Abstract
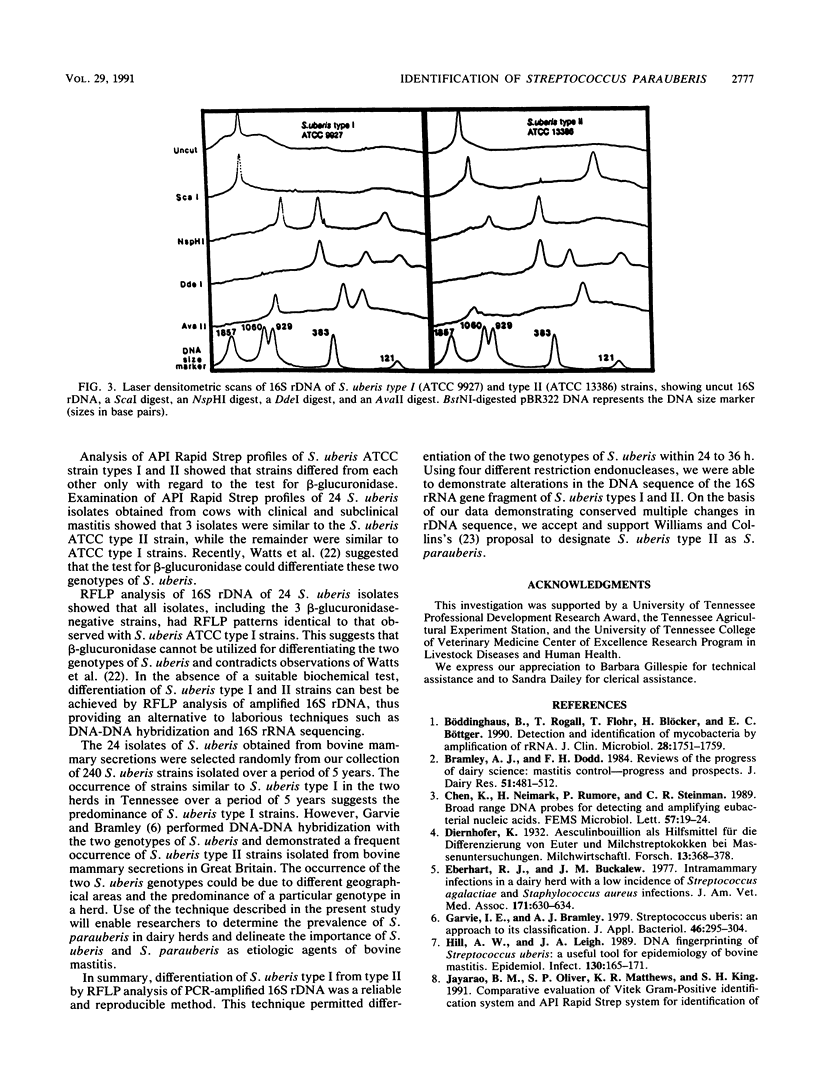
Streptococcus uberis type II has been proposed recently as a separate species designated Streptococcus parauberis (A. M. Williams and M. D. Collins, J. Appl. Bacteriol. 68:485-490, 1990). Differentiation of S. parauberis from S. uberis has been possible only by DNA-DNA hybridization or 16S rRNA sequencing, since the biochemical and serological characteristics of the two species are indistinguishable. A simple and reliable technique was developed for differentiating S. parauberis (S. uberis type II [ATCC 13386]) from S. uberis (S. uberis type I [ATCC 9927, ATCC 13387, and ATCC 27958]) by restriction fragment length polymorphism (RFLP) analysis of 1.4-kb 16S ribosomal DNA (rDNA). Oligonucleotide primers complementary to 16S rRNA genes were used to amplify 16S ribosomal gene fragments from genomic DNA by polymerase chain reaction. The 1.4-kb 16S rDNA fragment was digested with ScaI, NspI, DdeI, and AvaII restriction endonucleases. Restriction fragments produced by all four restriction endonucleases were characteristic for each species. RFLP analysis of 16S rDNA from 24 "S. uberis" isolates obtained from mammary secretions of dairy cows indicated that all 24 isolates were indeed S. uberis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bramley A. J., Dodd F. H. Reviews of the progress of dairy science: mastitis control--progress and prospects. J Dairy Res. 1984 Aug;51(3):481–512. doi: 10.1017/s0022029900023797. [DOI] [PubMed] [Google Scholar]
- Böddinghaus B., Rogall T., Flohr T., Blöcker H., Böttger E. C. Detection and identification of mycobacteria by amplification of rRNA. J Clin Microbiol. 1990 Aug;28(8):1751–1759. doi: 10.1128/jcm.28.8.1751-1759.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Neimark H., Rumore P., Steinman C. R. Broad range DNA probes for detecting and amplifying eubacterial nucleic acids. FEMS Microbiol Lett. 1989 Jan 1;48(1):19–24. doi: 10.1016/0378-1097(89)90139-0. [DOI] [PubMed] [Google Scholar]
- Eberhart R. J., Buckalew J. M. Intramammary infections in a dairy herd with a low incidence of Streptococcus agalactiae and Staphylococcus aureus infections. J Am Vet Med Assoc. 1977 Oct 1;171(7):630–634. [PubMed] [Google Scholar]
- Hill A. W., Leigh J. A. DNA fingerprinting of Streptococcus uberis: a useful tool for epidemiology of bovine mastitis. Epidemiol Infect. 1989 Aug;103(1):165–171. doi: 10.1017/s0950268800030466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J. S. Streptococcus uberis: a review of its role as a causative organism of bovine mastitis. I. Characteristics of the organism. Br Vet J. 1981 Jan;137(1):36–52. doi: 10.1016/s0007-1935(17)31786-4. [DOI] [PubMed] [Google Scholar]
