Abstract
Objective
It has been suggested that chondrocyte death by apoptosis may play a role in the pathogenesis of cartilage destruction in osteoarthritis, but the results of in vivo and in vitro investigations have been conflicting. To investigate further the cell death in our in vitro model for traumatic joint injury, we performed a quantitative analysis by electron microscopy (EM) of cell morphology after injurious compression. For comparison, the TUNEL assay was also performed.
Design
Articular cartilage explant disks were harvested from newborn calf femoropatellar groove. The disks were subjected to injurious compression (50% strain at a strain rate of 100%/s), incubated for 3 days, and then fixed for quantitative morphological analysis.
Results
By TUNEL, the cell apoptosis rate increased from 7 ± 2% in unloaded controls to 33 ± 6% after injury (p = 0.01; N = 8 animals). By EM, the apoptosis rate increased from 5 ± 1% in unloaded controls to 62 ± 10% in injured cartilage (p = 0.02, N = 5 animals). Analysis by EM also identified that of the dead cells in injured disks, 97% were apoptotic by morphology.
Conclusions
These results confirm a significant increase in cell death after injurious compression and suggest that most cell death observed here was by an apoptotic process.
Introduction
In osteoarthritis (OA), the factors leading to the degeneration of the cartilage are not well understood. The chondrocytes are the cells responsible for the maintenance of the cartilage extracellular matrix, which provides the mechanical integrity and function of the cartilage tissue. Loss of chondrocytes from the tissue, seen as the presence of empty lacunae on histology, has long been observed in late-stage osteoarthritic cartilage. The cause is not known, but as mature chondrocytes have limited capacity to repopulate cartilage,1 cell death may have permanent effects on the ability of the tissue to repair and maintain its matrix.
Recently, it has been proposed that cell death by apoptosis may be an important event in osteoarthritic cartilage. Several investigators have reported increased rates of apoptosis in OA cartilage, using the TUNEL staining method.2–4 It has further been proposed that apoptotic cell death is part of a process which promotes mineralization of the cartilage, and thus may contribute directly to the pathogenesis of OA.4,5 However, the relative importance of this process has remained controversial, as other investigators have failed to confirm the finding of large numbers of apoptotic cells in OA cartilage.6
Researchers investigating cell death with in vitro cartilage experiments have since emphasized that false-positive staining by the TUNEL assay can be a major limitation6–9 (and reviewed by Aigner and Kim10). In particular, Chen et al.9 found that cells in cartilage subjected to freeze-thaw cycles were 90% TUNEL positive after three days of culture, suggesting that TUNEL is not reliable for distinguishing apoptotic from necrotic cell death. In addition, it is increasingly clear that modes of cell death with features of both necrosis and apoptosis exist, and that the DNA fragments, which the TUNEL stain measures, may not give a complete picture of the ongoing cell death processes.11,12
In vitro models of cartilage mechanical injury have also been used to investigate chondrocyte cell death. Development of these models was motivated by the observation that traumatic joint injury is a risk factor for the subsequent development of OA,13 so investigation of the effects of mechanical injury on the cartilage tissue and cells may shed light on the events which lead to the development of OA in vivo. In these in vitro models, we and others have shown that one effect of mechanical injury is an increase in apoptotic cell death, as assessed by TUNEL staining and by nuclear morphology on light microscopy.14,15
In light of the questions regarding the interpretation of the TUNEL assay, we undertook a study to examine and quantify cell death by electron microscopy (EM) in our model of mechanical cartilage injury. Electron microscopy is the standard for morphological assessment of cell apoptosis, and has been used for confirmation of apoptosis in previous studies of chondrocyte cell death, but not for quantitative assessment. The aims of this study were therefore to i) quantify the relative contributions of apoptotic and necrotic/oncotic cell death after injurious compression in newborn bovine cartilage by EM and ii) directly compare the results of the EM analysis with TUNEL evaluation.
Methods
Cartilage Harvest
Newborn bovine articular cartilage explant disks were obtained from the femoropatellar grooves of 1 to 2-week-old calves, obtained from a local abattoir (Research ’87, Hopkinton, MA) on the day of slaughter, as previously described.16 In brief, cartilage-bone cylinders (9 mm in diameter) were drilled perpendicular to the cartilage surface and placed in a microtome holder. After creating a level surface by removal of the most superficial ~100 µm, the next 2 mm of cartilage were sliced by a microtome, producing two 1-mm-thick slices. Finally, four explant disks were punched out of each slice, resulting in cartilage disks that were 1 mm thick and 3 mm in diameter. Cartilage was then left in culture to equilibrate for 3 days in medium (low-glucose Dulbecco’s Modified Eagle’s Medium [DMEM], supplemented with 10% fetal bovine serum, 10 mM HEPES buffer, 0.1 mM nonessential amino acids, 0.4 mM proline, 20 µg/ml ascorbic acid, 100 U/ml penicillin G, 100 µg/ml streptomycin, and 0.25 µg/ml amphotericin B), in a 37 °C, 5% CO2 environment.
Injurious compression
Injurious compression was performed in a custom-designed incubator-housed loading apparatus.17 Cartilage disks were placed in a well in the center of a polysulfone chamber that allowed for unconfined compression of the disk. The lid of the chamber is attached to a load cell, and the displacement of the lid (equal to the displacement of the cartilage surface) is measured using a linear-voltage differential transducer. The tissue thickness just prior to injury was measured and recorded so that the zero-strain position coincided precisely where contact was made between the chamber lid and the top surface of the cartilage disk. The loading protocol applied during injurious compression was identical to that used in a prior report,18 consisting of a ramp compression at a velocity of 1 mm/s (a strain rate of 100%/s) to a final strain of 50%, followed by release of compression.
