Abstract
Carbon fixation at temperatures above 73°C, the upper limit for photosynthesis, is carried out by chemosynthetic thermophiles. Yellowstone National Park (YNP), Wyoming possesses many thermal features that, while too hot for photosynthesis, presumably support chemosynthetic-based carbon fixation. To our knowledge, in situ rates of chemosynthetic reactions at these high temperatures in YNP or other high-temperature terrestrial geothermal springs have not yet been reported. A microbial community attached to precipitated elemental sulfur (So floc) at the source of Dragon Spring (73°C, pH 3.1) in Norris Geyser Basin, YNP, exhibited a maximum rate of CO2 uptake of 21.3 ± 11.9 μg of C 107 cells−1 h−1. When extrapolated over the estimated total quantity of So floc at the spring's source, the So floc-associated microbial community accounted for the uptake of 121 mg of C h−1 at this site. On a per-cell basis, the rate was higher than that calculated for a photosynthetic mat microbial community dominated by Synechococcus spp. in alkaline springs at comparable temperatures. A portion of the carbon taken up as CO2 by the So floc-associated biomass was recovered in the cellular nucleic acid pool, demonstrating that uptake was coupled to fixation. The most abundant sequences in a 16S rRNA clone library of the So floc-associated community were related to chemolithoautotrophic Hydrogenobaculum strains previously isolated from springs in the Norris Geyser Basin. These microorganisms likely contributed to the uptake and fixation of CO2 in this geothermal habitat.
The upper temperature limit for primary production via photosynthesis is ∼73°C (7, 8, 11). At this temperature, photosynthesis is restricted to cyanobacteria of the genus Synechococcus, which generally inhabit alkaline environments (11). In acidic environments (pH < 4.0), the upper temperature limit for photosynthetic-based primary production is ∼56°C. Under these conditions, phototrophic activity is restricted to the unicellular eukaryotic red algae Cyanidium, Galdieria, and Cyanidioschyzon, collectively referred to as “cyanidia” (6, 12, 31, 48). Primary production above this temperature in acidic environments occurs through chemoautotrophy, a metabolism restricted to prokaryotes.
Yellowstone National Park (YNP), WY, possesses numerous high-temperature (73 to 93°C) geothermal environments that are thought to support communities of microorganisms through chemoautotrophic-based primary production. Evidence for chemosynthesis in these environments is based on the recovery of 16S rRNA gene sequences that are affiliated with cultivated representatives of the phyla Aquificae and Crenarchaeota, many of which are capable of CO2 fixation via the oxidation of hydrogen (H2) and/or sulfide (HS−) (15, 17, 21, 24, 26, 28, 41, 46). Surprisingly, CO2 fixation has yet to be demonstrated in situ in YNP hot spring environments (acidic or alkaline) where temperatures exceed the limits of photosynthesis and where primary production is thought to be driven by chemoautotrophic metabolism (14, 15, 28, 29).
Dragon Spring, an acid-sulfate-chloride (ASC) spring located in the Norris Geyser Basin of YNP, is a likely habitat for chemoautotrophic primary production. The pH of the water is ∼3.1, and the temperature of the water at the source fluctuates from 65 to 78°C, which is well above the upper temperature limit for photosynthesis under acidic conditions. Potential electron donors for chemolithoautotrophic growth in the source water include hydrogen (H2) and sulfide (S2−) at concentrations of 13 nM and 65 μM, respectively (15). In addition, submerged substrata at the spring's source are blanketed by precipitates of elemental sulfur (S°), hereafter referred to as So floc (23). Inventories of bacterial and archaeal 16S rRNA genes recovered from So floc collected from the source of Dragon Spring indicate the presence of Crenarchaeota and Aquificae (4, 15). The latter are related to chemolithoautotrophic Hydrogenobaculum spp., representatives of which have recently been isolated from the spring (15). In the present study, we demonstrate uptake and fixation of CO2 at a temperature of 73°C by a Hydrogenobaculum-dominated microbial community associated with So floc collected from the source of Dragon Spring. This is the first direct evidence of CO2 uptake in situ by a thermoacidophilic microbial community at a temperature that precludes photosynthesis in terrestrial geothermal springs.
MATERIALS AND METHODS
Collection and preparation of So floc.
Microorganisms that colonized So floc in Dragon Spring (44°43′54.8"N, 110°42′39.9"W) served as the source of biomass for CO2 uptake experiments. So floc was collected from the spring source with a sterile 50-ml syringe and transferred to a sterile 30-ml serum bottle purged with sterile N2 on the day of the experiment immediately prior to initiating the uptake reaction. The bottle was shaken to suspend and distribute the So floc uniformly throughout the aqueous phase and used immediately as a source of microbial biomass for CO2 uptake and fixation experiments as described below. Another portion of the So floc suspension was frozen on dry ice for subsequent DNA- and RNA-based analyses. A portion of the frozen So floc was later thawed and dried at 65°C for 48 h to determine volumetric solids content.
Static 14CO2 uptake studies.
Portions (10 ml) of spring source water and 0.25 ml of the So floc suspension containing 1.0 mg (dry weight) of floc solids were injected by syringe and needle through the septa of presterilized, N2-purged 12-ml serum bottles containing 0.1 ml of pH 3.0 citrate buffer (1 mM final concentration) unless otherwise indicated. An 18-gauge needle was used to introduce the So floc suspension to serum bottles to minimize floc disintegration. Serum bottles were then submerged in the spring at its source and equilibrated to the spring water temperature (73°C). The 14CO2 uptake reaction was initiated by syringe injection of NaH[14C]O3 (specific activity, 14.1 mCi/mmol; Sigma-Aldrich, St. Louis, MO). Killed controls were prepared by syringe injection of glutaraldehyde (0.5% final concentration) through the septa of serum bottles containing the same quantities of So floc and spring water as live samples prior to the addition of NaH[14C]O3. Four serum bottles, three containing live samples and one serving as a killed control, were prepared for each experimental condition. All serum bottles were submerged in 73°C water at the source of Dragon Spring during the CO2 uptake reaction. Unless otherwise noted, incubations were performed under ambient midday light. CO2 uptake in the dark was determined in serum bottles wrapped in aluminum foil.
Electron donor and acceptor limitation on CO2 uptake was investigated using the protocol described above with the following modifications. After 4 h of incubation of the So floc-associated community in the presence of 7.7 μM NaH[14C]O3, the reaction mixture was supplemented with either O2 (171 nM), H2 (750 nM), or NaS2 (65 μM) alone or H2 (750 nM) or NaS2 (65 μM) in combination with O2 (171 nM) and then incubated for an additional hour before termination. The 14CO2 uptake was compared to that of samples incubated for 5 h without electron donor/acceptor supplementation. The quantity of NaS2 added to the reaction mixture (65 μM final concentration) did not affect the pH of the citrate-buffered reaction. The amount of NaS2 added was intended to reflect the natural concentration of sulfide in the spring water (15). H2 was added to achieve a saturating aqueous-phase concentration of 750 nM and thus is higher than that which exists in the spring source water (13 nM) but similar to that (726 nM) shown previously to promote the highest rate of growth of Hydrogenobaculum sp. strain YNP 3684, previously isolated from Dragon Spring (15). Since the concentration of O2 in the source water is below the detection limit (13 μM) at this site and therefore not known (14), a quantity was added to the reaction mixture (171 nM final concentration) well below this limit. Because the various experiments described above were conducted at different times over a period of several years, the date of sampling, the quantity of NaH[14C]O3 used in incubations, and the incubation time are presented with the results of each experiment.
