Abstract
Francisella tularensis, a bacterium which causes tularemia in humans, is classified as a CDC category A bioterrorism agent. In this study, we demonstrate that celecoxib, an anti-inflammatory cyclooxygenase-2 inhibitor in clinical use, exhibits activity against a type A strain of F. tularensis (Schu S4), the live vaccine strain of F. tularensis (a type B strain), and F. novicida (“F. tularensis subsp. novicida”) directly in growth medium. This bacterial killing, however, was not noted with rofecoxib, despite its higher potency than that of celecoxib in inhibiting cyclooxygenase-2. The unique ability of celecoxib to inhibit the proliferation of F. tularensis could be pharmacologically exploited to develop novel anti-Francisella therapeutic agents, of which the proof of principle is demonstrated by compound 20, a celecoxib derivative identified through the screening of a celecoxib-based focused compound library. Compound 20 inhibited the intracellular proliferation of Francisella in macrophages without causing appreciable toxicity to these host cells. Together, these data support the translational potential of compound 20 for the further development of novel, potent anti-Francisella agents.
Francisella tularensis is a gram-negative, facultative, highly virulent bacterium that causes the zoonotic disease tularemia. Infection can occur through several routes, but pneumonic tularemia is the most severe clinical form, with a mortality rate of up to 60% in the absence of treatment (5, 17, 22). F. tularensis can invade a range of host cells, but its primary target in vivo is the macrophage (22). After being phagocytosed by macrophages, this intracellular pathogen can block the fusion of Francisella-containing phagosomes with lysosomes and escape from the phagosome into the cytosol, where it multiplies (2, 6, 20). Following proliferation within macrophages, F. tularensis induces host cell apoptosis or pyroptosis, leading to the release of bacteria and the subsequent infection of new cells (13, 14). Because of the ease with which aerosolized organisms could potentially be deliberately disseminated, inflicting substantial morbidity and mortality on large numbers of people, F. tularensis has been recognized as a potential biological warfare agent and, consequently, has been classified as a category A bioterrorism agent by the U.S. Centers for Disease Control and Prevention (CDC) (3, 18). The current live attenuated vaccine derived from a type B strain of F. tularensis has serious drawbacks and is of limited utility in the face of a bioterrorism threat (18). Moreover, it is believed that antibiotic-resistant strains of F. tularensis were created in the early 1990s as biological weapons (1, 3). Consequently, the development of novel, antibacterial agents against F. tularensis has become a priority.
In this study, we demonstrate that the cyclooxygenase-2 (COX-2)-specific inhibitor celecoxib exhibits activity against a virulent type A strain of F. tularensis (Schu S4), the live vaccine strain (LVS) of F. tularensis (a type B strain), and F. novicida (“F. tularensis subspecies novicida”; an avirulent species) directly in growth medium. This bacterial killing, however, was not noted with another COX-2-specific inhibitor, rofecoxib, despite its higher potency than that of celecoxib in COX-2 inhibition (23). From a drug discovery perspective, the unique ability of celecoxib to inhibit the proliferation of F. tularensis could be pharmacologically exploited as a molecular platform to develop novel anti-Francisella agents. The proof of principle of this premise is demonstrated by compound 20, a celecoxib derivative identified through the screening of a celecoxib-based focused compound library. Compound 20 not only inhibited the growth of Francisella in growth medium but also inhibited the intracellular proliferation of Francisella in macrophages at doses that do not cause appreciable toxicity to the host cells.
MATERIALS AND METHODS
Bacteria.
F. novicida strain U112 and F. tularensis LVS (type B) and strain Schu S4 (type A) were used throughout this study. Experiments involving Schu S4 were conducted in a CDC-approved select-agent biosafety level 3 laboratory at The Ohio State University. Bacteria were grown at 37°C on chocolate II agar (Becton, Dickinson and Company, Franklin Lakes, NJ) or in tryptic soy broth (TSB; Becton, Dickinson and Company) supplemented with 0.025% (wt/vol) iron(III) pyrophosphate (Sigma-Aldrich, St. Louis, MO) and 0.1% (wt/vol) cysteine hydrochloride (MP Biomedicals, Solon, OH).
Salmonella enterica serovar Typhimurium (ATCC 14028) and Escherichia coli (ATCC 25922) were grown on Luria-Bertani (LB) agar (Becton, Dickinson and Company) or in LB broth at 37°C. Experiments involving these bacteria were performed using biosafety level 2 laboratory procedures.