TUNEL staining
Cartilage was flash-frozen in liquid nitrogen and sectioned. TUNEL staining was performed as reported previously.14 At least 200 cells were assessed in each section for apoptotic cell death by TUNEL staining.
Electron microscopy
Cartilage was fixed in 5% glutaraldehyde (v/v) in 0.1 M sodium cacodylate buffer. The tissue was post-fixed for EM analysis in a solution of 1% (w/w) osmium tetroxide and then dehydrated in ethanol and embedded in Epon 812.19 Thin sections (65 nm) were then cut and stained with uranyl acetate and lead citrate. 20 The entire section was systematically sampled by scanning the section for centrally-cut cells (i.e., cells sectioned through the nucleus). From each section, approximately 100 cells from the central and edge portions of the section were classified by morphology as either normal, necrotic, apoptotic, or unknown. The classification criteria were developed from the literature and from the results of preliminary studies in newborn bovine tissue.21 Details of these criteria are summarized in Table 1.
Table 1.
Morphological criteria for cell death classification by electron microscopy
| Apoptosis | Necrosis |
|---|---|
| • Nuclear blebbing | • Cell swelling |
| • Presence of apoptotic bodies | • Lack of an intact cell membrane |
| • Cell shrinkage and retraction | • Disintegration or ruptures of the intracellular organelles |
| • Intense staining of the cytoplasm | |
| • Budding of the cell membrane | • Dispersed nuclear chromatin |
| • Condensation of the chromatin | |
Experimental Design
From each animal joint used, two cartilage explants (punched from the same cartilage slice) were used in this experiment. One explant was used as a free-swelling control, and the other was subjected to injurious compression. In addition, as a control for necrotic cell death, three cartilage disks were subjected to freeze-thaw cycles (flash-freeze in liquid nitrogen followed by thawing in a 60°C water bath, for three cycles). After injury or freeze-thaw, the cartilage explants were replaced in culture for an additional three days. Each explant was then removed from culture, and cut in half (across a diameter, forming a semicircle). From each explant, one half was processed for TUNEL staining, and the other half was processed for electron microscopic analysis.
Statistical Analysis
All descriptive statistics for results are given in the text as mean ± standard error and shown in figures as box plots. Each cartilage disk was from a different animal in order to allow statistical inferences over the general population of newborn calves. Differences between two population means were tested with the non-parametric Wilcoxon signed-rank test (SPlus, MathSoft Inc.; now Insightful Corp., Seattle WA).
Results
Injurious Compression
Cartilage was harvested from eight different bovine femoropatellar grooves. After three days of equilibration in culture, cartilage explants were subjected to injurious compression. The actual thickness of the disks immediately before injury was 1.03 ± 0.02 mm (mean ± SEM), reflecting a small amount of swelling during culture. Compression of the cartilage to 50% strain at 1 mm/s produced peak stresses of 20 ± 1 MPa. On visual inspection after injury, cartilage appeared unchanged in 4/8 disks, permanently deformed to an elliptical shape in 3/8 disks, and disrupted (fissured) in 1/8 disks.
Cellular Morphology on Electron Microscopy
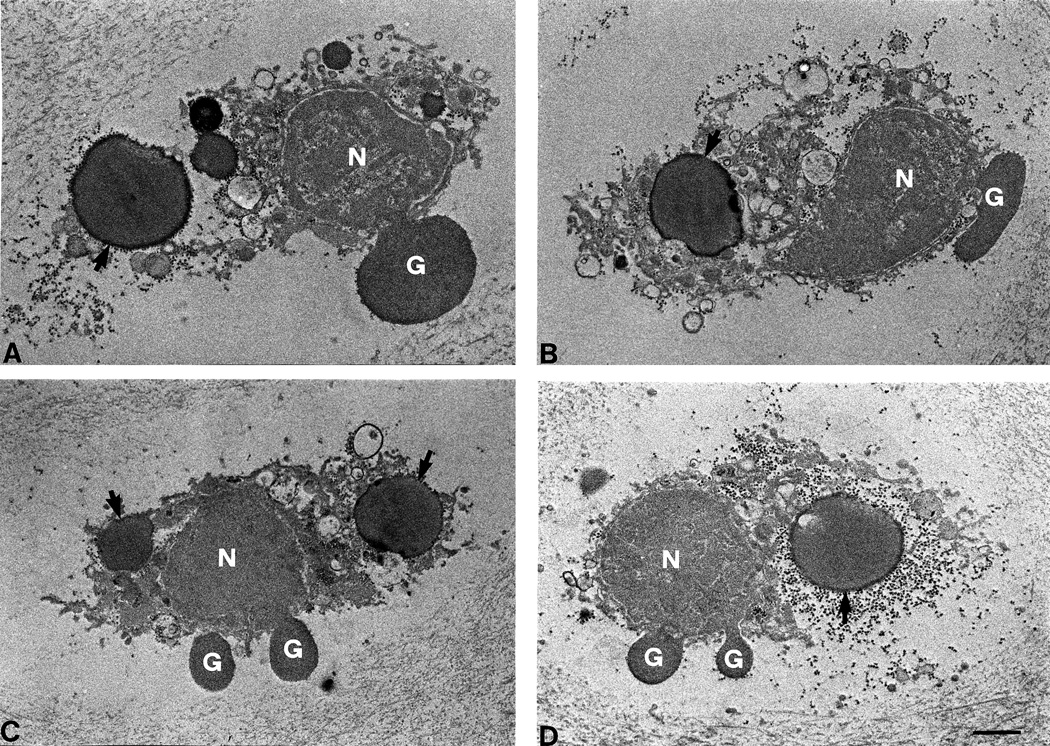
After fixation for EM, it was determined that three unloaded and three loaded samples were inadequately fixed and were excluded from the analysis. Examples of cellular morphology for cells classed as normal, necrotic, and apoptotic are shown in Fig. 1. In freeze-thawed cartilage, necrotic cells (Fig. 1, D–F) were characterized by amorphous granular debris throughout the lacunae, including at the matrix border, and general lack of membrane integrity. In contrast, apoptotic cells (Fig. 1, G–I) were shrunken and clearly retracted from the surrounding matrix. In general, the most frequently observed ultrastructural features of apoptotic cells observed here were the presence of nuclear blebbing, apoptotic bodies, and cell shrinkage. Infrequently observed features were an intensified staining of the cytoplasm, blebbing of the cell membrane, and condensation of the chromatin. To show these ultrastructural features more clearly, additional micrographs of apoptotic cells are shown at higher magnification in Fig. 2.
