantly reduced in MAR−/−OvAR+/+ (4.7 ± 2.4) and MAR+/+OvAR−/− (4.1 ± 1.7) female mice compared with control (MAR+/+OvAR+/+) (12.3 ± 1.6) mice (P < 0.05) (Fig. 2B). Histological analysis of ovarian transplant tissue showed the presence of follicles at all developmental stages as well as CL in all experimental groups (Fig. 2C).
mRNA expression of LHβ, FSHβ, and GnRH receptor in pituitaries of intact and ovary transplant mice
There was no significant difference in expression levels of GnRH receptor (supplemental Fig. S1A, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org), LHβ, or FSHβ (data not shown). There was no difference in expression levels of GnRH receptor (supplemental Fig. S1B), LHβ, or FSHβ (data not shown) between transplant groups collected at diestrus.
Real-time RT-PCR on pituitaries collected from intact mice at the proestrus stage revealed that there was no difference in expression levels of LHβ between genotypes (supplemental Fig. S1C).
Serum FSH and E2 concentrations at diestrus and estrus
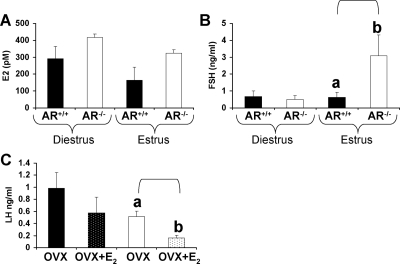
Analysis of E2 levels between AR+/+ and AR−/− females revealed a significant effect of genotype (P < 0.05) and marginal effect for estrous stage (P = 0.07) with no interaction (Fig. 3A). Levels of baseline FSH were not significantly different between intact AR+/+ and AR−/− at the diestrus stage of the estrous cycle (Fig. 3B). However, AR−/− females exhibited significantly elevated baseline levels of serum FSH (AR−/−: 3.08 ± 1.2 ng/ml, AR+/+: 0.62 ± 0.3 ng/ml) compared with AR+/+ females at estrus (P < 0.05) (Fig. 3B).
Figure 3.
Hormone levels in intact and OVX mice with and without E2 treatment. AR+/+, black bars; AR−/−, white bars. E2 (A) and FSH (B) levels (P < 0.05) at the diestrus and estrus stage in 12-wk-old females (n = 6/genotype). C, OVX AR+/+, black bars; OVX AR−/−, white bars; OVX AR+/++E2, black with white dots; AR−/−+E2, white with black dots. LH levels in AR+/+ and AR−/− (P < 0.01) OVX mice with and without E2 treatment are shown (n = 8/genotype). All data are expressed as the mean ± sem. Different superscript letters denote statistically significant differences.
LH levels after OVX and E2 treatment
LH levels were increased (P < 0.05) after OVX in AR+/+ (0.99 ± 0.3 ng/ml) and AR−/− (0.51 ± 0.1 ng/ml) females compared with intact AR+/+ females (0.05 ± 0.04 ng/ml) (3). After E2 treatment of OVX AR+/+ and AR−/− females, LH levels revealed a significant effect of genotype (P < 0.05) and marginal effect for E2 treatment (P = 0.06), with OVX AR−/− females (0.16 ± 0.04 ng/ml) exhibiting greater E2-induced suppression of LH compared with OVX AR+/+ females (0.58 ± 0.3 ng/ml) (P < 0.01) (Fig. 3C).
Verifying excision of AR exon 3 in the AR−/− uterus
Using RT-PCR, uterine RNA from AR−/− mice showed exclusively AR mRNA with a deleted exon 3 and no native AR mRNA with intact exon 3 (Fig. 4B).
Uterine weight and morphology
Uterine weights did not differ between AR−/− and AR+/+ females (Fig. 4C). AR−/− uteri exhibited thinner and longer uterine horns at diestrus (P < 0.01) and estrus (P < 0.01) (Fig. 4, D and E) compared with AR+/+ uteri. AR−/− females exhibited a significant reduction in uterine diameter at diestrus (P < 0.01) and estrus (P < 0.05) (Fig. 4F).