Reagents.
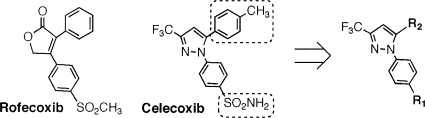
Celecoxib was extracted and purified from Celebrex capsules (Amerisource Health, Malvern, PA) with ethyl acetate and then recrystallized in a mixture of ethyl acetate and hexane. Rofecoxib was synthesized according to a previously described procedure (19). The celecoxib-based focused compound library consisted of 21 compounds (see Table 2). The synthesis of these compounds will be described elsewhere. The identities and purities (≥99%) of all compounds used in this study were verified by proton nuclear magnetic resonance spectroscopy (300 MHz), high-resolution mass spectrometry, and elemental analyses.
TABLE 2.
Structures and anti-Francisella activities of celecoxib, rofecoxib, and compounds 1 to 21
NE, no effect of the test agent at 64 μg/ml. Data for F. novicida and LVS were obtained after 24 and 48 h, respectively.
Macrophages.
The RAW 264.7 murine macrophage cell line and the THP-1 human monocytic leukemia cell line were obtained from the American Type Culture Collection (ATCC; Manassas, VA). The RAW 264.7 cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco-BRL, Invitrogen Corp., Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS; Gibco-BRL). The THP-1 cells were maintained in RPMI 1640 containing 10% FBS. THP-1 cells were differentiated by treatment with 20 nM 12-O-tetradecanoylphorbol-13-acetate (TPA; Sigma-Aldrich, St. Louis, MO) for 48 h. All culture of murine and human cells was performed at 37°C in a humidified incubator containing 5% CO2. Cells were seeded into 96- or 12-well tissue culture plates, and the plates were incubated for 8 to 12 h prior to experimentation.
Antibacterial assays.
The MICs of individual agents were determined by a broth microdilution method recommended by the Clinical and Laboratory Standards Institute (24), with the exception that TSB was used. Bacteria cells grown overnight on chocolate II agar plates were suspended in phosphate-buffered saline (PBS) to an optical density at 600 nm of 1.0, which was equivalent to 1010 CFU/ml, and the suspensions were then diluted in modified TSB to a final concentration of 5 × 105 CFU/ml. The bacterial suspensions were exposed to the test agents and chloramphenicol at escalating doses, ranging from 1 to 64 μg/ml, in triplicate in 96-well plates, and the plates were incubated at 37°C for 24 h (for F. novicida and Schu S4) or 48 h (for LVS). The MIC was derived from the concentration at which no growth of bacteria was visible. Subsequently, to analyze the viabilities of F. novicida and LVS cells after drug exposure, a 100-μl sample of the bacterial suspension from each well was serially diluted with PBS and the diluted samples were spread onto chocolate II agar plates. After 24 h (for F. novicida) or 48 h (for LVS) of incubation at 37°C, the bacterial colonies on each plate were counted and the results were expressed as the numbers of CFU per milliliter. The effects of test agents on the growth of two additional gram-negative bacteria, S. enterica serovar Typhimurium (ATCC 14028) and E. coli (ATCC 25922), in LB broth or modified TSB were assayed as described above for Francisella spp. To assess the protein binding effects on the anti-Francisella activities of celecoxib and compound 20, the MICs of these two agents for F. novicida were assayed in the presence of different concentrations of human AB serum (Valley Biomedical, VA) and FBS (Gibco-BRL) by the microdilution method as described above.
Macrophage viability assay.
The effects of individual agents on macrophage viability were assessed by using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay (4). RAW 264.7 cells were seeded into 96-well plates at 2.5 × 104 cells/well (with a minimum of 6 wells per test group) in DMEM supplemented with 10% FBS and 10 μg/ml of gentamicin, and then the plates were incubated overnight at 37°C in a humidified incubator containing 5% CO2. The medium from each well was removed and replaced with fresh 5% FBS-supplemented DMEM containing various concentrations of test agents dissolved in dimethyl sulfoxide (DMSO; final concentration of 0.1%). Controls received DMSO alone at a concentration equal to that in drug-treated cell samples. After 8 h of treatment, the medium was removed and replaced by 200 μl of 0.5-mg/ml MTT in 10% FBS-containing medium and the cells were incubated in the CO2 incubator at 37°C for 1 h. Supernatants were removed from the wells, and the reduced MTT dye was solubilized in 200 μl/well of DMSO. Absorbances at 570 nm were determined on a plate reader. The viability of drug-treated cells was calculated as a percentage of that of vehicle-treated control cells, and a 50% inhibitory concentration (IC50) for cells was determined by using CalcuSyn software (Biosoft, Cambridge, United Kingdom).