Figure 1.
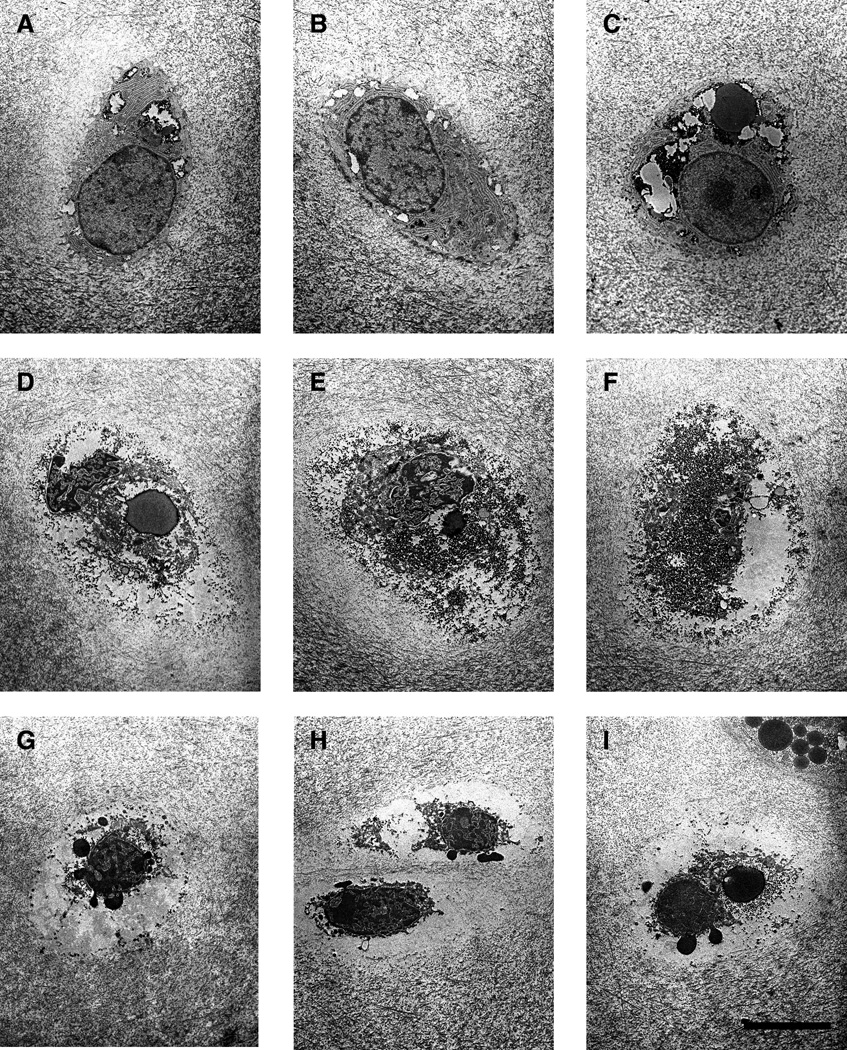
Typical examples of cell morphology on electron microscopy are shown for normal cells (A–C, from unloaded cartilage), necrotic cells (D–F, from cartilage subjected to freeze-thaw cycles), and apoptotic cells (G–I, from cartilage subjected to injurious compression to 50% strain at 1 mm/s). Tissue was fixed for EM analysis three days after the intervention and cut into thin (60-µm-thick) sections. All images are shown at the same magnification. The scale bar represents 5 µm.
Figure 2.
Higher-resolution electron micrographs of apoptotic chondrocytes (A–D) undergoing nuclear blebbing (G) and containing apoptotic bodies (arrows). These two features were regularly encountered in apoptotic cells. Note that panel D is a higher-resolution image of the chondrocyte shown in Figure 1, panel I. N = nucleus; the scale bar represents 1 µm.
Cell Death in Freeze-Thawed Cartilage
In cartilage subjected to repeated freeze-thaw cycles and fixed three days later, a mean of 65% of cells stained positive for TUNEL (Table 2). In contrast, a mean of 97% (128/132) of the cells were classified as necrotic on electron microscopic analysis of cellular morphology.
Table 2.