Uterine weight was nonsignificantly decreased in MAR−/−OvAR+/+ (55.5 ± 12.3 mg) compared with MAR+/+OvAR−/− (90.4 ± 39.5 mg) and control (MAR+/+OvAR+/+) females (141.9 ± 23.4 mg) (P = 0.09) (Fig. 4G). MAR−/−OvAR+/+females exhibited a significant increase in uterine horn length (P < 0.01) (Fig. 4H) and MAR+/+OvAR−/−, and MAR−/−OvAR+/+ females displayed a significant reduction in uterine diameter (P < 0.01) (Fig. 4I) compared with surgical controls (MAR+/+OvAR+/+).
Further cross-sectional analysis of the major compartment of the uterus (histological representation: intact AR+/+ and AR−/−, supplemental Fig. S2, A–H; ovary transplant females, supplemental Fig. S2I) identified a significant reduction in total uterine area at diestrus (P < 0.01) and estrus (P < 0.05) (Fig. 5A), endometrial area at diestrus (P < 0.05) (Fig. 5B), and myometrial area at diestrus (P < 0.01) and estrus (P < 0.01) in AR−/− uteri (Fig. 5C) compared with AR+/+ uteri. Luminal epithelial cell height did not differ between AR−/− and AR+/+ females (Fig. 5D). Compared with surgical controls, MAR+/+OvAR−/−and MAR−/−OvAR+/+ females exhibited a significant reduction in total uterine area (P < 0.01) (Fig. 5E), endometrial area (P < 0.01) (Fig. 5F), and myometrial area (P < 0.01) (Fig. 5G). There was no difference between genotypes in luminal epithelial cell height (Fig. 5H).
Figure 5.
Morphology of the uterus. AR+/+, black bars; AR−/−, white bars; MAR+/+OvAR+/+, black with white dots; MAR+/+OvAR−/−, black with white horizontal lines; MAR−/−OvAR+/+, white with black diagonal lines. A and E, Average total uterine area of AR+/+ and AR−/− females at diestrus (P < 0.01) and estrus (P < 0.05) and ovary transplants groups at diestrus (P < 0.01). B and F, Average endometrial area of AR+/+ and AR−/− females at diestrus (P < 0.05) and estrus (P = 0.08) and ovary transplants groups at diestrus (P < 0.01). C and G, Average myometrial area of AR+/+ and AR−/− females at diestrus (P < 0.01) and estrus (P < 0.01) and ovary transplants groups at diestrus (P < 0.01). D and H, Average luminal epithelial cell height of AR+/+ and AR−/− females at diestrus and estrus and ovary transplants groups at diestrus. Data are the mean ± sem (n = 5/genotype). Different superscript letters denote statistically significant differences.
Uterine morphology in relation to location
Within the AR−/− genotype, there was a significant difference in uterine diameter in relation to location, with the cervical end cross-section of the uterus having a significantly larger diameter than the fimbrial end cross-section at diestrous (P < 0.01) (data not shown). No such difference was apparent within wild-type uteri. There was no significant difference in any other morphological parameter in relation to location at diestrus or estrus in AR−/− and AR+/+ uteri (data not shown). After ovary transplantation there was no significant difference in uterine location for any of the morphological parameters (data not shown).
Uterine function and hormone levels during pregnancy
At 16 days gestation AR−/− ovaries exhibited fewer CL (AR−/−: 5.2 ± 0.4; AR+/+: 8.2 ± 0.5, P < 0.01) (Fig. 6A). Similarly, AR−/− females had a reduced number of implantation sites (AR−/−: 4.3 ± 0.8; AR+/+: 8.0 ± 0.5, P < 0.01) (Fig. 6B) and fetuses present in their uteri (AR−/−: 3.8 ± 0.8; AR+/+: 7.0 ± 0.8, P < 0.05) (Fig. 6C). However, there was no difference between AR−/− and AR+/+ females in the percentage of pre- (AR−/−: 13.9 ± 13.9; AR+/+: 2.8 ± 1.8) and postimplantation losses (AR−/−: 11.1 ± 8.2; AR+/+: 13.4 ± 5.9) (Fig. 6, D and E, respectively). AR−/− females displayed a normal gestation length (AR−/−: 22.2 ± 3.0; AR+/+: 19.0 ± 0.3) (Fig. 6F). At 16 d gestation, AR−/− females exhibited a lower serum P4 concentration (AR−/−: 65.0 nm ± 12.2; AR+/+: 138.2 nm ± 15.7, P < 0.01) (Fig. 6G) compared with AR+/+ females. Serum E2 concentrations did not differ between genotypes (Fig. 6H). Pup weights on the day of birth were not significantly different between intact (supplemental Fig. S3A) or ovary transplant groups (supplemental Fig. S3B). All the intact and ovary transplant groups had similar 4-d pup survival rates (supplemental Fig. S3, C and D, respectively) and 30-d pup growth rates (supplemental Fig. S3, E and F, respectively).