Preliminary studies performed in August 2005 indicated that reduction of sample pH from 3.1 to 2.0 by syringe injection of concentrated HCl through the septa of the serum bottle to drive residual aqueous phase CO2 into the headspace did not completely inhibit the biological CO2 uptake reaction. Nold and Ward (39) reported that freezing stopped carbon fixation in cyanobacterial mat samples. Samples were therefore flash frozen on dry ice immediately after acidification to stop biological 14CO2 uptake. Freezing may have resulted in some cell lysis, causing a portion of the fixed cell carbon to partition as dissolved organic carbon in the aqueous phase. This fraction of fixed carbon would likely have passed through the membrane during filtration and therefore would not have been included with the cell-associated fixed carbon. Since the extent of loss of fixed radiolabeled carbon was not determined, CO2 uptake rates reported here may underestimate actual rates.
Upon return to the laboratory at Montana State University (MSU), each sample was thawed under a stream of N2 to purge from the serum bottle any 14CO2 not taken up by cells. The samples were immediately filtered through a polycarbonate membrane (0.22-μm pore size; Fisher Scientific, Pittsburg, PA), and the particulate material retained by the membrane rinsed with 10 ml of citrate-buffered water (pH 3.0) to reduce the amount of residual 14CO2 associated with trapped particles and membrane surface that was not taken up by cells. The membranes were air dried and transferred to scintillation vials containing 10 ml of CytoScint scintillation cocktail (MP Biomedicals, Irvine, CA), and the radioactivity associated with the membranes was quantified by using a 1900CA Tri-Carb liquid scintillation counter (Packard, Downers Grove, IL).
14CO2 uptake under flowthrough conditions.
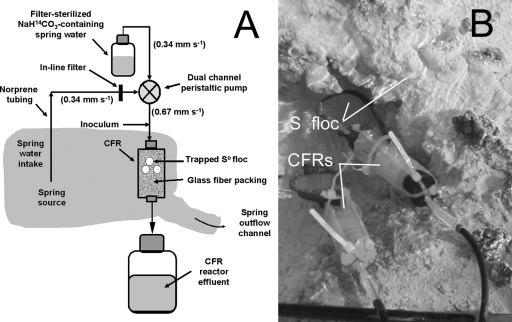
Continuous-flow reactors (CFRs) were used to evaluate CO2 uptake by the So floc-associated microbial community during continuous replenishment of fresh spring source water on 14 September 2005 and repeated on 7 October 2006. The CFRs were constructed of rigid plastic cylinders (25-mm length, 9-mm radius) packed with 0.4 g of combusted glass wool fibers (550°C, 6 h) and capped with butyl rubber stoppers (Fig. 1). After assembly and submersion in the spring at the source, CFRs were perfused with 50 ml of spring source water using gas-impermeable Norprene tubing and a battery-powered peristaltic pump to equilibrate CFR contents to the surrounding spring source water temperature (73°C) and flow rate (0.67 mm s−1). S° floc (1.0 mg [dry weight] of floc solids in 0.25 ml) prepared as described above for the static incubations was then injected with a syringe and 18-gauge needle through the wall of the feed tubing at the CFR inlet while maintaining flow of source water through the reactor. The glass fiber packing served to retain the So floc and associated microbial community in the CFR as water was pumped through the reactor.
FIG. 1.
Schematic (A) and image (B) of the CFR system used to measure CO2 uptake by the So floc-associated microbial community under conditions of continuous replenishment of fresh spring water.
A separate volume of water was transferred from the spring's source into a N2-purged reservoir using gas-impermeable Norprene tubing containing an in-line filter (to remove suspended So floc) and a battery-powered peristaltic pump. The water was supplemented with NaH[14C]O3 to achieve a final concentration of 7.7 μM (4 MBq liter−1), and the pH was adjusted to 3.0. The water in the reservoir was then fed into the CFR after mixing the contents 1:1 (vol/vol) with the untreated water delivered directly from the spring's source by using a multichannel peristaltic pump in a “once through” continuous manner over a 2-h period. The mixture was pumped through the CFR at the same flow rate (0.67 mm s−1) used during introduction of So floc (Fig. 1A). The effluent of the CFR was collected in a waste reservoir and transported to MSU for disposal. A separate set of CFRs inoculated with the same quantity of So floc but fed a mixture of NaH[14C]O3-supplemented spring water containing 0.5% (vol/vol) glutaraldehyde (final concentration) were included as killed controls.
CO2 uptake by the So floc-associated microbial community was terminated after the 2-h reaction period by flash freezing the CFRs and their contents on dry ice. Immediately prior to termination of the reaction, a 20-ml volume of CFR effluent from both live and killed experiments was collected in sterile Falcon tubes and frozen on dry ice for subsequent evaluation of efficiency of retention of So floc-associated biomass by the reactors over the 2-h incubation. These samples were later thawed and filtered through 0.22-mm-pore-size polycarbonate membranes to collect the particulate phase. The membranes were rinsed with 1 mM citrate buffer (pH 3.0), air dried, and transferred to cocktail-containing scintillation vials, and the radioactivity was determined as described above. CFRs were thawed in the laboratory, and the glass fiber packing containing the So floc-associated biomass was aseptically recovered and split into two 0.2-g subsamples. One subsample was dried and transferred to a scintillation vial containing scintillation cocktail. The radioactivity was measured as described above. The other 0.2-g subsample was used to characterize the glass fiber-associated microbial community as described below. The entire experiment was repeated, and the results (n = 2) were used to determine the statistical parameters as described below.
CO2 uptake calculations.
Biological CO2 uptake rates were calculated by using the approach of Lizotte et al. (32) (see also the supplemental material). The approach is based on an increase in the rate of 14CO2 uptake with increasing concentration of 14CO2 when the added substrate represents an insignificant fraction of the total CO2 present in the reaction mixture. The NaH[14C]O3 concentrations used in the uptake studies reported here ranged from 3.8 to 15.3 μM, while the concentration of unlabeled total dissolved inorganic carbon (DIC) in the spring water used for the uptake reaction was 4.4 mM (23, 29). Thus, the amount of radioactive NaH[14C]O3 added to the serum bottles represented an insignificant portion (≤0.35%) of the total CO2 present.
The amount of 14CO2 taken up by the So floc-associated community was based on the amount of radioactivity retained by the membrane filter after sample filtration (static incubations) or from the amount of radioactivity associated with the glass fiber matrix recovered from the CFRs after the radioactivity associated with killed controls was subtracted. The amount of 14CO2 taken up by the So floc-associated community, the amount of NaH[14C]O3 present in the system, and the concentration of unlabeled DIC in the system were used to calculate total CO2 uptake by the community (32). For experiments involving static incubations, the concentration of NaH[14C]O3 in the system was based on the amount added to initiate the reaction at t = 0. For experiments using CFRs, the concentration of NaH[14C]O3 in the system at any point in time during the 2-h incubation was calculated from the concentration of NaH[14C]O3 in the feed reservoir and the dilution factor due to mixing with water pumped directly from the spring's source before entering the reactors.
