Abstract
IκBα is an inhibitor of the transcriptional factor NF-κB, and it is an essential component of the signaling pathways that lead to expression of inflammatory molecules. These include cytokines and costimulatory molecules associated with antigen presentation in an inflammatory immune response. In this study, we report that antigen-presenting cells exposed to TGF-β induce peripheral tolerance by increasing IκBα expression. Exposure of antigen presenting cells (APCs) to TGF-β is known to impair their ability to secrete IL-12, and such impairment correlated with reduced NF-κB activity as indicated by significantly reduced nuclear levels of p50, an essential subunit of NF-κB for IL-12 transcription. Blockade of increased nuclear IκBα in APCs by expression of small interfering RNA molecules (siRNAs) targeting IκBα transcripts prevented IL-12 impairment and the decline in nuclear p50 levels. Furthermore, such IκBα blockade also interfered with the tolerogenic property of TGF-β- exposed APCs. However, increased expression of IκBα in APCs, independent of TGF-β exposure, reduced nuclear p50 levels and permitted tolerance induction by APCs. Thus, our findings attribute a direct and significant role to IκBα in the tolerogenic potential of APCs. Increased IκBα expression in APCs may therefore offer a therapeutic approach to achieve antigen-specific immunomodulation.—Ghafoori, P., Yoshimura, T., Turpie, B., Masli, S. Increased IκBα expression is essential for the tolerogenic property of TGF-β-exposed APCs.
Keywords: monocytes, macrophages, antigen presentation, antigen processing, tolerance
The nf-κb family of transcription factors is known to regulate inflammatory immune responses by promoting transcription of many proinflammatory molecules. In some models of autoimmune disorders, elevated NF-κB activity in antigen presenting cells (APCs) has been linked to the pathogenesis of the disease (1). Many studies have reported the significance of NF-κB activity in the antigen presentation by APCs in vitro as well as in vivo. In some reports, selective inhibition of NF-κB activity in APCs has impaired their ability to stimulate T cell proliferation and secretion of proinflammatory cytokines (2,3,4). However, whether such APCs with reduced NF-κB activity induce tolerance remains to be examined.
The ability of F4/80+ APCs to induce peripheral immunological tolerance on their exposure to TGF-β is well established (5,6,7,8). Their ability to induce regulatory T cells results in peripheral tolerance. The effector cells activated by TGF-β-treated APCs produce reduced IL-2 and IFN-γ (7, 9). Such tolerogenic APCs can produce their own TGF-β and thereby amplify the effect of the original exposure (10, 11). In response to TGF-β, APCs are known to down-regulate their expression of the accessory molecules CD40 and IL-12 (9, 11). However, such APCs secrete increased levels of TNF-α, which also contributes to the tolerance induction (12). In fact, increased expression of molecules such as thrombospondin and TNF-α by TGF-β-exposed APCs contributes to their impaired ability to secrete IL-12 (13, 14). While transcription of most of these molecules previously mentioned involves NF-κB, it is also known that ligation of receptors like CD40 by its ligand and binding of their respective receptors by IL-12 and TNF-α can result in activation of NF-κB (15,16,17,18). Thus, the net tolerogenic property of TGF-β-exposed APCs appears to be regulated by a very fine balance in activation of various components of the NF-κB family.
Members of the Rel family of proteins (p105/p50, p100/p52, p65, RelB, and cRel) form homo- or heterodimers in the cytoplasm to form the transcriptionally active NF-κB complex. The biological activity of these complexes is regulated by inhibitor IκB proteins. The two isoforms, IκBα and IκBβ, are known to bind predominantly to p50-p65 heterodimers in the cytoplasm to form stable trimers. In response to various external stimuli, IκB proteins are phosphorylated and subsequently degraded, which allows for the release and nuclear translocation of the heterodimers to initiate transcription of various genes regulated by NF-κB. IκBα is functionally distinct in that regulation of NF-κB by this isoform is rapid and transient (19), and in turn, NF-κB activation up-regulates expression of IκBα (20). Newly synthesized IκBα can enter the nucleus and dissociate transcriptionally competent NF-κB bound to DNA, thereby repressing NF-κB function (21). Newly formed NF-κB-IκBα complexes are exported out of the nucleus to reestablish a cytoplasmic pool of inactive NF-κB dimers (22). Both IκBα and NF-κB bound to IκBα are known to shuttle between the nucleus and the cytoplasm (20). Thus, newly synthesized IκBα may effectively terminate transcription of an inflammatory gene.
Previously, we reported that TGF-β exposure of APCs down-regulates expression of the NF-κB gene-precursor protein p105 (source of p50 subunit), which reflects a potential decrease in NF-κB activity (11, 23). Consistent with this finding, in microarray analysis, we detected increased expression of IκBα transcripts in TGF-β-exposed APCs. In this study, we report that this newly synthesized IκBα results in increased nuclear levels of this protein, which correlates with the impaired ability of these APCs to express CD40 and IL-12. Although the IL-12b gene is known to contain binding sites for p50 and cRel, some reports assign a nonredundant and pivotal role to the p50 subunit in the regulation of IL-12 (24). Accordingly, we detected reduced NF-κB activity as well as nuclear p50 levels in the APCs exposed to TGF-β. Blockade of the increase in IκBα with small interfering RNA molecules (siRNAs) in these APCs prevented the decline in nuclear p50 levels as well as IL-12 secretion. Furthermore, these APCs lacking the ability to up-regulate IκBα failed to induce tolerance on their exposure to TGF-β. However, APCs overexpressing IκBα successfully induced tolerance independent of their TGF-β exposure. Thus our results strongly support an important role of IκBα in the tolerance-inducing properties of TGF-β-exposed APCs and further indicate that many gene products expressed in response to TGF-β may contribute to an increased IκBα that leads to the tolerogenic potential of APCs.
MATERIALS AND METHODS
Mice
C3D2F1/J (H-2k/d) (hybrid strain C3H/HeJ x DBA/2J) mice, 6 to 8 wk old, were purchased from Jackson Laboratories (Bar Harbor, ME, USA). All animal experiments were conducted in accordance with institutional guidelines.