Figure 6.
Uterine reproductive function. AR+/+, black bars; AR−/−, white bars. A, Average number of CL (P < 0.01). B, Average number of implantation sites (P < 0.01). C, Average number of fetuses in utero (P < 0.05). D, Percentage of preimplantation losses. E, Percentage of postimplantation losses. F, Average gestation length. G, P4 levels at d 16 of gestation (P < 0.01). H, E2 levels at d 16 of gestation. Data are the mean ± sem (n ≥ 5/genotype). Different superscript letters denote statistically significant differences.
Discussion
We have demonstrated that AR-mediated actions are important in maintaining female fertility in mice via both extraovarian, in particular, neuroendocrine regulation of ovarian function, and local intraovarian mechanism. Furthermore, we have identified that AR-mediated actions have a direct effect on the growth and development of the uterus but are not essential for normal implantation and fetal development.
In the present study, all control surgical mice displayed normal estrus cycling and fertility corresponding with observations from intact control mice (AR+/+) (3), confirming the viability of this surgical technique in our mouse colony. OVX AR+/+ hosts with AR−/− ovaries also displayed normal estrus cycling; however, OVX AR−/− hosts with AR+/+ ovary transplants displayed abnormal estrus cycles, similar to those observed in intact AR−/− females. This indicates that hypothalamic-pituitary-gonadal function is defective in the AR−/− female, even in the presence of a competent ovary. The overall fertility (percentage of females to produce a litter) of control and OVX AR+/+ hosts with AR−/− ovaries females was normal. However, OVX AR−/− hosts with a normal AR+/+ ovary, again similar to that observed in the intact AR−/− females (3), had a significant reduction in fertility, further supporting our hypothesis that AR inactivation leads to extraovarian defects. We hypothesize that these extraovarian defects are due to a dysfunctional hypothalamic-pituitary unit because genomic AR-mediated actions are not essential for the normal reproductive function of the uterus. AR−/− females exhibited similar pre- and postimplantation losses to AR+/+ mice and normal gestation lengths and pup weights on day of birth. Hence, the loss of AR did not disrupt the ability of the AR−/− uterus to support implantation and growth of a pup to full term. Lactation also appeared not to be altered by the loss of AR function because all experimental groups produced pups with normal postnatal growth.
Although AR-mediated actions were not essential in the reproductive function of the uterus, our data revealed that AR actions have a function in the physiological growth and development of the uterus, which is in agreement with previous studies that hypothesis a role for androgens in uterine diseases associated with dysfunctional cell proliferation (41,42,43,44). The AR−/− uterus has a significant reduction in diameter, total uterine area, endometrial area, and myometrial area, indicating that disruption of genomic AR signaling leads to abnormal uterine development. These findings of AR action playing a role in uterine growth are consistent with previous studies in which the addition of nonaromatizable AR-selective agonists promoted growth and differentiation of the uterus, whereas an antiandrogen inhibited this effect (30). Both ovary transplant groups displayed a significant reduction in uterine diameter, total uterine area, endometrial area, and myometrial area compared with control females. The finding that OVX AR+/+ host females with a transplanted AR−/− ovary were unable to maintain similar uterine morphology as the control females implies that a loss of AR-mediated actions in the ovary has a significant effect on the growth and development of the uterus, possibly due to a disruption in E2 production. However, OVX AR−/− hosts in the presence of a normal ovary exhibited defective uterine growth to a greater extent than MAR+/+OvAR−/− mice, indicating a direct role for intrauterine AR-mediated action in uterine physiology. In agreement with these findings, another AR−/− mouse model generated by a non-in-frame targeted deletion of exon 2 of the Ar (5,45) implied a role for AR actions in uterine function by reporting a decrease in uterine response to estrus and a decrease in uterine diameter at estrus. A direct role for androgens in regulating morphological changes in the uterus is also supported by the findings that androgens can inhibit the growth of endometrial epithelial cells in vitro (28) and inhibit their matrix metalloproteinase production (46): matrix metalloproteinases are important in regulating structural changes in the uterus and in particular the initiation of menstruation in women (47). Moreover, the expression of IGF-I, one of the major growth factors involved in stimulating cellular proliferation (48), is regulated by androgens in the uterus (33,49).