Cell death classification in freeze-thawed cartilage (% of total cells)
| Apoptosis | Necrosis | |
|---|---|---|
| TUNEL Assay | 65% | |
| Electron Microscopy | 4/132 (3%) | 128/132 (97%) |
Apoptosis after Injurious Compression
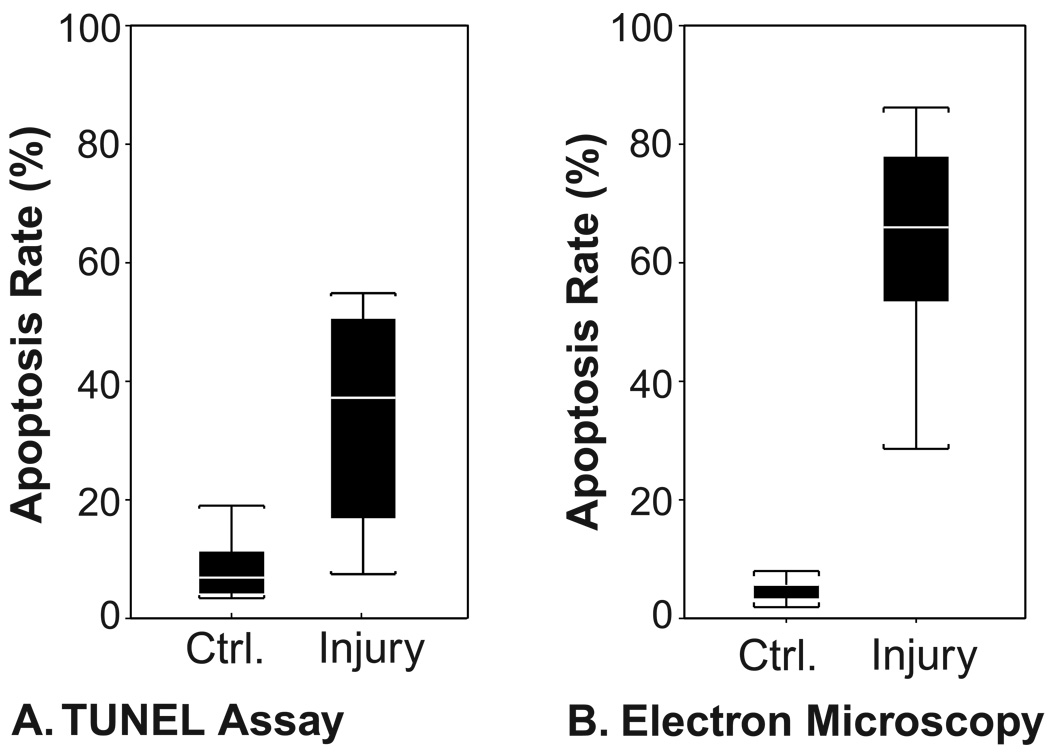
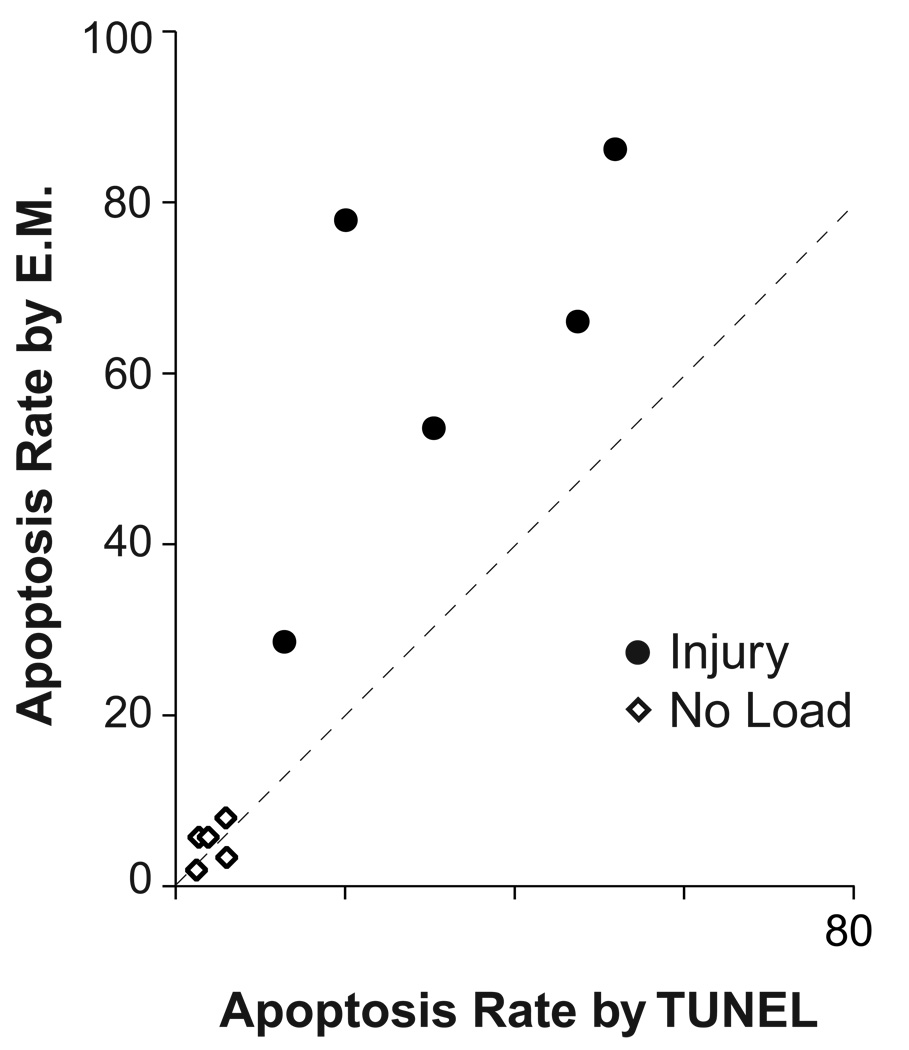
Unloaded and injured cartilage explants were fixed three days later and analyzed by both TUNEL and EM (Fig. 3). The TUNEL assay showed a significant increase in the percentage of apoptotic cells, from 7 ± 2 % in unloaded controls to 33 ± 6 % in injured cartilage (N = 8, p = 0.01). The EM analysis of the second half of the same cartilage explants also showed a significant increase in apoptotic cells, from 5 ± 1 % in unloaded controls to 62 ± 10 % in injured cartilage (N = 5, p = 0.02). The TUNEL and EM analyses of the same cartilage disks were also compared directly (Fig. 4). In all injured cartilage samples, apoptosis rates were higher by EM than by TUNEL (N = 5, p = 0.045). The correlation coefficient between the two measures in injured cartilage was good (ρ = 0.67) but the linear relationship between TUNEL and EM measurements did not reach statistical significance (p = 0.06) due to the small sample size.
