Abstract
Background and Purpose
To elucidate how the motor pathways rewire the denervated tissue after stroke, we investigated remodeling of the corticospinal tract (CST) in transgenic mice with Yellow Fluorescent Protein (YFP) CST labeling in conjunction with trans-synaptic pseudorabies virus (PRV) retrograde tracing.
Methods
Adult male CST-YFP mice were subjected to permanent right middle cerebral artery occlusion (MCAo, n=8/group). Foot-Fault test was performed to monitor functional deficit and recovery. PRV tracer was injected into the left forelimb muscles at 1 or 4 weeks after MCAo (4 days before sacrifice), respectively. A third group of CST-YFP mice without MCAo was used for normal control (n=6). The YFP labeling of CST in the cervical cord and PRV labeling of pyramidal neurons in the bilateral cortices were measured on vibratome sections using a confocal imaging system.
Results
Compared with normal animals, axonal density in the stroke-affected side of the cervical cord was significantly decreased at 11 days (p<0.001) and significantly increased at 32 days after stroke compared to the day 11 values (p<0.05). PRV labeling was significantly decreased in the ischemic hemisphere 11 days after MCAo (p<0.001). In contrast, a significant increase was observed in PRV labeling of bilateral cortices 32 days after stroke compared to 11 days (p<0.05). The CST axonal density in the denervated spinal cord and pyramidal neuron labeling in the bilateral cortices were significantly correlated with behavioral recovery (p<0.05).
Conclusions
Spontaneous functional recovery after stroke may, at least in part, be attributed to neuronal remodeling in the corticospinal system.
Keywords: functional recovery, middle cerebral artery occlusion, neuronal plasticity, mice
In the early stage after stroke, functional recovery may be attributable to the resolution of brain edema, absorption of damaged tissue or reperfusion of the ischemic penumbra, while the recovery after the initial week is likely due to neuronal plasticity and substantial structural reorganization of the remaining intact brain tissue.1 With the advance of acute stroke treatment, the issues of functional restoration and post-stroke rehabilitation have become increasingly important. Unfortunately, our understanding of the mechanisms of neuronal plasticity, and their relation to behavioral and functional recovery remain poor.
The corticospinal tract (CST), long axons of the cortical pyramidal neurons extending to the spinal cord, connecting with the spinal motoneurons directly or indirectly, is the primary transmission tract from the sensorimotor cortex, and thus, forms the neuroanatomical basis for brain controlled voluntary movements of the peripheral muscles.2 One of the most common impairments after stroke is hemiparesis of the contralateral body side to the affected cerebral hemisphere. As the hemiparesis is a consequence of interruption of neuronal signals from the cortical pyramidal neurons onto the spinal motoneurons, we hypothesized that the remodeling of the CST axons to rewire the denervated spinal cord is a key element contributing to neurological recovery after stroke. In this study, a transgenic mouse strain, in which the CST is specifically and completely labeled by yellow fluorescent protein (YFP),3 was employed to directly monitor the axonal morphological change in the spinal gray matter with fluorescent microscopy after middle cerebral artery occlusion (MCAo). Additionally, pseudorabies virus (PRV)-Bartha, an attenuated strain of PRV,4 was used for retrograde trans-synaptic neuronal tracing.5, 6 Using a PRV recombinant that expresses monomeric red fluorescent protein (PRV-614-mRFP) 7 injected into stroke-impaired forelimb muscles, we examined neuronal reorganization of the cortical pyramidal neurons in bilateral hemispheres having synaptic connections with the stroke-impaired forelimb.