Although T stimulates behavioral estrus and ovulation in ewes (50), it is proposed that androgen action in the brain is largely due to aromatization of T to E2 and thereby estrogen receptor-mediated effects (51,52). We identified no significant difference in the female mating behavior between any of the experimental groups, as indicated by the detection of vaginal plugs. Thus, we conclude that AR actions do not play a vital role in regulating normal female mouse mating behavior, although this does not exclude effects of T mediated via aromatization.
The progesterone receptor, AR’s most closely related member of the nuclear receptor gene superfamily (53), is a requirement for the transmission of E2-induced signals leading to gonadotrophin surges (38,54). Support for a direct role for androgen actions in the brain, mediated via the AR, comes from a recent study that identified that ovulation in hens is blocked by treatment with the AR antagonist flutamide, with preovulatory surges of E2 and LH being absent, implementing AR-mediated actions in the endocrine control of ovulation (55). Several studies in women show that low doses of T or dihydrotestosterone do not alter LH levels, whereas high levels of T repress LH pulse frequency and/or mean LH secretion (56,57,58). Furthermore, treatment of women with polycystic ovary syndrome using the anitiandrogen, cyproterone acetate, failed to decrease LH pulse amplitude (59), although a nonsteroidal antiandrogen, flutamide, restores the sensitivity of the GnRH pulse generator to feedback inhibition by E2 and P4 (60). Previously no difference was identified in LH levels of AR−/− mice at the proestrus (preovulatory) stage (4). We identified a significant neuroendocrine effect of genotype for baseline E2 levels, an increase in FSH levels in AR−/− females at estrus as well as increased sensitivity to negative E2 feedback on LH secretion consistent with a neuroendocrine defect of negative feedback signaling, supporting our hypothesis that AR plays a role in regulating female fertility via central neuroendocrine mechanisms.
During late pregnancy, AR−/− females exhibited a reduction in CL numbers and serum P4 and uteri with reduced numbers of implantation sites and fetuses. However, pre- and postimplantation losses were similar to that observed in AR+/+ females; hence, the differences were a consequence of reduced ovulations as CL numbers were proportional to implantation site and fetus numbers. Moreover, we observed a reduction in pups per litter in both experimental ovary transplant groups, compared with controls. The finding that ovariectomized AR+/+ hosts with AR−/− ovaries were unable to maintain similar fertility to the control mice, despite normal estrus cycling, implies that intraovarian functional defects play a role in maintaining optimal fertility in addition to extraovarian regulation. As fewer CL numbers is indicative of a reduction in ovulation, the reduced CL numbers in both experimental ovary transplant groups infer that both AR-mediated intra- and extraovarian influences are important in maintaining normal ovulation rates. It is unlikely that reduced histoincompatibility between AR+/+ hosts and AR−/− ovary transplants explains the intraovarian defects because all transplants were carried out within a single mouse strain (61), and transplanted ovaries had no lymphocytic infiltration 3 months after transfer.
In conclusion, this study is the first to conclusively provide direct evidence that both extraovarian, in particular neuroendocrine regulation of the ovary, and local intraovarian AR-mediated actions are important in maintaining female fertility in mice. We propose that the reduction in ovulation observed in AR−/− mice is due to a decrease in follicle health (3,5), presumably leading to impaired follicle development and follicular response, in combination with dysfunctional hypothalamic-pituitary regulation of gonadotrophin secretion. Further analysis of specific intra- and extraovarian mechanisms involved in the ovulatory process will provide a better understanding of mechanisms of female infertility and may provide AR regulated targets for the treatment of androgen-associated reproductive disorders such as polycystic ovary syndrome, which is associated with an aberration in gonadotrophin secretion and anovulation (62,63). We also confirm a role for AR-mediated androgen action in uterine growth. The loss of AR predisposes the uterus to aberrant uterine growth, implying that apart from the synergistic role of androgens with E2 (31,64) in the uterus, AR-mediated actions also have a distinct role in the regulation of uterine physiology.
Supplementary Material
Acknowledgments
We thank Jeff Zajac (University of Melbourne) for access to the ARflox mice and Jenny Spaliviero, Ellen Gao, and Danny Liske for technical support.