Figure 3.
Quantification of cell apoptosis after injurious compression by TUNEL and by electron microscopy. Three days after injurious compression was applied, unloaded control disks and injured cartilage disks were sliced in half. From each disk, one half was flash-frozen in preparation for analysis by TUNEL staining (A), and the other half fixed for EM analysis (B). Box plots display values as range (brackets), interquartiles (solid bars), and median (white stripes). Apoptosis rates were significantly higher in injured cartilage as assessed by either TUNEL staining (p = 0.01, N = 8) or by EM (p = 0.01, N = 5).
Figure 4.
Apoptosis rates measured by TUNEL compared to apoptosis rates measured by electron microscopy on the same cartilage explants three days after either free-swelling culture (No Load) or injurious compression (Injury). Correlation between the two measurements was good (ρ = 0.67) but did not reach statistical significance (p = 0.06; N = 5 injured cartilage disks).
Cell Morphology after Injurious Compression by Electron Microscopy
In the five injured cartilage samples, approximately 100 cells in each sample were examined by EM. On average, 35% were normal in morphology. Of the remaining cells, 94% (range: 84–100%, N = 5) were classified as apoptotic, 1% (range: 0–5%) were classified as necrotic, and 5% (range: 0–11%) were left unclassified because the correct classification was not clear.
Discussion
We demonstrate here the quantitative investigation of chondrocyte cell death after injurious compression by cell morphology using electron microscopic analysis. In addition to supporting previous evidence that injurious compression can result in a dramatic increase in apoptotic chondrocyte cell death, we show that unexpectedly, the majority of dead cells were apoptotic in morphology. Unlike the TUNEL staining assay, analysis of cellular morphology did not produce false positives for apoptosis in freeze-thawed cartilage.
We have previously reported a significant increase in apoptotic cell death after injurious compression using TUNEL staining and nuclear morphology on light microscopy.14 Other researchers have also shown apoptotic cell death in models which cause several different kinds of mechanical cartilage damage or injury,9,15,22,23 and confirmed the presence of apoptosis by electron microscopy. However, quantification of apoptosis has generally relied on the TUNEL assay. Multiple concerns regarding the use of TUNEL staining have been reported. First, TUNEL staining can be nonspecific, staining freeze-thawed cartilage9 and hypertrophic chondrocytes.6,8 Second, false-positives have been reported as an artifact of histological sectioning.24 Third, quantification of the absolute numbers of apoptotic cells may be unreliable due to the dependence of the assay on details of the staining protocol.6 Analysis of freeze-thawed cells in this experiment confirmed that necrotic cells were frequently stained by TUNEL, whereas morphological analysis of the same sample confirmed that nearly all cell death was by necrosis.
Surprisingly, the results of quantitative electron microscopic analysis here not only confirmed the increase in cell death after injury but also showed more cell death than the TUNEL assay. The high level of cell death seen here is probably related to the young age of the newborn calves from which the tissue was taken. Kurz et al. 25 have shown that with increasing age of the animal, there is a significant decrease in the level of apoptosis induced by injury. They injured cartilage at the same strain and strain rate used here (50% strain at 1 mm/s velocity) and found cell apoptosis rates decreased from 22% in cartilage from two-week-old calves to ~5% cartilage from animals over 6 months old. Nevertheless, the increase in apoptotic cell death after injury remained statistically significant in cartilage from the skeletally mature animals. Similarly, Tew et al.23 also report a decrease in apoptotic response with age at the margins of cut bovine articular cartilage. The explanation is unclear but newborn calf cartilage chondrocytes are in general more metabolically active and more responsive to a variety of stimuli than chondrocytes in adult cartilage.1,26–28 In addition to the age of the tissue, our in vitro injury model does not attempt to simulate the precise three-dimensional forces and deformation patterns that cartilage would experience in a clinical joint injury. For these reasons, the absolute rate of cell death observed in vitro should not be precisely extrapolated to cell death after in vivo events.
The finding that cell death after mechanical injury can occur predominantly by apoptosis is nonetheless interesting from both mechanistic and therapeutic perspectives. It has been reported in diverse settings that mechanical forces can cause cell apoptosis (e.g., elevated hydrostatic pressure in glaucoma,29 decreased lumen flow in vascular endothelium,30,31 and traumatic brain11 and spinal cord32 injury). However, despite the progress in detailed understanding of the signaling pathways and the molecular machinery responsible for apoptosis, little is known of the mechanism for mechanical induction of apoptosis. Since the level of injurious compression applied to the cartilage here (50% strain at 100%/s) causes visible damage in ~50% of injured cartilage, it seems reasonable to hypothesize that significant alterations in cell-ECM interactions occur after injury and are responsible for signaling the apoptotic response. This would also be consistent with the apoptosis observed at the margins of cut cartilage.23 Integrin receptor-mediated signaling is important in maintaining cell survival in a range of situations,33–36 and disruption of the integrin receptor complex has been reported to mediate mechanically induced apoptosis in endothelial cells.37,38 Alternatively, generation of apoptosis may involve changes in the oxidative status of the tissue. A diet high in antioxidants can reduce mechanically induced OA in an animal model,39 possibly related to the finding that thioredoxin interacting protein (Txnip, also known as vitamin-D3-upregulated protein-1) is mechanically responsive.40
Regardless of the precise mechanism involved here, in interpreting published results for cell death after in vitro injury, the evidence suggests it may be useful to separate the mechanisms involved in injury models that consist of rapid or impact-like single compressive loads15,41–43 from very slow or creep-dominated loads that result in high local strain.9,22,44 In the latter category, Quinn et al.45 suggest that cell death can result from high-strain deformation at very low strain rates, which would be expected to cause substantial loss of water from the tissue but little or no matrix damage. Lucchinetti et al.44 note that in their model, cell death is seen first in the superficial zone chondrocytes and that this appears to be necrotic cell death. Although the strain is not reported, this is consistent with the higher local strain that the chondrocytes would be predicted to experience during the creep-like loading that was applied, and this may also explain the lack of evidence for apoptosis. Similarly, although Chen et al.9 do not measure strain during loading, their load-controlled compression appears to be creep-dominated and produce high total strain. They demonstrate convincing evidence by nuclear morphology for the presence of some apoptosis in their repetitively loaded cartilage, but overall, their results are again consistent with mostly necrotic cell death. In contrast, significant cell death was not observed by Thibault et al.46 with repetitive loading to fixed strains (i.e., without creep), despite evidence of damage to collagen fibrils.