Materials and Methods
Animals
Adult CST-YFP mice (2 months-old, body weight 25-30 g) were generated by our in-house breeding colony using two transgenic mouse strains of B6.Cg-Tg(Thy1-EYFP)15Jrs/J and B6.129-Emx1tm1(cre)Krj/J obtained from Jackson Laboratories (Bar Harbor, Maine, USA). In the Thy1-STOP-YFP mice, YFP expression is driven by neuron-specific regulatory elements of the Thy1 promoter after Cre-mediated excision of STOP sequences. In the Emx-Cre mice, Cre recombinase is specifically expressed in the embryonic forebrain, the area of origin of the CST. Therefore, in CST-YFP mice generated by mating Thy1-STOP-YFP with Emx-Cre strain, YFP expression is limited to the forebrain and CST.3 All experimental procedures were approved by the Institutional Animal Care and Use Committee of Henry Ford Hospital.
MCAo Model
For ischemic stroke, 22 mice were subjected to permanent MCAo by advancing a 6-0 surgical nylon suture with an expanded (heated) tip from the right external carotid artery into the lumen of the internal carotid artery, to block the origin of the MCA.8 Six mice died within the first 5-days. The remaining animals were randomly divided into 2 groups, and sacrificed at 11 or 32 days after MCAo, respectively (n=8 per group). A third group of naive CST-YFP mice without surgery was used for normal control (n=6).
Behavioral Tests
To evaluate the motor functional recovery, a Foot-Fault test was performed at 1 day after MCAo and weekly thereafter. This test measures the accuracy of forepaw placement on a non-equidistant grid as the percentage of foot faults of the left forepaw to total steps.9
Retrograde PRV Tracing
To confirm the neuronal wiring between the motor cortex and the stroke-impaired peripheral target tissues, a trans-synaptic tracer, PRV-614-mRFP (Gift from Dr. Lynn Enquist, Princeton University, Princeton, NJ) was used to retrogradely label the cortical pyramidal neurons from the left forelimb muscles.6 At 4 days before sacrifice, a 10 μl total volume of PRV-164-mRFP, divided into multiple injections of 1-2 μl, was injected into muscles of the left forelimb (radioulnar flexor) with a 10 μl Hamilton syringe through a skin incision. The animals were then transferred to a Biosafety Level-2 room to survive for an additional 94-96 hrs for the tracer transport from axonal terminals in muscles to the cell body of pyramidal neurons in the cortex.
Tissue Preparation
At 11 or 32 days after MCAo, animals were perfused transcardially with saline, followed by 4% paraformaldehyde. The entire brain and the spinal cord were removed, and immersed in 4% paraformaldehyde overnight. Every first 100 μm of each 500 μm thickness in the forebrain was sectioned using a vibratome to examine the mRFP-positive pyramidal neurons. The remaining 400 μm brain blocks were embedded in paraffin. A series of adjacent 6 μm-thick paraffin sections were cut from each block and stained with Hematoxylin and Eosin for lesion volume measurement. The cervical spinal cord segment of C4-7 were cut into consecutive 100 μm-thick vibratome sections to detect the YFP positive CST axons with fluorescent microscopy.
Data Analysis and Statistics
The behavior outcomes were evaluated by the numbers of the stroke-impaired left forepaw missteps between the wires with time after stroke. Lesion volume was measured by NIH imaging software (Image J) and presented as a volume percentage of the lesion area compared with the contralesional hemisphere, as previously reported.10 A Bio-Rad MRC 1024 (argon and krypton) laser-scanning confocal imaging system mounted onto a Zeiss microscope (Bio-Rad, Cambridge, MA, USA) was used to examine PRV labeling of pyramidal neurons in the bilateral cerebral cortices and the YFP labeling of CST axons in the cervical cord. The number of mRFP-positive cells was presented in 0.5 mm granularity to the bregma. The CST axonal densities in both sides of the gray matter of cervical cord were measured on 30 consecutive transverse sections with Image J. To avoid variation on fluorescent measurements, the percentage of CST density in the stroke-impaired side to the contralateral side on same sections was calculated as an index to assess axonal remodeling in the spinal cord after stroke.
All data are presented as mean±SD. A value of p<0.05 is taken as significant. One-way analysis of variance (ANOVA) was used to evaluate functional recovery, numbers of PRV-positive pyramidal neurons, and the index of axonal remodeling. To test the correlation between behavioral outcome and neuronal reorganization, the correlation coefficients between the left forepaw motor performance and the index of axonal density in the denervated side of the cervical cord or total PRV positive cell numbers in the bilateral cortices were calculated by Pearson's correlation coefficients after MCAo with r>0.70 indicating a good correlation.