- King J. S. Streptococcus uberis: a review of its role as a causative organism of bovine mastitis. II. Control of infection. Br Vet J. 1981 Mar-Apr;137(2):160–165. doi: 10.1016/s0007-1935(17)31733-5. [DOI] [PubMed] [Google Scholar]
- Medlin L., Elwood H. J., Stickel S., Sogin M. L. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene. 1988 Nov 30;71(2):491–499. doi: 10.1016/0378-1119(88)90066-2. [DOI] [PubMed] [Google Scholar]
- Oliver S. P. Frequency of isolation of environmental mastitis-causing pathogens and incidence of new intramammary infection during the nonlactating period. Am J Vet Res. 1988 Nov;49(11):1789–1793. [PubMed] [Google Scholar]
- Oliver S. P., Mitchell B. A. Prevalence of mastitis pathogens in herds participating in a mastitis control program. J Dairy Sci. 1984 Oct;67(10):2436–2440. doi: 10.3168/jds.S0022-0302(84)81592-1. [DOI] [PubMed] [Google Scholar]
- Oliver S. P., Sordillo L. M. Udder health in the periparturient period. J Dairy Sci. 1988 Sep;71(9):2584–2606. doi: 10.3168/jds.S0022-0302(88)79847-1. [DOI] [PubMed] [Google Scholar]
- Pustell J., Kafatos F. C. A high speed, high capacity homology matrix: zooming through SV40 and polyoma. Nucleic Acids Res. 1982 Aug 11;10(15):4765–4782. doi: 10.1093/nar/10.15.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relman D. A., Loutit J. S., Schmidt T. M., Falkow S., Tompkins L. S. The agent of bacillary angiomatosis. An approach to the identification of uncultured pathogens. N Engl J Med. 1990 Dec 6;323(23):1573–1580. doi: 10.1056/NEJM199012063232301. [DOI] [PubMed] [Google Scholar]
- Smith K. L., Todhunter D. A., Schoenberger P. S. Environmental pathogens and intramammary infection during the dry period. J Dairy Sci. 1985 Feb;68(2):402–417. doi: 10.3168/jds.s0022-0302(85)80838-9. [DOI] [PubMed] [Google Scholar]
- Sogin M. L., Gunderson J. H., Elwood H. J., Alonso R. A., Peattie D. A. Phylogenetic meaning of the kingdom concept: an unusual ribosomal RNA from Giardia lamblia. Science. 1989 Jan 6;243(4887):75–77. doi: 10.1126/science.2911720. [DOI] [PubMed] [Google Scholar]
- Williams A. M., Collins M. D. Molecular taxonomic studies on Streptococcus uberis types I and II. Description of Streptococcus parauberis sp. nov. J Appl Bacteriol. 1990 May;68(5):485–490. doi: 10.1111/j.1365-2672.1990.tb02900.x. [DOI] [PubMed] [Google Scholar]
- Wilson K. H., Blitchington R. B., Greene R. C. Amplification of bacterial 16S ribosomal DNA with polymerase chain reaction. J Clin Microbiol. 1990 Sep;28(9):1942–1946. doi: 10.1128/jcm.28.9.1942-1946.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]