From a clinical perspective, the induction of apoptosis after a discrete event such as traumatic joint injury would be important to investigate as a potential target for therapeutic intervention even without the benefit of understanding the precise mechanism involved. Several investigators have recently reported that apoptotic cell death is substantially increased in cartilage fragments obtained after intra-articular fracture47,48 and in cartilage biopsies obtained after joint injury.49 However, these initial reports appear to have relied primarily on TUNEL staining, and it will therefore be important for future studies to confirm these findings by other methods for quantification of apoptosis.
Although this study was not designed to characterize the morphology of the apoptotic cells, it may be useful to note that our results provide some support for the hypothesis that aspects of cell morphology in apoptotic chondrocytes may differ from the classical description of apoptosis in other cell types.50 For example, clear examples for condensation of the chromatin were infrequently observed, and this appears to be consistent with previously reported images of apoptotic chondrocytes.23 Some differences in the processes involved in cell death and the resulting morphology may be attributable to the atypical characteristics of cartilage tissue. Cartilage is adapted to a hypoxic environment, does not recruit inflammatory or scavenger cells, and partially restrains cells from swelling by the surrounding ECM. Thus of coagulation necrosis, ischemic necrosis, and oncosis, none seem to be an ideal term for description of non-programmed cell death in this tissue. An investigation of apoptotic and necrotic cell morphology in death caused by other stimuli could be useful to help further characterize this issue in chondrocytes.
Acknowledgments
This research was supported in part by NIH grant AR45779, a grant from GlaxoSmithKline, and the Whitaker Foundation.
References
- 1.Mankin HJ. Localization of tritiated thymidine in articular cartilage of rabbits. III: Mature articular cartilage. J Bone Joint Surg Am. 1963;45:529–540. [Google Scholar]
- 2.Blanco FJ, Guitian R, Vazquez-Martul E, de Toro FJ, Galdo F. Osteoarthritis chondrocytes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthritis Rheum. 1998;41:284–289. doi: 10.1002/1529-0131(199802)41:2<284::AID-ART12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 3.Hashimoto S, Ochs RL, Komiya S, Lotz M. Linkage of chondrocyte apoptosis and cartilage degradation in human osteoarthritis. Arthritis Rheum. 1998;41:1632–1638. doi: 10.1002/1529-0131(199809)41:9<1632::AID-ART14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 4.Kirsch T, Swoboda B, Nah H. Activation of annexin II and V expression, terminal differentiation, mineralization and apoptosis in human osteoarthritic cartilage. Osteoarthritis Cartilage. 2000;8:294–302. doi: 10.1053/joca.1999.0304. [DOI] [PubMed] [Google Scholar]
- 5.Hashimoto S, Ochs RL, Rosen F, et al. Chondrocyte-derived apoptotic bodies and calcification of articular cartilage. Proc Natl Acad Sci U S A. 1998;95:3094–3099. doi: 10.1073/pnas.95.6.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aigner T, Hemmel M, Neureiter D, et al. Apoptotic cell death is not a widespread phenomenon in normal aging and osteoarthritis human articular knee cartilage: a study of proliferation, programmed cell death (apoptosis), and viability of chondrocytes in normal and osteoarthritic human knee cartilage. Arthritis Rheum. 2001;44:1304–1312. doi: 10.1002/1529-0131(200106)44:6<1304::AID-ART222>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 7.Grogan SP, Aklin B, Frenz M, Brunner T, Schaffner T, Mainil-Varlet P. In vitro model for the study of necrosis and apoptosis in native cartilage. J Pathol. 2002;198:5–13. doi: 10.1002/path.1169. [DOI] [PubMed] [Google Scholar]
- 8.Doi T, Nishida K, Matsuo M, Yoshida A, Murakami T, Inoue H. Evidence of oncotic cell death and DNA fragmentation in human hypertrophic chondrocytes in chondro-osteophyte. Osteoarthritis Cartilage. 2002;10:270–276. doi: 10.1053/joca.2001.0503. [DOI] [PubMed] [Google Scholar]
- 9.Chen CT, Burton-Wurster N, Borden C, Hueffer K, Bloom SE, Lust G. Chondrocyte necrosis and apoptosis in impact damaged articular cartilage. J Orthop Res. 2001;19:703–711. doi: 10.1016/S0736-0266(00)00066-8. [DOI] [PubMed] [Google Scholar]
- 10.Aigner T, Kim HA. Apoptosis and cellular vitality: issues in osteoarthritic cartilage degeneration. Arthritis Rheum. 2002;46:1986–1996. doi: 10.1002/art.10554. [DOI] [PubMed] [Google Scholar]
- 11.Colicos MA, Dash PK. Apoptotic morphology of dentate gyrus granule cells following experimental cortical impact injury in rats: possible role in spatial memory deficits. Brain Res. 1996;739:120–131. doi: 10.1016/s0006-8993(96)00824-4. [DOI] [PubMed] [Google Scholar]