The rate of CO2 uptake by the So floc-associated microbial community was normalized to DNA as previously described (4). Briefly, DNA was extracted from 1.0 mg of So floc, reacted with the DNA-binding fluorophore SYBR Gold I (Invitrogen, Carlsbad, CA), and quantified with a NanoDrop 3300 fluorimeter (Thermo Scientific, Wilmington, DE) using purified lambda phage DNA (Promega, Madison, WI) as a standard. This approach yielded 2.44 ± 0.53 μg of DNA g−1 (dry weight) of So floc−1. DNA-normalized CO2 uptake rates were converted to uptake on a per-cell basis to facilitate comparison to rates reported previously for phototrophs. The conversion factor (7.0 fg of DNA cell−1) was based on the size of the genome (3.2 Mb) of Hydrogenobaculum sp. strain 3684, a chemolithoautotroph belonging to the Aquificaceae within the Aquificae previously isolated from Dragon Spring (15). An average of 2.0 genome equivalents per cell for thermophilic microorganisms at stationary phase growth (1, 33, 35) and an average molecular weight of double-stranded DNA of 660 Da (45) were also used to determine the conversion factor. Exponential-phase cultures of thermophiles can contain a greater number of genome equivalents per cell (≥4) than stationary-phase cultures (35). Thus, the conversion factor used in the present study may result in an underestimate of the actual CO2 uptake rate per cell. Details of CO2 uptake calculations are presented in the supplemental material.
Statistical analyses.
The standard deviation among replicates was calculated according to the formula: SD = √(A2 + B2), where A is the average standard deviation of replicate CO2 uptake measurements (n = 3 for static experiments; n = 2 for CFR experiments, unless otherwise stated) and B is the standard deviation of replicate DNA determinations on different subsamples of S° floc (n = 3 for static or CFR experiments). P values were obtained by using a two-tailed Student t test. Differences in CO2 uptake under the various conditions tested were considered significant if P values were ≤0.05.
Incorporation of inorganic carbon into cellular organic material.
Incorporation of radiolabeled substrate into cellular carbon pools followed the approach of Brock and Brock (10). So floc suspension (5 ml) and fresh unfiltered spring source water (45 ml) were added by syringe and needle to six sterile N2-purged 150-ml serum bottles sealed with serum stoppers and equilibrated in the source water of Dragon Spring. After equilibration, 5 ml of air (171 nM final dissolved O2 concentration) and 100 μl of NaH[14C]O3 (16.4 MBq ml−1, 2.11 GBq mmol−1) were introduced with syringe and needle to the serum bottles. Three serum bottles were immediately chilled on ice to inhibit biological carbon fixation and serve as killed controls. The remaining serum bottles were returned to the spring and incubated for 4 h at spring source water temperature (70°C) under ambient light during the peak photoperiod on 12 November 2008. After incubation, the serum bottles were chilled on ice to stop biological carbon fixation. All serum bottles were stored on ice during transport to the laboratory at MSU. Upon return to the laboratory, the contents of each serum bottle were mixed, evenly distributed into three centrifuge tubes, and centrifuged at 5,000 × g for 10 min. The supernatant was decanted and replaced with either 2 ml of cold 0.5 N HClO4 to extract the low-molecular-weight pool, 2 ml of hot 0.5 N HClO4 to extract nucleic acids and acid-labile peptides, or 2 ml of hot 1 N NaOH to extract protein. The tubes containing hot HClO4 or NaOH were boiled (∼93°C) for 20 min, while the tubes containing cold HClO4 were chilled (0 to 1°C) on ice for 20 min. The tubes were then centrifuged at 5,000 × g for 10 min, and the supernatant was decanted into scintillation vials containing scintillation cocktail. The radioactivity was counted in a scintillation counter as described above. The amount of 14CO2 incorporated into the various cellular pools was calculated after the subtraction of uptake associated with killed controls.
RT-PCR.
Triplicate 2-mg quantities of S° floc (500 μl) used to inoculate serum bottles and CFRs and triplicate 50-mg quantities of glass fiber packing material recovered from the glutaraldehyde-free CFRs after the 2-h incubation were subjected to bead beating-sodium dodecyl sulfate RNA extraction as previously described (2). The replicate extracts for each set of subsamples were pooled and subjected to reverse transcription PCR (RT-PCR) using an Access RT-PCR kit (Promega) as previously described (4). Primers used in RT-PCR (1070F [bacterial] or 931F [archaeal] and 1492R-GC) targeted the community 16S rRNA. Negative control reactions were performed in the absence of reverse transcriptase.
DGGE.
The 16S cDNA generated by RT-PCR was evaluated by denaturing gradient gel electrophoresis (DGGE) using a 7% polyacrylamide gel containing a vertical gradient of 50 to 70% denaturant (bacterial 16S cDNA amplicons) or 35 to 55% denaturant (archaeal 16S cDNA amplicons) (4).
Cloning, sequencing, and analysis of cDNA.
Cloning, sequencing, and analysis of bacterial 16S cDNA amplicons was performed as previously described (3). The single DGGE band obtained from the archaeal amplicons was excised, purified, and sequenced as previously described (20). CLUSTAL X (version 2.0.8) (30) was used to align sequences and to create distance matrices for use in identifying and clustering operational taxonomic units (OTU) using DOTUR (42). DOTUR calculations used the furthest-neighbor algorithm and a precision of 0.01. A representative of each OTU was subjected to BLASTn to determine the phylogeny of the closest affiliated sequence type.
Nucleotide sequence accession numbers.
All 16S cDNA sequences have been deposited in the GenBank, DDBJ, and EMBL databases under the accession numbers DQ3984487 to DQ398805.
RESULTS
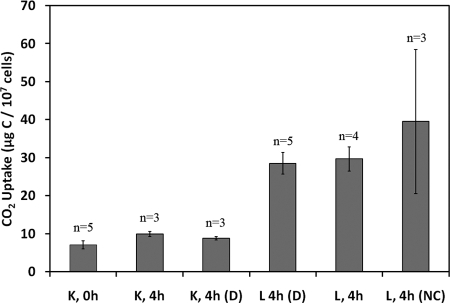
The results from a series of experiments performed on 15 July 2006 indicated that glutaraldehyde treatment inhibited uptake of CO2 by the So floc-associated microbial community in pH 3.0 Dragon Spring source water at 73°C. The quantity of radiolabeled substrate taken up by the community after 4 h of incubation in glutaraldehyde-treated source water containing 15.3 μM NaH[14C]O3 was not significantly different from that taken up after no incubation upon exposure to source water containing the same supplements (Fig. 2). This was the case regardless of whether the incubations were performed in the light or the dark (P = 0.11 and 0.08, respectively). The amount of radioactivity recovered from samples of spring water without added So floc incubated for 4 h in the presence of 15.3 μM NaH[14C]O3 was not significantly different (P = 0.43) from that recovered from glutaraldehyde-killed So floc-containing source water incubated in the presence of the same quantity of NaH[14C]O3 for the same period of time (data not shown). This result suggests that the residual radioactivity associated with the glutaraldehyde-treated So floc-containing samples, hereafter referred to as killed controls, was NaH[14C]O3 that was bound to the membrane or associated with material in the source water other than the added floc trapped by the membrane.