Assay for intracellular survival of Francisella in macrophages.
F. novicida and F. tularensis (type A strain Schu S4) cells grown overnight on chocolate II agar plates were suspended in PBS to a concentration of approximately 1010 CFU/ml (as estimated by measuring an optical density at 600 nm of 1.0). RAW 264.7 murine macrophages and TPA-differentiated THP-1 cells were seeded into 12-well plates at 5 × 105 cells/well, and bacteria were added at a multiplicity of infection of 50 (16). After centrifugation of the plates at 800 × g for 15 min to facilitate infection, macrophages were incubated at 37°C in a humidified incubator containing 5% CO2 for 2 h, exposed to 50 μg/ml of gentamicin for 30 min, and washed with prewarmed PBS twice to remove killed extracellular bacteria (14). Infected macrophages were then treated in triplicate with various concentrations of test agents for 8 h, after which culture medium was collected from each well and macrophages were lysed with 500 μl of 0.1% sodium deoxycholate in PBS at 37°C for 5 min to release intracellular bacteria (15). Bacteria present in the collected culture medium, either as free bacteria or as cells within floating macrophages, were harvested by centrifugation at 16,000 × g for 5 min, followed by the resuspension of the pellet in 500 μl of 0.1% sodium deoxycholate in PBS. Combined lysates were serially diluted with PBS and spread onto chocolate II agar plates. The numbers of CFU were calculated after the incubation of the plates for 24 h at 37°C. The rates of survival of intracellular bacteria in drug-treated macrophages were calculated as percentages of the number of surviving control (untreated) cells.
Statistical analyses.
Data are expressed as means ± standard deviations (SD). Group means were compared using a two-tailed t test for independent samples. Differences were considered significant at P of <0.05. Statistical analyses were performed using SPSS for Windows (version 16.0; SPSS, Inc., Chicago, IL).
RESULTS
Differential inhibitory effects of celecoxib and rofecoxib on the growth of F. tularensis in broth culture.
As part of our effort to identify lead agents with activities against F. tularensis, we examined the effects of a panel of pharmaceuticals in clinical use on the growth of F. novicida and LVS in modified TSB. Of the drugs examined, the COX-2 inhibitor celecoxib exhibited a unique ability to inhibit bacterial growth, with MICs of 32 and 16 μg/ml for F. novicida and LVS, respectively (Table 1). The treatment of these bacteria with celecoxib at the respective MICs led to 3- and 1.5-log decreases in CFU of F. novicida and LVS, respectively (data not shown). F. novicida and LVS were completely eliminated by 64 and 32 μg/ml of celecoxib, respectively (data not shown). Importantly, celecoxib was equipotent in suppressing the growth of the human virulent type A strain of F. tularensis, Schu S4, with an MIC of 16 μg/ml (Table 1). Moreover, this suppressive effect was highly specific for Francisella since celecoxib was inactive against two other gram-negative bacteria examined, namely, S. enterica serovar Typhimurium (ATCC 14028) and E. coli (ATCC 25922). In contrast, at 64 μg/ml, the structurally distinct but more potent COX-2 inhibitor rofecoxib had no appreciable effect on any of the bacteria examined (Table 1), indicating that the antibacterial effect of celecoxib was attributable to an “off-target” mechanism independent of the inhibition of a putative COX-2-like enzyme in Francisella.
TABLE 1.
MICs of celecoxib and rofecoxib
| Gram-negative bacterium | MICa (μg/ml) of:
|
|
|---|---|---|
| Celecoxib | Rofecoxib | |
| F. novicida | 32 | NE |
| F. tularensis LVS | 16 | NE |
| F. tularensis Schu S4 | 16 | NE |
| S. enterica serovar Typhimurium | NE | NE |
| E. coli | NE | NE |
NE, no effect of the test agent at 64 μg/ml.
Pharmacological exploitation of the anti-Francisella activity of celecoxib.