Results
Lesion Volume and Behavioral Recovery
The mean lesion volume in mice sacrificed at 11 and 32 days after permanent suture MCAo was 29.5±4.2% (Range 21.9 to 36.2%; 30.3±3.9% for day 11 and 28.8±4.6 for day 21; Supplemental Figure A). As assessed with Foot-Fault test (Figure 1), severe behavioral deficits were evident in all animals 1 day after stroke, which partially recovered during the first week (p<0.001). A significant progressive recovery was observed with time (p<0.001 vs 1 week).
Figure 1. Functional outcome after MCAo.
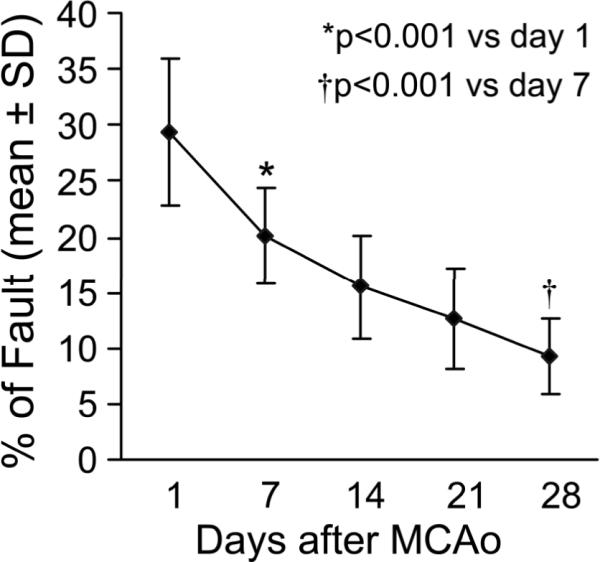
Line graph shows the temporal profile of functional recovery measured by the Foot-Fault test. The animals show recovery from functional disability with time after stroke.
Axonal Remodeling of the CST in the Denervated Side of the Cervical Cord
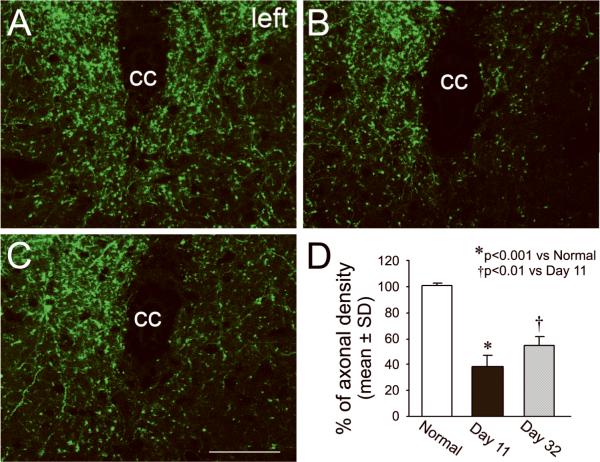
CST axons innervate the spinal motoneurons and interneurons by synaptic connections in the spinal gray matter for peripheral motor control. In CST-YFP mice, the cortical neurons in the forebrain and their axons, the CST, were specifically labeled with YFP, and therefore, were visible on vibratome sections under a fluorescent microscope (Supplemental Figure B). To examine the neuroanatomical basis of behavioral disability and recovery, we measured axonal density in the central area of the cervical gray matter in adult normal mice and mice with stroke 11 or 32 days later (Figure 2). Compared with a normal animal (A), the axonal density in the stroke-impaired side was decreased 11 days after stroke (B), consistent with previous data.3 However, increased axonal density was observed at the 32 day, i.e., late time point (C) compared with day 11 animals. To avoid subtle inter-section differences induced by fluorescent imaging sensitivity, we calculated the ratio of axonal density in the denervated side to the intact side on the same sections in each animal as an index to assess the axonal remodeling. Statistical data showed that more than 60% of CST axons were eliminated in the stroke-impaired side 11 days after stroke (p<0.001), while a significant recovery in CST density in the denervated side of the spinal cord was present at 32 days compared with 11 days after MCAo (p<0.01).