FIG. 2.
Uptake of 14CO2 by a So floc-associated microbial community under static conditions in the presence of 15.3 μM NaH[14C]O3 after no incubation (0 h) or after 4 h (4 h) of incubation. All incubations were performed in the light with the exception of those labeled D, denoting incubations performed in the dark. Live (L) and glutaraldehyde-killed control (K) reactions are indicated. All incubations contained 1 mM citrate buffer (pH 3.0) unless otherwise indicated (NC), which contained no citrate. n, Number of biological replicates.
The quantity of radiolabeled substrate taken up by the So floc-associated microbial community incubated in glutaraldehyde-free source water containing 15.3 μM NaH[14C]O3 under mid-day ambient light was significantly greater (P = 0.01) than that taken up by the floc-associated community in glutaraldehyde-treated source water incubated under otherwise similar conditions (Fig. 2). The difference represents uptake of CO2 by the So floc-associated microbial community. Other microbial populations present in the source water did not appear to contribute significantly to CO2 uptake under these conditions. The amount of radioactivity recovered from NaH[14C]O3-supplemented source water incubated for 4 h without added So floc was not significantly different (P = 0.28) from that recovered from source water receiving neither NaH[14C]O3 or So floc and incubated for the same period of time (data not shown).
Although photosynthesis is not thought to occur at the combined low pH and high temperature of Dragon Spring source water (5, 6, 12), a dark incubation was performed to compare CO2 uptake under this condition to that reported above in the light. CO2 uptake in the dark was not significantly different (P = 0.89) from that in the light during peak photoperiod under otherwise similar conditions (15.3 μM NaH[14C]O3, 4-h incubation) (Fig. 2). This indicates that essentially all CO2 uptake by the So floc-associated microbial community at 73°C in the presence of light can be attributed to the activities of chemolithoautotrophs that do not depend on photosynthesis. In view of these results, all of the studies reported below were conducted under ambient light.
CO2 uptake by the So floc-associated community in the studies described above was determined in the presence of citrate buffer to maintain the reaction mixture at the source water pH (3.0). An additional incubation was performed on this date to determine the effect of citrate on CO2 uptake by comparing 14CO2 uptake in the presence or absence of the buffer. The presence of buffer had no significant effect (P = 0.50) on 14CO2 uptake other than to reduce variability among replicates (Fig. 2).
A portion of the CO2 taken up by the So floc-associated microbial community was fixed into the cell biomass. Significantly more radioactivity (introduced as NaH[14C]O3) was recovered in the hot HClO4 extract (nucleic acid and acid-labile peptide cellular pool) of samples incubated for 4 h at 70°C than from extracts of NaH[14C]O3-amended samples that were immediately chilled on ice (P = 0.006). The total amount of CO2 incorporated into this pool was 0.38 μg of C g (dry weight) of So floc−1 based on the previously determined spring water DIC concentration of 4.4 mM (23, 29). The quantities of radioactivity associated with the low-molecular-weight (cold HClO4) and protein (NaOH) pools of incubated samples were not significantly different from those of the killed controls (data not shown).
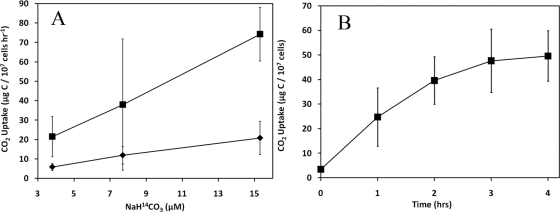
The rate of 14CO2 uptake in live samples over a 2-h period increased linearly with NaH[14C]O3 concentration (3.8 to 15.3 μM) for different amounts (0.5 and 1.0 mg) of added So floc in an experiment performed on 2 August 2005 (Fig. 3A). These results qualify the data for use in calculating the rate of uptake of unlabeled CO2 in the samples by the approach of Lizotte et al. (32). In the presence of 1.0 mg of S° floc and 7.7 μM NaH[14C]O3, detectable 14CO2 uptake occurred for up to 3 h, but the rate decreased over this period of time (Fig. 3B). A maximum rate of 14CO2 uptake (21.3 ± 11.9 μg of C 107 cells−1 h−1) was observed during the first hour of incubation, decreasing to 18.1 ± 9.7 μg of C 107 cells−1 h−1 when averaged over a 2-h incubation period. These results indicate that the reaction was limited shortly after initiation.
FIG. 3.
CO2 uptake under static conditions by the microbial community associated with 0.5 mg (⧫) or 1.0 mg (▪) of So floc after 2 h of incubation in Dragon Spring source water supplemented with a range of NaH[14C]O3 concentrations (A) or associated with 1.0 mg of So floc over a 4-h incubation in Dragon Spring source water supplemented with 7.7 μM NaH[14C]O3 (B). Means and standard deviations are based on results from experiments performed in triplicate (n = 3).
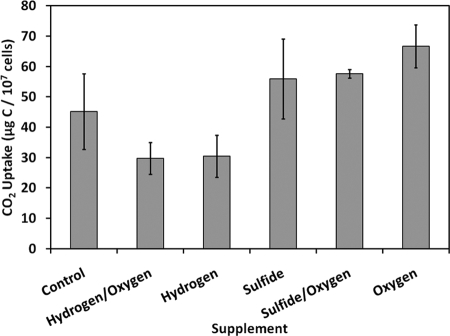
Electron donor or acceptor limitation of CO2 uptake under the same conditions described above (7.7 μM NaH[14C]O3) was evaluated through supplementation experiments on 20 August 2005. Supplementation of O2 (171 nM) alone significantly (P = 0.016) stimulated CO2 uptake relative to the unsupplemented control, suggesting that the reaction was limited by this electron acceptor (Fig. 4). Supplementation of NaS2 (65 μM) alone or in combination with O2 (171 nM) did not significantly stimulate (P = 0.37 and P = 0.10, respectively) CO2 uptake (Fig. 4). H2 supplementation (750 nM) appeared to significantly inhibit (P = 0.05) CO2 uptake relative to unsupplemented controls (Fig. 4). Furthermore, H2 inhibition was not alleviated by the addition of O2 (Fig. 4).
FIG. 4.
CO2 uptake by microbial community associated with 1.0 mg of So floc after a 5-h incubation under static conditions in Dragon Spring source water supplemented with 7.7 μM NaH[14C]O3 after addition in the final hour of either 750 nM hydrogen (hydrogen), 65 μM sulfide (sulfide), 191 nM O2 (oxygen), 750 nM hydrogen plus 191 nM O2 (hydrogen/oxygen), 65 μM sulfide plus 191 nM O2 (sulfide/oxygen), or no electron donor or acceptor (control). Means and standard deviations are based on results from experiments performed in triplicate (n = 3).
In view of the evidence of O2 limitation of CO2 uptake by the So floc-associated community in samples incubated under static conditions, the reaction was subsequently carried out in a CFR containing 1.0 mg of So floc exposed to 7.7 μM NaH[14C]O3 in the presence of a fresh supply of flowing spring source water. A total CO2 uptake rate of 13.5 ± 0.9 μg of C 107 cells−1 h−1 was obtained when averaged over a 2-h incubation period. The uptake rate under these conditions was not significantly different (P = 0.23) from the rate (18.1 ± 9.7 μg of C 107 cells−1 h−1) averaged over the same 2-h incubation period in static incubations containing the same quantity of S° floc and concentration of radiolabeled substrate. Comparison of the volume and radioactivity of solution in the feed reservoir to that of the CFR effluent indicated that the concentration of radiolabeled substrate in the CFRs did not differ by more than 7% over the 2-h incubation period (data not shown). Furthermore, the amount of radioactivity associated with the particulate phase of the effluent from the live and killed CFRs was not significantly different. This result indicates that the inoculum (So floc-associated biomass) introduced at the beginning of the incubation that participated in the uptake of radiolabeled substrate was retained in the CFRs over the duration of the experiment (data not shown).
The composition of the microbial community associated with the glass fiber matrix in the CFRs at the end of the 2-h incubation was compared to that associated with the So floc used to inoculate the reactors. DGGE analysis of bacterial 16S cDNA from RNA recovered from the So floc used as CFR inoculums and from the CFR glass fiber matrix yielded 14 and 12 bands (phylotypes), respectively. All 12 bands from the CFR glass fiber matrix were present in the So floc inoculum. All 12 DGGE phylotypes that were shared between communities migrated to a similar position in a 50 to 70% denaturing gel (data not shown), suggesting that these 16S cDNA sequences have similar mol% G+C content. Similarly, DGGE analysis of archaeal 16S rRNA indicated a single, shared phylotype in both the inoculum and the material recovered from the CFR glass fiber matrix. The results suggest that the microbial community did not change significantly during the uptake reaction as fresh filtered spring water was continuously pumped through the reactors. The results also indicate that the So floc-associated microbial community used for the CO2 uptake reaction in the static incubation studies was not significantly different from that responsible for the reaction in the CFRs.
A clone library was constructed from bacterial 16S cDNA derived from RNA extracted from the So floc used as the CFR inoculum. At a 100% sequence identify threshold, a total of 14 OTU were present, 6 of which contained multiple clones and 8 of which represented singlets (Table 1). Analysis of representatives from each OTU indicated that 12 of the 14 OTUs exhibited high affiliation (>98% sequence identities) to chemolithoautotrophic Hydrogenobaculum sequences previously isolated from the Norris Geyser Basin belonging to the Aquificaceae within the Aquaficae (15-17, 19). A smaller fraction of the OTUs were affiliated with Cupriavidus sp. strain JB1B4 and Ralstonia taiwanensis LMG 19424, both of which are members of the Betaproteobacteria (Table 1). The sole archaeal 16S rRNA sequence (as assessed by DGGE) was affiliated (97.6%) with Thermocladium modestius, a crenarchaeon isolated from a geothermal area in Japan (24).
TABLE 1.
Diversity of RT-PCR-amplified bacterial and archaeal 16S cDNA recovered from sulfur flocs
| OTUa | Frequencyb | Closest affiliated organismc | Phylum | % Identityd |
|---|---|---|---|---|
| B1 | 18 | Hydrogenobaculum sp. strain NOR3L3B (ZAJ320225) | Aquificae | 99.8 |
| B2 | 4 | Hydrogenobaculum sp. strain Y04ANC1 (AM259505) | Aquificae | 100.0 |
| B3 | 7 | Hydrogenobaculum sp. strain Y04ANC1 (AM259505) | Aquificae | 99.8 |
| B4 | 1 | Hydrogenobaculum sp. strain Y04AAS1 (AM259504) | Aquificae | 99.3 |
| B5 | 5 | Hydrogenobaculum sp. strain Y04AAS1 (AM259504) | Aquificae | 100.0 |
| B6 | 1 | Hydrogenobaculum sp. strain Y04AAS1 (AM259504) | Aquificae | 99.5 |
| B7 | 2 | Hydrogenobaculum sp. strain NOR3L3B (ZAJ320225) | Aquificae | 99.8 |
| B8 | 2 | Hydrogenobaculum sp. strain Y04ANC1 (AM259505) | Aquificae | 99.8 |
| B9 | 1 | Hydrogenobaculum sp. strain NOR3L3B (ZAJ320225) | Aquificae | 99.1 |
| B10 | 1 | Hydrogenobaculum sp. strain Y04ANC1 (AM259505) | Aquificae | 98.8 |
| B11 | 1 | Hydrogenobaculum acidophilum H55 (AY268103) | Aquificae | 99.0 |
| B12 | 1 | Hydrogenobaculum acidophilum H55 (AY268103) | Aquificae | 99.2 |
| B13 | 1 | Cupriavidus sp. strain JB1B4 (EU375663) | Betaproteobacteria | 86.7 |
| B14 | 1 | Ralstonia taiwanensis LMG 19424 (AF300324) | Betaproteobacteria | 100.0 |
| A1 | NA | Thermocladium modestius (AB005296) | Themoprotei | 97.6 |
OTU were as defined by DOTUR using a sequence identity threshold of 100%. B, bacteria; A, archaea.
That is, the OTU frequency in the bacterial 16S cDNA library. NA, not applicable.
That is, the cultured organism with the closest affiliated 16S rRNA gene sequence. The accession number is given in parentheses.
That is, the percent identity with the closest affiliated sequence.
DISCUSSION
The results confirm earlier predictions that carbon fixation occurs at temperatures above the maximum temperature for photosynthesis in habitats of YNP containing alternative sources of energy that support chemosynthesis. If phototrophs had contributed to CO2 uptake by the So floc-associated microbial community at 73°C, one would predict the rate of uptake in the light to be greater than that in the dark. In habitats with comparable temperatures but higher pH where photosynthetic prokaryotes of the genus Synechococcus outcompete other life forms, dark CO2 uptake typically represents less than 10% of the total CO2 taken up (8, 9, 38). The insignificant difference in uptake rates in the light and dark and evidence of incorporation of radiolabeled inorganic carbon into cellular DNA indicates that fixation of carbon is coupled to So floc-associated chemosynthesis rather than photosynthesis at the source of Dragon Spring. To our knowledge, this is the first direct evidence of in situ CO2 uptake and fixation by a thermoacidophilic microbial community at a temperature above that which supports photosynthesis.
The limited size bacterial 16S cDNA library constructed from So floc-associated RNA was dominated (92% of total clones) by sequences exhibiting close affiliation (>98% identity) to Hydrogenobaculum spp. The 12 OTU present in the bacterial 16S rRNA clone library that are related to Hydrogenobaculum spp. are genetically similar (average sequence divergence of 0.0087%) and thus may correspond to the 12 shared phylotypes that migrated to nearly the same position in the DGGE acrylamide gel. Members of this genus are acidophilic chemolithoautotrophs that generally use H2, S°, S2O3− or HS− as electron donor and O2 or oxidized sulfur compounds such as S° or thiosulfate as electron acceptor to fix CO2 (17, 41, 43, 44, 47). In Dragon Spring, as well as other acidic springs, representatives of this genus have been proposed to obtain their cell carbon through CO2 fixation (22, 23, 25, 34, 46). They have also been shown to physically attach to sulfur (36). The populations of Hydrogenobaculum spp. detected in the clone library may therefore contribute to the chemoautotrophic CO2 uptake and fixation by the So floc-associated microbial community at the source of Dragon Spring.