Footnotes
This work was supported by the National Health Medical and Research Council (NHMRC). L.A.S. is supported by a fellowship from NHMRC of Australia (no. 388901).
Disclosure Summary: All authors have nothing to disclose.
First Published Online April 9, 2009
Abbreviations: AR, Androgen receptor; CL, corpora lutea; E2, estradiol; M, mouse; Ov, ovary; OVX, ovariectomy; P4, progesterone; T, testosterone.
References
- Lubahn DB, Joseph DR, Sullivan PM, Willard HF, French FS, Wilson EM 1988 Cloning of human androgen receptor complementary DNA and localization to the X chromosome. Science 240:327–330 [DOI] [PubMed] [Google Scholar]
- Hillier SG, Whitelaw PF, Smyth CD 1994 Follicular oestrogen synthesis: the ‘two-cell, two-gonadotrophin’ model revisited. Mol Cell Endocrinol 100:51–54 [DOI] [PubMed] [Google Scholar]
- Walters KA, Allan CM, Jimenez M, Lim PR, Davey RA, Zajac JD, Illingworth P, Handelsman DJ 2007 Female mice haploinsufficient for an inactivated androgen receptor (AR) exhibit age-dependent defects that resemble the AR null phenotype of dysfunctional late follicle development, ovulation, and fertility. Endocrinology 148:3674–3684 [DOI] [PubMed] [Google Scholar]
- Shiina H, Matsumoto T, Sato T, Igarashi K, Miyamoto J, Takemasa S, Sakari M, Takada I, Nakamura T, Metzger D, Chambon P, Kanno J, Yoshikawa H, Kato S 2006 Premature ovarian failure in androgen receptor-deficient mice. Proc Natl Acad Sci USA 103:224–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YC, Wang PH, Yeh S, Wang RS, Xie C, Xu Q, Zhou X, Chao HT, Tsai MY, Chang C 2004 Subfertility and defective folliculogenesis in female mice lacking androgen receptor. Proc Natl Acad Sci USA 101:11209–11214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Weihua Z, Mäkinen S, Mäkelä S, Saji S, Warner M, Gustafsson JA, Hovatta O 2002 A role for the androgen receptor in follicular atresia of estrogen receptor β knockout mouse ovary. Biol Reprod 66:77–84 [DOI] [PubMed] [Google Scholar]
- Tetsuka M, Whitelaw PF, Bremner WJ, Millar MR, Smyth CD, Hillier SG 1995 Developmental regulation of androgen receptor in rat ovary. J Endocrinol 145:535–543 [DOI] [PubMed] [Google Scholar]
- Campo SM, Carson RS, Findlay JK 1985 Distribution of specific androgen binding sites within the ovine ovarian follicle. Mol Cell Endocrinol 39:255–265 [DOI] [PubMed] [Google Scholar]
- Hampton JH, Manikkam M, Lubahn DB, Smith MF, Garverick HA 2004 Androgen receptor mRNA expression in the bovine ovary. Domest Anim Endocrinol 27:81–88 [DOI] [PubMed] [Google Scholar]
- Horie K, Takakura K, Fujiwara H, Suginami H, Liao S, Mori T 1992 Immunohistochemical localization of androgen receptor in the human ovary throughout the menstrual cycle in relation to oestrogen and progesterone receptor expression. Hum Reprod 7:184–190 [DOI] [PubMed] [Google Scholar]
- Weil SJ, Vendola K, Zhou J, Adesanya OO, Wang J, Okafor J, Bondy CA 1998 Androgen receptor gene expression in the primate ovary: cellular localization, regulation, and functional correlations. J Clin Endocrinol Metab 83:2479–2485 [DOI] [PubMed] [Google Scholar]
- Murray AA, Gosden RG, Allison V, Spears N 1998 Effect of androgens on the development of mouse follicles growing in vitro. J Reprod Fertil 113:27–33 [DOI] [PubMed] [Google Scholar]
- Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA 1998 Androgens stimulate early stages of follicular growth in the primate ovary. J Clin Invest 101:2622–2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendola K, Zhou J, Wang J, Famuyiwa OA, Bievre M, Bondy CA 1999 Androgens promote oocyte insulin-like growth factor I expression and initiation of follicle development in the primate ovary. Biol Reprod 61:353–357 [DOI] [PubMed] [Google Scholar]