Figure 2. Single layer confocal images of CST axons in the gray matter of the cervical cord.
In normal CST-YFP mice, the CST axons are visible in the spinal gray matter on vibratome traverse sections with fluorescent microscopy (A). In mice with right MCAo, damaged axons were degenerated in the left side of spinal cord 11 days after stroke (B and D, p<0.001). However, axonal density significantly recovered 32 days after stroke (C and D, p<0.01). cc: central canal. Scale bar=50 μm.
Neuronal Reorganization in the Bilateral Cortices
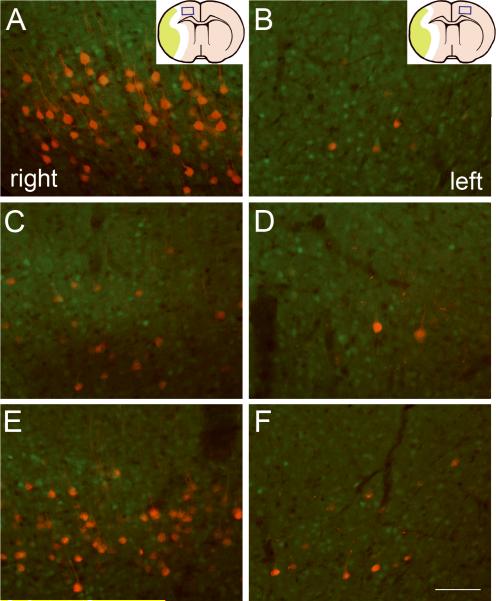
To confirm the establishment of neuronal connections between the bilateral hemispheres and the stroke-impaired peripheral tissue, we retrogradely labeled the neural pathways from the left forelimb muscles to the motor cortices with a trans-synaptic fluorescent viral tracer of PRV-614-mRFP in CST-YFP mice (Figure 3). In the normal mouse, 4 days after tracer injection into the left forelimb muscles, most PRV-positive pyramidal neurons were found in layer V of the forelimb motor areas in the right cerebral cortex with fluorescent labeling on the cell body and dendrites (A), while only few pyramidal cells in the symmetrical areas in the left hemisphere were labeled with mRFP (B). Eleven days after right MCAo, the number of PRV-positive pyramidal neurons was reduced in the ischemic cortex (C) and was unchanged in the contralesional cortex (D). Interestingly, the number of PRV labeled cells increased within the forelimb areas in both ipsilesional and contralesional cortices 32 days compared with animals 11 days after stroke (E and F). We counted the numbers of PRV-positive neurons on each one of five 100 μm-thick coronal sections of rostral forebrain (Table 1). Quantitative data showed that in the right ischemic hemisphere, the number of pyramidal neurons connecting with the stroke impaired left forelimb was significantly decreased after MCAo compared with normal animals in both caudal forelimb area (-0.5~0 mm rostral to the bregma) and rostral forelimb area (1.5~2.0 mm rostral to the bregma; p<0.001). However, significant increases of PRV positive neurons were observed over the caudal and rostral forelimb areas in both hemispheres at 32 days after stroke (p<0.05 vs day 11).
Figure 3. PRV labeling of pyramidal neurons in the bilateral cerebral cortices at the bregma level.
PRV-614-mRFP (red) was injected into the left forelimb muscles. Single layer confocal images on coronal brain sections at the bregma level show that in a normal mouse, the pyramidal neurons were labeled with PRV primarily in the corresponding area of the right cortex (A) and sparsely in the left cortex (B). PRV-positive pyramidal cells in the right cortex were reduced 11 days after MCAo (C), while the number PRV-labeled cells was little increased in the contralesional cortex (D). In contrast, the PRV-positive cells were increased 32 days after stroke in both ipsilesional (E) and contralesional hemispheres (F) compared with 11 days. Inserts in A and B, schematic drawing of the brain coronal sections showing the position of the photomicrograph appearing in the left and right panels, respectively. Scale bar=100 μm.