The above-described findings of the off-target activity of celecoxib against F. tularensis could be exploited by using celecoxib as a molecular platform to develop potent anti-Francisella agents for therapeutic use. Accordingly, we established a focused compound library consisting of 21 celecoxib derivatives by replacing the sulfonamide (R1) and methylphenyl (R2) fragments of celecoxib with various functionalities (Table 2). The activities of these celecoxib derivatives against F. novicida and LVS were examined. Of these derivatives, compounds 2, 11, 12, 16, and 20 exhibited MICs of no greater than 4 μg/ml for both strains. In particular, compound 12 was able to suppress the growth of F. novicida and LVS at 2 and 1 μg/ml, respectively. This multifold increase in antibacterial activity provided a proof of principle that celecoxib could be structurally optimized to develop potent anti-Francisella agents.
Differential cytotoxicities of lead agents for macrophages.
Since the primary target in vivo for F. tularensis is the macrophage, we further assessed the cytotoxicities of celecoxib and these lead agents in RAW 264.7 murine macrophage cells. Figure 1 depicts the dose-response curves for individual agents and samples of RAW 264.7 cells in 5% FBS-containing DMEM medium after 8 h of treatment, demonstrating the following order of relative potencies: compound 12 > compound 11 > compound 2 > compound 16 > celecoxib > compound 20. Serum was an important variable in this assay, as prior studies had shown that serum can suppress the activities of these agents (C. S. Chen, unpublished data). Although compound 12 at 2 μg/ml was highly effective at inhibiting bacterial growth, it also showed cytotoxicity for macrophages at the same concentration, i.e., the IC50/MIC ratio was 1.2 (Table 3). On the other hand, compound 20 exhibited the highest IC50/MIC ratio, 11.5, indicating desirable selectivity in drug-induced bacterial growth inhibition relative to cytotoxicity for host cells. Moreover, like that of celecoxib, the inhibitory activity of compound 20 was specific for Francisella, as this compound was inactive against the gram-negative bacteria S. enterica serovar Typhimurium and E. coli (data not shown).
FIG. 1.
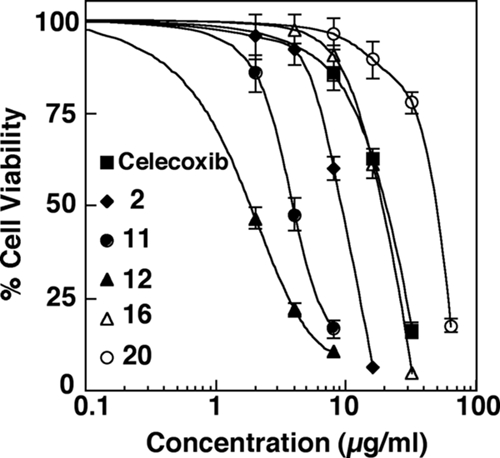
The cytotoxic effects of celecoxib and selected agents (compounds 2, 11, 12, 16, and 20) on RAW 264.7 cells were measured using the MTT cell viability assay, and the results are expressed as the percentages of viable cells relative to the number of surviving vehicle control (DMSO)-treated cells. Points indicate means, and bars indicate SD (n = 3).
TABLE 3.
Comparison of cytotoxicities and antimicrobial efficacies of selected agents
| Compound | MIC (μg/ml) for F. novicida | IC50 (μg/ml) for RAW 264.7 cells | IC50/MIC ratio |
|---|---|---|---|
| Celecoxib | 32 | 17 | 0.5 |
| 2 | 4 | 9 | 2.3 |
| 11 | 4 | 4.8 | 1.2 |
| 12 | 2 | 2.4 | 1.2 |
| 16 | 4 | 15 | 3.8 |
| 20 | 4 | 46 | 11.5 |
Compound 20 inhibits the growth of intracellular F. tularensis in murine and human macrophages.
Based on these results, compound 20 was studied further for its effect on the intracellular survival of F. novicida in murine RAW 264.7 and TPA-differentiated human THP-1 macrophages. After the infection of macrophages and the removal of extracellular bacteria, the infected samples were treated with 4, 8, and 16 μg/ml of compound 20 in 5% FBS-containing DMEM medium for 8 h. Intracellular bacteria were then harvested and enumerated by calculating the numbers of CFU after growth on agar. As shown in Fig. 2A, compound 20 effectively inhibits intracellular F. novicida at 16 μg/ml (P < 0.05). Subsequently, the effect of compound 20 on F. tularensis (type A strain Schu S4) in TPA-treated THP-1 cells was assessed. At 4 μg/ml, the lowest concentration tested, compound 20 significantly inhibited the intracellular survival of Schu S4 (P < 0.01), indicating the greater susceptibility of Schu S4 than of F. novicida to compound 20 (Fig. 2B).