- 12.Sperandio S, de Belle I, Bredesen DE. An alternative, nonapoptotic form of programmed cell death. Proc Natl Acad Sci U S A. 2000;97:14376–14381. doi: 10.1073/pnas.97.26.14376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gelber AC, Hochberg MC, Mead LA, Wang NY, Wigley FM, Klag MJ. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann Intern Med. 2000;133:321–328. doi: 10.7326/0003-4819-133-5-200009050-00007. [DOI] [PubMed] [Google Scholar]
- 14.Loening AM, James IE, Levenston ME, et al. Injurious mechanical compression of bovine articular cartilage induces chondrocyte apoptosis. Arch Biochem Biophys. 2000;381:205–212. doi: 10.1006/abbi.2000.1988. [DOI] [PubMed] [Google Scholar]
- 15.D'Lima DD, Hashimoto S, Chen PC, Colwell CW, Jr, Lotz MK. Human chondrocyte apoptosis in response to mechanical injury. Osteoarthritis Cartilage. 2001;9:712–719. doi: 10.1053/joca.2001.0468. [DOI] [PubMed] [Google Scholar]
- 16.Sah RL, Kim YJ, Doong JY, Grodzinsky AJ, Plaas AH, Sandy JD. Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res. 1989;7:619–636. doi: 10.1002/jor.1100070502. [DOI] [PubMed] [Google Scholar]
- 17.Frank EH, Jin M, Loening AM, Levenston ME, Grodzinsky AJ. A versatile shear and compression apparatus for mechanical stimulation of tissue culture explants. J Biomech. 2000;33:1523–1527. doi: 10.1016/s0021-9290(00)00100-7. [DOI] [PubMed] [Google Scholar]
- 18.Patwari P, Cook MN, DiMicco MA, et al. Proteoglycan degradation after injurious compression of bovine and human articular cartilage in vitro: interaction with exogenous cytokines. Arthritis Rheum. 2003;48:1292–1301. doi: 10.1002/art.10892. [DOI] [PubMed] [Google Scholar]
- 19.Buschmann MD, Maurer AM, Berger E, Hunziker EB. A method of quantitative autoradiography for the spatial localization of proteoglycan synthesis rates in cartilage. J Histochem Cytochem. 1996;44:423–431. doi: 10.1177/44.5.8627000. [DOI] [PubMed] [Google Scholar]
- 20.Reynolds E. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patwari P, Kurz B, Berger E, et al. Comparative evaluation of apoptotic and necrotic cell death after injurious compression of bovine articular cartilage using electron microscopy and TUNEL staining. Transactions of the Orthopaedic Research Society. 2002;27:108. (abstract) [Google Scholar]
- 22.Clements KM, Bee ZC, Crossingham GV, Adams MA, Sharif M. How severe must repetitive loading be to kill chondrocytes in articular cartilage? Osteoarthritis Cartilage. 2001;9:499–507. doi: 10.1053/joca.2000.0417. [DOI] [PubMed] [Google Scholar]
- 23.Tew SR, Kwan AP, Hann A, Thomson BM, Archer CW. The reactions of articular cartilage to experimental wounding: role of apoptosis. Arthritis Rheum. 2000;43:215–225. doi: 10.1002/1529-0131(200001)43:1<215::AID-ANR26>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 24.Sloop GD, Roa JC, Delgado AG, Balart JT, Hines MO, 3rd, Hill JM. Histologic sectioning produces TUNEL reactivity. A potential cause of false-positive staining. Arch Pathol Lab Med. 1999;123:529–532. doi: 10.5858/1999-123-0529-HSPTR. [DOI] [PubMed] [Google Scholar]
- 25.Kurz B, Lemke A, Kehn M, et al. Tissue maturation and antioxidants alter the apoptotic response of articular cartilage after injurious compression. Arthritis and Rheumatism. 2004;50:123–130. doi: 10.1002/art.11438. [DOI] [PubMed] [Google Scholar]
- 26.Plaas AH, Sandy JD. Age-related decrease in the link-stability of proteoglycan aggregates formed by articular chondrocytes. Biochem J. 1984;220:337–340. doi: 10.1042/bj2200337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barone-Varelas J, Schnitzer TJ, Meng Q, Otten L, Thonar EJ. Age-related differences in the metabolism of proteoglycans in bovine articular cartilage explants maintained in the presence of insulin-like growth factor I. Connect Tissue Res. 1991;26:101–120. doi: 10.3109/03008209109152167. [DOI] [PubMed] [Google Scholar]
- 28.DeGroot J, Verzijl N, Bank RA, Lafeber FP, Bijlsma JW, TeKoppele JM. Age-related decrease in proteoglycan synthesis of human articular chondrocytes: the role of nonenzymatic glycation. Arthritis Rheum. 1999;42:1003–1009. doi: 10.1002/1529-0131(199905)42:5<1003::AID-ANR20>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 29.Agar A, Yip SS, Hill MA, Coroneo MT. Pressure related apoptosis in neuronal cell lines. J Neurosci Res. 2000;60:495–503. doi: 10.1002/(SICI)1097-4547(20000515)60:4<495::AID-JNR8>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 30.Kaiser D, Freyberg MA, Friedl P. Lack of hemodynamic forces triggers apoptosis in vascular endothelial cells. Biochem Biophys Res Commun. 1997;231:586–590. doi: 10.1006/bbrc.1997.6146. [DOI] [PubMed] [Google Scholar]