Table 1.
Numbers of PRV-positive pyramidal neurons in bilateral cortices
| Group | mm to bregma | -0.5 | 0 | 0.5 | 1.0 | 1.5 | 2.0 | Total |
| Normal | Left | 2.0±1.4 | 5.7±2.5 | 0.3±0.8 | 0.3±0.8 | 3.5±2.9 | 3.3±1.4 | 15.2±4.1 |
| Right | 81.2±9.2 | 134.0±11.1 | 19.0±4.3 | 2.7±2.5 | 24.2±5.8 | 30.5±6.3 | 291.5±22.0 | |
| Day 11 | Left | 4.4±3.6 | 6.6±3.8 | 1.9±1.8 | 0.9±1.5 | 4.8±2.7 | 3.5±2.1 | 23.0±10.0 |
| Right | 34.0±13.8 ** | 59.8±18.7 ** | 13.8±4.4 * | 2.0±1.9 | 14.1±7.2 * | 14.5±5.6 ** | 138.1±32.8** | |
| Day 32 | Left | 12.6±5.0 ‡ | 14.8±4.0 ‡ | 8.9±4.0 ‡ | 1.0±1.2 | 9.3±3.1 ‡ | 7.8±3.4 † | 54.3±12.5 ‡ |
| right | 57.9±17.6 † | 78.3±19.7 | 15.5±5.2 | 2.8±1.8 | 23.5±6.8 † | 22.6±5.5 ‡ | 200.5±45.0 ‡ | |
Numbers are mean±SD.
p<0.01,
p<0.001 vs normal;
p<0.05,
p<0.01 vs day 11.
Correlation between Functional Recovery and Neural Remodeling
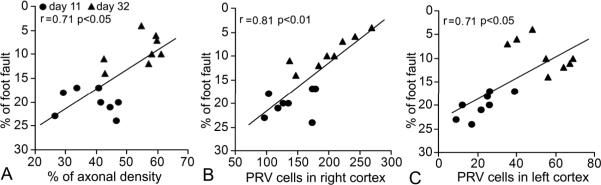
To test the hypothesis that neuronal remodeling contributes to functional outcome in the subacute and chronic phases after stroke, we determined the correlation of behavioral performance with the neuronal morphological status in mice sacrificed at 11 or 32 days after MCAo. Our data showed that functional scores were highly correlated with the index of axonal density in the denervated side of the spinal cord (Figure 4A) and the numbers of neurons connecting with the stroke-impaired forelimb in both ischemic (B) and contralesional (C) cortices (p<0.05). In addition, at day 11 post stroke, a significant correlation was found between functional behavior and PRV positive cells in the contralesional left cortex (Table 2, p<0.05), with no significant correlation of neurological function and PRV cells in the right ischemic cortex or axonal density in the spinal cord. In contrast, at 32 days after stroke, significant correlations were evident between functional outcome and both axonal density (p<0.05) and PRV labeling in the right cortex (p<0.01), while a marginal negative correlation was found between functional outcome and the PRV cells in the left cortex (p=0.08), suggesting that functional recovery at day 32 is related to remodeling in the ipsilesional cortex and spinal cord, while at the early time point (i.e. day 11) functional recovery is associated with axonal changes in the contralesional intact cortex.
Figure 4. Sketch graphs of correlations between neural remodeling and behavioral recovery.
The functional recovery assessed by Foot-Fault test was highly correlated with the CST axon density in the denervated side of the spinal cord (A) and numbers of PRV-positive pyramidal neurons in the bilateral cortices (B and C, p<0.05).
Table 2.
