Abstract
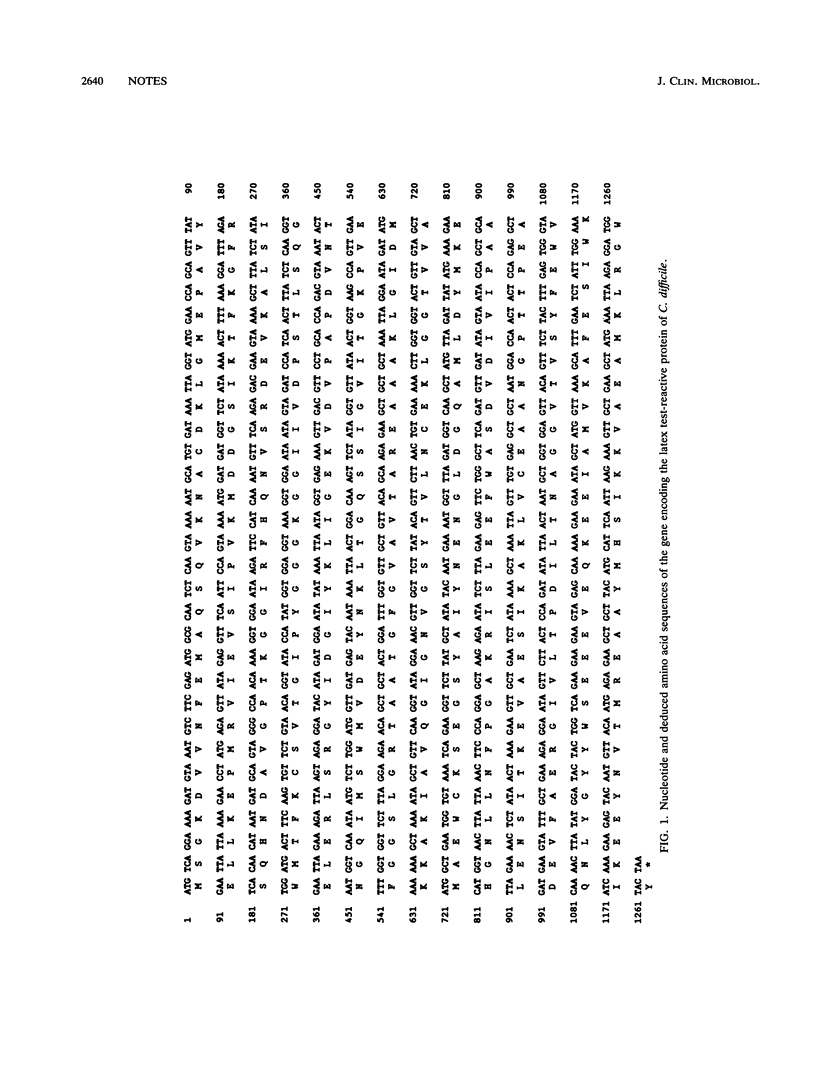
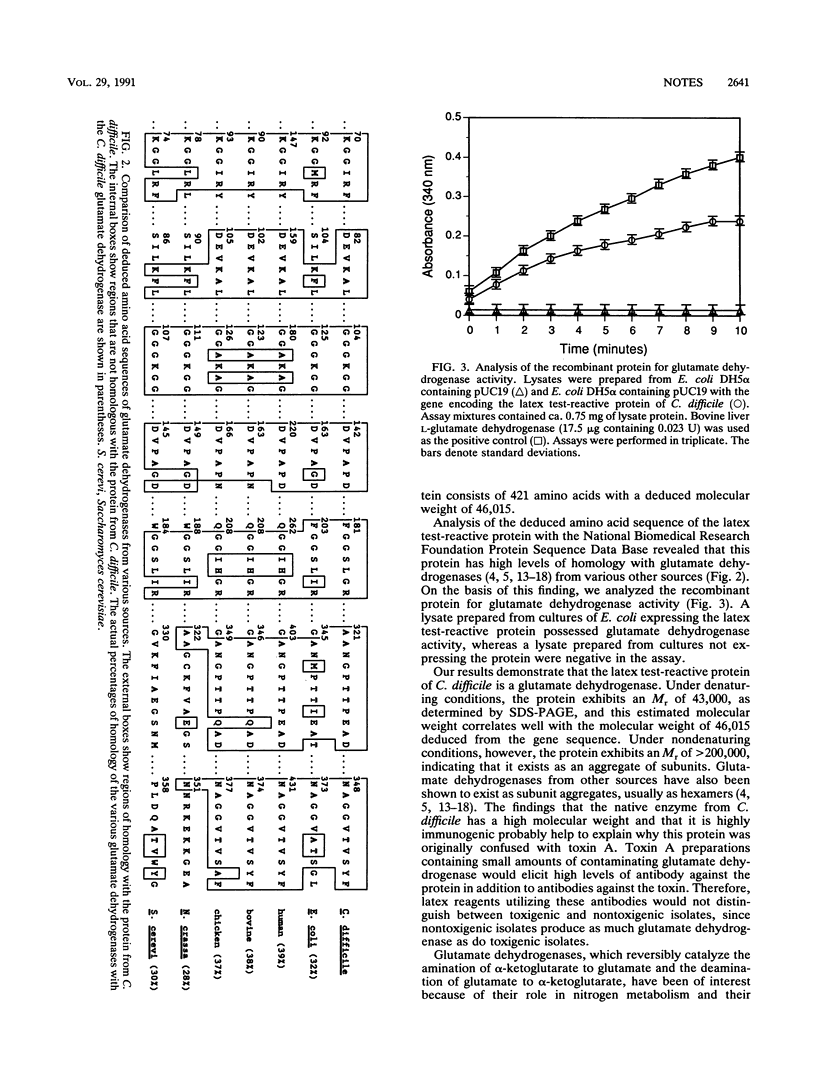
Computer analysis showed that the gene encoding the latex test-reactive protein of Clostridium difficile exhibited high levels of homology with glutamate dehydrogenases from various sources. Further analysis demonstrated that the recombinant protein possessed glutamate dehydrogenase activity. Our results show that the protein that reacts in commercial latex tests for C. difficile is a glutamate dehydrogenase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dove C. H., Wang S. Z., Price S. B., Phelps C. J., Lyerly D. M., Wilkins T. D., Johnson J. L. Molecular characterization of the Clostridium difficile toxin A gene. Infect Immun. 1990 Feb;58(2):480–488. doi: 10.1128/iai.58.2.480-488.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder A. A., Wootton J. C., Baron A. J., Chambers G. K., Fincham J. R. The amino acid sequence of Neurospora NADP-specific glutamate dehydrogenase. Peptic and chymotryptic peptides and the complete sequence. Biochem J. 1975 Sep;149(3):757–773. doi: 10.1042/bj1490757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julliard J. H., Smith E. L. Partial amino acid sequence of the glutamate dehydrogenase of human liver and a revision of the sequence of the bovine enzyme. J Biol Chem. 1979 May 10;254(9):3427–3438. [PubMed] [Google Scholar]
- Kanamori K., Weiss R. L., Roberts J. D. Ammonia assimilation pathways in nitrogen-fixing Clostridium kluyverii and Clostridium butyricum. J Bacteriol. 1989 Apr;171(4):2148–2154. doi: 10.1128/jb.171.4.2148-2154.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Ball D. W., Toth J., Wilkins T. D. Characterization of cross-reactive proteins detected by Culturette Brand Rapid Latex Test for Clostridium difficile. J Clin Microbiol. 1988 Mar;26(3):397–400. doi: 10.1128/jcm.26.3.397-400.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Krivan H. C., Wilkins T. D. Clostridium difficile: its disease and toxins. Clin Microbiol Rev. 1988 Jan;1(1):1–18. doi: 10.1128/cmr.