FIG. 2.
Effects of compound 20 on the survival of intracellular F. novicida and F. tularensis (type A strain Schu S4) in macrophages. Compound 20 inhibits the intracellular survival of F. novicida in RAW 264.7 and THP-1 cells (A) and that of F. tularensis (type A strain Schu S4) in THP-1 cells (B). Surviving intracellular bacteria were enumerated by determining the numbers of CFU after treatments, and the results are expressed as percentages of CFU relative to the CFU in control (DMSO)-treated groups. The absolute CFU values for F. novicida from control-treated RAW 264.7 and THP-1 cells were 2,466,667 and 160,000, respectively. The absolute CFU value for Schu S4 from control-treated THP-1 cells was 113,666. Columns indicate means; bars indicate SD (n = 3). *, P < 0.05 for the difference between groups treated with 16 μg/ml compound 20 and the control group; **, P < 0.01 for the difference between each drug-treated group and the control group.
DISCUSSION
Several drugs that were not originally developed for the treatment of bacterial infections have been demonstrated to possess antimicrobial activities in vitro (8-12). For example, statins, a group of cholesterol-lowering drugs, were shown previously to inhibit the in vitro growth of Staphylococcus aureus (8). Here, we have demonstrated that celecoxib, a broadly used anti-inflammatory agent, exhibits off-target activity against F. tularensis in vitro. It is particularly noteworthy that the activity of celecoxib against F. tularensis is more potent than that against F. novicida. These differential effects are reflected in the disparate MICs for F. novicida versus F. tularensis type A strain Schu S4 and LVS. Moreover, the assessment of the anti-Francisella activities of new celecoxib derivatives revealed that F. novicida and LVS showed a marked difference in their susceptibilities to celecoxib and its derivatives, especially compound 17, which had no measurable inhibitory effect on F. novicida but was a potent inhibitor of LVS growth in modified TSB (Table 2). This finding indicates that the interactions between the drug and its putative bacterial target protein differ among Francisella spp. One possibility is that the binding sites for celecoxib on its putative target protein differ, leading to higher binding affinities in F. tularensis strains than in other Francisella strains and stronger growth inhibition of F. tularensis in vitro.
Among the celecoxib derivatives synthesized and evaluated, compound 20 was identified as having the best selectivity for bacterial growth inhibition relative to its cytotoxicity for macrophages. Equally important, it could inhibit the survival of both intracellular F. novicida and intracellular F. tularensis (type A strain Schu S4) in macrophages. Nevertheless, there is a difference between the MICs for intracellular F. novicida and bacteria grown in broth culture (16 versus 4 μg/ml). This discrepancy reflects many factors that limit the access of antibacterial agents to intracellular pathogens, including serum protein binding and physical barriers imposed by biological membranes. As shown in Table 4, the presence of either human serum or FBS in growth broth decreased the inhibitory activities of both celecoxib and compound 20 on F. novicida growth in broth. This issue identifies some of the challenges that need to be addressed for the continued development of this class of anti-Francisella agents. These include the development of compounds with greater antibacterial potencies and lower host toxicities and methods for increasing cytosolic levels of compound 20 and future compounds. Also, because Francisella is located primarily in macrophages of the infected host, methods for targeted drug delivery to macrophages should be considered in the development of future anti-Francisella agents. Thus, strategies that couple compound 20 with a carrier that can be actively phagocytosed by macrophages may prove to be promising means to attain both increased intracellular drug concentrations and specificity of drug delivery. One possible approach in this regard is to utilize the mannose receptor that is expressed abundantly on macrophages, which has been broadly used to enhance the specific delivery of drugs, oligonucleotides, and proteins to intracellular compartments in macrophages (7).
TABLE 4.
Effect of sera on MICs of celecoxib and compound 20 for F. novicida
| Serum type | Serum concn (%) | MICa (μg/ml) of:
|
|
|---|---|---|---|
| Celecoxib | Compound 20 | ||
| Human AB serum | 0 | 32 | 4 |
| 10 | NE | 16 | |
| 50 | NE | NE | |
| 80 | NE | NE | |
| FBS | 0 | 32 | 4 |
| 10 | 64 | 8 | |
| 50 | NE | NE | |
| 80 | NE | NE | |
NE, no effect of the test agent at 64 μg/ml.