Within group comparisons between functional outcome and neuronal states
| Group | % of CST density | Right PRV labeling | Left PRV labeling |
|---|---|---|---|
| Day 11 | r=0.20 | r=0.27 | r=0.74 |
| p=0.64 | p=0.52 | p<0.05 | |
| Day 32 | r=0.71 | r=-0.66 | r=0.91 |
| p<0.05 | p=0.08 | p<0.01 | |
Discussion
In the present study, using transgenic and viral fluorescent labeling to the cortical pyramidal neurons in adult mice, we directly demonstrated that the neuronal rewiring in the stroke-impaired side of the cervical spinal cord originating from bilateral cortices significantly recovered during subacute and chronic phases after stroke. Additionally, spontaneous motor behavioral recovery after cerebral ischemic stroke is time dependent and highly correlated with the CST axonal remodeling in the spinal cord and the pyramidal neuronal reorganization in the bilateral cortices.Compared with traditional dye methods, the use of transgenic labeling in CST-YFP mice has salient advantages of invariability, noninvasiveness and sensitivity.3 Additionally, the PRV is a powerful neural tracer for multi-synaptic pathways without dilution in the transport process. After PRV injection into forelimb muscles, the virus replicates in the infected neurons and infectious particles are released and taken up at synapses, thus spreading along neuronal hierarchical chains.5 Moreover, transport of the attenuated PRV-Bartha strain between CNS neurons occurs only at points of synaptic contact and proceeds in the retrograde direction.11 Our findings demonstrate that the corticospinal innervation is impaired early after stroke, while the descending motor signaling pathways are functionally rewired through the increased CST axons detected with YFP labeling in the denervated spinal cord 32 days after MCAo. Therefore, high correlations between functional outcome and axonal density of the CST and PRV labeling of the cortical pyramidal neurons suggest that the behavioral recovery in the late phase after stroke may, at least in part, be attributed to the neuronal remodeling in the spinal cord and bilateral cortices. An important observation from this study was that the neuronal remodeling occurred widely in extensive cerebral areas after unilateral ischemic stroke, including remote regions in the infarct hemisphere and the contralateral intact hemisphere. Although the adult mammalian corticospinal system exhibits essentially an unilateral innervation pattern, if the motor cortex is extensively damaged, an alternative network outside the damaged area, either in the ipsilateral hemisphere or contralateral hemisphere, arises to compensate for the loss of function in the damaged system.12-15 Our data demonstrate a dynamic rewiring in the denervated gray matter of the spinal cord after stroke. Acutely, within 11 days post stroke, there is a prominent reduction of CST connections between the forelimb and the ipsilesional cortex, with a significant increase of CST axons in bilateral cortices originating from the forelimb at day 32. We also found that functional recovery post stroke is highly correlated with contralesional cortical wiring, only acutely (i.e., 11 days), with a negative correlation at the late time point (32 days). Similar data has also been reported in the human, with increasing activation in the sensorimotor cortex from early contralesional activity to late ipsilesional activity during recovery from hemiparesis.16 Thus, the cortical rewiring formed in the mouse and its correlation with neurological function parallels observations in the stroke patient. Our data, for the first time, demonstrate a robust anatomical correlation between the CST and functional recovery. Thus, spontaneous recovery after stroke may depend on a time dependent rewiring of the CST.
The present finding of CST axonal remodeling provides neuroanatomical evidence for brain functional reorganization after stroke. Namely, the cortical pyramidal neurons residing outside the infarct region extend their arborization via axonal outgrowth in the spinal cord to take over the lost innervation from neurons in the ischemic infarct area or neurons with CST axonal disruption on spinal motoneurons to reestablish the corticospinal motor control. Moreover, with time post stroke, the increase of PRV labeling was mostly found in the ischemic hemisphere, suggesting that the ischemic cortex may be the main source of the neuronal recovery. Interestingly, in the day 32 group, animals having higher contralesional PRV labeling showed worse behavioral performances, supporting a previous report that functional recruitment from the intact cortex is greatest in the more impaired patients.17 However, the functional contribution of the contralesional cortex requires further validation. Additional studies on the relationship of specific deficits/recovery with anatomical sets of neuronal remodeling and demonstrating causality by selective inhibition of CST remodeling, as well as studies using different stroke models and therapeutic interventions are also warranted.