Thermophilic chemolithoautotrophs isolated from high-temperature environments have been shown to use O2, NO3−, Fe3+, and various oxidized sulfur species as the terminal electron acceptor (14-17, 27, 47). Past studies indicate that the source water of Dragon Spring contains 17 μM NO3− and an O2 concentration that is below detection (13 μM) (14, 15, 23, 29). The stimulation in the rate of CO2 uptake by the So floc-associated microbial community under static conditions upon addition of O2 indicates that uptake under these conditions was O2 limited. Dissolved O2 is maintained at undetectable concentrations at the source of Dragon Spring through a reaction with HS− in the source water to form polysulfide (Sn) and S2O3−, which subsequently disproportionate to So and HS− (40). Hydrogenobaculum acidophilum (formerly Hydrogenobacter acidophilis), one of the few strains of this genus for which the oxygen requirements have been determined, exhibits optimum growth at partial pressures (pO2) between 2 to 10%, well above the ambient concentration in Dragon Spring source water (43). The threshold concentration of O2 for carbon fixation in populations of the So floc-associated community may therefore be lower than that of the few isolates examined to date. Supporting this hypothesis is evidence that As(III) oxidation by Hydrogenobaculum sp. strain H55 isolated from Dragon Spring was only detected under microaerophilic (∼1% O2) conditions (16). The So floc-associated microbial populations may also be capable of coupling carbon fixation to alternative electron acceptors present in the source water such as nitrate. However, their involvement in chemoautotrophic carbon fixation under conditions of O2 limitation is beyond the scope of the present study.
Thermophilic chemolithoautotrophs isolated from high-temperature environments have been shown to use H2 and various reduced sulfur species as electron donors (energy source) (14-17, 27, 47). Past studies indicate that the source water of Dragon Spring contains 13 nM H2 and 60 to 80 μM H2S (14, 15, 23, 29). CO2 uptake by the So floc-associated community was inhibited by high concentrations of H2. Whereas H2 has been shown to inhibit heterotrophic metabolism in thermophiles (13, 18, 49, 50), this is, to our knowledge, the first report of H2 inhibiting a reaction associated with chemoautotrophic metabolism at a high temperature. The concentration of H2 used to evaluate H2 limitation (750 nM), while over an order of magnitude greater than the ambient concentration (13 nM) in the source water of the spring, was similar to that (726 nM) shown previously to promote the highest rate of growth of isolate YNP 3684, a chemolithoautotrophic Hydrogenobaculum sp. isolated from Dragon Spring (15). The inhibitory effect of 750 nM H2 on CO2 uptake in the present study suggests that the response of this isolate to H2 is not representative of that of the So floc-associated community. Further investigation of the effect of H2 on chemosynthetic processes at the source of Dragon Spring is clearly warranted but is beyond the scope of the present study.
Sequences related to the crenarchaeon T. modestius, the only archaea detected in the So floc-associated community by DGGE analysis of 16S cDNA, have previously been recovered from Succession Spring, a nearby thermal feature in the Norris Geyser Basin that has water chemistry similar to that of Dragon Spring (23, 35). T. modestius does not grow autotrophically using H2 and CO2, although heterotrophic growth under anaerobic conditions at 75°C over the pH range of 4.0 to 4.5 is stimulated by addition of CO2 to the culture headspace (24). Although the limited community analysis reported here does not indicate involvement of archaea in CO2 uptake by the So floc-associated microbial community, deeper sampling of 16S rRNA gene sequences with more diverse primer sets or 454 V6 tag sequencing may reveal autotrophic archaeal populations associated with the microbial community at this site.
Rates of CO2 uptake normalized to chlorophyll (Chl) content have been reported for photosynthetic organisms at temperatures comparable to that used here to investigate chemosynthetic CO2 uptake (8, 9, 38). The photosynthetic efficiency and Chl a content of cells vary with the temperature and radiation level in Synechococcus (7, 38), the only known phototroph capable of growth at temperatures approaching that (73°C) used for incubations in the present study. Brock and Brock (9) reported a cellular Chl a content of 81 fg of Chl a cell−1 for Synechcoccus spp. taking up 14CO2 at 71°C at a rate of 1,533 cpm μg−1 Chl a h−1. Based on these values and a previously reported spring water total DIC concentration of 55 μg ml−1 (8), a total CO2 uptake rate of 0.47 μg of C 107 cells−1 h−1 was obtained when cpm was converted to dpm using the counting efficiency (65.5%) determined for the present study. A higher total CO2 uptake rate (1.9 μg of C 107 cells−1 h−1) was calculated from the 14CO2 uptake rate (2.3 × 108 cpm 186 mg of Chl a−1 m−1 h−1) measured in an earlier study at the same site (8). More recently, Miller et al. (38) reported a 14CO2 uptake rate of 2,304 cpm μg of Chl a−1 h−1 for dispersed cells of a Synechococcus mat from another alkaline spring incubated at 70°C in water containing 67.5 μg of DIC ml−1. This reflects a total CO2 uptake rate of 1.1 μg of C 107 cells−1 h−1 based on the experimentally determined value of 64 fg of Chl a cell−1 from a 45°C exponential culture of a Synechococcus sp. isolated from the mat (37). The rates calculated above for the alkalinophilic phototrophic Synechococcus communities are at least an order of magnitude less than the 18.1 and 13.5 μg of C 107 cells−1 h−1 calculated for the So floc-associated microbial community over 2-h incubations under static and flowing conditions, respectively. The higher CO2 uptake rates by the So floc-associated microbial community suggests that chemoautotrophic acidophiles are better adapted than photoautotrophic alkalinophiles to take up CO2 at the upper temperature limit for photosynthesis.
So floc forms an ∼0.5-cm-thick blanket over an area of ∼300 cm by 50 cm at the spring's source (Fig. 1B). Extrapolation of the maximum CO2 uptake rate exhibited by the microbial community associated with 0.25 ml of So floc in the static incubation experiments to the total volume of So floc at the source yielded a total CO2 uptake rate of 121 mg of C h−1. Additional studies are needed to determine the biomass production resulting from this rate of CO2 uptake before the significance of chemosynthesis under these conditions can be established.
In summary, a microbial community associated with So floc at the source of Dragon Spring, YNP, coupled the uptake and fixation of inorganic carbon into cellular organic matter using light-independent chemosynthetic metabolism at a temperature and pH that do not support photosynthesis. Uptake rates measured in situ at 73°C and pH 3.1 were at least an order of magnitude higher than those reported for photosynthetic Synechococcus microbial mats in alkaline springs at slightly lower temperatures coinciding with the upper limits for photosynthesis. Chemolithoautotrophic Hydrogenobaculum spp. appeared to be important members of the So floc-associated microbial community and likely contributed to the CO2 uptake rates measured under the conditions of the present study.
Supplementary Material
Acknowledgments
This research was supported by National Science Foundation grant MCB-0132022 to G.G.G. E.S.B. was supported by an Inland Northwest Research Alliance Graduate Fellowship.
We thank Seth D'Imperio for the unpublished data on the genome size of Hydrogenobaculum sp. strain 3684. We thank Christie Hendrix at Yellowstone Center for Resources, YNP, WY, for assistance with the permitting process.
Footnotes
Published ahead of print on 8 May 2009.
REFERENCES
- 1.Bernander, R., and A. Poplawski. 1997. Cell cycle characteristics of thermophilic Archaea. J. Bacteriol. 179:4963-4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Botero, L. M., S. D'Imperio, M. Burr, T. R. McDermott, M. Young, and D. J. Hassett. 2005. Poly(A) polymerase modification and reverse transcriptase PCR amplification of environmental RNA. Appl. Environ. Microbiol. 71:1267-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyd, E. S., D. E. Cummings, and G. G. Geesey. 2007. Mineralogy influences structure and diversity of bacterial communities associated with geological substrata in a pristine aquifer. Microb. Ecol. 54:170-182. [DOI] [PubMed] [Google Scholar]
- 4.Boyd, E. S., R. A. Jackson, G. Encarnacion, J. A. Zahn, T. Beard, W. D. Leavitt, Y. Pi, C. L. Zhang, A. Pearson, and G. G. Geesey. 2007. Isolation, characterization, and ecology of sulfur-respiring Crenarchaea inhabiting acid-sulfate-chloride geothermal springs in Yellowstone National Park. Appl. Environ. Microbiol. 73:6669-6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brock, T. D. 1967. Life at high temperatures. Science 158:1012-1019. [DOI] [PubMed] [Google Scholar]
- 6.Brock, T. D. 1973. Lower pH limit for the existence of blue-green algae: evolutionary and ecological implications. Science 179:480-483. [DOI] [PubMed] [Google Scholar]
- 7.Brock, T. D. 1967. Micro-organisms adapted to high temperature. Nature 214:882-885. [DOI] [PubMed] [Google Scholar]
- 8.Brock, T. D. 1967. Relationship between standing stock and primary productivity along a hot spring thermal gradient. Ecology 48:566-571. [Google Scholar]
- 9.Brock, T. D., and M. L. Brock. 1969. Effect of light intensity on photosynthesis by thermal algae adapted to natural and reduced sunlight. Limnol. Oceanogr. 14:334-341. [Google Scholar]
- 10.Brock, T. D., and M. L. Brock. 1967. The measurement of chlorophyll, primary production, photophosphorylation, and macromolecules in benthic algal mats. Limnol. Oceanogr. 12:600-605. [Google Scholar]
- 11.Brock, T. D., and M. L. Brock. 1968. Measurement of steady-state growth rates of a thermophilic alga in nature. J. Bacteriol. 95:811-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brock, T. D., and G. K. Darland. 1970. Limits of microbial existence: temperature and pH. Science 169:1316-1318. [DOI] [PubMed] [Google Scholar]
- 13.Childers, S. E., M. Vargas, and K. M. Noll. 1992. Improved methods for cultivation of the extremely thermophilic bacterium Thermotoga neapolitana. Appl. Environ. Microbiol. 58:3949-3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Imperio, S., C. R. Lehr, M. Breary, and T. R. McDermott. 2007. Autoecology of an arsenite chemolithotroph: sulfide constraints on function and distribution in a geothermal spring. Appl. Environ. Microbiol. 73:7067-7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Imperio, S., C. R. Lehr, H. Oduro, G. Druschel, M. Kuhl, and T. R. McDermott. 2008. Relative importance of H2 and H2S as energy sources for primary production in geothermal springs. Appl. Environ. Microbiol. 74:5802-5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donahoe-Christiansen, J., S. D'Imperio, C. R. Jackson, W. P. Inskeep, and T. R. McDermott. 2004. Arsenite-oxidizing Hydrogenobaculum strain isolated from an acid-sulfate-chloride geothermal spring in Yellowstone National Park. Appl. Environ. Microbiol. 70:1865-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eder, W., and R. Huber. 2002. New isolates and physiological properties of the Aquificales and description of Thermocrinis albus sp. nov. Extremophiles 6:309-318. [DOI] [PubMed] [Google Scholar]
- 18.Erauso, G., A.-L. Reysenbach, A. Godfroy, J.-R. Meunier, B. Crump, F. Partensky, J. A. Baross, V. Marteinsson, G. Barbier, N. R. Pace, and D. Prieur. 1993. Pyrococcus abyssi sp. nov., a new hyperthermophilic archaeon isolated from a deep-sea hydrothermal vent. Arch. Microbiol. 160:338-349. [Google Scholar]
- 19.Ferrera, I., S. Longhorn, A. Banta, Y. Liu, D. Preston, and A. L. Reysenbach. 2007. Diversity of 16S rRNA gene, ITS region and aclB gene of the Aquificales. Extremophiles 11:57-64. [DOI] [PubMed] [Google Scholar]
- 20.Ferris, M. J., G. Muyzer, and D. M. Ward. 1996. Denaturing gradient gel electrophoresis profiles of 16S rRNA-defined populations inhabiting a hot spring microbial mat community. Appl. Environ. Microbiol. 62:340-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hügler, M., H. Huber, S. J. Molyneaux, C. Vetriani, and S. M. Sievert. 2007. Autotrophic CO2 fixation via the reductive tricarboxylic acid cycle in different lineages within the phylum Aquificae: evidence for two ways of citrate cleavage. Environ. Microbiol. 9:81-92. [DOI] [PubMed] [Google Scholar]
- 22.Inskeep, W. P., G. G. Ackerman, W. P. Taylor, M. Kozubal, S. Korf, and R. E. Macur. 2005. On the energetics of chemolithotrophy in nonequilibrium systems: case studies of geothermal springs in Yellowstone National Park. Geobiology 3:297-317. [Google Scholar]
- 23.Inskeep, W. P., and T. R. McDermott. 2005. Geomicrobiology of acid-sulfate-chloride springs in Yellowstone National Park, p. 143-162. In W. P. Inskeep and T. R. McDermott (ed.), Geothermal biology and geochemistry in Yellowstone National Park. Montana State University Press, Bozeman.