- Wang H, Andoh K, Hagiwara H, Xiaowei L, Kikuchi N, Abe Y, Yamada K, Fatima R, Mizunuma H 2001 Effect of adrenal and ovarian androgens on type 4 follicles unresponsive to FSH in immature mice. Endocrinology 142:4930–4936 [DOI] [PubMed] [Google Scholar]
- Lu SF, McKenna SE, Cologer-Clifford A, Nau EA, Simon NG 1998 Androgen receptor in mouse brain: sex differences and similarities in autoregulation. Endocrinology 139:1594–1601 [DOI] [PubMed] [Google Scholar]
- Roselli CE 1991 Sex differences in androgen receptors and aromatase activity in microdissected regions of the rat brain. Endocrinology 128:1310–1316 [DOI] [PubMed] [Google Scholar]
- Scott CJ, Clarke IJ, Rao A, Tilbrook AJ 2004 Sex differences in the distribution and abundance of androgen receptor mRNA-containing cells in the preoptic area and hypothalamus of the ram and ewe. J Neuroendocrinol 16:956–963 [DOI] [PubMed] [Google Scholar]
- Fernández-Guasti A, Kruijver FP, Fodor M, Swaab DF 2000 Sex differences in the distribution of androgen receptors in the human hypothalamus. J Comp Neurol 425:422–435 [DOI] [PubMed] [Google Scholar]
- Sar M, Stumpf WE 1977 Distribution of androgen target cells in rat forebrain and pituitary after [3H]-dihydrotestosterone administration. J Steroid Biochem 8:1131–1135 [DOI] [PubMed] [Google Scholar]
- Scheithauer BW, Kovacs K, Zorludemir S, Lloyd RV, Erdogan S, Slezak J 2009 Immunoexpression of androgen receptor in the nontumorous pituitary and in adenomas. Endocr Pathol 19:27–33 [DOI] [PubMed] [Google Scholar]
- Kumar RC, Thakur MK 2004 Androgen receptor mRNA is inversely regulated by testosterone and estradiol in adult mouse brain. Neurobiol Aging 25:925–933 [DOI] [PubMed] [Google Scholar]
- Pelletier G, Luu-The V, Li S, Labrie F 2004 Localization and estrogenic regulation of androgen receptor mRNA expression in the mouse uterus and vagina. J Endocrinol 180:77–85 [DOI] [PubMed] [Google Scholar]
- Hirai M, Hirata S, Osada T, Hagihara K, Kato J 1994 Androgen receptor mRNA in the rat ovary and uterus. J Steroid Biochem Mol Biol 49:1–7 [DOI] [PubMed] [Google Scholar]
- Pelletier G 2000 Localization of androgen and estrogen receptors in rat and primate tissues. Histol Histopathol 15:1261–1270 [DOI] [PubMed] [Google Scholar]
- Adesanya-Famuyiwa OO, Zhou J, Wu G, Bondy C 1999 Localization and sex steroid regulation of androgen receptor gene expression in rhesus monkey uterus. Obstet Gynecol 93:265–270 [DOI] [PubMed] [Google Scholar]
- Kimura N, Mizokami A, Oonuma T, Sasano H, Nagura H 1993 Immunocytochemical localization of androgen receptor with polyclonal antibody in paraffin-embedded human tissues. J Histochem Cytochem 41:671–678 [DOI] [PubMed] [Google Scholar]
- Tuckerman EM, Okon MA, Li T, Laird SM 2000 Do androgens have a direct effect on endometrial function? An in vitro study. Fertil Steril 74:771–779 [DOI] [PubMed] [Google Scholar]
- Armstrong DT, Moon YS, Leung PC 1976 Uterotrophic effects of testosterone and 5α-dihydrotestosterone in intact and ovariectomized immature female rats. Biol Reprod 15:107–114 [DOI] [PubMed] [Google Scholar]
- Nantermet PV, Masarachia P, Gentile MA, Pennypacker B, Xu J, Holder D, Gerhold D, Towler D, Schmidt A, Kimmel DB, Freedman LP, Harada S, Ray WJ 2005 Androgenic induction of growth and differentiation in the rodent uterus involves the modulation of estrogen-regulated genetic pathways. Endocrinology 146:564–578 [DOI] [PubMed] [Google Scholar]