In the adult mammal, the injured CNS is a highly inhibitory environment for axonal regeneration. However, following ischemic lesions, the adult CNS can induce cellular responses needed for neurite growth and synaptic formation.18, 19 Such molecules might support CST axonal sprouting in a time window of 2 to 3 weeks post stroke.20 Indeed, CST plasticity in the spinal cord associated with enhanced functional outcome has recently been found in animals after experimental stroke.21-23 Furthermore, our previous study demonstrated that the increase of CST axons in the spinal cord is attributed to increased arborization of neighboring uninjured fibers via short-range axonal sprouting within the spinal gray matter, a region without any direct ischemic damage.24 Such local short-range sprouting has important benefit to overcome glial inhibition in the CNS.25 Therefore, the CST axonal remodeling in the spinal cord may be considered as a potential target for cerebral stroke treatment, by enhancing axonal growth stimulative factors in the brain, and/or reducing axonal growth inhibitory factors in the spinal cord.
Summary
Using transgenic CST-YFP labeling and retrograde trans-synaptic PRV tracing in adult mice, we demonstrated that CST axonal remodeling in the spinal cord and neuronal reorganization in the bilateral cortices are time dependent and highly correlated with spontaneous behavioral recovery after ischemic stroke.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the gifts of pseudorabies viral tracers from Dr. Lynn W. Enquist, Princeton University, Princeton, NJ. This work was supported by American Heart Association National Scientist Development Grant 0835397N, National Institute of Neurological Diseases and Stroke grant PO1 NS42345 and the Mandell and Madeleine H. Berman Foundation.
References
- 1.Nudo RJ. Mechanisms for recovery of motor function following cortical damage. Curr Opin Neurobiol. 2006;16:638–644. doi: 10.1016/j.conb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Fromm C, Evarts EV. Pyramidal tract neurons in somatosensory cortex: Central and peripheral inputs during voluntary movement. Brain Res. 1982;238:186–191. doi: 10.1016/0006-8993(82)90781-8. [DOI] [PubMed] [Google Scholar]
- 3.Bareyre FM, Kerschensteiner M, Misgeld T, Sanes JR. Transgenic labeling of the corticospinal tract for monitoring axonal responses to spinal cord injury. Nat Med. 2005;11:1355–1360. doi: 10.1038/nm1331. [DOI] [PubMed] [Google Scholar]
- 4.Bartha A. Experimental reduction of virulence of aujesky's disease. Magy Allatorv Lapja. 1961;16:42–45. [Google Scholar]
- 5.Enquist LW, Husak PJ, Banfield BW, Smith GA. Infection and spread of alphaherpesviruses in the nervous system. Adv Virus Res. 1998;51:237–347. doi: 10.1016/s0065-3527(08)60787-3. [DOI] [PubMed] [Google Scholar]
- 6.Song CK, Enquist LW, Bartness TJ. New developments in tracing neural circuits with herpesviruses. Virus Res. 2005;111:235–249. doi: 10.1016/j.virusres.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 7.Banfield BW, Kaufman JD, Randall JA, Pickard GE. Development of pseudorabies virus strains expressing red fluorescent proteins: New tools for multisynaptic labeling applications. J Virol. 2003;77:10106–10112. doi: 10.1128/JVI.77.18.10106-10112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, Katakowski M, Lu M, Chopp M. Atorvastatin induction of vegf and bdnf promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25:281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernandez TD, Schallert T. Seizures and recovery from experimental brain damage. Exp Neurol. 1988;102:318–324. doi: 10.1016/0014-4886(88)90226-9. [DOI] [PubMed] [Google Scholar]