- 24.Itoh, T., K. Suzuki, and T. Nakase. 1998. Thermocladium modestius gen. nov., sp. nov., a new genus of rod-shaped, extremely thermophilic crenarchaeote. Int. J. Syst. Bacteriol. 48:879-887. [DOI] [PubMed] [Google Scholar]
- 25.Jackson, C. R., H. W. Langner, J. Donahoe-Christiansen, W. P. Inskeep, and T. R. McDermott. 2001. Molecular analysis of microbial community structure in an arsenite-oxidizing acidic thermal spring. Environ. Microbiol. 3:532-542. [DOI] [PubMed] [Google Scholar]
- 26.Jochimsen, B., S. Peinemann-Simon, H. Völker, D. Stüben, R. Botz, P. Stoffers, P. R. Dando, and M. Thomm. 1997. Stetteria hydrogenophila, gen. nov. and sp. nov., a novel mixotrophic sulfur-dependent crenarchaeote isolated from Milos, Greece. Extremophiles 1:67-73. [DOI] [PubMed] [Google Scholar]
- 27.Kashefi, K., J. M. Tor, D. E. Holmes, C. V. Gaw Van Praagh, A. L. Reysenbach, and D. R. Lovley. 2002. Geoglobus ahangari gen. nov., sp. nov., a novel hyperthermophilic archaeon capable of oxidizing organic acids and growing autotrophically on hydrogen with Fe(III) serving as the sole electron acceptor. Int. J. Syst. Evol. Microbiol. 52:719-728. [DOI] [PubMed] [Google Scholar]
- 28.Kozubal, M., R. E. Macur, S. Korf, W. P. Taylor, G. G. Ackerman, A. Nagy, and W. P. Inskeep. 2008. Isolation and distribution of a novel iron-oxidizing crenarchaeon from acidic geothermal springs in Yellowstone National Park. Appl. Environ. Microbiol. 74:942-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langner, H. W., C. R. Jackson, T. R. McDermott, and W. P. Inskeep. 2001. Rapid oxidation of arsenite in a hot spring ecosystem, Yellowstone National Park. Environ. Sci. Technol. 35:3302-3309. [DOI] [PubMed] [Google Scholar]
- 30.Larkin, M. A., G. Blackshields, N. P. Brown, R. Chenna, P. A. McGettigan, H. McWilliam, F. Valentin, I. M. Wallace, A. Wilm, R. Lopez, J. D. Thompson, T. J. Gibson, and D. G. Higgins. 2007. CLUSTAL W and CLUSTAL X version 2.0. Bioinformatics 23:2947-2948. [DOI] [PubMed] [Google Scholar]
- 31.Lehr, C. R., S. D. Frank, T. Norris, S. D'Imperio, A. V. Kalanin, J. A. Toplin, R. W. Castenholz, and T. R. McDermott. 2007. Cyanidia (Cyanidiales) population diversity and dynamics in an acid-sulfate-chloride spring in Yellowstone National Park. J. Phycol. 43:3-14. [Google Scholar]
- 32.Lizotte, M. P., T. R. Sharp, and J. C. Priscu. 1996. Phytoplankton dynamics in the stratified water column of Lake Bonney, Antarctica. I. Biomass and productivity during the winter-spring transition. Polar Biol. 16:155-162. [Google Scholar]
- 33.Lundgren, M., L. Malandrin, S. Eriksson, H. Huber, and R. Bernander. 2008. Cell cycle characteristics of crenarchaeota: unity among diversity. J. Bacteriol. 190:5362-5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macur, R. E., H. W. Langner, B. D. Kocar, and W. P. Inskeep. 2004. Linking geochemical processes with microbial community analysis: successional dynamics in an arsenic-rich, acid-sulfate-chloride geothermal spring. Geobiology 2:163-177. [Google Scholar]
- 35.Malandrin, L., H. Huber, and R. Bernander. 1999. Nucleoid structure and partition in Methanococcus jannaschii: an archaeon with multiple copies of the chromosome. Genetics 152:1315-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mathur, J., R. W. Bizzoco, D. G. Ellis, D. A. Lipson, A. W. Poole, R. Levine, and S. T. Kelley. 2007. Effects of abiotic factors on the phylogenetic diversity of bacterial communities in acidic thermal springs. Appl. Environ. Microbiol. 73:2612-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller, S. R., M. Martin, J. Touchton, and R. W. Castenholz. 2002. Effects of nitrogen availability on pigmentation and carbon assimilation in the cyanobacterium Synechococcus sp. strain SH-94-5. Arch. Microbiol. 177:392-400. [DOI] [PubMed] [Google Scholar]
- 38.Miller, S. R., E. Wingard, and R. W. Castenholz. 1998. Effects of visible light and UV radiation on photosynthesis in a population of a hot spring cyanobacterium, a Synechococcus sp., subjected to high-temperature stress. Appl. Environ. Microbiol. 64:3893-3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nold, S. C., and D. M. Ward. 1996. Photosynthate partitioning and fermentation in hot spring microbial mat communities. Appl. Environ. Microbiol. 62:4598-4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nordstrom, D. K., J. W. Ball, and R. B. McCleskey. 2005. Ground water to surface water: chemistry of thermal outflows in Yellowstone National Park, p. 73-94. In W. P. Inskeep and T. R. McDermott (ed.), Geothermal biology and geochemistry in Yellowstone National Park. Montana State University Press, Bozeman.
- 41.Reysenbach, A.-L., A. Banta, S. Civello, J. Daly, K. Mitchel, S. Lalonde, K. Konhauser, A. Rodman, K. Rusterholtz, and C. Takacs-Vesbach. 2005. Aquificales in Yellowstone National Park, p. 129-142. In W. P. Inskeep and T. R. McDermott (ed.), Geothermal biology and geochemistry in Yellowstone National Park. Montana State University Press, Bozeman.
- 42.Schloss, P. D., and J. Handelsman. 2005. Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl. Environ. Microbiol. 71:1501-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shima, S., and K.-I. Suzuki. 1993. Hydrogenobacter acidophilis sp. nov., a thermoacidophilic, aerobic, hydrogen-oxidizing bacterium requiring elemental sulfur for growth. Int. J. Syst. Bacteriol. 43:703-708. [Google Scholar]
- 44.Skirnisdottir, S., G. O. Hreggvidsson, S. Hjorleifsdottir, V. T. Marteinsson, S. K. Petursdottir, O. Holst, and J. K. Kristjansson. 2000. Influence of sulfide and temperature on species composition and community structure of hot spring microbial mats. Appl. Environ. Microbiol. 66:2835-2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smits, T. H. M., C. Devenoges, K. Szynalski, J. Maillard, and C. Holliger. 2004. Development of a real-time PCR method for quantification of the three genera Dehalobacter, Dehalococcoides, and Desulfitobacterium in microbial communities. J. Microbiol. Methods 57:369-378. [DOI] [PubMed] [Google Scholar]
- 46.Spear, J. R., J. J. Walker, T. M. McCollom, and N. R. Pace. 2005. Hydrogen and bioenergetics in the Yellowstone geothermal ecosystem. Proc. Natl. Acad. Sci. USA 102:2555-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stohr, R., A. Waberski, W. Liesack, H. Volker, U. Wehmeyer, and M. Thomm. 2001. Hydrogenophilus hirschii sp. nov., a novel thermophilic hydrogen-oxidizing beta-proteobacterium isolated from Yellowstone National Park. Int. J. Syst. Evol. Microbiol. 51:481-488. [DOI] [PubMed] [Google Scholar]
- 48.Toplin, J. A., T. Norris, C. R. Lehr, T. R. McDermott, and R. W. Castenholz. 2008. Biogeographic and phylogenetic diversity of thermoacidophilic Cyanidiales in Yellowstone National Park, Japan, and New Zealand. Appl. Environ. Microbiol. 74:2822-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Niel, E. W. J., P. A. M. Claassen, and A. J. M. Stams. 2003. Substrate and product inhibition of hydrogen production by the extreme thermophile, Caldicellulosiruptor saccharolyticus. Biotechnol. Bioeng. 81:255-262. [DOI] [PubMed] [Google Scholar]
- 50.Xue, Y., Y. Xu, Y. Liu, Y. Ma, and P. Zhou. 2001. Thermoanaerobacter tengcongensis sp. nov., a novel anaerobic, saccharolytic, thermophilic bacterium isolated from a hot spring in Tengcong, China. Int. J. Syst. Evol. Microbiol. 51:1335-1341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.