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Wilkins T. D. Commercial latex test for Clostridium difficile toxin A does not detect toxin A. J Clin Microbiol. 1986 Mar;23(3):622–623. doi: 10.1128/jcm.23.3.622-623.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Mattaj I. W., McPherson M. J., Wootton J. C. Localisation of a strongly conserved section of coding sequence in glutamate dehydrogenase genes. FEBS Lett. 1982 Oct 4;147(1):21–25. doi: 10.1016/0014-5793(82)81003-x. [DOI] [PubMed] [Google Scholar]
- McPherson M. J., Wootton J. C. Complete nucleotide sequence of the Escherichia coli gdhA gene. Nucleic Acids Res. 1983 Aug 11;11(15):5257–5266. doi: 10.1093/nar/11.15.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon K., Piszkiewicz D., Smith E. L. Amino acd sequence of chicken liver glutamate dehydrogenase. J Biol Chem. 1973 May 10;248(9):3093–3107. [PubMed] [Google Scholar]
- Moon K., Smith E. L. Sequence of bovine liver glutamate dehydrogenase. 8. Peptides produced by specific chemical cleavages; the complete sequence of the protein. J Biol Chem. 1973 May 10;248(9):3082–3088. [PubMed] [Google Scholar]
- Nagasu T., Hall B. D. Nucleotide sequence of the GDH gene coding for the NADP-specific glutamate dehydrogenase of Saccharomyces cerevisiae. Gene. 1985;37(1-3):247–253. doi: 10.1016/0378-1119(85)90279-3. [DOI] [PubMed] [Google Scholar]
- Rasched I., Jörnvall H., Sund H. Studies of glutamate dehydrogenase. Identification of an amino group involved in the substrate binding. Eur J Biochem. 1974 Feb 1;41(3):603–606. doi: 10.1111/j.1432-1033.1974.tb03302.x. [DOI] [PubMed] [Google Scholar]
- Syed S. E., Engel P. C. A pH-dependent activation-inactivation equilibrium in glutamate dehydrogenase of Clostridium symbiosum. Biochem J. 1990 Oct 15;271(2):351–355. doi: 10.1042/bj2710351. [DOI] [PMC free article] [PubMed] [Google Scholar]