Celecoxib and rofecoxib are potent COX-2 inhibitors that have been shown previously to interact with the same binding pocket of the COX-2 enzyme with IC50s in the submicromolar range. Nonetheless, our data show that only celecoxib possessed activity against Francisella and that the MIC of celecoxib for Francisella (32 μg/ml) is much higher than its reported IC50 for COX-2 (0.21 μg/ml) (19). These findings suggest that the antimicrobial activity of celecoxib is independent of the structural features that dictate its binding to COX-2. Thus, we postulate that the putative bacterial target of celecoxib in F. tularensis is structurally distinct from the COX-2 enzyme. In addition to COX-2, celecoxib has been reported to possess inhibitory activities against other mammalian enzymes, including phosphoinositide-dependent kinase-1, carbonic anhydrase, sarcoplasmic/endoplasmic reticulum calcium ATPase, and COX-1 (21). These mammalian enzymes may serve as leads to identify structurally similar bacterial proteins, one of which may be the putative antibacterial target of celecoxib in F. tularensis. Based on this assumption, the protein sequences of these celecoxib-targeted enzymes were used to search for homologous protein sequences in the published proteomes of F. tularensis Schu S4, F. novicida, and F. tularensis LVS at the National Center of Biotechnology Information (http://www.ncbi.nlm.nih.gov/). Our preliminary results have identified several proteins of F. novicida and LVS that show homology to carbonic anhydrase, sarcoplasmic/endoplasmic reticulum calcium ATPase, and COX-1, including superoxide dismutase, FGAM (phosphoribosylformylglycinamidine) synthase, and a cation transport ATPase (FTF1738c). Although further experiments must be performed to validate the roles of these bacterial proteins in celecoxib-induced growth inhibition of Francisella spp., these preliminary findings suggest that such an approach to identifying bacterial drug targets is feasible and will facilitate the development of more potent and specific, celecoxib-derived anti-Francisella agents.
Acknowledgments
This work was sponsored by the NIH/NIAID Regional Center of Excellence for Bio-defense and Emerging Infectious Diseases Research (RCE) Program. We acknowledge membership within and support from the Region V “Great Lakes” RCE (NIH award 1-U54-AI-057153).
Footnotes
Published ahead of print on 27 April 2009.
REFERENCES
- 1.Alibek, K. 1999. Biohazard. Random House, New York, NY.
- 2.Clemens, D. L., B. Y. Lee, and M. A. Horwitz. 2004. Virulent and avirulent strains of Francisella tularensis prevent acidification and maturation of their phagosomes and escape into the cytoplasm in human macrophages. Infect. Immun. 72:3204-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dennis, D. T., T. V. Inglesby, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. D. Fine, A. M. Friedlander, J. Hauer, M. Layton, S. R. Lillibridge, J. E. McDade, M. T. Osterholm, T. O'Toole, G. Parker, T. M. Perl, P. K. Russell, and K. Tonat. 2001. Tularemia as a biological weapon: medical and public health management. JAMA 285:2763-2773. [DOI] [PubMed] [Google Scholar]
- 4.Edmondson, J. M., L. S. Armstrong, and A. O. Martinez. 1988. A rapid and simple MTT-based spectrophotometric assay for determining drug sensitivity in monolayer cultures. Methods Cell Sci. 11:15-17. [Google Scholar]
- 5.Ellis, J., P. C. Oyston, M. Green, and R. W. Titball. 2002. Tularemia. Clin. Microbiol. Rev. 15:631-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golovliov, I., V. Baranov, Z. Krocova, H. Kovarova, and A. Sjostedt. 2003. An attenuated strain of the facultative intracellular bacterium Francisella tularensis can escape the phagosome of monocytic cells. Infect. Immun. 71:5940-5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irache, J. M., H. H. Salman, C. Gamazo, and S. Espuelas. 2008. Mannose-targeted systems for the delivery of therapeutics. Expert Opin. Drug Deliv. 5:703-724. [DOI] [PubMed] [Google Scholar]