Cells
Macrophage hybridoma clone 59 cells were originally produced by fusing splenic adherent cells from CKB mice (H-2k) with the macrophage-like murine mastocytoma cell line P388D1 (H-2d) by Uchida et al. (25) and Kuchroo et al. (26). These cells were characterized extensively for their ability to present antigen under the influence of various cytokines and their ability to induce effectors in vitro as well as in vivo (27, 28). These cells were maintained in complete RPMI 1640 (Lonza, Basel, Switzerland) containing 10 mM HEPES, 0.1 mM NEAA, 1 mM sodium pyruvate, 2 mM l-glutamine, 100 U/ml penicillin, 100 mg/ml streptomycin (Lonza), and 10% fetal calf serum (FCS). For experiments described in this study, these cells were cultured in serum-free RPMI 1640 in which 10% FCS was replaced with ITS+ culture supplement [1 μg/ml iron-free transferrin, 10 ng/ml linoleic acid, 0.3 ng/ml Na2se, and 0.2 μg/ml Fe(NO3)3] (Sigma, St. Louis, MO, USA) and 0.1% BSA (Sigma). The ability of these cells to modulate their function on their exposure to ocular cell-derived factors has been reported previously (12, 27). In this study, these cells were used as antigen-presenting cells. For TGF-β treatment, cells were cultured in serum-free medium containing TGF-β2 (5 ng/ml) (R&D Systems, Minneapolis, MN, USA) for the periods indicated in each experiment. For some experiments, cells were pulsed with soluble antigen ovalbumin (OVA) (7 mg/ml) (Sigma).
Western blotting
Cytoplasmic and nuclear extracts were prepared using a Nuclear Extract Kit (Active Motif, Carlsbad, CA, USA). Protein concentration was determined in a BCA assay (Pierce, Rockford, IL, USA). Equal amounts of protein from various samples were subjected to SDS-PAGE in 4–12% Bis-Tris gradient gel (Invitrogen Inc., Carlsbad, CA, USA) followed by electrophoretic transfer of separated proteins to nitrocellulose membranes (Pierce). Western blot analysis was performed using anti-IκBα, antip21 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-b-actin antibodies (AbCam, Cambridge, MA, USA). Antibodies bound to proteins on the membrane were detected using horseradish peroxidase (HRP)-conjugated secondary antibody (Santa Cruz Biotechnology) and a chemiluminescent substrate (ECL detection reagents, Amersham Pharmacia Biotech, Piscataway, NJ, USA). Membranes were then exposed to BioMax light film (Eastman Kodak Company, Rochester, NY, USA) to detect the chemiluminescent signal. The band intensity was analyzed using the NIH Image software program (U.S. National Institutes of Health, Bethesda, MD, USA).
Electrophoretic mobility shift assay (EMSA)
EMSA was performed on nuclear extracts from untreated or TGF-β-treated APCs to compare the levels of NF-κB activation. DNA binding of NF-κB was assessed using the consensus oligonucleotide of NF-κB (5′-AGT TGA GGG GAC TTT CCC AGG C-3′ and 3′-TCA ACT CCC CTG AAA GGG TCC G-5′) (Promega, Madison, WI, USA). The oligonucleotides were used as probes and were radiolabeled with [γ-32P] ATP (Perkin-Elmer, Waltham, MA, USA) using T4 polynucleotide kinase (Promega). The labeled probe was purified using the microspin G-50 columns (Amersham). For the binding reactions, 20 μg of nuclear proteins was incubated in binding buffer (10 mM HEPES, 250 mM KCl, 3 mM DTT, and 2.5% glycerol) with 0.15U poly(dI-dC) (Sigma). After 10 min, the labeled oligonucleotide was added and incubated for another 45 min at room temperature (RT) in a final volume of 30 μl. DNA-protein complexes were then loaded onto a 4% nondenaturing polyacrylamide gel in Tris-glycine buffer (Invitrogen). Dried gels were exposed overnight at −80°C to X-Omat autoradiograph film (Eastman Kodak) for detection.
NF-κB binding activity assay
Activation of NF-κB p50 was determined using TransAM assay (Active Motif) according to the manufacturer’s instructions. Briefly, nuclear extracts were suspended in TransAm lysis buffer (Active Motif); nuclear proteins (5 μg total proteins) were incubated in 96-well plates with immobilized oligonucleotides containing the NF- κB consensus DNA binding site (5′-GGG ACT TTC C-3′) for 1 h at room temperature. Wells were then washed 3 times. To each well 100 μl of p50 subunit monoclonal antibody (1:1000) was added, followed by incubation for 1 h at room temperature. Wells were washed 3 times. Then 100 μl of HRP-conjugated secondary antibody (1:1000) was added to each well for 1 h at room temperature. Chemiluminscence of the HRP substrate was determined using a Bio-Plex system (Bio-Rad, Hercules, CA, USA). Activity of p50 containing dimers was reported as luminescence units. Triplicate samples for each nuclear extract were analyzed. Specificity of the assay was monitored using mutated consensus oligonucleotide provided in the kit as per the manufacturer’s instructions.
IκBα inhibition with siRNAs
Using the IκBα gene sequence siRNA oligos were designed with the software program offered by OligoEngine, Inc. (Seattle, WA, USA; http://www.oligoengine.com) and synthesized from Sigma Genosys. Annealed oligos were cloned using a retroviral vector pSUPER.retro.puro (OligoEngine) according to the manufacturer’s instructions. For each oligo, 2–3 positive clones were sequenced to ensure absence of any mutations, followed by large-scale plasmid preparation using Qiagen Endofree Plasmid Mega kit (Qiagen, Duesseldorf, Germany). Retroviral vectors were generated by transfecting plasmids in 293T cells using Lipofectamine and Plus reagent (Invitrogen) according to the manufacturer’s instructions (OligoEngine). Macrophage hybridoma cells were infected with siRNA-expressing retroviral vectors and cultured in puromycin (2.5 μg/ml) containing complete medium. To confirm siRNA-dependent inhibition of IκBα, Western blots were performed on nuclear extracts derived from the infected cells.
Immunofluorescent staining of nuclear IκBα
Cells expressing empty vector or siRNA were cultured with or without TGF-β as described earlier in serum-free medium in chamber slide wells (3×105/well) (Fisher Scientific, Pittsburgh, PA, USA). After 18 h of culture, cells were washed with PBS and fixed with 4% paraformaldehyde. The slide with fixed cells was then covered with cold methanol for 10 min (−20°C), followed by washes with PBS. Next, the slide was covered with 0.1% Triton-X-100, 0.1% sodium citrate (Sigma) for 3 min at 4°C. After washes with PBS, cells were incubated with anti-IκBα or isotype control antibody (Santa Cruz Biotechnology) for 1 h at RT, followed by Alexa Fluor conjugated secondary antibody (Invitrogen) for 1 h at RT. Cells were then washed thoroughly with PBS before being placed in the mounting medium (Vectashield-DAPI; Vector Labs, Burlingame, CA, USA) and coverslip. Stained cells were analyzed using a laser scanning cytometer (CompuCyte, Westwood, MA, USA) and WinCyte 3.6 software (CompuCyte).
Inhibition of IL-12 secretion
Culture supernatants were collected from antigen OVA-pulsed APCs (1×105) cocultured with T cells (2×105) harvested from OVA-immunized C3D2F1 mice or DO11.10 mice (T cells with OVA-specific transgenic TCR) in serum-free medium for 48 h. Cytokine IL-12 content of this supernatant was determined in an ELISA using a kit according to manufacturer’s instructions (R&D Systems Inc.). Inhibition of IL-12 secretion was calculated as (IL-12 secreted by TGF-β-treated APCs/IL-12 secreted by untreated APCs) × 100. Representative data of 2–3 independent experiments are presented.
IκBα overexpression
Inducible IκBα degradation is mediated by phosphorylation of serine residues 32 and 36, located in the N-terminal domain of the protein, and mutation of these two amino acids has been proven to inhibit IκBα degradation and prevent NF-κB activation (29,30,31,32,33,34). A mutant form of IκBα in which residues 32 and 36 are replaced with alanine (S32A/S36A) has been shown to act as a super-repressor of NF-κB activity (35). An adenoviral vector was constructed bearing this mutant form of IκBα, and its infectibility and antiinflammatory properties were proven (36). Macrophage hybridoma cells were infected with adenoviral vectors containing mutant IκBα or the GFP gene (generous gifts from Dr. Robert Scheinman, University of Colorado Cancer Center, Aurora, CO, USA). Cells infected with adenoviral-GFP were analyzed by flow cytometry to determine the appropriate MOI. At an MOI of 50, over 90% of cells were found to express GFP.
In vivo assay to determine tolerogenic function of APCs
Untreated or TGF-β-treated APCs pulsed with OVA (2–5×10 (3) cells/mouse) were infused intravenously in naive C3D2F1 mice (n=5 per group). Seven days later, mice were immunized subcutaneously into the nape of the neck with OVA/CFA (50 μg). A week later, these mice and a group of mice that did not receive any cells or immunization (negative control group) were inoculated intradermally with OVA (200 μg/20 μl) into the right ear pinna. The left ear served as an untreated control. The thickness of both ears was measured immediately before and at 24 h after the OVA injection using a micrometer (Mitutoyo 227-101; MTI Corp., Paramus, NJ, USA). The measurements were performed in triplicates on each mouse. Delayed-type hypersensitivity (DTH) was measured as change in ear swelling [(24 h − 0 h measurement in treated ear) − (24 h − 0 h measurement in control ear)]. Tolerance induction was detected as suppression of DTH in groups infused with TGF-β-treated APCs as compared to those seen in recipients of untreated APCs. A two-tailed Student’s t test was used, with significance assumed at P ≤ 0.05. Results of DTH assays presented in this report are representative of 2 to 3 experiments.
RESULTS
TGF-β exposure of APCs increases nuclear levels of IκBα
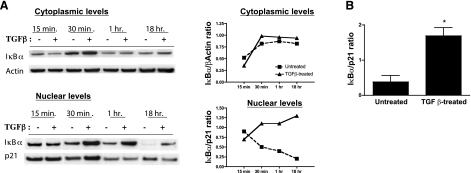
To test whether increased IκBα transcript levels detected in TGF-β-exposed APCs as compared to the untreated APCs correspond with a matching increase in protein, we determined IκBα protein levels in these APCs. Exposure of APCs to TGF-β for a period of 18–21 h in vitro has been demonstrated to render them tolerogenic. We determined IκBα protein levels at various time points during this TGF-β exposure period. Untreated and TGF-β-treated APCs were harvested at 15 min, 30 min, 1 h, and 18 h after the initial culture with or without TGF-β. Both cytoplasmic and nuclear extracts were prepared and subjected to Western blot analysis using anti-IκBα antibodies to detect the protein. As shown in Fig. 1A cytoplasmic IκBα levels were normalized against cytoplasmic levels of β-actin, whereas nuclear levels of IκBα were normalized against nuclear levels of p21. While cytoplasmic levels of IκBα did not change significantly at various time intervals, we detected a steady increase in nuclear IκBα levels beginning as early as 30 min after TGF-β exposure. At the 18-h interval, when APCs are typically known to have acquired the tolerogenic property, nuclear IκBα levels were significantly increased as compared to those in untreated APCs (Fig. 1B). These results clearly demonstrate that the increased IκBα message levels detected in microarray analysis of TGF-β-treated APCs vs. untreated APCs corresponds to an increase in IκBα protein synthesis. Similar increase in nuclear IκBα levels were also detected in TGF-β-exposed macrophages derived from thioglycollate-elicited peritoneal exudates (data not shown). Thus, our results indicate that increased IκBα levels in TGF-β-exposed APCs are detected in the nucleus, suggesting that nuclear translocation of the newly synthesized IκBα in these APCs takes place.
Figure 1.
TGF-β-exposed APCs increase their nuclear IκBα expression. A) Cytoplasmic and nuclear extracts of APCs cultured with or without TGF[b]-β (5 ng/ml) for indicated times were analyzed by Western blot analysis. Densitometric analysis was performed to determine ratio of IκBα with β-actin for cytoplasmic levels and with p21 for nuclear levels. B) Mean ± se ratio of nuclear IκBα levels at 18 h post-TGF-β exposure from 3 independent experiments. *P < 0.05.
TGF-β exposure of APCs results in decreased NF-κB activity
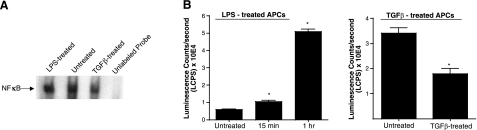
Activity of the nuclear transcription factor NF-κB is known to be regulated by inhibitor IκB proteins. It is therefore possible that an increase in the levels of such inhibitor proteins reflects a reduction in NF-κB activity. It was reported previously that TGF-β exposure of APCs for a period of 18–21 h results in reduced expression of accessory molecules such as CD40 and IL-12, which coincides with the tolerance-inducing property of such APCs (9). As it is known that NF-κB regulates transcription of both CD40 and IL-12, we speculated that at 18 h after TGF-β exposure, NF-κB activity in APCs is likely to be reduced. To investigate this possibility, we tested nuclear extracts isolated from untreated and TGF-β-treated APCs for NF-κB activity in an EMSA. As seen in Fig. 2A, NF-κB activity detected in the nuclear extracts derived from TGF-β-exposed APCs appeared relatively reduced as compared to the untreated control APCs. Although the NF-κB family is represented by several subunits that can form various homo- or heterodimers, it has been demonstrated by other investigators that the p50 subunit is pivotal for the transcription of IL-12 (24). This reduction in NF-κB activity is consistent with our previous reports of decreased IL-12 production in TGF-β-exposed APCs, and accordingly we anticipated a decline in nuclear p50 levels in TGF-β-exposed APCs. Independently, other investigators have reported that following increased nuclear IκBα levels, a dramatic reduction in the nuclear p50 levels occurs, indicating clearance of NF-κB:IκBα complexes from the nucleus (21). To test such a possibility in TGF-β-treated APCs, we used an ELISA-based assay with a chemiluminescent substrate as described in Materials and Methods. In this assay, nuclear extracts from untreated and TGF-β-treated APCs were compared for their levels of p50 activity. As shown in Fig. 2B, significantly decreased levels of p50 were detected in nuclear extracts derived from TGF-β-treated APCs as compared to the untreated control APCs. These results suggest that TGF-β exposure of APCs results in reduced NF-κB activity in a way that lowers IL-12 transcription in these APCs. Furthermore, these data also correlate with the increased nuclear IκBα levels in TGF-β-exposed APCs. Together, these results are consistent with the tolerogenic property of these APCs.
Figure 2.
Decreased NF-κB activity in TGF-β-exposed APCs. A) Nuclear extracts prepared from APCs untreated or treated with LPS (1 μg/ml) or TGF-β (5 ng/ml) 18 h were subjected to EMSA as described in Materials and Methods. B) NFκBp50 activity was measured in nuclear extracts prepared at different time intervals after control LPS (15 min and 1 h) or TGF-β (18 h) treatment of APCs using an ELISA-based assay (see Materials and Methods) to determine p50 levels. Data represent mean ± se luminescence count per second detected in 4 wells. *P < 0.05.
Increase in nuclear IκBα in TGF-β-exposed APCs is blocked by siRNAs
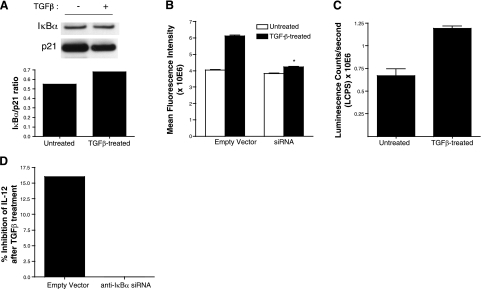
We next examined whether expression of siRNAs that target the IκBα message could block the increase in the nuclear levels of IκBα. To test this possibility, we selected several siRNA oligonucleotides and expressed these using a retroviral vector in our APCs. We achieved varying levels of knockdown of both nuclear and cytoplasmic levels of IκBα and out of four oligonucleotide sets selected one (oligonucleotide 4) that resulted in most reduction in the nuclear IκBα protein levels as determined by Western blot analysis (>80% reduction, data not shown). These APCs expressing anti-IκBα siRNAs (oligonucleotide 4) were exposed to TGF-β, and nuclear extracts were prepared from these and untreated control APCs that expressed an empty vector. An equal amount of nuclear protein was subjected to Western blot analysis to determine nuclear levels of IκBα. As shown in Fig. 3A, after exposure of siRNA-expressing APCs to TGF-β, the nuclear levels of IκBα remained unaltered. We also performed immunofluorescent staining of untreated or TGF-β-exposed siRNA-expressing APCs and control APCs expressing an empty vector. Nuclear staining of IκBα was then analyzed by laser scanning cytometry (LSC), and mean fluorescence intensity (MFI) was determined. Significantly reduced MFI in siRNA-expressing APCs exposed to TGF-β as compared to similarly treated control APCs (Fig. 3C) reflected decline in nuclear IκBα levels in the presence of siRNA. These results indicated successful blockade of the increase in nuclear IκBα in TGF-β-exposed APCs using a siRNA strategy. Since our results indicated a reverse correlation between nuclear IκBα and levels of p50 (a subunit important in IL-12 transcription), we tested nuclear p50 levels in the siRNA-expressing APCs on their exposure to TGF-β. As expected, and consistent with the knockdown of IκBα expression, we detected significantly increased levels of nuclear p50 in TGF-β-exposed APCs that also expressed the siRNAs (Fig. 3B). To determine further whether the reversal of nuclear p50 levels associated with the knockdown of IκBα also reflects a reversal of IL-12 inhibition that is normally detectable in TGF-β-exposed APCs, we collected culture supernatants collected from either control or siRNA-expressing APCs that were cultured with or without TGF-β. These cells were stimulated using anti-CD40 antibodies as described in Materials and Methods. As reported previously, such stimulation of APCs that do not express any vector and that have been exposed to TGF-β results in significant inhibition of IL-12 secretion (untreated vs. TGF-β-treated, 53±6.7 vs. 28±0.5 pg/ml, P<0.05, 47% inhibition). Expression of empty vector in these APCs although reduced levels of IL-12 secretion did not alter the inhibitory effect of TGF-β (untreated vs. TGF-β-treated, 19+0.4 vs. 16+0.4 pg/ml, P<0.05, 16% inhibition). However, expression of siRNA in APCs left IL-12 secretion unaltered after their TGF-β exposure (untreated vs. TGF-β-treated, 26±4.2 vs. 27±2.7 pg/ml, no inhibition). The significant increase in the IL-12 secreted by untreated APCs expressing siRNA compared to those expressing empty vector is consistent with the changes in the constitutive levels of IκBα expressed in these APCs. As shown in Fig. 3D, the inhibitory effect of TGF-β on IL-12 expression was lost in APCs that expressed anti-IκBα siRNAs. Together, these results indicate that increased nuclear IκBα in TGF-β-exposed APCs regulates IL-12 transcription by inhibiting NF-κB activity through modulation of nuclear p50 levels.
Figure 3.
Expression of siRNAs blocks the nuclear increase in IκBα in TGF-β-exposed APCs. A) Nuclear extracts derived from siRNA-expressing APCs treated with or without TGF-β were analyzed to determine levels of IκBα by Western blot. B) Densitometric analysis was performed to determine ratio of IκBα and p21 and to determine NF-κB activity by measuring p50 levels in an ELISA-based TransAM assay, as described in Materials and Methods. Data represent mean ± se luminescence count per second detected in 4 wells. *P < 0.05. C) Immunofluorescent staining of nuclear IκBα in cells expressing empty vector (control) or siRNA was performed as described in Materials and Methods, and mean fluorescence intensity of stained cells was determined using laser scanning cytometry. Data are means + se. *P < 0.05 vs. corresponding control. D) Culture supernatants collected from untreated or TGF-β-treated APCs stimulated with anti-CD40 ligation as described in Materials and Methods were assayed for IL-12 content by ELISA. Percentage inhibition of IL-12 after TGF-β exposure of APCs expressing empty retroviral vector or siRNAs targeting IκBα is presented.
Blockade of increased IκBα prevents tolerogenic ability of TGF-β-exposed APCs
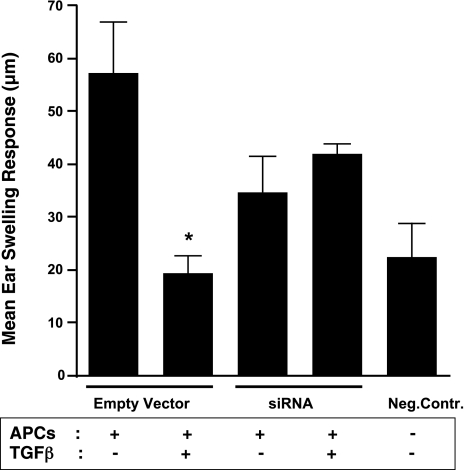
The role of IL-12 in the ability of APCs to induce an inflammatory immune response is well known. Absence of IL-12 production by APCs has been linked to their tolerogenic potential (37,38,39). Therefore, the tolerogenic ability of TGF-β-exposed APCs and their impaired ability to secrete IL-12 are findings, which are consistent with these reports. After achieving successful reversal of IL-12 inhibition in TGF-β-treated APCs by effective blockade of increased nuclear IκBα levels, we examined whether such APCs retained their ability to induce tolerance. To test this possibility we performed an in vivo assay in which tolerance induction results in suppression of a typical Th1-mediated inflammatory response, such as the DTH response. As described in Materials and Methods, APCs with siRNAs or empty vector were pulsed with the soluble antigen OVA in the presence or absence of TGF-β. After overnight culture, cells were harvested, washed, and injected intravenously in groups of mice (n=5/group). A group of negative control mice did not receive any APCs. Seven days after APC infusion, animals in all the experimental groups, except the negative control, were immunized with OVA in complete Freund’s adjuvant (OVA/CFA). A week later, all the animals received intradermal injections of soluble OVA in the ear pinna as an ear challenge. Ear thickness of all the animals was determined 24 h after the ear challenge. As shown in Fig. 4, a significantly reduced ear swelling response was detected in the group of animals that received TGF-β-treated control APCs expressing empty vectors as compared to those animals that were infused with untreated control APCs. The reduced ear swelling response in this assay represents the suppressed DTH response and therefore the ability of TGF-β-exposed APCs to induce tolerance. However, such a reduction in ear swelling response was not detected if the infused APCs expressed siRNAs and were exposed to TGF-β. The failure of these APCs to suppress the DTH response corresponds with their inability to induce tolerance. Thus TGF-β-exposed APCs expressing anti-IκBα siRNAs appear to lose their tolerogenic property. These results suggest that increased nuclear IκBα levels in TGF-β-exposed APCs contribute significantly to their tolerogenic ability and blockade of such an increase results in a reversal of this functional phenotype.
Figure 4.
siRNA-mediated knockdown of IκBα prevents tolerance induction by TGF-β-exposed APCs. Tolerance-inducing ability of APCs was evaluated in a DTH suppression assay. Briefly, OVA-pulsed untreated or TGF-β-treated control (empty vector) or siRNA-expressing APCs were infused i.v. in groups of recipients (n=5/group). Negative control group did not receive any APCs. Seven days later, mice in all the groups, except the negative control, were immunized with OVA/CFA; 1 wk later, all mice received ear challenge (intradermal) of OVA. Ear swelling response representing DTH response was measured at 24 h after ear challenge (means±se). Significantly suppressed DTH response in recipients of TGF-β-treated control APCs as compared to those infused with untreated control APCs indicated tolerance induction. *P < 0.05.
Overexpression of IκBα confers tolerance-inducing property on APCs
Exposure of APCs to TGF-β has previously been demonstrated to increase expression of molecules such as thrombspondin and TNF-α (11, 12). While TNF-α is well known as an NF-κB regulated gene, the presence of binding sites for this transcription factor in the promoter region of THBS1 gene makes thrombospondin also a likely candidate gene regulated by NF-κB (40, 41). Therefore, increased expression of such molecules is in contrast with the detected reduced NF-κB activity. Thus, it is quite likely that increased IκBα is a downstream event resulting from the initial NF-κB activity. As the increase in IκBα expression appears to be one of the central events in these TGF-β-exposed APCs, we sought to determine whether this event is sufficient to allow for the expression of tolerogenic property of APCs, we elected to induce IκBα overexpression in APCs independent of their TGF-β exposure. We utilized an adenoviral vector expressing the mutant form of IκBα to infect APCs, and as a control gene we used an adenoviral vector expressing GFP. Nuclear extracts derived from these cells were tested for their levels of p50 subunit. As shown in Fig. 5A, significantly increased levels of p50 were detectable in nuclear extracts prepared from IκBα expressing APCs as compared to their control counterparts. This result is consistent with our earlier observations. We then examined the tolerogenic capacity of these APCs in an in vivo assay, as described earlier in this report. Briefly, OVA-pulsed APCs were infused in two groups of mice intravenously while negative control group of mice did not receive any cells. All the mice, except those in negative control group, were immunized with OVA/CFA 7 d after APC infusion followed by ear challenge 1 wk later. Ear swelling response measured at 24 h after ear challenge is presented in Fig. 5B. A significantly reduced ear swelling response was detected in mice that received IκBα-expressing APCs as compared to mice that received control APCs. These results indicate that APCs that express increased IκBα can suppress a DTH response and therefore induce tolerance. Thus, the tolerogenic property of APCs appears to be linked to increased IκBα expression, which is likely to represent a point of convergence of various signaling pathways activated either directly by TGF-β or by the molecules expressed in response to TGF-β exposure of APCs.
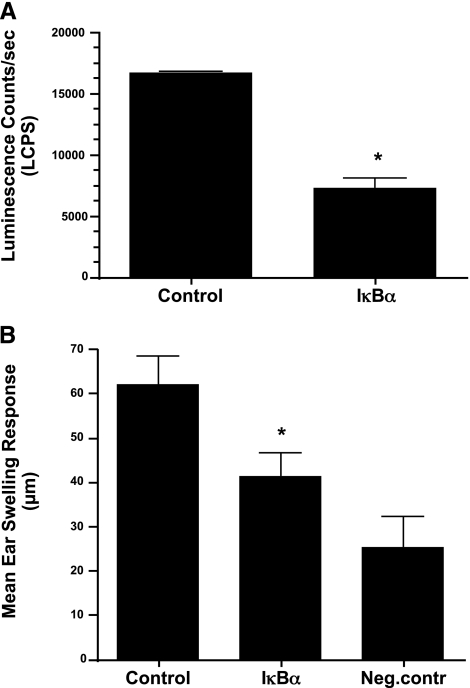
Figure 5.
Increased expression of IκBα in APCs allows tolerance induction. A) Nuclear extracts derived from APCs infected with adenoviral vector containing mutant IκBα or control gene (GFP) were analyzed for NF-κB activity by measuring p50 levels using TransAM assay, as described in Materials and Methods. Results represent mean ± se luminescence per second. B) Antigen-presenting cells infected with control or IκBα containing adenoviral vector were pulsed with OVA and tested for ability to induce tolerance in a DTH suppression assay, as described earlier. Results represent mean ± se ear swelling response. *P < 0.05.
DISCUSSION
Several reports have demonstrated the significance of nuclear transcription factor NF-κB in inflammation as well as in antigen presentation by APCs (4, 42). Many signaling pathways initiated by ligation of receptors on the cell surface are known to result in degradation of IκB family members in the cytoplasm permitting nuclear translocation of NF-κB dimers and subsequent transcription of genes, which include molecules involved in inflammatory responses (16, 18, 43). Regulation of this nuclear factor is considered a valuable therapeutic strategy that will help limit inflammatory damage. In this study, we addressed a role of IκBα, an inhibitor of NF-κB, in the tolerance-inducing ability of APCs. Our findings support a critical role of IκBα in tolerance induction, and this has implications in developing antiinflammatory therapeutic strategies.
Consistent with the reports that newly synthesized IκBα accumulates in the nucleus, we noted that TGF-β exposure of APCs results in increased nuclear levels of IκBα (21). Typically members of the inhibitor IκB family are known to inhibit NF-κB activity by retaining NF-κB dimers in the cytoplasm, and this is accomplished by masking the nuclear localization signals (NLS) on each subunit of the dimers (44). However, IκBα is known to accomplish inhibition of NF-κB activity by a distinct mechanism. It is reported that unlike IκBβ, IκBα fails to mask one of the two NLS sites, allowing shuttling of NF-κB-IκBα complexes between the cytoplasm and the nucleus (44). Also, the presence of a nuclear export signal (NES) on IκBα allows export of this molecule out of the nucleus. Thus, IκBα could dissociate DNA bound NF-κB and export it out of the nucleus, thereby returning it to the cytoplasmic pool (20, 44). Therefore, IκBα has the unique ability to inhibit transcriptionally competent NF-κB bound to DNA. Based on this unique ability of IκBα and previous reports of impaired expression of CD40 and IL-12 in TGF-β-exposed APCs, we speculated that increased nuclear IκBα in these APCs is likely involved in interrupting NF-κB-dependent transcription of these genes.
The significance of IL-12 in antigen presentation is well established. Absence of IL-12 secretion by APCs is often correlated with their ability to induce tolerance (38, 39, 45, 46). Therefore, we explored whether increased IκBα in TGF-β-exposed APCs targeted IL-12 transcription to contribute to their tolerogenic property. Other investigators have demonstrated a nonredundant and pivotal role of the p50 subunit of NF-κB in IL-12 transcription (24). These reports led us to examine nuclear p50 levels in TGF-β-exposed APCs; consistent with their reduced ability to secrete IL-12, we detected lowered nuclear p50 levels in these cells as compared to controls. These results correlate with the ability of IκBα to dissociate DNA-bound, transcriptionally competent NF-κB. The reduced p50 levels are also consistent with our earlier observation of differentially expressed genes in TGF-β-exposed APCs in that the expression of p105, a precursor of p50, is down-regulated in TGF-β-treated APCs (11). Although the signals that lead to reduced p105 expression are not clearly known yet, together our results indicate that TGF-β-exposure of APCs results in a significant decline in the levels of the p50 subunit of NF-κB that is important for the transcription of IL-12 and correlate with the impaired expression of IL-12 in these APCs.
In response to TGF-β exposure, APCs are known to alter their expression of many genes, including TNF-α, transcription of which involves NF-κB activity (47). Enhanced expression of such a NF-κB-dependent gene juxtaposed with the down-regulation of others such as IL-12 and CD40 within the same cell, although appearing contradictory, is plausible considering the postinduction repression of NF-κB activity mediated by IκBα. It is known that due to the presence of NF-κB consensus binding sites within the IκBα promoter, NF-κB activity up-regulates IκBα (20). Such autoregulatory induction by NF-κB is not known to occur for IκBβ, therefore giving IκBα a unique functional role in NF-κB regulation. We speculate that increased IκBα detected in TGF-β-exposed APCs is likely to be a downstream event resulting from the initial increase in NF-κB activity that is involved in the expression of genes like TNF-α. The significance of this IκBα in TGF-β-treated APCs is made clear by the results of our experiments in which IκBα expression was knocked down by the expression of siRNAs. Our experimental results clearly show that blockade of the increase in IκBα expression leads to not only the loss of IL-12 impairment but also the tolerance induction by APCs. We have reported increased expression of various molecules in response to TGF-β exposure, such as TNF-α, thrombospondin, and IFN-β (11, 13, 14), all of which can directly inhibit IL-12 secretion by APCs. Therefore, our findings in this study suggest that numerous changes induced in APCs in response to TGF-β culminate in a nuclear event that regulates the key molecules associated with tolerance induction.
Other investigators have demonstrated that in APCs like dendritic cells, inhibition of NF-κB, using either a proteosome inhibitor or adenoviral gene transfer of IκBα, results in inhibition of antigen presentation in vitro as well as in vivo (2,3,4). Induction of immunological tolerance by such dendritic cells was demonstrated in vitro, as T cells stimulated by these APCs failed to respond on restimulation by normal APCs. Our findings are consistent with this report in that we demonstrate induction of immunological tolerance by APCs with enhanced expression of IκBα. In our in vivo experiments, recipients of intravenous APC infusion are immunized, and yet the effectors generated by this immunization fail to mount a DTH response. Such a suppressed DTH response cannot be expected with a mere lack of antigen presentation but highlights the development of an active mechanism that prevents inflammatory effectors from mounting a DTH response. In the case of TGF-β-exposed APCs, it has been demonstrated that regulatory T cells induced by these APCs mediate such DTH suppression (7). It remains to be explored further whether such regulatory T cells are generated in response to APCs that overexpress IκBα independent of TGF-β exposure and whether their phenotype resembles that induced by TGF-β-exposed APCs.
Our findings in this study support a significant role played by IκBα in tolerance induction by APCs. Increased nuclear levels of IκBα are essential for the ability of TGF-β-exposed APCs to induce tolerance. Furthermore, it is noteworthy that APCs capable of enhanced IκBα expression independent of their TGF-β exposure also develop tolerogenic potential. Such an approach is likely to be useful in developing antiinflammatory therapies with a much sought-after option of antigen specificity in regulating immune responses.
Acknowledgments
This research was supported by U.S. National Institutes of Health grant EY015472.
References
- Poligone B, Weaver D J, Jr, Sen P, Baldwin A S, Jr, Tisch R. Elevated NF-kappaB activation in nonobese diabetic mouse dendritic cells results in enhanced APC function. J Immunol. 2002;168:188–196. doi: 10.4049/jimmunol.168.1.188. [DOI] [PubMed] [Google Scholar]
- Zhou L F, Zhang M S, Yin K S, Ji Y, Xie W P, Cui X F, Ji X H. Effects of adenoviral gene transfer of mutated IkappaBalpha, a novel inhibitor of NF-kappaB, on human monocyte-derived dendritic cells. Acta Pharmacol Sin. 2006;27:609–616. doi: 10.1111/j.1745-7254.2006.00310.x. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Bondeson J, Brennan F M, Foxwell B M, Feldmann M. Antigen presentation by murine dendritic cells is nuclear factor-kappa B dependent both in vitro and in vivo. Scand J Immunol. 2003;58:165–172. doi: 10.1046/j.1365-3083.2003.01246.x. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Bondeson J, Foxwell B M, Brennan F M, Feldmann M. Effective antigen presentation by dendritic cells is NF-kappaB dependent: coordinate regulation of MHC, co-stimulatory molecules and cytokines. Int Immunol. 2001;13:675–683. doi: 10.1093/intimm/13.5.675. [DOI] [PubMed] [Google Scholar]
- Hara Y, Okamoto S, Rouse B, Streilein J W. Evidence that peritoneal exudate cells cultured with eye-derived fluids are the proximate antigen-presenting cells in immune deviation of the ocular type. J Immunol. 1993;151:5162–5171. [PubMed] [Google Scholar]
- Wilbanks G A, Streilein J W. Fluids from immune privileged sites endow macrophages with the capacity to induce antigen-specific immune deviation via a mechanism involving transforming growth factor-beta. Eur J Immunol. 1992;22:1031–1036. doi: 10.1002/eji.1830220423. [DOI] [PubMed] [Google Scholar]
- Kezuka T, Streilein J W. Analysis of in vivo regulatory properties of T cells activated in vitro by TGFbeta2-treated antigen presenting cells. Invest Ophthalmol Vis Sci. 2000;41:1410–1421. [PubMed] [Google Scholar]
- Zhang-Hoover J, Stein-Streilein J. Tolerogenic APC generate CD8+ T regulatory cells that modulate pulmonary interstitial fibrosis. J Immunol. 2004;172:178–185. doi: 10.4049/jimmunol.172.1.178. [DOI] [PubMed] [Google Scholar]
- Takeuchi M, Alard P, Streilein J W. TGF-beta promotes immune deviation by altering accessory signals of antigen-presenting cells. J Immunol. 1998;160:1589–1597. [PubMed] [Google Scholar]
- Takeuchi M, Kosiewicz M M, Alard P, Streilein J W. On the mechanisms by which transforming growth factor-beta 2 alters antigen-presenting abilities of macrophages on T cell activation. Eur J Immunol. 1997;27:1648–1656. doi: 10.1002/eji.1830270709. [DOI] [PubMed] [Google Scholar]
- Masli S, Turpie B, Hecker K H, Streilein J W. Expression of thrombospondin in TGFbeta-treated APCs and its relevance to their immune deviation-promoting properties. J Immunol. 2002;168:2264–2273. doi: 10.4049/jimmunol.168.5.2264. [DOI] [PubMed] [Google Scholar]
- Hecker K H, Niizeki H, Streilein J W. Distinct roles for transforming growth factor-beta2 and tumour necrosis factor-alpha in immune deviation elicited by hapten-derivatized antigen-presenting cells. Immunology. 1999;96:372–380. doi: 10.1046/j.1365-2567.1999.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masli S, Turpie B, Streilein J W. Thrombospondin orchestrates the tolerance-promoting properties of TGFbeta-treated antigen-presenting cells. Int Immunol. 2006;18:689–699. doi: 10.1093/intimm/dxl006. [DOI] [PubMed] [Google Scholar]
- Masli S, Turpie B. Anti-inflammatory effects of TNF-alpha are mediated via TNF-R2 (p75) in tolerogenic TGFbeta-treated antigen presenting cells [E-pub ahead of print] Immunology. 2008 doi: 10.1111/j.1365-2567.2008.02933.x. doi: 10.1111/j.1365–2567.2008.2933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto N, Kobayashi N, Azuma S, Yamamoto T, Inoue J. Two differently regulated nuclear factor kappaB activation pathways triggered by the cytoplasmic tail of CD40. Proc Natl Acad Sci U S A. 1999;96:1234–1239. doi: 10.1073/pnas.96.4.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taoufik Y, de Goer de Herve M G, Giron-Michel J, Durali D, Cazes E, Tardieu M, Azzarone B, Delfraissy J F. Human microglial cells express a functional IL-12 receptor and produce IL-12 following IL-12 stimulation. Eur J Immunol. 2001;31:3228–3239. doi: 10.1002/1521-4141(200111)31:11<3228::aid-immu3228>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Na S Y, Kang B Y, Chung S W, Han S J, Ma X, Trinchieri G, Im S Y, Lee J W, Kim T S. Retinoids inhibit interleukin-12 production in macrophages through physical associations of retinoid X receptor and NFkappaB. J Biol Chem. 1999;274:7674–7680. doi: 10.1074/jbc.274.12.7674. [DOI] [PubMed] [Google Scholar]
- Thommesen L, Laegreid A. Distinct differences between TNF receptor 1- and TNF receptor 2-mediated activation of NFkappaB. J Biochem Mol Biol. 2005;38:281–289. doi: 10.5483/bmbrep.2005.38.3.281. [DOI] [PubMed] [Google Scholar]
- Thompson J E, Phillips R J, Erdjument-Bromage H, Tempst P, Ghosh S. I kappa B-beta regulates the persistent response in a biphasic activation of NF-kappa B. Cell. 1995;80:573–582. doi: 10.1016/0092-8674(95)90511-1. [DOI] [PubMed] [Google Scholar]
- Huang T T, Miyamoto S. Postrepression activation of NF-kappaB requires the amino-terminal nuclear export signal specific to IkappaBalpha. Mol Cell Biol. 2001;21:4737–4747. doi: 10.1128/MCB.21.14.4737-4747.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenzana-Seisdedos F, Thompson J, Rodriguez M S, Bachelerie F, Thomas D, Hay R T. Inducible nuclear expression of newly synthesized I kappa B alpha negatively regulates DNA-binding and transcriptional activities of NF-kappa B. Mol Cell Biol. 1995;15:2689–2696. doi: 10.1128/mcb.15.5.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenzana-Seisdedos F, Turpin P, Rodriguez M, Thomas D, Hay R T, Virelizier J L, Dargemont C. Nuclear localization of I kappa B alpha promotes active transport of NF-kappa B from the nucleus to the cytoplasm. J Cell Sci. 1997;110:369–378. doi: 10.1242/jcs.110.3.369. [DOI] [PubMed] [Google Scholar]
- Streilein J W, Masli S, Takeuchi M, Kezuka T. The eye’s view of antigen presentation. Hum Immunol. 2002;63:435–443. doi: 10.1016/s0198-8859(02)00393-2. [DOI] [PubMed] [Google Scholar]
- Laderach D, Compagno D, Danos O, Vainchenker W, Galy A. RNA interference shows critical requirement for NF-kappa B p50 in the production of IL-12 by human dendritic cells. J Immunol. 2003;171:1750–1757. doi: 10.4049/jimmunol.171.4.1750. [DOI] [PubMed] [Google Scholar]
- Uchida T, Ju S, Fay A, Liu Y, Dorf M E. Functional analysis of macrophage hybridomas. I. Production and initial characterization. J Immunol. 1985;134:772–778. [PubMed] [Google Scholar]
- Kuchroo V K, Minami M, Diamond B, Dorf M E. Functional analysis of cloned macrophage hybridomas. VI. Differential ability to induce immunity or suppression. J Immunol. 1988;141:10–16. [PubMed] [Google Scholar]
- Hara Y, Caspi R R, Wiggert B, Dorf M, Streilein J W. Analysis of an in vitro-generated signal that induces systemic immune deviation similar to that elicited by antigen injected into the anterior chamber of the eye. J Immunol. 1992;149:1531–1538. [PubMed] [Google Scholar]
- Ishikura H, Jayaraman S, Kuchroo V, Diamond B, Saito S, Dorf M E. Functional analysis of cloned macrophage hybridomas. VII. Modulation of suppressor T cell-inducing activity. J Immunol. 1989;143:414–419. [PubMed] [Google Scholar]
- Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267:1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- Chen Z, Hagler J, Palombella V J, Melandri F, Scherer D, Ballard D, Maniatis T. Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes Dev. 1995;9:1586–1597. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- Chen Z J, Parent L, Maniatis T. Site-specific phosphorylation of IkappaBalpha by a novel ubiquitination-dependent protein kinase activity. Cell. 1996;84:853–862. doi: 10.1016/s0092-8674(00)81064-8. [DOI] [PubMed] [Google Scholar]
- Scherer D C, Brockman J A, Chen Z, Maniatis T, Ballard D W. Signal-induced degradation of I kappa B alpha requires site-specific ubiquitination. Proc Natl Acad Sci U S A. 1995;92:11259–11263. doi: 10.1073/pnas.92.24.11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traenckner E B, Wilk S, Baeuerle P A. A proteasome inhibitor prevents activation of NF-kappa B and stabilizes a newly phosphorylated form of I kappa B-alpha that is still bound to NF-kappa B. EMBO J. 1994;13:5433–5441. doi: 10.1002/j.1460-2075.1994.tb06878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman J A, Scherer D C, McKinsey T A, Hall S M, Qi X, Lee W Y, Ballard D W. Coupling of a signal response domain in I kappa B alpha to multiple pathways for NF-kappa B activation. Mol Cell Biol. 1995;15:2809–2818. doi: 10.1128/mcb.15.5.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C Y, Mayo M W, Baldwin A S., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- Jobin C, Panja A, Hellerbrand C, Iimuro Y, Didonato J, Brenner D A, Sartor R B. Inhibition of proinflammatory molecule production by adenovirus-mediated expression of a nuclear factor kappaB super-repressor in human intestinal epithelial cells. J Immunol. 1998;160:410–418. [PubMed] [Google Scholar]
- Li G, Kim Y J, Broxmeyer H E. Macrophage colony-stimulating factor drives cord blood monocyte differentiation into IL-10(high)IL-12absent dendritic cells with tolerogenic potential. J Immunol. 2005;174:4706–4717. doi: 10.4049/jimmunol.174.8.4706. [DOI] [PubMed] [Google Scholar]
- Xu H, Zhang Y, Hua Y, Chen T, Wang H, Wu W. IL-12 p35 silenced dendritic cells modulate immune responses by blocking IL-12 signaling through JAK-STAT pathway in T lymphocytes. Biochem Biophys Res Commun. 2007;353:812–816. doi: 10.1016/j.bbrc.2006.12.108. [DOI] [PubMed] [Google Scholar]
- Hill J A, Ichim T E, Kusznieruk K P, Li M, Huang X, Yan X, Zhong R, Cairns E, Bell D A, Min W P. Immune modulation by silencing IL-12 production in dendritic cells using small interfering RNA. J Immunol. 2003;171:691–696. doi: 10.4049/jimmunol.171.2.691. [DOI] [PubMed] [Google Scholar]
- McLaughlin J N, Mazzoni M R, Cleator J H, Earls L, Perdigoto A L, Brooks J D, Muldowney J A, 3rd, Vaughan D E, Hamm H E. Thrombin modulates the expression of a set of genes including thrombospondin-1 in human microvascular endothelial cells. J Biol Chem. 2005;280:22172–22180. doi: 10.1074/jbc.M500721200. [DOI] [PubMed] [Google Scholar]
- Dabir P, Marinic T E, Krukovets I, Stenina O I. Aryl hydrocarbon receptor is activated by glucose and regulates the thrombospondin-1 gene promoter in endothelial cells. Circ Res. 2008;102:1558–1565. doi: 10.1161/CIRCRESAHA.108.176990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura S, Bondeson J, Brennan F M, Foxwell B M, Feldmann M. Role of NFkappaB in antigen presentation and development of regulatory T cells elucidated by treatment of dendritic cells with the proteasome inhibitor PSI. Eur J Immunol. 2001;31:1883–1893. doi: 10.1002/1521-4141(200106)31:6<1883::aid-immu1883>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Blair P, Freedman J E. CD40-40L signaling in vascular inflammation. J Biol Chem. 2007;282:18307–18317. doi: 10.1074/jbc.M700211200. [DOI] [PubMed] [Google Scholar]
- Malek S, Chen Y, Huxford T, Ghosh G. IkappaBbeta, but not IkappaBalpha, functions as a classical cytoplasmic inhibitor of NF-kappaB dimers by masking both NF-kappaB nuclear localization sequences in resting cells. J Biol Chem. 2001;276:45225–45235. doi: 10.1074/jbc.M105865200. [DOI] [PubMed] [Google Scholar]
- Gao J X, Madrenas J, Zeng W, Cameron M J, Zhang Z, Wang J J, Zhong R, Grant D. CD40-deficient dendritic cells producing interleukin-10, but not interleukin-12, induce T-cell hyporesponsiveness in vitro and prevent acute allograft rejection. Immunology. 1999;98:159–170. doi: 10.1046/j.1365-2567.1999.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt D A, Owen-Schaub L, Ullrich S E. Effect of IL-12 on immune suppression and suppressor cell induction by ultraviolet radiation. J Immunol. 1995;154:5114–5120. [PubMed] [Google Scholar]
- Liu H, Sidiropoulos P, Song G, Pagliari L J, Birrer M J, Stein B, Anrather J, Pope R M. TNF-alpha gene expression in macrophages: regulation by NF-kappa B is independent of c-Jun or C/EBP beta. J Immunol. 2000;164:4277–4285. doi: 10.4049/jimmunol.164.8.4277. [DOI] [PubMed] [Google Scholar]