Abstract
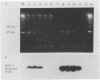
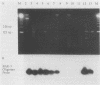
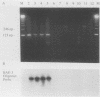
Culture and the polymerase chain reaction (PCR) were compared for detection of Borrelia burgdorferi infection in wild-caught Peromyscus leucopus and experimentally inoculated C.B-17 scid/scid (severe combined immunodeficient) mice. PCR targeted highly conserved regions of the ospA gene and could detect one to five cultured organisms and 10 to 50 copies of molecularly cloned ospA DNA. Organs (kidney, spleen, and urinary bladder) and/or ear biopsy samples were obtained from 108 captured P. leucopus mice, and tissues were obtained from 7 experimentally inoculated mice. A simple sample-processing procedure with proteinase K and detergent treatment was used in the PCR analysis. Overall, B. burgdorferi was detected in 29 of 108 (27%) P. leucopus mice by culture and in 31 of 108 (29%) mice by PCR. As assessed by the kappa statistic, agreement between PCR and culture was high for ear and bladder (kappa = 0.80 and 0.65, respectively) and low for kidney and spleen (kappa = 0.37 and 0.03, respectively). While concordant results were obtained from 98 animals, PCR detected B. burgdorferi from 6 additional mice for which cultures were negative and culture detected B. burgdorferi from 4 animals which were PCR negative. Further phenol-chloroform extraction of DNA in a limited number of samples improved the sensitivity of PCR compared with that of culture. These results indicate that PCR may be as sensitive as culture for detecting B. burgdorferi in ear samples and that PCR analysis is suitable for establishing the infection status of animals in mark-release-recapture studies.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. F., Duray P. H., Magnarelli L. A. Prevalence of Borrelia burgdorferi in white-footed mice and Ixodes dammini at Fort McCoy, Wis. J Clin Microbiol. 1987 Aug;25(8):1495–1497. doi: 10.1128/jcm.25.8.1495-1497.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. F., Johnson R. C., Magnarelli L. A. Seasonal prevalence of Borrelia burgdorferi in natural populations of white-footed mice, Peromyscus leucopus. J Clin Microbiol. 1987 Aug;25(8):1564–1566. doi: 10.1128/jcm.25.8.1564-1566.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. F., Magnarelli L. A., Burgdorfer W., Barbour A. G. Spirochetes in Ixodes dammini and mammals from Connecticut. Am J Trop Med Hyg. 1983 Jul;32(4):818–824. doi: 10.4269/ajtmh.1983.32.818. [DOI] [PubMed] [Google Scholar]
- Barbour A. G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):521–525. [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Todd W. J. Lyme disease spirochetes and ixodid tick spirochetes share a common surface antigenic determinant defined by a monoclonal antibody. Infect Immun. 1983 Aug;41(2):795–804. doi: 10.1128/iai.41.2.795-804.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthold S. W., Moody K. D., Terwilliger G. A., Duray P. H., Jacoby R. O., Steere A. C. Experimental Lyme arthritis in rats infected with Borrelia burgdorferi. J Infect Dis. 1988 Apr;157(4):842–846. doi: 10.1093/infdis/157.4.842. [DOI] [PubMed] [Google Scholar]
- Benach J. L., Bosler E. M., Hanrahan J. P., Coleman J. L., Habicht G. S., Bast T. F., Cameron D. J., Ziegler J. L., Barbour A. G., Burgdorfer W. Spirochetes isolated from the blood of two patients with Lyme disease. N Engl J Med. 1983 Mar 31;308(13):740–742. doi: 10.1056/NEJM198303313081302. [DOI] [PubMed] [Google Scholar]
- Bergström S., Bundoc V. G., Barbour A. G. Molecular analysis of linear plasmid-encoded major surface proteins, OspA and OspB, of the Lyme disease spirochaete Borrelia burgdorferi. Mol Microbiol. 1989 Apr;3(4):479–486. doi: 10.1111/j.1365-2958.1989.tb00194.x. [DOI] [PubMed] [Google Scholar]
- Bosler E. M., Coleman J. L., Benach J. L., Massey D. A., Hanrahan J. P., Burgdorfer W., Barbour A. G. Natural Distribution of the Ixodes dammini spirochete. Science. 1983 Apr 15;220(4594):321–322. doi: 10.1126/science.6836274. [DOI] [PubMed] [Google Scholar]
- Bosma G. C., Fried M., Custer R. P., Carroll A., Gibson D. M., Bosma M. J. Evidence of functional lymphocytes in some (leaky) scid mice. J Exp Med. 1988 Mar 1;167(3):1016–1033. doi: 10.1084/jem.167.3.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W., Barbour A. G., Hayes S. F., Benach J. L., Grunwaldt E., Davis J. P. Lyme disease-a tick-borne spirochetosis? Science. 1982 Jun 18;216(4552):1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Burgess E. C., Amundson T. E., Davis J. P., Kaslow R. A., Edelman R. Experimental inoculation of Peromyscus spp. with Borrelia burgdorferi: evidence of contact transmission. Am J Trop Med Hyg. 1986 Mar;35(2):355–359. doi: 10.4269/ajtmh.1986.35.355. [DOI] [PubMed] [Google Scholar]