- 8.Jerwood, S., and J. Cohen. 2008. Unexpected antimicrobial effect of statins. J. Antimicrob. Chemother. 61:362-364. [DOI] [PubMed] [Google Scholar]
- 9.Kruszewska, H., T. Zareba, and S. Tyski. 2000. Antimicrobial activity of selected non-antibiotics—activity of methotrexate against Staphylococcus aureus strains. Acta Pol. Pharm. 57(Suppl.):117-119. [PubMed] [Google Scholar]
- 10.Kruszewska, H., T. Zareba, and S. Tyski. 2006. Estimation of antimicrobial activity of selected non-antibiotic products. Acta Pol. Pharm. 63:457-460. [PubMed] [Google Scholar]
- 11.Kruszewska, H., T. Zareba, and S. Tyski. 2004. Examination of antimicrobial activity of selected non-antibiotic drugs. Acta Pol. Pharm. 61(Suppl.):18-21. [PubMed] [Google Scholar]
- 12.Kruszewska, H., T. Zareba, and S. Tyski. 2002. Search of antimicrobial activity of selected non-antibiotic drugs. Acta Pol. Pharm. 59:436-439. [PubMed] [Google Scholar]
- 13.Lai, X. H., I. Golovliov, and A. Sjostedt. 2001. Francisella tularensis induces cytopathogenicity and apoptosis in murine macrophages via a mechanism that requires intracellular bacterial multiplication. Infect. Immun. 69:4691-4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mariathasan, S., D. S. Weiss, V. M. Dixit, and D. M. Monack. 2005. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J. Exp. Med. 202:1043-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohapatra, N. P., A. Balagopal, S. Soni, L. S. Schlesinger, and J. S. Gunn. 2007. AcpA is a Francisella acid phosphatase that affects intramacrophage survival and virulence. Infect. Immun. 75:390-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohapatra, N. P., S. Soni, T. J. Reilly, J. Liu, K. E. Klose, and J. S. Gunn. 2008. Combined deletion of four Francisella novicida acid phosphatases attenuates virulence and macrophage vacuolar escape. Infect. Immun. 76:3690-3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oyston, P. C. 2008. Francisella tularensis: unravelling the secrets of an intracellular pathogen. J. Med. Microbiol. 57:921-930. [DOI] [PubMed] [Google Scholar]
- 18.Oyston, P. C., A. Sjostedt, and R. W. Titball. 2004. Tularaemia: bioterrorism defence renews interest in Francisella tularensis. Nat. Rev. Microbiol. 2:967-978. [DOI] [PubMed] [Google Scholar]
- 19.Prasit, P., Z. Wang, C. Brideau, C. C. Chan, S. Charleson, W. Cromlish, D. Ethier, J. F. Evans, A. W. Ford-Hutchinson, J. Y. Gauthier, R. Gordon, J. Guay, M. Gresser, S. Kargman, B. Kennedy, Y. Leblanc, S. Leger, J. Mancini, G. P. O'Neill, M. Ouellet, M. D. Percival, H. Perrier, D. Riendeau, I. Rodger, R. Zamboni, et al. 1999. The discovery of rofecoxib, [MK 966, Vioxx, 4-(4′-methylsulfonylphenyl)-3-phenyl-2(5H)-furanone], an orally active cyclooxygenase-2-inhibitor. Bioorg. Med. Chem. Lett. 9:1773-1778. [DOI] [PubMed] [Google Scholar]
- 20.Santic, M., R. Asare, I. Skrobonja, S. Jones, and Y. Abu Kwaik. 2008. Acquisition of the vacuolar ATPase proton pump and phagosome acidification are essential for escape of Francisella tularensis into the macrophage cytosol. Infect. Immun. 76:2671-2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schonthal, A. H. 2007. Direct non-cyclooxygenase-2 targets of celecoxib and their potential relevance for cancer therapy. Br. J. Cancer 97:1465-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sjostedt, A. 2003. Virulence determinants and protective antigens of Francisella tularensis. Curr. Opin. Microbiol. 6:66-71. [DOI] [PubMed] [Google Scholar]
- 23.Tacconelli, S., M. L. Capone, M. G. Sciulli, E. Ricciotti, and P. Patrignani. 2002. The biochemical selectivity of novel COX-2 inhibitors in whole blood assays of COX-isozyme activity. Curr. Med. Res. Opin. 18:503-511. [DOI] [PubMed] [Google Scholar]
- 24.Wikler, M. A. 2008. Performance standards for antimicrobial susceptibility testing; 18th informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA.