Abstract
Human immunodeficiency virus type 1 (HIV-1) Nef interferes with the endocytic machinery to modulate the cell surface expression of CD4. However, the basal trafficking of CD4 is governed by different rules in the target cells of HIV-1: whereas CD4 is rapidly internalized from the cell surface in myeloid cells, CD4 is stabilized at the plasma membrane through its interaction with the p56lck kinase in lymphoid cells. In this study, we showed that Nef was able to downregulate CD4 in both lymphoid and myeloid cell lines but that an increase in the internalization rate of CD4 could be observed only in lymphoid cells. Expression of p56lck in nonlymphoid CD4-expressing cells restores the ability of Nef in order to increase the internalization rate of CD4. Concurrent with this observation, the expression of a p56lck-binding-deficient mutant of CD4 in lymphoid cells abrogates the Nef-induced acceleration of CD4 internalization. We also show that the expression of Nef causes a decrease in the association of p56lck with cell surface-expressed CD4. Regardless of the presence of p56lck, the downregulation of CD4 by Nef was followed by CD4 degradation. Our results imply that Nef uses distinct mechanisms to downregulate the cell surface expression levels of CD4 in either lymphoid or myeloid target cells of HIV-1.
Besides proteins that are essential for proper virus processing and assembly, the genomes of primate lentiviruses such as human immunodeficiency virus type 1 (HIV-1) encode auxiliary proteins that modulate viral infectivity. The 27-kDa auxiliary protein Nef is a key element in the progression of primary HIV-1 infection toward AIDS. Cases of patients infected with HIV-1 strains harboring a deletion in the nef gene or a defective nef allele have been reported. Some of these patients exhibit asymptomatic or slow progression toward the disease (6, 17, 37). In vitro, Nef facilitates viral replication and enhances the infectivity of viral particles (13, 47, 69). The mechanisms involved in the Nef-induced increase of viral infectivity remain elusive; however, it is a multifactorial process related to the ability of Nef to alter the trafficking of host cell proteins.
Indeed, the most documented effect of Nef during the course of viral infection is its ability to disturb the clathrin-dependent trafficking machinery involved in the transport of transmembrane proteins through endosomal compartments. This leads to the modulation of the level of cell surface expression for some receptors, including CD4, which is the primary receptor of HIV-1 (35) and major histocompatibility complex class I (reviewed in references 22 and 27). The downregulation of CD4, which results in the impairment of the immunological synapse (72) and the downregulation of major histocompatibility complex class I molecules (reviewed in reference 16), is believed to contribute to the escape of HIV-1-infected cells from immunosurveillance. Moreover, the downregulation of CD4 helps avoid superinfection of cells, which would be deleterious to the virus (reviewed in reference 21), and has a direct impact on viral fitness by allowing better incorporation of the functional envelope in viral particles produced from CD4-expressing cells (3, 36, 53).
Nef-induced cell surface downregulation of CD4 is efficient in all CD4-expressing cells and depends on the integrity of a di-Leu motif at position 164/165 of the C-terminal flexible loop of HIV-1 Nef (2, 9, 25). This di-Leu motif allows for the interaction with clathrin-associated adaptor protein (AP) complexes that participate in the clathrin-dependent vesicular transport within the endocytic pathway. The AP type 2 (AP-2) complex is localized at the plasma membrane and is essential to the assembly and function of clathrin-coated pits involved in the internalization of receptors from the cell surface (59). The interaction of Nef with AP-2 is well delineated and has been proposed to enhance the targeting of CD4 to clathrin-coated pits and its internalization (10, 12, 26, 32, 39).
Helper T lymphocytes are the predominant cell type that expresses CD4; however, CD4 is also present at the surfaces of monocytes and macrophages (70), where its function is yet to be elucidated. Whereas cell surface CD4 is rapidly internalized in myeloid cells, CD4 is stabilized at the plasma membrane in lymphoid cells through its interaction with the Src family protein tyrosine kinase p56lck. Cys residues located at positions 420/422 in the CD4 cytoplasmic tail are essential to the constitutive association with p56lck (73). Besides its role in signal transduction, this interaction also correlates with an accumulation of CD4 in lipid rafts and enhanced exclusion of CD4 from clathrin-coated pits (50).
In T cells, treatment with phorbol esters such as phorbol 12-myristate 13-acetate (PMA) provokes the phosphorylation of Ser residues found in the cytoplasmic tail of CD4. This correlates with a decreased association of p56lck with CD4 and the internalization of the receptor (24, 32-34, 41, 45, 48, 52, 56, 61, 66-68). Nef-induced CD4 downregulation is known to be independent of Ser phosphorylation (20) and is therefore governed by mechanisms different from those involved in PMA-induced CD4 downregulation. However, the Leu-based sorting motif in the CD4 cytoplasmic tail is critical for both PMA and Nef-induced CD4 downregulation (2, 5, 24, 31, 56, 60, 68), thus indicating that despite being different, the mechanisms involved in Nef- and PMA-induced CD4 downregulation partially overlap.
In the present study, we investigated whether the mechanisms used by Nef to downregulate CD4 are cell type-dependent processes. We looked at the trafficking and steady-state expression of CD4 in the main target cells of HIV-1, CD4-positive T lymphocytes, and cells of the monocyte/macrophage lineage. Our results demonstrate that the presence of p56lck has a direct impact on the mechanisms used by Nef to downregulate CD4 from the cell surface of T lymphocytes. They also reveal that Nef uses distinct pathways to decrease levels of cell surface expression of CD4 in lymphoid or myeloid target cells of HIV-1.
MATERIALS AND METHODS
Plasmids.
The plasmid encoding human CD4 was described previously (8). CD4 mutations of Ser408 to Ala (CD4 S/A) and of both Cys420 and Cys422 to Ser (CD4 CC/SS) were performed using the QuikChange site-directed mutagenesis kit (Stratagene). The CD4 mutant deleted from the cytoplasmic tail (CD4ΔCT) was previously described (4). CD4 mutants were subcloned into pMSCVhyg (Clontech), and the corresponding constructs were used to establish stable cell lines. The p56lck-encoding plasmid was a kind gift from G. Bismuth (Institut Cochin, Paris, France). The HIV-1 NL4-3 proviral constructs in which env and vpu genes are defective, carrying an intact or disrupted nef gene followed by an internal ribosomal entry sequence (IRES) and the enhanced green fluorescent protein (EGFP) (pBR-NL43-env*nef+vpu*-IRES-EGFP and pBR-NL43-env*nef-vpu*-IRES-EGFP, respectively), were obtained from F. Kirchhoff (University of Ulm, Germany) (11, 63, 64). The plasmid encoding wild-type (WT) Nef fused to the influenza hemagglutinin epitope was obtained from H. Gottlinger (University of Massachusetts Medical School, MA). The Nef-Stop was generated by introducing a frameshift at the unique XhoI site in the nef gene. Vectors encoding green fluorescent protein (GFP)-tagged HIV-1 NL4-3 Nef (Nef-GFP) as well as the Nef mutant in which an Ala substitution was performed for Leu 164/165 (Nef LL/AA-GFP) were described elsewhere (43).
Antibodies.
The monoclonal antibody OKT4 (ATCC) specific for the extracellular domain of CD4 was used to immunoprecipitate CD4. The immunodetection of CD4 following Western blotting was performed with an anti-CD4 polyclonal rabbit antibody (H-370; Santa Cruz) or a monoclonal antibody (1F6; Novocastra). p56lck was detected with a rabbit polyclonal antibody (2102; Santa Cruz). GFP was detected with a rabbit polyclonal antibody (sc-8334; Santa Cruz). HIV-1 p24-specific antibodies were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. Secondary reagents were peroxidase-coupled antibodies directed against mouse or rabbit immunoglobulin G (Sigma). Fluorescence-activated cell sorting (FACS) analysis of cell surface CD4 was performed with phycoerythrin-cyanine 5-coupled anti-human CD4 (PE-Cy5 anti-human CD4; BD Pharmingen) or OKT4 and Alexa 647-coupled antibodies directed against mouse immunoglobulin G (Invitrogen).
Cell culture and transduction.
All cell culture reagents were purchased from Invitrogen, unless indicated otherwise. Adherent cell lines (HeLa and 293T cells) were grown in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum (FCS), 100 IU/ml penicillin, and 0.1 mg/ml streptomycin. The A2.01 human T-cell line and THP-1 human monocytic cell line were obtained through the NIH AIDS Research and Reference Reagent Program. U937, HPB-ALL, and J.CaM cells were kind gifts from S. Marullo, G. Bismuth (Institut Cochin, Paris, France), and O. Schwartz (Institut Pasteur, Paris, France), respectively. These nonadherent cells were cultured in RPMI 1640 supplemented with 10 mM HEPES, 10% FCS, 100 IU/ml penicillin, and 0.1 mg/ml streptomycin. All cell lines were maintained in a humidified atmosphere at 37°C with 5% CO2.
Transduction of the A2.01 cell line was carried out using the pVPack vector system (Stratagene). Recombinant viruses were obtained as described previously (4). Briefly, 293T cells were cotransfected by the calcium phosphate precipitation technique, with pVPack-GP, pVPack-VSV-G, and pMSCVhyg containing the appropriate CD4 mutant. Cells were washed 7 h and 24 h posttransfection. Supernatants (40 ml) were harvested 48 h later, filtered through 0.45-μm sterile filters, and ultracentrifuged at 27,000 × g for 90 min at 4°C. Viruses were then resuspended in 200 μl phosphate-buffered saline (PBS) and stored at −80°C. Transduction was carried out using ∼5 × 105 A2.01 cells in 200 μl complete RPMI medium, to which 50 μl of concentrated virus was added. Cells were expanded and selected in complete RPMI medium supplemented with 0.8 mg/ml hygromycin B (PAA).
The expression of Nef in CD4-expressing A2.01 cell lines was achieved using an HIV-1-based retroviral transduction system. 293T cells were cotransfected by the calcium phosphate precipitation technique, with pBR-NL43-env*nef+vpu*-IRES-EGFP or pBR-NL43-env*nef-vpu*-IRES-EGFP and pHCMV-G to allow for vesicular stomatitis virus G glycoprotein (VSV-G) expression. Transfected cells were treated as described above, and supernatant were assayed for reverse transcriptase activity, as described elsewhere (4). A total of ∼5 × 105 A2.01 cells were transduced, with 200,000 reverse transcriptase counts at a final volume of 250 μl, and cell surface CD4 expression was analyzed by FACS 48 h posttransduction. When indicated, HeLa cells stably expressing CD4 (HeLa-CD4) were also transduced to achieve Nef expression. Viral dilutions resulting in 30% and 100% of transduction efficiencies were used in cell surface CD4 staining and CD4 degradation experiments, respectively.
Transfections.
HeLa cells were transfected with the plasmids mentioned in the text using Lipofectamine 2000 (Invitrogen). THP-1 cells were nucleofected using the Amaxa kit V, according to the instructions of the manufacturer. J.CaM, HPB-ALL, and U937 cells were transfected with 10 μg DNA, as follows: ∼10 × 106 cells were electroporated at 250 V and 950 μF in complete media supplemented with 40 mM NaCl at a final volume of 250 μl. 293T cells were seeded at a density of ∼2.5 × 106 cells/T75 and transfected 16 h later by the calcium phosphate precipitation technique. Cells were harvested and analyzed 48 h posttransfection.
Analysis of cell surface CD4 expression by FACS.
Cells transfected with a plasmid encoding Nef (or Nef mutants) fused to GFP or Nef in combination with a GFP-encoding plasmid were harvested in PBS supplemented with 5 mM EDTA, washed with PBS, and incubated for 30 min on ice in PBS supplemented with 2% FCS (PBS-FCS). The following staining procedure was then carried out at 4°C, unless otherwise mentioned.
For cell surface CD4 expression analysis at steady state, ∼1 × 106 cells were pelleted and resuspended in 20 μl of PE-Cy5-anti-CD4 (diluted 1:2 in PBS-FCS) and incubated for 45 min. Cells were then washed twice with PBS-FCS, washed once with PBS, fixed in PBS supplemented with 3.7% formaldehyde, and analyzed by FACS.
For the endocytosis assay, ∼10 × 106 cells in 100 μl of PBS-FCS were incubated with 5 μg/ml OKT4 for 45 min under gentle agitation. Cells were washed three times with PBS-FCS, resuspended either in complete medium or in complete medium containing 100 ng/ml PMA (Sigma), and incubated at 37°C for the indicated periods of time. CD4 internalization was stopped by washing cells twice in ice-cold PBS-FCS. Cells were then stained with Alexa 647-coupled anti-mouse antibody and washed twice with PBS-FCS and once with PBS prior to fixation in PBS supplemented with 3.7% formaldehyde and FACS analysis. All FACS experiments were performed with a Cytomics FC 500 cytometer, and data were analyzed with Cytomics RXP analysis software. Transfected cells were gated on the basis of GFP expression. The percentage of internalized CD4 at each time point was calculated from the GFP-positive population, as follows: (CD4t0 − CD4t)/CD4t0 × 100, in which CD4t0 and CD4t represent the mean fluorescence intensity of cell surface CD4 before and after internalization, respectively.
Cell surface biotinylation and immunoprecipitation.
All of the following steps were carried out at 4°C, unless otherwise mentioned. For PMA treatment, cells were resuspended in complete media in the presence of 100 ng/ml PMA and incubated at 37°C for 5 min. Cells were then washed three times with PBS, resuspended in 1 mg/ml N-hydroxysuccinimide-LC-biotin, and incubated for 1 h in the dark. The reaction was quenched by resuspension in 100 mM glycin in PBS, pH 7.5. Unbound biotin was removed by washing cells three times with PBS. Cells were then resuspended in solubilization buffer containing 1% NP-40 (Sigma), 0.1 M (NH4)2SO4, 20 mM Tris (pH 7.5), 10% glycerol, and 1× protease inhibitor (Roche). After 30 min of incubation under gentle agitation, cell lysates were centrifuged at 14,000 × g for 30 min. The soluble fraction was then assayed for protein content with the DC protein assay kit (Bio-Rad). Capture of biotinylated proteins was performed at a final volume of 500 μl, with 15 μl of packed ImmunoPure streptavidin-immobilized agarose beads (Pierce), using 240 μg of protein extract. Incubation was carried out under gentle agitation for 1 h. Beads were then washed three times in lysis buffer. Captured biotinylated proteins were then eluted from the beads by incubation for 5 min at 95°C in Laemmli sample buffer containing 5% of β-mercaptoethanol. Proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 10% acrylamide NuPAGE Novex bis-Tris precast gels (Invitrogen). Immunoprecipitations of CD4 were performed as described elsewhere (4).
CD4 degradation.
CD4 degradation was measured in HeLa-CD4 cells. Cells were transfected with a GFP-encoding plasmid in combination with p56lck- and/or Nef-encoding plasmids. The CD4 expression level at the cell surface was measured by flow cytometry analysis of the GFP-positive population. Alternatively, transfected cells were sorted based on GFP expression (MoFlo XPD cell sorter; Beckman Coulter). Cell lysates were prepared as described above, and 20 μg of proteins was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 12% acrylamide NuPAGE Novex bis-Tris precast gels (Invitrogen). When MG132 and NH4Cl treatments were performed, cells were transduced with the indicated viruses and treated 36 h later for a 6-hour period of time. Nef, CD4, p56lck, GFP, and p24 were visualized by Western blotting, followed by immunodetection. All quantifications were performed with ImageReader LAS-3000 software on enhanced chemiluminescence signals acquired with a charge-coupled-device camera (FujiFilm LAS-3000). Nef-induced CD4 degradation was calculated by normalizing the amount of CD4 detected in cells expressing Nef to that detected in the absence of Nef.
RESULTS
Nef and PMA differentially induce CD4 downregulation in lymphoid and myeloid cells.
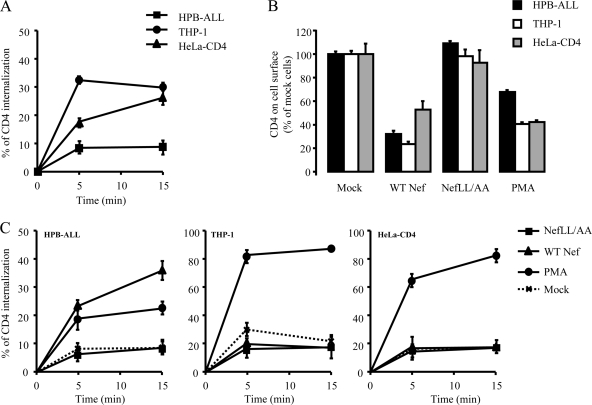
We first measured the basal internalization rate of CD4 in myeloid (THP-1 and U937), lymphoid (HPB-ALL), and CD4-expressing epithelial (HeLa-CD4) cells using a flow cytometry-based assay. Consistent with published results (18, 49-51, 68), Fig. 1A and Table 1 show that CD4 internalization in HPB-ALL cells followed slow kinetics (1.6%/min) compared with U937, THP-1, and HeLa-CD4 cells, in which the rate of CD4 internalization was two- to fourfold higher (4.5, 6.5, and 3.5%/min, respectively). We then investigated whether these differences could have an impact on the ability of WT HIV-1 Nef (WT Nef) to promote cell surface CD4 downregulation. HPB-ALL, THP-1, and HeLa-CD4 cells were transfected with a Nef-GFP construct, and the steady-state level of CD4 expressed at the cell surface was analyzed 24 h posttransfection. As shown in Fig. 1B, CD4 downregulation was efficient in the three cell lines. Nef induced a 70% decrease of cell surface CD4 in both HPB-ALL and THP-1 cells that displayed low and high CD4 internalization rates, respectively. CD4 downregulation was less efficient in HeLa-CD4 cells that displayed an intermediate basal CD4 internalization rate. As expected, no significant difference in CD4 expression levels was observed between mock-transfected cells and cells expressing Nef LL/AA, a Nef mutant which is deficient for the interaction with clathrin-associated AP complexes (9, 10, 15, 25). Similarly, treatment of THP-1, HPB-ALL, and HeLa-CD4 cells with PMA also resulted in CD4 downregulation (Fig. 1B). Of note, the effect of PMA was less pronounced in HPB-ALL cells (40% decrease) than in THP-1 and HeLa-CD4 cells (60% decrease). Overall, our data show that Nef and PMA efficiently downregulate CD4 in the three cell types studied, independent of the basal rate of CD4 internalization.
FIG. 1.
Effect of Nef and PMA on CD4 internalization in lymphoid, myeloid, and HeLa-CD4 cells. Cell surface expression of CD4 at steady state (B) as well as the CD4 internalization rate (A, C) were analyzed by FACS in lymphoid (HPB-ALL), myeloid (THP-1), and HeLa-CD4 cells expressing WT or mutant Nef-GFP or treated with PMA. (A) Cells were stained at 4°C with an anti-CD4 antibody and incubated at 37°C for 0, 5, and 15 min to allow for CD4 internalization. Cells were then cooled down and stained with Cy5-coupled secondary antibody. The percentage of internalized CD4 at each time point was calculated as described in Materials and Methods. Transfected cells were gated on the basis of GFP expression. (B) Steady-state levels of cell surface CD4 in cells expressing Nef-GFP (gated on the basis of GFP expression) or treated with PMA. Results are expressed as the percentage of cell surface CD4 on mock-treated/transfected cells. (C) CD4 internalization was assessed, as described in panel A, on transfected or PMA-treated cells. Values are the means of three independent experiments; error bars represent 1 standard deviation from the mean.
TABLE 1.
Initial rate of CD4 internalization in various cell lines
| Treatment | Initial rate of internalization ± SD (%) in indicated cell line
|
||||
|---|---|---|---|---|---|
| HPB-ALL | THP-1 | HeLa-CD4 | U937 | J.CaM | |
| Mocka | 1.6 ± 0.3b | 6.5 ± 0.2 | 3.5 ± 0.4 | 4.5 ± 1.6 | 2.9 ± 0.2 |
| WT Nef | 4.3 ± 0.7 | 4.5 ± 0.6 | 3.8 ± 0.4 | 5.6 ± 0.5 | 3.8 ± 1.2 |
| Nef LL/AA | 1.1 ± 0.3 | 4.1 ± 1.3 | 3.7 ± 1.3 | NDc | ND |
| PMAd | 3.6 ± 1.0 | 14.9 ± 0.2 | 12.9 ± 0.8 | ND | ND |
Nontransfected cells were incubated at 37°C for 5 min in the absence of PMA.
The percentage of CD4 internalized per minute was calculated over the first 5 minutes of internalization.
ND, not determined.
Cells were incubated at 37°C for 5 min in the presence of 100 ng/ml PMA.
Since it has been reported that Nef-induced CD4 downregulation can primarily result from an increase in the internalization rate of CD4 (2, 38, 44, 55), we investigated the impact of both Nef and PMA on the kinetics of CD4 internalization in HPB-ALL, THP-1, U937, and HeLa-CD4 cells. Nef LL/AA, which had no effect on CD4 cell surface expression at steady state, had no significant influence on the time course of CD4 internalization (Fig. 1C). As previously described (10), HPB-ALL cells expressing WT Nef displayed a higher rate of CD4 internalization than mock-transfected cells (Fig. 1C, left). On the contrary, the kinetics of CD4 internalization in THP-1, HeLa-CD4, and U937 cells were not affected by the expression of WT Nef (Fig. 1C, middle and right; Table 1), in spite of efficient downregulation of cell surface CD4 at steady state in these cell lines (Fig. 1B and data not shown). Interestingly, when cells were treated with PMA, an increase in the rate of CD4 internalization was seen in all three cell types (Fig. 1C). These results show that Nef is able to downregulate CD4 in the three cell types but that an increase of CD4 internalization rate is observed only in lymphoid cells (Fig. 1C; see Fig. S5 in the supplemental material).
Characterization of lymphoid T cells expressing CD4 mutants.
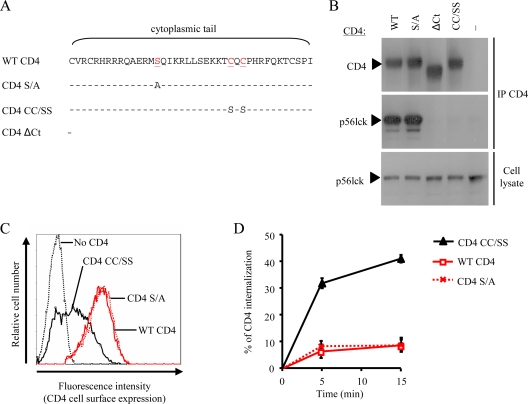
Given that p56lck is expressed in lymphoid cells but not in myeloid cells, we investigated whether this could account for the differential susceptibility of these cells to Nef-induced CD4 internalization. A mutant of CD4 unable to interact with p56lck was generated by introducing Ser substitutions for Cys420 and Cys422 that are required for p56lck binding (CD4 CC/SS) (Fig. 2A). Since phosphorylation of the cytoplasmic tail of CD4 has been reported to modulate both interaction with p56lck and recognition of its Leu-based sorting signal (52, 56), the contribution of Ser408 to CD4 was also investigated through substitution of this residue by Ala (CD4 S/A) (Fig. 2A).
FIG. 2.
Characterization of lymphoid cells stably expressing CD4 mutants. (A) Schematic representation of the human CD4 mutants. The amino acid sequences of the cytoplasmic tail of CD4 mutants are aligned with that of WT CD4. Dashes indicate amino acid identities with the WT protein, and red letters identify amino acid substitutions. (B) p56lck binding of CD4 mutants. Protein extracts were prepared 48 h posttransfection from 293T cells coexpressing p56lck together with WT CD4, CD4 S/A, CD4ΔCT, CD4 CC/SS, or no CD4. Crude extracts were subjected to CD4 immunoprecipitation (IP), followed by Western blotting and immunodetection analysis with anti-CD4 and anti-p56lck (top and middle, respectively). The p56lck expression level was also analyzed in whole-cell lysate (20 μg of protein/lane; bottom). (C) Cell surface expression of CD4 in A2.01 T cells stably expressing WT or CD4 mutants. (D) Kinetics of internalization for WT or CD4 mutants in transduced A2.01 T cells. Values are the means of three independent experiments; error bars represent 1 standard deviation from the mean.
We first checked the ability of the CD4 mutants to interact with p56lck by coimmunoprecipitation in 293T cells (Fig. 2B). As expected, p56lck was efficiently coprecipitated with WT CD4 but not with CD4ΔCT (Fig. 2B, middle), a mutant lacking the cytoplasmic tail of CD4 (Fig. 2A), in spite of having similar amounts of p56lck expressed in the cells (Fig. 2B, bottom). Similarly, substitution of Cys420 and Cys422 totally abolished the ability of CD4 to interact with p56lck. In contrast, substitution of Ser408 had no apparent effect on the ability of p56lck to coprecipitate with CD4 (CD4 S/A), indicating that this residue does not play a major role in the CD4/p56lck interaction.
To evaluate the impact of these mutations on the endocytosis of CD4, WT CD4 and the CD4 CC/SS and S/A mutants were stably expressed in a T-lymphoid cell line (A2.01) that expresses p56lck but lacks detectable endogenous CD4 expression (data not shown). Figure 2C shows that WT CD4 and CD4 S/A T-cell lines displayed similar levels of CD4 at the cell surface, whereas the CD4 CC/SS cell line had lower levels of cell surface CD4 at steady state. The kinetics of CD4 endocytosis were also analyzed and revealed that the rate of internalization was higher in CD4 CC/SS-expressing cells than in WT CD4- and CD4 S/A-expressing cells (6%/min and 2%/min, respectively) (Fig. 2D). Interestingly, the kinetics of CD4 CC/SS internalization in A2.01 T cells resembled those of WT CD4 expressed in nonlymphoid cells lacking p56lck expression, such as THP-1 and HeLa-CD4 cells (Table 1). These data confirm that the interaction of p56lck with CD4 results in the stabilization of CD4 at the plasma membrane. In addition, they indicate that Ser408 has no influence on the internalization of CD4 in T lymphoid cells.
Impact of p56lck expression on Nef-induced CD4 downregulation in T lymphoid cells.
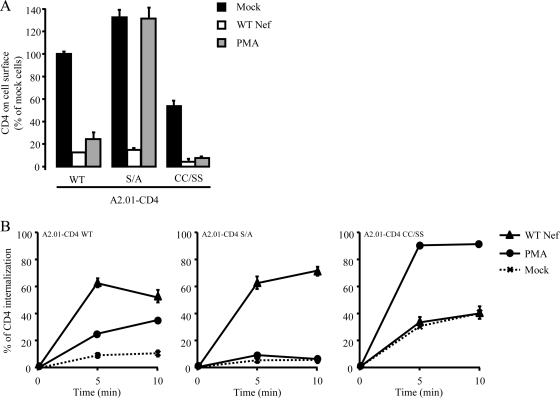
The A2.01 T-cell lines described above are valuable tools for the study of CD4 trafficking in a lymphoid background. These cells were used to analyze the impact of the interaction between p56lck and CD4 on PMA- and Nef-induced CD4 downregulation. An HIV-1-based transduction system was used to ensure efficient delivery of the Nef-IRES-EGFP sequence into the cell lines (75). Cell surface levels of CD4 were then analyzed by flow cytometry. As shown in Fig. 3A, Nef equally downregulated cell surface CD4, regardless of the CD4 mutant expressed. The effect of PMA was also analyzed and revealed that WT CD4 and CD4 CC/SS were equally susceptible to PMA-induced CD4 downregulation (Fig. 3A). PMA treatment had no effect on the cell surface levels of the CD4 S/A mutant, confirming that Ser408 is necessary for PMA-induced downregulation.
FIG. 3.
Nef- and PMA-induced CD4 downregulation analysis in lymphoid cell lines expressing CD4 mutants. A2.01 cell lines stably expressing WT CD4, CD4 S/A, or CD4 CC/SS were transduced to achieve Nef expression or treated with PMA prior to analysis of cell surface CD4 levels (A) and internalization (B), as described in the legend to Fig. 1. Values are the means of three independent experiments; error bars represent 1 standard deviation from the mean.
Kinetic analysis of CD4 internalization was also performed on the three A2.01-CD4 cell lines. Consistent with results obtained with HPB-ALL cells (Fig. 1C), Fig. 3B shows that both Nef and PMA increased the rate of CD4 internalization in A2.01 cells expressing WT CD4 (left). The rate of CD4 S/A internalization was also increased by Nef, but PMA had no effect on the trafficking of this mutant (Fig. 3B, middle). In contrast, PMA was fully efficient at increasing the rate of internalization of CD4 CC/SS, whereas Nef had no effect on the internalization rate of this mutant (Fig. 3B, right). This latter observation recapitulates the effect of Nef and PMA on THP-1 and HeLa-CD4 cells that lack p56lck (Fig. 1C).
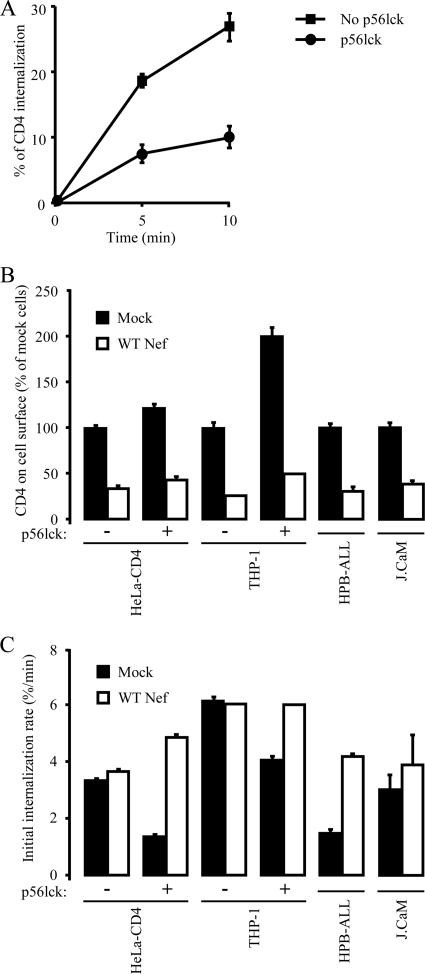
In order to determine whether p56lck expression was sufficient to reveal the effect of Nef on the increase of the rate of CD4 internalization, Nef-induced CD4 downregulation was analyzed in p56lck-negative cell lines reconstituted with p56lck. Because it has been reported that overexpression of p56lck could counteract the ability of Nef to downregulate cell surface CD4 (23) (see Fig. S1 in the supplemental material), p56lck was expressed at levels that do not inhibit the activity of Nef. Expression of p56lck in HeLa-CD4 cells resulted in a 25% increase in the cell surface level of CD4 at steady state and provoked a marked decrease in the basal rate of CD4 internalization from 3.6 to 1.5%/min (Fig. 4A and B), indicating that the expression of p56lck is sufficient to stabilize CD4 at the cell surface. In this context, Nef was still able to downregulate cell surface CD4 as efficiently as in HeLa-CD4 cells that did not express p56lck (Fig. 4B). We then investigated the impact of p56lck expression in HeLa-CD4 cells on the ability of Nef to increase the rate of CD4 internalization. As previously shown (Fig. 1), expression of Nef in HeLa-CD4 cells resulted in the efficient downregulation of cell surface CD4 (Fig. 4B), but Nef did not affect the rate of CD4 internalization in this cell line (Fig. 4C). In contrast, Nef significantly increased the rate of CD4 endocytosis from 1.5 to 5%/min in p56lck-expressing HeLa-CD4 cells (Fig. 4C). Similarly, expression of p56lck in THP-1 myeloid cells resulted in the stabilization of CD4 at the cell surface and enabled Nef to increase the rate of CD4 internalization (Fig. 4B and C). Experiments performed in T-cell lines that expressed or did not express functional p56lck such as HPB-ALL and J.CaM cell lines, respectively, also demonstrated that in the absence of a CD4/p56lck interaction that stabilizes CD4 at the plasma membrane, Nef did not increase the rate of CD4 internalization (Fig. 4B and C). All together, these results indicate that the ability of Nef to increase the rate of CD4 internalization strictly depends on the CD4/p56lck interaction and does not require additional T-cell-specific factors.
FIG. 4.
Nef-induced CD4 downregulation analysis of THP-1 and HeLa-CD4 cells reconstituted with p56lck. (A) The kinetics of CD4 internalization were measured in HeLa-CD4 cells expressing or not expressing p56lck, as described in the legend to Fig. 1B. (B and C) The indicated cell lines were transfected to achieve Nef and p56lck expression. Steady-state levels of cell surface CD4 (B) and the rate of CD4 internalization (C) were measured, as described in the legend to Fig. 1. Data plotted in panel C represent the rate of CD4 internalization calculated over the first 5 min of internalization. Values are the means of three independent experiments; error bars represent 1 standard deviation from the mean.
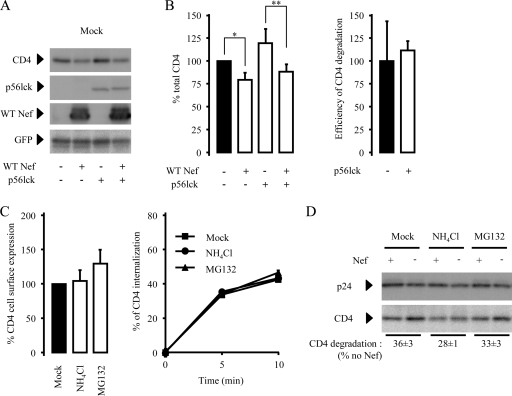
Nef induces degradation of CD4, regardless of the presence of p56lck.
We investigated the fate of CD4 molecules in Nef-expressing cells in the presence or absence of p56lck. HeLa-CD4 cells were cotransfected with constructs encoding GFP along with constructs encoding either WT Nef or p56lck or both constructs, as indicated in Fig. 5A. As previously noticed in HeLa cells, p56lck induced a significant increase in cell surface levels of CD4, and Nef downregulated cell surface levels of CD4 at steady state, regardless of the presence of p56lck (not shown). GFP-positive cells were sorted 24 h posttransfection to recover Nef- and p56lck-expressing cells. Whole-cell lysates were prepared and analyzed by Western blotting, followed by immunodetection. As shown in Fig. 5A and B, there was a reproducible ∼25% decrease in the total amount of CD4 in Nef-expressing cells compared with that in mock-transfected cells (P < 0.05), confirming that CD4 internalization induced by Nef leads to CD4 degradation. Similar results were obtained in p56lck-expressing cells (P < 0.01), indicating that expression of p56lck has no influence on the efficiency of CD4 degradation by Nef (Fig. 5B, right). We next investigated the mechanisms responsible for Nef-induced CD4 degradation. Cells were treated with 100 mM NH4Cl in order to buffer lysosome acidification and to inhibit pH-dependent proteases or with 10 μM MG132 in order to inhibit the proteasome-dependent degradation pathway. To check the efficiency of such treatments, cells were incubated with VSV-G-pseudotyped GFP reporter viruses in the presence of NH4Cl or MG132. In agreement with published results, a 6-hour treatment with NH4Cl inhibited lysosome acidification and VSV-G-pseudotyped virus entry into treated cells, whereas MG132 slightly increased virus entry (see Fig. S2 in the supplemental material) (1, 46, 65, 74). We next analyzed the impact of a 6-hour treatment of HeLa-CD4 cells with NH4Cl or MG132 on the trafficking of CD4. Figure 5C shows that neither cell surface levels of CD4 at steady state nor the kinetics of CD4 internalization were affected by such treatments. The effect of NH4Cl and MG132 on Nef-induced CD4 degradation was then investigated. HeLa-CD4 cells were transduced with viruses containing a Nef-IRES-EGFP or IRES-EGFP sequence (Fig. 3) and treated 16 h later with NH4Cl or MG132 for 6 h, as indicated in Fig. 5D. While Nef expression provoked a ∼36% decrease of whole-cell CD4 levels in mock-treated cells, a weaker decrease was measured when cells were treated with NH4Cl (∼28%). This means that a 6-hour incubation time with NH4Cl reverts ∼22% of CD4 degradation induced by Nef. When cells were treated with MG132, Nef-induced CD4 degradation was also slightly inhibited but not to the same extent as when cells were treated with NH4Cl. Together, these results indicate that Nef-induced CD4 degradation relies mostly on lysosomal activity (34, 40, 58).
FIG. 5.
Degradation of CD4 in Nef-expressing cells. (A) HeLa-CD4 cells were cotransfected with a construct encoding GFP along with the indicated constructs. Cells were then sorted by FACS based on GFP expression and solubilized to analyze the expression levels of CD4, Nef, p56lck, and GFP in cleared lysates. (B) CD4 levels were normalized to that of GFP and set to 100% in cells expressing neither Nef nor p56lck (left). CD4 degradation calculated as (CD4no Nef − CD4Nef)/CD4no Nef was set to 100% in cells expressing no p56lck (right). An average of three independent experiments is shown (t test: *, P of <0.05; **, P of <0.01). (C) The impact of a 6-hour treatment with NH4Cl (100 mM) and MG132 (20 μM) on the levels of cell surface CD4 (left) and the rate of CD4 internalization (right) was measured, as described in the legend to Fig. 1. (D) HeLa-CD4 cells were incubated with VSV-G-pseudotyped viruses expressing or not expressing Nef (+ and −, respectively) to achieve 100% of transduction efficiency. Cells were then treated for 6 h with the indicated chemicals and solubilized to analyze the expression level of CD4 (bottom) and p24 (top). CD4 levels were normalized to that of p24, and CD4 degradation was calculated for each treatment as 100 × (CD4no Nef − CD4Nef)/CD4no Nef. Immunodetection signals were acquired as indicated in Materials and Methods. Error bars represent 1 standard deviation from the mean.
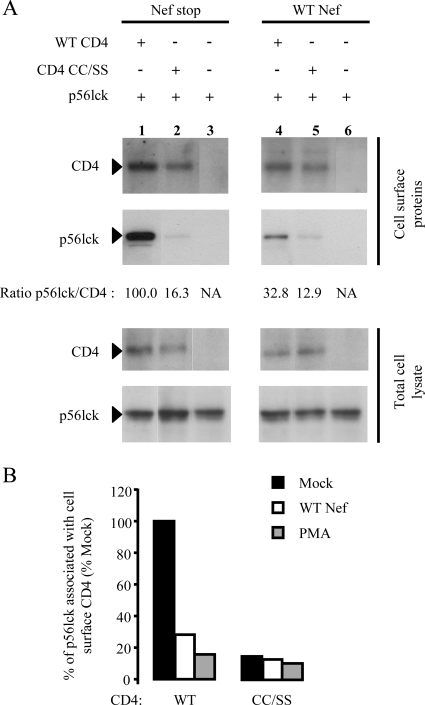
The Nef-induced acceleration of CD4 internalization is related to a reduction of CD4/p56lck association at the plasma membrane.
According to the results reported in Fig. 3 and 4, the dissociation of the CD4/p56lck complex at the cell surface is a conceivable hypothesis to explain the Nef- or PMA-dependent increase in the rate of CD4 internalization. We investigated the impact of Nef on the CD4/p56lck complex at the plasma membrane using a cell surface protein biotinylation assay. Since the kinetics of CD4 endocytosis in HeLa-CD4 cells expressing p56lck and T cells are comparable, with respect to basal and Nef-induced endocytosis, the analysis of the CD4/p56lck complex at the cell surface was performed with HeLa cells coexpressing the two proteins. As shown in Fig. 6A, cell surface protein biotinylation of cells expressing both WT CD4 and p56lck allowed the coprecipitation of CD4 and p56lck (top, lane 1). No p56lck was precipitated from HeLa cells expressing p56lck alone (Fig. 6A, lane 3). This confirms that the precipitation of p56lck from HeLa cells expressing both WT CD4 and p56lck reflects the interaction of CD4 and p56lck at the plasma membrane. Moreover, it indicates that no protein at the plasma membrane of HeLa cells can mediate an interaction with p56lck. When HeLa cells were cotransfected with p56lck and CD4 CC/SS, only background levels of p56lck were coprecipitated with CD4 (Fig. 6A, lane 2). In agreement with the results showing that the cell surface level of CD4 CC/SS was lower in A2.01 T cells (Fig. 2C and 3A), densitometry analysis indicated that CD4 CC/SS was less abundant at the plasma membrane than WT CD4. As expected, less WT CD4 was also retrieved from the plasma membranes of cells coexpressing Nef, p56lck, and CD4 compared with that retrieved from the plasma membranes of cells expressing only CD4 and p56lck (Fig. 6, top, compare lanes 1 and 4). Analysis of the material precipitated with cell surface CD4 revealed that the expression of Nef induced a 70% decrease of the amount of p56lck associated with CD4 at the cell surface (Fig. 6B). As previously reported (52), PMA treatment also severely affected the amount of p56lck associated with cell surface CD4 (Fig. 6B). All together, these results show that the Nef-induced increase in the CD4 internalization rate is concomitant to a reduced association of p56lck with CD4 at the plasma membrane.
FIG. 6.
Nef-induced decrease of CD4/p56lck association at the plasma membrane. (A) HeLa cells coexpressing p56lck and either WT CD4 or CD4 CC/SS were transfected to achieve Nef expression or treated with PMA. After biotinylation of the cell surface proteins, biotinylated CD4 was immunoprecipitated, as described in Materials and Methods, and both CD4 and p56lck were visualized by Western blotting, followed by immunodetection in the precipitated material (top) or in cleared lysates (20 μg of protein/lane; bottom). The intensity of the CD4 and p56lck bands was quantified by densitometry using NIH Image software, and the p56lck signal was normalized to that of cell surface CD4 (NA, not applicable). (B) Plotted data represent the percentage of the CD4-associated p56lck signal intensity relative to that of mock-transfected or mock-treated cells expressing WT CD4 (100%) and are representative of three independent experiments.
DISCUSSION
In spite of extensive study, the molecular mechanisms involved in Nef-induced CD4 downregulation remain poorly understood. In lymphoid cells where CD4 interacts with p56lck, it is questioned whether Nef causes a decrease in the level of CD4 cell surface expression through downregulation of the CD4/p56lck complex or through disruption of the interaction between CD4 and p56lck (24, 34, 45, 48, 52, 61, 68). In this paper, we investigated how Nef downregulates CD4 from the surface of HIV-1 host cells. Steady-state analysis revealed that Nef causes downregulation of cell surface CD4 in all cell types; however, Nef caused an increase in the rate of CD4 internalization in lymphoid cells but not in myeloid cells. Our results demonstrate that this phenomenon strictly depends on the ability of p56lck to interact with CD4 and is independent of any other specific component of the lymphoid lineage. We also observed that the expression of Nef causes a net decrease in the association of p56lck with cell surface CD4 and reroutes internalized CD4 toward the degradation pathway. Taken together, our data indicate that Nef uses different mechanisms in lymphoid and myeloid cells to downregulate CD4 from the cell surface.
It was previously established that p56lck expression causes the stabilization of CD4 at the cell surface of T cells by targeting CD4 to lipid rafts and promoting its exclusion from clathrin-coated pits (50, 52). In agreement with previous results (18, 49-51, 68), we observed that the basal rate of CD4 internalization was lower in p56lck-positive cells of the lymphoid lineage than in p56lck-negative cells of the myeloid lineage or in HeLa cells stably expressing CD4. Expression levels of p56lck in HeLa-CD4 and THP-1 cells were sufficient to decrease the rate of CD4 internalization. In addition, Nef was able to increase the rate of CD4 internalization in THP-1 and HeLa-CD4 cells only when p56lck was expressed. Conversely, the rate of internalization of CD4 CC/SS, a mutant unable to interact with p56lck, was higher than that of WT CD4 and was not increased further by Nef, even in a T-cell background. Similarly, no significant increase in the CD4 internalization rate was induced by Nef in J.CaM T cells that lack p56lck expression. These results demonstrate that the interaction between p56lck and CD4 is responsible for the different basal and Nef-induced alterations of CD4 trafficking observed between lymphoid and myeloid cells. Our results also suggest that, in the absence of p56lck, Nef does not affect the rate of CD4 internalization but induces cell surface CD4 downregulation by promoting the degradation of internalized CD4 molecules. This is in agreement with published data showing that Nef inhibits the recycling of internalized CD4 back to the plasma membrane (55, 58, 62). Similarly, we previously showed that Nef downregulates cell surface expression of the transferrin receptor, without affecting its internalization, by inhibiting its recycling to the plasma membrane (43).
In p56lck-negative cells that naturally express CD4 (macrophages and monocytes) or exogenous CD4 (HeLa and 293T cells), the rate of internalization for CD4 is constitutively high, independent of Nef (18, 49-51, 68; this paper). The ability of Nef to redirect internalized CD4 to the lysosomal degradation compartments can thus result in an overall decrease in cell surface levels of CD4 at steady state in these cells. On the contrary, in cells that express p56lck, CD4 is stabilized at the plasma membrane (50, 52; this paper), and the lysosomal targeting of internalized CD4 would not suffice to decrease cell surface levels of CD4 at steady state. It is thus conceivable that Nef first needs to increase the rate of CD4 internalization in p56lck-expressing lymphoid cells before promoting the lysosomal degradation of internalized CD4.
Of note, the p56lck complementation assays in HeLa-CD4 and THP-1 cells revealed that cell surface CD4 downregulation by Nef was equally efficient, regardless of p56lck expression. In addition, the proportion of Nef-induced CD4 degradation in HeLa-CD4 cells was identical (∼25%), regardless of p56lck expression. The fact that Nef causes an increase in CD4 internalization in p56lck-positive cells but not in p56lck-negative cells strongly suggests that there is no component in HeLa or myeloid cells that can compensate for the absence of p56lck. p59hck, another member of the Src kinase family specifically expressed in myeloid cells, was shown to interact with CD4 (42), but there is poor understanding of both the physiological role of CD4 and the biological relevance of the p59hck/CD4 interaction in myeloid cells. The fact that CD4 is rapidly internalized in myeloid cells argues against a role for p59hck in the exclusion of CD4 from clathrin-coated pits. In addition, the absence of Nef-induced acceleration of CD4 internalization in these cells further confirms that p59hck and p56lck have a different impact on the trafficking of CD4.
CD4 trafficking was also examined in cells treated with PMA. The activation of PKC by treating cells with PMA is believed to promote the phosphorylation of the cytoplasmic tail of CD4 on Ser residues (Ser408, Ser415, and Ser431); these phosphorylations are concomitant to an increase in the rate of CD4 internalization (32, 33, 41, 48, 52, 56, 66, 67). If there is a general consensus regarding a minor role for Ser431 phosphorylation in CD4 internalization, the importance of Ser408 and Ser415 in this process is still debated. The analysis of the cell surface levels of various CD4 mutants expressed in HeLa cells revealed that Ala substitution for the three Ser residues (Ser408, Ser 415, and Ser431) was necessary to abrogate the ability of PMA to downregulate CD4 (66). Surface plasmon resonance analysis also showed that a peptide derived from the cytoplasmic tail of CD4 in which Ser408 or Ser415 are phosphorylated binds more efficiently to purified AP-2 complexes than the unphosphorylated peptide. This suggests that Ser415 phosphorylation can compensate for the Ser408/Ala substitution (56). On the contrary, our results show that an Ala substitution for Ser408 (CD4 S/A mutant) was sufficient to render CD4 refractory to PMA-induced downregulation, indicating that Ser415 and Ser431 cannot compensate for the absence of Ser408 in T cells. Differences in the experimental systems might account for these discrepancies. The fact that Nef equally affected the trafficking of WT CD4 and CD4 S/A confirms that Nef-induced CD4 downregulation is independent of Ser408 phosphorylation (20) and that Nef and PMA use distinct mechanisms to promote cell surface CD4 internalization (33).
In spite of these differences, both Nef- and PMA-induced CD4 downregulation require the integrity of a Leu-based sorting motif located in the cytoplasmic tail of CD4 that is masked by p56lck in T lymphoid cells (2, 5, 24, 31, 56, 60, 68). In addition, there is evidence that the dissociation of the CD4/p56lck complex occurs during PMA-induced internalization of CD4 in T cells (52). In order to investigate whether a similar mechanism can occur during Nef-induced CD4 downregulation, we designed a cell surface biotinylation assay which renders possible the analysis of the CD4/p56lck complex at the cell surface. This assay enabled us to confirm that upon treatment with PMA, less p56lck is coprecipitated with cell surface CD4. We also observed that the level of p56lck associated with CD4 at the cell surface is decreased in the presence of Nef. This could explain why upon Nef expression, the kinetics of cell surface CD4 internalization are similar to those of WT CD4 in p56lck-negative cells in the absence of Nef.
Several mechanisms can be put forward to explain how Nef could disrupt the CD4/p56lck complex. Nef could displace this interaction through binding to p56lck. Such an interaction between the poly-proline 72PxxP75 motif of Nef and the SH3 domain of p56lck has been described (28). Moreover, it was also recently reported that Nef affects the localization of p56lck and that this function requires the integrity of the 72PxxP75 motif of Nef (30, 72). Since the ability of Nef to increase the rate of CD4 internalization in T cells does not depend on the 72PxxP75 motif (10) (see Fig. S3 in the supplemental material), the disruption of the CD4/p56lck complex by a Nef/p56lck interaction is unlikely to explain this phenotype. Alternatively, the disruption of this complex could be explained by a direct interaction between Nef and the cytoplasmic domain of CD4 (5, 14, 29, 57). In this model, the Nef-induced internalization of CD4 would be a two-step process, as follows: first, Nef would disrupt the CD4/p56lck interaction at the plasma membrane, and second, the Leu-based motif of Nef would then drive the CD4/Nef complex to the clathrin-dependent endocytic machinery (10, 12, 32), leading to CD4 internalization. This “connector” model is largely in agreement with the results reported in the present study. However, there is evidence that the interaction between p56lck and CD4 is strong, as opposed to the weak interaction detected between Nef and CD4 (29), and occurs early during the biosynthesis of the two proteins Therefore, additional investigations are required to understand exactly how Nef could dissociate the CD4/p56lck complex at the plasma membrane.
Alternatively, the dissociation of the CD4/p56lck complex could also be a consequence of, and not a prerequisite for, the targeting of CD4 to endocytic structures such as clathrin-coated pits. The fact that PMA treatment results in the increase of CD4 internalization, regardless of the expression of p56lck, suggests that the increase in the CD4 internalization rate does not necessarily require the dissociation of a preexisting CD4/p56lck complex. It can thus be speculated that the presence of Nef at the plasma membrane (10, 19) locally increases the pool of AP-2 complexes in the vicinity of CD4. In T lymphocytes where the Leu-based sorting motif of CD4 targeted by AP-2 is hidden by the interaction with p56lck, the enrichment of AP-2 could compensate for this steric hindrance, allowing AP-2 to access this motif and to promote CD4 internalization. In nonlymphoid cells that lack p56lck, the accessibility of the Leu-based AP-binding site in CD4 is already optimal, and the Nef-dependent enrichment of AP-2 at the plasma membrane would not further increase the rate of CD4 internalization. Interestingly, the affinity of simian immunodeficiency virus Nef for AP-2 is higher than that of HIV-1 Nef (54, 60); however, it did not further increase the rate of CD4 internalization in p56lck-negative cells (see Fig. S4 in the supplemental material). This suggests that the limiting factor in CD4 internalization is the availability of AP-2 in the vicinity of CD4 and that HIV-1 Nef overcomes this limiting factor.
The fact that Nef might increase the availability of AP-2 for the di-Leu motif of CD4 while PMA-induced phosphorylation of CD4 increases the affinity of this motif for AP-2 could explain the stronger effect of PMA: PMA increases CD4 internalization, regardless of the presence of p56lck, whereas a Nef-dependent increase in the rate of CD4 endocytosis is seen only in the presence of p56lck. In either case, the requirement of AP-2 in the whole process remains undeniable.
Interestingly, published work reported that under certain circumstances, Nef could increase the rate of CD4 internalization in cells that do not express p56lck. Blagoveshchenskaya et al. used a vaccinia virus-based expression system to achieve expression of Nef in HeLa-CD4 cells and found a significant increase in the rate of CD4 internalization in Nef-expressing cells (7). The high levels of expression for Nef achieved in this study, as opposed to the HIV-1 long terminal repeat-driven expression of Nef used in our study, might explain these differences. Of note, similar levels of vaccinia virus-based expression for HIV-1 Nef used by others revealed no or a modest increase of the rate of CD4 internalization in p56lck-negative cells (39, 60). In addition, this increase is measurable within the first minutes of the kinetics and disappears after 5 min of internalization (39). These data are in agreement with our results and confirm that physiological levels of Nef cannot significantly increase the rate of CD4 internalization in cells that do not express p56lck.
The ability of Nef to downregulate the cell surface expression of CD4 is an important feature regarding the impact of Nef on infectivity and pathogenesis, both in infected patients and in animal models (71). The results reported herein give new insight into this function of Nef in the natural target cells of HIV-1. Our results demonstrate that the presence of p56lck has a direct impact on the mechanism used by Nef to downregulate the expression of CD4 at the cell surfaces of T lymphocytes. They reveal that Nef uses distinct mechanisms to reduce cell surface expression of CD4 in either lymphoid or myeloid target cells of HIV-1.
Supplementary Material
Acknowledgments
We thank Heinrich Gottlinger for providing us with the plasmid encoding WT Nef fused to the hemagglutinin epitope. We are grateful to Frank Kirchhoff for providing us with the HIV-1 NL4-3 proviral constructs used in this study. We also thank Stefano Marullo, Georges Bismuth, and Olivier Schwartz for providing us with the U937, HPB-ALL, and J.CaM cell lines, respectively. Finally, we thank Mark Marsh for the gift of CD4-expressing HeLa cells. We are indebted to Brigitte Chanaud, Laurence Stouvenel, and Martine De Sousa of the flow cytometry core facility for expert technical assistance.
This work was supported by grants from Agence Nationale de Recherches sur le SIDA et les Hépatites Virales (ANRS) and Sidaction to S. Benichou. S. Basmaciogullari was supported successively by ANRS and INSERM fellowships. N. Laguette is supported by a Ph.D. fellowship from the French Ministry of Research.
Footnotes
Published ahead of print on 13 May 2009.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Aiken, C. 1997. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J. Virol. 715871-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aiken, C., J. Konner, N. R. Landau, M. E. Lenburg, and D. Trono. 1994. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell 76853-864. [DOI] [PubMed] [Google Scholar]
- 3.Arganaraz, E. R., M. Schindler, F. Kirchhoff, M. J. Cortes, and J. Lama. 2003. Enhanced CD4 down-modulation by late stage HIV-1 nef alleles is associated with increased Env incorporation and viral replication. J. Biol. Chem. 27833912-33919. [DOI] [PubMed] [Google Scholar]
- 4.Basmaciogullari, S., B. Pacheco, S. Bour, and J. Sodroski. 2006. Specific interaction of CXCR4 with CD4 and CD8alpha: functional analysis of the CD4/CXCR4 interaction in the context of HIV-1 envelope glycoprotein-mediated membrane fusion. Virology 35352-67. [DOI] [PubMed] [Google Scholar]
- 5.Bentham, M., S. Mazaleyrat, and M. Harris. 2003. The di-leucine motif in the cytoplasmic tail of CD4 is not required for binding to human immunodeficiency virus type 1 Nef, but is critical for CD4 down-modulation. J. Gen. Virol. 842705-2713. [DOI] [PubMed] [Google Scholar]
- 6.Birch, M. R., J. C. Learmont, W. B. Dyer, N. J. Deacon, J. J. Zaunders, N. Saksena, A. L. Cunningham, J. Mills, and J. S. Sullivan. 2001. An examination of signs of disease progression in survivors of the Sydney Blood Bank Cohort (SBBC). J. Clin. Virol. 22263-270. [DOI] [PubMed] [Google Scholar]
- 7.Blagoveshchenskaya, A. D., L. Thomas, S. F. Feliciangeli, C. H. Hung, and G. Thomas. 2002. HIV-1 Nef downregulates MHC-I by a PACS-1- and PI3K-regulated ARF6 endocytic pathway. Cell 111853-866. [DOI] [PubMed] [Google Scholar]
- 8.Bour, S., C. Perrin, and K. Strebel. 1999. Cell surface CD4 inhibits HIV-1 particle release by interfering with Vpu activity. J. Biol. Chem. 27433800-33806. [DOI] [PubMed] [Google Scholar]
- 9.Bresnahan, P. A., W. Yonemoto, S. Ferrell, D. Williams-Herman, R. Geleziunas, and W. C. Greene. 1998. A dileucine motif in HIV-1 Nef acts as an internalization signal for CD4 downregulation and binds the AP-1 clathrin adaptor. Curr. Biol. 81235-1238. [DOI] [PubMed] [Google Scholar]
- 10.Burtey, A., J. Z. Rappoport, J. Bouchet, S. Basmaciogullari, J. Guatelli, S. M. Simon, S. Benichou, and A. Benmerah. 2007. Dynamic interaction of HIV-1 Nef with the clathrin-mediated endocytic pathway at the plasma membrane. Traffic 861-76. [DOI] [PubMed] [Google Scholar]
- 11.Carl, S., T. C. Greenough, M. Krumbiegel, M. Greenberg, J. Skowronski, J. L. Sullivan, and F. Kirchhoff. 2001. Modulation of different human immunodeficiency virus type 1 Nef functions during progression to AIDS. J. Virol. 753657-3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaudhuri, R., O. W. Lindwasser, W. J. Smith, J. H. Hurley, and J. S. Bonifacino. 2007. Downregulation of CD4 by human immunodeficiency virus type 1 Nef is dependent on clathrin and involves direct interaction of Nef with the AP2 clathrin adaptor. J. Virol. 813877-3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chowers, M. Y., C. A. Spina, T. J. Kwoh, N. J. Fitch, D. D. Richman, and J. C. Guatelli. 1994. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J. Virol. 682906-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cluet, D., C. Bertsch, C. Beyer, L. Gloeckler, M. Erhardt, J. P. Gut, J. L. Galzi, and A. M. Aubertin. 2005. Detection of human immunodeficiency virus type 1 Nef and CD4 physical interaction in living human cells by using bioluminescence resonance energy transfer. J. Virol. 798629-8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coleman, S. H., N. Van Damme, J. R. Day, C. M. Noviello, D. Hitchin, R. Madrid, S. Benichou, and J. C. Guatelli. 2005. Leucine-specific, functional interactions between human immunodeficiency virus type 1 Nef and adaptor protein complexes. J. Virol. 792066-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins, K. L., and D. Baltimore. 1999. HIV's evasion of the cellular immune response. Immunol. Rev. 16865-74. [DOI] [PubMed] [Google Scholar]
- 17.Dyer, W. B., A. F. Geczy, S. J. Kent, L. B. McIntyre, S. A. Blasdall, J. C. Learmont, and J. S. Sullivan. 1997. Lymphoproliferative immune function in the Sydney Blood Bank Cohort, infected with natural nef/long terminal repeat mutants, and in other long-term survivors of transfusion-acquired HIV-1 infection. AIDS 111565-1574. [DOI] [PubMed] [Google Scholar]
- 18.Foti, M., L. Cartier, V. Piguet, D. P. Lew, J. L. Carpentier, D. Trono, and K. H. Krause. 1999. The HIV Nef protein alters Ca(2+) signaling in myelomonocytic cells through SH3-mediated protein-protein interactions. J. Biol. Chem. 27434765-34772. [DOI] [PubMed] [Google Scholar]
- 19.Foti, M., A. Mangasarian, V. Piguet, D. P. Lew, K. H. Krause, D. Trono, and J. L. Carpentier. 1997. Nef-mediated clathrin-coated pit formation. J. Cell Biol. 13937-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia, J. V., and A. D. Miller. 1991. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature 350508-511. [DOI] [PubMed] [Google Scholar]
- 21.Geleziunas, R., S. Bour, and M. A. Wainberg. 1994. Cell surface down-modulation of CD4 after infection by HIV-1. FASEB J. 8593-600. [DOI] [PubMed] [Google Scholar]
- 22.Geyer, M., O. T. Fackler, and B. M. Peterlin. 2001. Structure-function relationships in HIV-1 Nef. EMBO Rep. 2580-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldsmith, M. A., M. T. Warmerdam, R. E. Atchison, M. D. Miller, and W. C. Greene. 1995. Dissociation of the CD4 downregulation and viral infectivity enhancement functions of human immunodeficiency virus type 1 Nef. J. Virol. 694112-4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gratton, S., X. J. Yao, S. Venkatesan, E. A. Cohen, and R. P. Sekaly. 1996. Molecular analysis of the cytoplasmic domain of CD4: overlapping but noncompetitive requirement for lck association and down-regulation by Nef. J. Immunol. 1573305-3311. [PubMed] [Google Scholar]
- 25.Greenberg, M., L. DeTulleo, I. Rapoport, J. Skowronski, and T. Kirchhausen. 1998. A dileucine motif in HIV-1 Nef is essential for sorting into clathrin-coated pits and for downregulation of CD4. Curr. Biol. 81239-1242. [DOI] [PubMed] [Google Scholar]
- 26.Greenberg, M. E., S. Bronson, M. Lock, M. Neumann, G. N. Pavlakis, and J. Skowronski. 1997. Co-localization of HIV-1 Nef with the AP-2 adaptor protein complex correlates with Nef-induced CD4 down-regulation. EMBO J. 166964-6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greene, W. C., and B. M. Peterlin. 2002. Charting HIV's remarkable voyage through the cell: basic science as a passport to future therapy. Nat. Med. 8673-680. [DOI] [PubMed] [Google Scholar]
- 28.Greenway, A., A. Azad, J. Mills, and D. McPhee. 1996. Human immunodeficiency virus type 1 Nef binds directly to Lck and mitogen-activated protein kinase, inhibiting kinase activity. J. Virol. 706701-6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grzesiek, S., S. J. Stahl, P. T. Wingfield, and A. Bax. 1996. The CD4 determinant for downregulation by HIV-1 Nef directly binds to Nef. Mapping of the Nef binding surface by NMR. Biochemistry 3510256-10261. [DOI] [PubMed] [Google Scholar]
- 30.Haller, C., S. Rauch, and O. T. Fackler. 2007. HIV-1 Nef employs two distinct mechanisms to modulate Lck subcellular localization and TCR induced actin remodeling. PLoS ONE 2e1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janvier, K., H. Craig, S. Le Gall, R. Benarous, J. Guatelli, O. Schwartz, and S. Benichou. 2001. Nef-induced CD4 downregulation: a diacidic sequence in human immunodeficiency virus type 1 Nef does not function as a protein sorting motif through direct binding to beta-COP. J. Virol. 753971-3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin, Y. J., C. Y. Cai, X. Zhang, H. T. Zhang, J. A. Hirst, and S. J. Burakoff. 2005. HIV Nef-mediated CD4 down-regulation is adaptor protein complex 2 dependent. J. Immunol. 1753157-3164. [DOI] [PubMed] [Google Scholar]
- 33.Jin, Y. J., X. Zhang, J. G. Boursiquot, and S. J. Burakoff. 2004. CD4 phosphorylation partially reverses Nef down-regulation of CD4. J. Immunol. 1735495-5500. [DOI] [PubMed] [Google Scholar]
- 34.Kim, Y. H., S. H. Chang, J. H. Kwon, and S. S. Rhee. 1999. HIV-1 Nef plays an essential role in two independent processes in CD4 down-regulation: dissociation of the CD4-p56(lck) complex and targeting of CD4 to lysosomes. Virology 257208-219. [DOI] [PubMed] [Google Scholar]
- 35.Klatzmann, D., E. Champagne, S. Chamaret, J. Gruest, D. Guetard, T. Hercend, J. C. Gluckman, and L. Montagnier. 1984. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature 312767-768. [DOI] [PubMed] [Google Scholar]
- 36.Lama, J., A. Mangasarian, and D. Trono. 1999. Cell-surface expression of CD4 reduces HIV-1 infectivity by blocking Env incorporation in a Nef- and Vpu-inhibitable manner. Curr. Biol. 9622-631. [DOI] [PubMed] [Google Scholar]
- 37.Learmont, J. C., A. F. Geczy, J. Mills, L. J. Ashton, C. H. Raynes-Greenow, R. J. Garsia, W. B. Dyer, L. McIntyre, R. B. Oelrichs, D. I. Rhodes, N. J. Deacon, and J. S. Sullivan. 1999. Immunologic and virologic status after 14 to 18 years of infection with an attenuated strain of HIV-1. A report from the Sydney Blood Bank Cohort. N. Engl. J. Med. 3401715-1722. [DOI] [PubMed] [Google Scholar]
- 38.Le Gall, S., L. Erdtmann, S. Benichou, C. Berlioz-Torrent, L. Liu, R. Benarous, J. M. Heard, and O. Schwartz. 1998. Nef interacts with the mu subunit of clathrin adaptor complexes and reveals a cryptic sorting signal in MHC I molecules. Immunity 8483-495. [DOI] [PubMed] [Google Scholar]
- 39.Lindwasser, O. W., W. J. Smith, R. Chaudhuri, P. Yang, J. H. Hurley, and J. S. Bonifacino. 2008. A diacidic motif in human immunodeficiency virus type 1 Nef is a novel determinant of binding to AP-2. J. Virol. 821166-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo, T., S. J. Anderson, and J. V. Garcia. 1996. Inhibition of Nef- and phorbol ester-induced CD4 degradation by macrolide antibiotics. J. Virol. 701527-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo, T., J. R. Downing, and J. V. Garcia. 1997. Induction of phosphorylation of human immunodeficiency virus type 1 Nef and enhancement of CD4 downregulation by phorbol myristate acetate. J. Virol. 712535-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lynch, G. W., S. Turville, B. Carter, A. J. Sloane, A. Chan, N. Muljadi, S. Li, L. Low, P. Armati, R. Raison, H. Zoellner, P. Williamson, A. Cunningham, and W. B. Church. 2006. Marked differences in the structures and protein associations of lymphocyte and monocyte CD4: resolution of a novel CD4 isoform. Immunol. Cell Biol. 84154-165. [DOI] [PubMed] [Google Scholar]
- 43.Madrid, R., K. Janvier, D. Hitchin, J. Day, S. Coleman, C. Noviello, J. Bouchet, A. Benmerah, J. Guatelli, and S. Benichou. 2005. Nef-induced alteration of the early/recycling endosomal compartment correlates with enhancement of HIV-1 infectivity. J. Biol. Chem. 2805032-5044. [DOI] [PubMed] [Google Scholar]
- 44.Mangasarian, A., M. Foti, C. Aiken, D. Chin, J. L. Carpentier, and D. Trono. 1997. The HIV-1 Nef protein acts as a connector with sorting pathways in the Golgi and at the plasma membrane. Immunity 667-77. [DOI] [PubMed] [Google Scholar]
- 45.Marracci, G. H., W. E. Marquardt, A. Strehlow, G. P. McKeon, J. Gross, D. C. Buck, L. B. Kozell, and D. N. Bourdette. 2006. Lipoic acid downmodulates CD4 from human T lymphocytes by dissociation of p56(Lck). Biochem. Biophys. Res. Commun. 344963-971. [DOI] [PubMed] [Google Scholar]
- 46.Matlin, K. S., H. Reggio, A. Helenius, and K. Simons. 1982. Pathway of vesicular stomatitis virus entry leading to infection. J. Mol. Biol. 156609-631. [DOI] [PubMed] [Google Scholar]
- 47.Miller, M. D., M. T. Warmerdam, K. A. Page, M. B. Feinberg, and W. C. Greene. 1995. Expression of the human immunodeficiency virus type 1 (HIV-1) nef gene during HIV-1 production increases progeny particle infectivity independently of gp160 or viral entry. J. Virol. 69579-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parolini, I., S. Topa, M. Sorice, A. Pace, P. Ceddia, E. Montesoro, A. Pavan, M. P. Lisanti, C. Peschle, and M. Sargiacomo. 1999. Phorbol ester-induced disruption of the CD4-Lck complex occurs within a detergent-resistant microdomain of the plasma membrane. Involvement of the translocation of activated protein kinase C isoforms. J. Biol. Chem. 27414176-14187. [DOI] [PubMed] [Google Scholar]
- 49.Pelchen-Matthews, A., J. E. Armes, and M. Marsh. 1989. Internalization and recycling of CD4 transfected into HeLa and NIH3T3 cells. EMBO J. 83641-3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pelchen-Matthews, A., I. Boulet, D. R. Littman, R. Fagard, and M. Marsh. 1992. The protein tyrosine kinase p56lck inhibits CD4 endocytosis by preventing entry of CD4 into coated pits. J. Cell Biol. 117279-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pelchen-Matthews, A., R. P. da Silva, M. J. Bijlmakers, N. Signoret, S. Gordon, and M. Marsh. 1998. Lack of p56lck expression correlates with CD4 endocytosis in primary lymphoid and myeloid cells. Eur. J. Immunol. 283639-3647. [DOI] [PubMed] [Google Scholar]
- 52.Pelchen-Matthews, A., I. J. Parsons, and M. Marsh. 1993. Phorbol ester-induced downregulation of CD4 is a multistep process involving dissociation from p56lck, increased association with clathrin-coated pits, and altered endosomal sorting. J. Exp. Med. 1781209-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pham, H. M., E. R. Arganaraz, B. Groschel, D. Trono, and J. Lama. 2004. Lentiviral vectors interfering with virus-induced CD4 down-modulation potently block human immunodeficiency virus type 1 replication in primary lymphocytes. J. Virol. 7813072-13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Piguet, V., Y. L. Chen, A. Mangasarian, M. Foti, J. L. Carpentier, and D. Trono. 1998. Mechanism of Nef-induced CD4 endocytosis: Nef connects CD4 with the mu chain of adaptor complexes. EMBO J. 172472-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Piguet, V., F. Gu, M. Foti, N. Demaurex, J. Gruenberg, J. L. Carpentier, and D. Trono. 1999. Nef-induced CD4 degradation: a diacidic-based motif in Nef functions as a lysosomal targeting signal through the binding of beta-COP in endosomes. Cell 9763-73. [DOI] [PubMed] [Google Scholar]
- 56.Pitcher, C., S. Honing, A. Fingerhut, K. Bowers, and M. Marsh. 1999. Cluster of differentiation antigen 4 (CD4) endocytosis and adaptor complex binding require activation of the CD4 endocytosis signal by serine phosphorylation. Mol. Biol. Cell 10677-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Preusser, A., L. Briese, A. S. Baur, and D. Willbold. 2001. Direct in vitro binding of full-length human immunodeficiency virus type 1 Nef protein to CD4 cytoplasmic domain. J. Virol. 753960-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rhee, S. S., and J. W. Marsh. 1994. Human immunodeficiency virus type 1 Nef-induced down-modulation of CD4 is due to rapid internalization and degradation of surface CD4. J. Virol. 685156-5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robinson, M. S. 2004. Adaptable adaptors for coated vesicles. Trends Cell Biol. 14167-174. [DOI] [PubMed] [Google Scholar]
- 60.Rose, J. J., K. Janvier, S. Chandrasekhar, R. P. Sekaly, J. S. Bonifacino, and S. Venkatesan. 2005. CD4 down-regulation by HIV-1 and simian immunodeficiency virus (SIV) Nef proteins involves both internalization and intracellular retention mechanisms. J. Biol. Chem. 2807413-7426. [DOI] [PubMed] [Google Scholar]
- 61.Salghetti, S., R. Mariani, and J. Skowronski. 1995. Human immunodeficiency virus type 1 Nef and p56lck protein-tyrosine kinase interact with a common element in CD4 cytoplasmic tail. Proc. Natl. Acad. Sci. USA 92349-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sanfridson, A., B. R. Cullen, and C. Doyle. 1994. The simian immunodeficiency virus Nef protein promotes degradation of CD4 in human T cells. J. Biol. Chem. 2693917-3920. [PubMed] [Google Scholar]
- 63.Schindler, M., J. Munch, and F. Kirchhoff. 2005. Human immunodeficiency virus type 1 inhibits DNA damage-triggered apoptosis by a Nef-independent mechanism. J. Virol. 795489-5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schindler, M., S. Wurfl, P. Benaroch, T. C. Greenough, R. Daniels, P. Easterbrook, M. Brenner, J. Munch, and F. Kirchhoff. 2003. Down-modulation of mature major histocompatibility complex class II and up-regulation of invariant chain cell surface expression are well-conserved functions of human and simian immunodeficiency virus nef alleles. J. Virol. 7710548-10556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schwartz, O., V. Marechal, B. Friguet, F. Arenzana-Seisdedos, and J. M. Heard. 1998. Antiviral activity of the proteasome on incoming human immunodeficiency virus type 1. J. Virol. 723845-3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shin, J., C. Doyle, Z. Yang, D. Kappes, and J. L. Strominger. 1990. Structural features of the cytoplasmic region of CD4 required for internalization. EMBO J. 9425-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shin, J., R. L. Dunbrack, Jr., S. Lee, and J. L. Strominger. 1991. Phosphorylation-dependent down-modulation of CD4 requires a specific structure within the cytoplasmic domain of CD4. J. Biol. Chem. 26610658-10665. [PubMed] [Google Scholar]
- 68.Sleckman, B. P., J. Shin, V. E. Igras, T. L. Collins, J. L. Strominger, and S. J. Burakoff. 1992. Disruption of the CD4-p56lck complex is required for rapid internalization of CD4. Proc. Natl. Acad. Sci. USA 897566-7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spina, C. A., T. J. Kwoh, M. Y. Chowers, J. C. Guatelli, and D. D. Richman. 1994. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J. Exp. Med. 179115-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stewart, S. J., J. Fujimoto, and R. Levy. 1986. Human T lymphocytes and monocytes bear the same Leu-3(T4) antigen. J. Immunol. 1363773-3778. [PubMed] [Google Scholar]
- 71.Stoddart, C. A., R. Geleziunas, S. Ferrell, V. Linquist-Stepps, M. E. Moreno, C. Bare, W. Xu, W. Yonemoto, P. A. Bresnahan, J. M. McCune, and W. C. Greene. 2003. Human immunodeficiency virus type 1 Nef-mediated downregulation of CD4 correlates with Nef enhancement of viral pathogenesis. J. Virol. 772124-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thoulouze, M. I., N. Sol-Foulon, F. Blanchet, A. Dautry-Varsat, O. Schwartz, and A. Alcover. 2006. Human immunodeficiency virus type-1 infection impairs the formation of the immunological synapse. Immunity 24547-561. [DOI] [PubMed] [Google Scholar]
- 73.Turner, J. M., M. H. Brodsky, B. A. Irving, S. D. Levin, R. M. Perlmutter, and D. R. Littman. 1990. Interaction of the unique N-terminal region of tyrosine kinase p56lck with cytoplasmic domains of CD4 and CD8 is mediated by cysteine motifs. Cell 60755-765. [DOI] [PubMed] [Google Scholar]
- 74.Wei, B. L., P. W. Denton, E. O'Neill, T. Luo, J. L. Foster, and J. V. Garcia. 2005. Inhibition of lysosome and proteasome function enhances human immunodeficiency virus type 1 infection. J. Virol. 795705-5712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wildum, S., M. Schindler, J. Munch, and F. Kirchhoff. 2006. Contribution of Vpu, Env, and Nef to CD4 down-modulation and resistance of human immunodeficiency virus type 1-infected T cells to superinfection. J. Virol. 808047-8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.