- Zhang J, Sun Y, Liu Y, Sun Y, Liao DJ 2004 Synergistic effects of androgen and estrogen on the mouse uterus and mammary gland. Oncol Rep 12:709–716 [PubMed] [Google Scholar]
- Schmidt WN, Katzenellenbogen BS 1979 Androgen-uterine interactions: an assessment of androgen interaction with the testosterone- and estrogen-receptor systems and stimulation of uterine growth and progesterone-receptor synthesis. Mol Cell Endocrinol 15:91–108 [DOI] [PubMed] [Google Scholar]
- Kowalski AA, Vale-Cruz DS, Simmen FA, Simmen RC 2004 Uterine androgen receptors: roles in estrogen-mediated gene expression and DNA synthesis. Biol Reprod 70:1349–1357 [DOI] [PubMed] [Google Scholar]
- Zhang X, Croy BA 1996 Maintenance of decidual cell reaction by androgens in the mouse. Biol Reprod 55:519–524 [DOI] [PubMed] [Google Scholar]
- Diao HL, Su RW, Tan HN, Li SJ, Lei W, Deng WB, Yang ZM 2008 Effects of androgen on embryo implantation in the mouse delayed-implantation model. Fertil Steril 90(Suppl 4):1376–1383 [DOI] [PubMed] [Google Scholar]
- Cargill SL, Medrano JF, Anderson GB 1999 Infertility in a line of mice with the high growth mutation is due to luteal insufficiency resulting from disruption at the hypothalamic-pituitary axis. Biol Reprod 61:283–287 [DOI] [PubMed] [Google Scholar]
- Salewski E 2007 Ferbemethode zum makroskopischen nachweis von implantationsstellen am uterus der ratte. Arch Exp Pathol Pharmakol 247:367–372 [Google Scholar]
- Chappell PE, Schneider JS, Kim P, Xu M, Lydon JP, O'Malley BW, Levine JE 1999 Absence of gonadotropin surges and gonadotropin-releasing hormone self-priming in ovariectomized (OVX), estrogen (E2)-treated, progesterone receptor knockout (PRKO) mice. Endocrinology 140:3653–3658 [DOI] [PubMed] [Google Scholar]
- Glidewell-Kenney C, Hurley LA, Pfaff L, Weiss J, Levine JE, Jameson JL 2007 Nonclassical estrogen receptor α signaling mediates negative feedback in the female mouse reproductive axis. Proc Natl Acad Sci USA 104:8173–8177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco JJ, Handelsman DJ, Pedersen JS, Risbridger GP 2002 Direct response of the murine prostate gland and seminal vesicles to estradiol. Endocrinology 143:4922–4933 [DOI] [PubMed] [Google Scholar]
- Crosignani P, Olive D, Bergqvist A, Luciano A 2006 Advances in the management of endometriosis: an update for clinicians. Hum Reprod Update 12:179–189 [DOI] [PubMed] [Google Scholar]
- Vercellini P, Somigliana E, Viganò P, Abbiati A, Daguati R, Crosignani PG 2008 Endometriosis: current and future medical therapies. Best Pract Res Clin Obstet Gynaecol 22:275–306 [DOI] [PubMed] [Google Scholar]
- Terakawa N, Inoue M, Shimizu I, Ikegami H, Mizutani T, Sakata M, Tanizawa O, Matsumoto K 1988 Preliminary report on the use of danazol in the treatment of endometrial adenomatous hyperplasia. Cancer 62:2618–2621 [DOI] [PubMed] [Google Scholar]
- McGrath M, Lee IM, Hankinson SE, Kraft P, Hunter DJ, Buring J, De Vivo I 2006 Androgen receptor polymorphisms and endometrial cancer risk. Int J Cancer 118:1261–1268 [DOI] [PubMed] [Google Scholar]
- Walters KA, Allan CM, Handelsman DJ 2008 Androgen actions and the ovary. Biol Reprod 78:380–389 [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Harada T, Kubota T, Aso T 2007 Testosterone inhibits matrix metalloproteinase-1 production in human endometrial stromal cells in vitro. Reproduction 133:1233–1239 [DOI] [PubMed] [Google Scholar]
- Hampton AL, Salamonsen LA 1994 Expression of messenger ribonucleic acid encoding matrix metalloproteinases and their tissue inhibitors is related to menstruation. J Endocrinol 141:R1–R3 [DOI] [PubMed] [Google Scholar]
- Rutanen EM 1998 Insulin-like growth factors in endometrial function. Gynecol Endocrinol 12:399–406 [DOI] [PubMed] [Google Scholar]
- Sahlin L, Norstedt G, Eriksson H 1994 Androgen regulation of the insulin-like growth factor-I and the estrogen receptor in rat uterus and liver. J Steroid Biochem Mol Biol 51:57–66 [DOI] [PubMed] [Google Scholar]
- Pant HC 1977 Effect of androgens on concentration of LH and FSH in the peripheral plasma of anoestrous ewes. J Reprod Fertil 50:133–136 [DOI] [PubMed] [Google Scholar]
- Schanbacher BD 1984 Regulation of luteinizing hormone secretion in male sheep by endogenous estrogen. Endocrinology 115:944–950 [DOI] [PubMed] [Google Scholar]
- Pinckard KL, Stellflug J, Resko JA, Roselli CE, Stormshak F 2000 Review: brain aromatization and other factors affecting male reproductive behavior with emphasis on the sexual orientation of rams. Domest Anim Endocrinol 18:83–96 [DOI] [PubMed] [Google Scholar]
- Marhefka CA, Moore 2nd BM, Bishop TC, Kirkovsky L, Mukherjee A, Dalton JT, Miller DD 2001 Homology modeling using multiple molecular dynamics simulations and docking studies of the human androgen receptor ligand binding domain bound to testosterone and nonsteroidal ligands. J Med Chem 44:1729–1740 [DOI] [PubMed] [Google Scholar]
- Chappell PE, Lydon JP, Conneely OM, O'Malley BW, Levine JE 1997 Endocrine defects in mice carrying a null mutation for the progesterone receptor gene. Endocrinology 138:4147–4152 [DOI] [PubMed] [Google Scholar]
- Rangel PL, Sharp PJ, Gutierrez CG 2006 Testosterone antagonist (flutamide) blocks ovulation and preovulatory surges of progesterone, luteinizing hormone and oestradiol in laying hens. Reproduction 131:1109–1114 [DOI] [PubMed] [Google Scholar]
- Dunaif A 1986 Do androgens directly regulate gonadotropin secretion in the polycystic ovary syndrome? J Clin Endocrinol Metab 63:215–221 [DOI] [PubMed] [Google Scholar]
- Serafini P, Silva PD, Paulson RJ, Elkind-Hirsch K, Hernandez M, Lobo RA 1986 Acute modulation of the hypothalamic-pituitary axis by intravenous testosterone in normal women. Am J Obstet Gynecol 155:1288–1292 [DOI] [PubMed] [Google Scholar]
- Spinder T, Spijkstra JJ, van den Tweel JG, Burger CW, van Kessel H, Hompes PG, Gooren LJ 1989 The effects of long term testosterone administration on pulsatile luteinizing hormone secretion and on ovarian histology in eugonadal female to male transsexual subjects. J Clin Endocrinol Metab 69:151–157 [DOI] [PubMed] [Google Scholar]
- Couzinet B, Le SN, Brailly S, Schaison G 1986 Comparative effects of cyproterone acetate or a long-acting gonadotropin-releasing hormone agonist in polycystic ovarian disease. J Clin Endocrinol Metab 63:1031–1035 [DOI] [PubMed] [Google Scholar]
- Eagleson CA, Gingrich MB, Pastor CL, Arora TK, Burt CM, Evans WS, Marshall JC 2000 Polycystic ovarian syndrome: evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab 85:4047–4052 [DOI] [PubMed] [Google Scholar]
- Hicken P, Krohn PL 1960 The histocompatibility requirements of ovarian grafts in mice. Proc R Soc Lond B Biol Sci 151:419–433 [DOI] [PubMed] [Google Scholar]
- Franks S 1995 Polycystic ovary syndrome. N Engl J Med 333:853–861 [DOI] [PubMed] [Google Scholar]
- Ehrmann DA 2005 Polycystic ovary syndrome. N Engl J Med 352:1223–1236 [DOI] [PubMed] [Google Scholar]
- Weihua Z, Ekman J, Almkvist A, Saji S, Wang L, Warner M, Gustafsson JA 2002 Involvement of androgen receptor in 17β-estradiol-induced cell proliferation in rat uterus. Biol Reprod 67:616–623 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.