- 10.Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 11.Pickard GE, Smeraski CA, Tomlinson CC, Banfield BW, Kaufman J, Wilcox CL, Enquist LW, Sollars PJ. Intravitreal injection of the attenuated pseudorabies virus prv bartha results in infection of the hamster suprachiasmatic nucleus only by retrograde transsynaptic transport via autonomic circuits. J Neurosci. 2002;22:2701–2710. doi: 10.1523/JNEUROSCI.22-07-02701.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seitz RJ, Hoflich P, Binkofski F, Tellmann L, Herzog H, Freund HJ. Role of the premotor cortex in recovery from middle cerebral artery infarction. Arch Neurol. 1998;55:1081–1088. doi: 10.1001/archneur.55.8.1081. [DOI] [PubMed] [Google Scholar]
- 13.Werhahn KJ, Conforto AB, Kadom N, Hallett M, Cohen LG. Contribution of the ipsilateral motor cortex to recovery after chronic stroke. Ann Neurol. 2003;54:464–472. doi: 10.1002/ana.10686. [DOI] [PubMed] [Google Scholar]
- 14.Fridman EA, Hanakawa T, Chung M, Hummel F, Leiguarda RC, Cohen LG. Reorganization of the human ipsilesional premotor cortex after stroke. Brain. 2004;127:747–758. doi: 10.1093/brain/awh082. [DOI] [PubMed] [Google Scholar]
- 15.Schaechter JD, Perdue KL, Wang R. Structural damage to the corticospinal tract correlates with bilateral sensorimotor cortex reorganization in stroke patients. Neuroimage. 2008;39:1370–1382. doi: 10.1016/j.neuroimage.2007.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marshall RS, Perera GM, Lazar RM, Krakauer JW, Constantine RC, DeLaPaz RL. Evolution of cortical activation during recovery from corticospinal tract infarction. Stroke. 2000;31:656–661. doi: 10.1161/01.str.31.3.656. [DOI] [PubMed] [Google Scholar]
- 17.Johansen-Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci U S A. 2002;99:14518–14523. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cramer SC, Chopp M. Recovery recapitulates ontogeny. Trends Neurosci. 2000;23:265–271. doi: 10.1016/s0166-2236(00)01562-9. [DOI] [PubMed] [Google Scholar]
- 19.Lu A, Tang Y, Ran R, Clark JF, Aronow BJ, Sharp FR. Genomics of the periinfarction cortex after focal cerebral ischemia. J Cereb Blood Flow Metab. 2003;23:786–810. doi: 10.1097/01.WCB.0000062340.80057.06. [DOI] [PubMed] [Google Scholar]
- 20.Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: Making waves. Ann Neurol. 2006;59:735–742. doi: 10.1002/ana.20845. [DOI] [PubMed] [Google Scholar]
- 21.Chen P, Goldberg DE, Kolb B, Lanser M, Benowitz LI. Inosine induces axonal rewiring and improves behavioral outcome after stroke. Proc Natl Acad Sci U S A. 2002;99:9031–9036. doi: 10.1073/pnas.132076299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiessner C, Bareyre FM, Allegrini PR, Mir AK, Frentzel S, Zurini M, Schnell L, Oertle T, Schwab ME. Anti-nogo-a antibody infusion 24 hours after experimental stroke improved behavioral outcome and corticospinal plasticity in normotensive and spontaneously hypertensive rats. J Cereb Blood Flow Metab. 2003;23:154–165. doi: 10.1097/01.WCB.0000040400.30600.AF. [DOI] [PubMed] [Google Scholar]
- 23.Lee JK, Kim JE, Sivula M, Strittmatter SM. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J Neurosci. 2004;24:6209–6217. doi: 10.1523/JNEUROSCI.1643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Z, Li Y, Zhang X, Savant-Bhonsale S, Chopp M. Contralesional axonal remodeling of the corticospinal system in adult rats following stroke and bone marrow stromal cell treatment. Stroke. 2008;39:2571–2577. doi: 10.1161/STROKEAHA.107.511659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yiu G, He Z. Glial inhibition of cns axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.