- Callister S. M., Agger W. A., Schell R. F., Brand K. M. Efficacy of the urinary bladder for isolation of Borrelia burgdorferi from naturally infected, wild Peromyscus leucopus. J Clin Microbiol. 1989 Apr;27(4):773–774. doi: 10.1128/jcm.27.4.773-774.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debue M., Gautier P., Hackel C., Van Elsen A., Herzog A., Bigaignon G., Bollen A. Detection of Borrelia burgdorferi in biological samples using the polymerase chain reaction assay. Res Microbiol. 1991 Jun;142(5):565–572. doi: 10.1016/0923-2508(91)90189-h. [DOI] [PubMed] [Google Scholar]
- Duray P. H., Johnson R. C. The histopathology of experimentally infected hamsters with the Lyme disease spirochete, Borrelia burgdorferi. Proc Soc Exp Biol Med. 1986 Feb;181(2):263–269. doi: 10.3181/00379727-181-42251. [DOI] [PubMed] [Google Scholar]
- Goodman J. L., Jurkovich P., Kramber J. M., Johnson R. C. Molecular detection of persistent Borrelia burgdorferi in the urine of patients with active Lyme disease. Infect Immun. 1991 Jan;59(1):269–278. doi: 10.1128/iai.59.1.269-278.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy E. C., Stanek G. Detection of Borrelia burgdorferi in patients with Lyme disease by the polymerase chain reaction. J Clin Pathol. 1991 Jul;44(7):610–611. doi: 10.1136/jcp.44.7.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe T. R., LaQuier F. W., Barbour A. G. Organization of genes encoding two outer membrane proteins of the Lyme disease agent Borrelia burgdorferi within a single transcriptional unit. Infect Immun. 1986 Oct;54(1):207–212. doi: 10.1128/iai.54.1.207-212.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebech A. M., Hindersson P., Vuust J., Hansen K. Comparison of in vitro culture and polymerase chain reaction for detection of Borrelia burgdorferi in tissue from experimentally infected animals. J Clin Microbiol. 1991 Apr;29(4):731–737. doi: 10.1128/jcm.29.4.731-737.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnarelli L. A., Anderson J. F., Hyland K. E., Fish D., Mcaninch J. B. Serologic analyses of Peromyscus leucopus, a rodent reservoir for Borrelia burgdorferi, in northeastern United States. J Clin Microbiol. 1988 Jun;26(6):1138–1141. doi: 10.1128/jcm.26.6.1138-1141.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy D. C., Nauman R. K., Paxton H. Detection of Borrelia burgdorferi using the polymerase chain reaction. J Clin Microbiol. 1990 Jun;28(6):1089–1093. doi: 10.1128/jcm.28.6.1089-1093.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather T. N., Wilson M. L., Moore S. I., Ribeiro J. M., Spielman A. Comparing the relative potential of rodents as reservoirs of the Lyme disease spirochete (Borrelia burgdorferi). Am J Epidemiol. 1989 Jul;130(1):143–150. doi: 10.1093/oxfordjournals.aje.a115306. [DOI] [PubMed] [Google Scholar]
- Nielsen S. L., Young K. K., Barbour A. G. Detection of Borrelia burgdorferi DNA by the polymerase chain reaction. Mol Cell Probes. 1990 Feb;4(1):73–79. doi: 10.1016/0890-8508(90)90041-w. [DOI] [PubMed] [Google Scholar]
- Persing D. H., Telford S. R., 3rd, Spielman A., Barthold S. W. Detection of Borrelia burgdorferi infection in Ixodes dammini ticks with the polymerase chain reaction. J Clin Microbiol. 1990 Mar;28(3):566–572. doi: 10.1128/jcm.28.3.566-572.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa P. A., Schwan T. G. A specific and sensitive assay for the Lyme disease spirochete Borrelia burgdorferi using the polymerase chain reaction. J Infect Dis. 1989 Dec;160(6):1018–1029. doi: 10.1093/infdis/160.6.1018. [DOI] [PubMed] [Google Scholar]
- Schaible U. E., Kramer M. D., Museteanu C., Zimmer G., Mossmann H., Simon M. M. The severe combined immunodeficiency (scid) mouse. A laboratory model for the analysis of Lyme arthritis and carditis. J Exp Med. 1989 Oct 1;170(4):1427–1432. doi: 10.1084/jem.170.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinsky R. J., Piesman J. Ear punch biopsy method for detection and isolation of Borrelia burgdorferi from rodents. J Clin Microbiol. 1989 Aug;27(8):1723–1727. doi: 10.1128/jcm.27.8.1723-1727.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Telford S. R., 3rd, Mather T. N., Adler G. H., Spielman A. Short-tailed shrews as reservoirs of the agents of Lyme disease and human babesiosis. J Parasitol. 1990 Oct;76(5):681–683. [PubMed] [Google Scholar]
- Wise D. J., Weaver T. L. Detection of the Lyme disease bacterium, Borrelia burgdorferi, by using the polymerase chain reaction and a nonradioisotopic gene probe. J Clin Microbiol. 1991 Jul;29(7):1523–1526. doi: 10.1128/jcm.29.7.1523-1526.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Nielsen S. W. Experimental infection of the white-footed mouse with Borrelia burgdorferi. Am J Vet Res. 1990 Dec;51(12):1980–1987. [PubMed] [Google Scholar]