- 31.Sho E, Sho M, Singh TM, Xu C, Zarins CK, Masuda H. Blood flow decrease induces apoptosis of endothelial cells in previously dilated arteries resulting from chronic high blood flow. Arterioscler Thromb Vasc Biol. 2001;21:1139–1145. doi: 10.1161/hq0701.092118. [DOI] [PubMed] [Google Scholar]
- 32.Beattie MS, Hermann GE, Rogers RC, Bresnahan JC. Cell death in models of spinal cord injury. Prog Brain Res. 2002;137:37–47. doi: 10.1016/s0079-6123(02)37006-7. [DOI] [PubMed] [Google Scholar]
- 33.Montgomery AM, Reisfeld RA, Cheresh DA. Integrin alpha v beta 3 rescues melanoma cells from apoptosis in three-dimensional dermal collagen. Proc Natl Acad Sci U S A. 1994;91:8856–8860. doi: 10.1073/pnas.91.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao L, Lee V, Adams ME, et al. beta-Integrin-collagen interaction reduces chondrocyte apoptosis. Matrix Biol. 1999;18:343–355. doi: 10.1016/s0945-053x(99)00027-x. [DOI] [PubMed] [Google Scholar]
- 36.Pulai JI, Del Carlo M, Jr, Loeser RF. The alpha5beta1 integrin provides matrix survival signals for normal and osteoarthritic human articular chondrocytes in vitro. Arthritis Rheum. 2002;46:1528–1535. doi: 10.1002/art.10334. [DOI] [PubMed] [Google Scholar]
- 37.Wernig F, Mayr M, Xu Q. Mechanical stretch-induced apoptosis in smooth muscle cells is mediated by beta1-integrin signaling pathways. Hypertension. 2003;41:903–911. doi: 10.1161/01.HYP.0000062882.42265.88. [DOI] [PubMed] [Google Scholar]
- 38.Freyberg MA, Kaiser D, Graf R, Buttenbender J, Friedl P. Proatherogenic flow conditions initiate endothelial apoptosis via thrombospondin-1 and the integrin-associated protein. Biochem Biophys Res Commun. 2001;286:141–149. doi: 10.1006/bbrc.2001.5314. [DOI] [PubMed] [Google Scholar]
- 39.Kurz B, Jost B, Schunke M. Dietary vitamins and selenium diminish the development of mechanically induced osteoarthritis and increase the expression of antioxidative enzymes in the knee joint of STR/1N mice. Osteoarthritis Cartilage. 2002;10:119–126. doi: 10.1053/joca.2001.0489. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, De Keulenaer GW, Lee RT. Vitamin D(3)-up-regulated protein-1 is a stress-responsive gene that regulates cardiomyocyte viability through interaction with thioredoxin. J Biol Chem. 2002;277:26496–26500. doi: 10.1074/jbc.M202133200. [DOI] [PubMed] [Google Scholar]
- 41.Kurz B, Jin M, Patwari P, Cheng DM, Lark MW, Grodzinsky AJ. Biosynthetic response and mechanical properties of articular cartilage after injurious compression. J Orthop Res. 2001;19:1140–1146. doi: 10.1016/S0736-0266(01)00033-X. [DOI] [PubMed] [Google Scholar]
- 42.Duda GN, Eilers M, Loh L, Hoffman JE, Kaab M, Schaser K. Chondrocyte death precedes structural damage in blunt impact trauma. Clin Orthop. 2001;393:302–309. doi: 10.1097/00003086-200112000-00035. [DOI] [PubMed] [Google Scholar]
- 43.Jeffrey JE, Gregory DW, Aspden RM. Matrix damage and chondrocyte viability following a single impact load on articular cartilage. Arch Biochem Biophys. 1995;322:87–96. doi: 10.1006/abbi.1995.1439. [DOI] [PubMed] [Google Scholar]
- 44.Lucchinetti E, Adams CS, Horton WE, Jr, Torzilli PA. Cartilage viability after repetitive loading: a preliminary report. Osteoarthritis Cartilage. 2002;10:71–81. doi: 10.1053/joca.2001.0483. [DOI] [PubMed] [Google Scholar]
- 45.Quinn TM, Allen RG, Schalet BJ, Perumbuli P, Hunziker EB. Matrix and cell injury due to sub-impact loading of adult bovine articular cartilage explants: effects of strain rate and peak stress. J Orthop Res. 2001;19:242–249. doi: 10.1016/S0736-0266(00)00025-5. [DOI] [PubMed] [Google Scholar]
- 46.Thibault M, Poole AR, Buschmann MD. Cyclic compression of cartilage/bone explants in vitro leads to physical weakening, mechanical breakdown of collagen and release of matrix fragments. J Orthop Res. 2002;20:1265–1273. doi: 10.1016/S0736-0266(02)00070-0. [DOI] [PubMed] [Google Scholar]
- 47.Murray M, Vrahas M. Chondrocyte apoptosis after articular fracture in humans. Transactions of the Orthopaedic Research Society. 2002;27:424. (abstract) [Google Scholar]
- 48.Kim HT, Lo MY, Pillarisetty R. Chondrocyte apoptosis following intraarticular fracture in humans. Osteoarthritis Cartilage. 2002;10:747–749. doi: 10.1053/joca.2002.0828. [DOI] [PubMed] [Google Scholar]
- 49.Colwell CW, Jr, D'Lima DD, Hoenecke HR, et al. In vivo changes after mechanical injury. Clin Orthop. 2001;391(Suppl):S116–S123. doi: 10.1097/00003086-200110001-00012. [DOI] [PubMed] [Google Scholar]
- 50.Kouri JB, Abbud-Lozoya K. Criteria for TUNEL labeling in determining apoptosis in human osteoarthritis cartilage: Comment on the article by Aigner et al. Arthritis Rheum. 2002;46:2260–2261. doi: 10.1002/art.10375. [DOI] [PubMed] [Google Scholar]