Abstract
Study Objectives:
(1) To describe the prevalence of and risk factors for postpartum maternal sleep problems and depressive symptoms simultaneously, (2) identify factors independently associated with either condition, and (3) explore associations between specific postpartum sleep components and depression.
Design:
Cross-sectional.
Setting:
Population-based.
Participants:
All women (n = 4191) who had delivered at Stavanger University Hospital from October 2005 to September 2006 were mailed a questionnaire seven weeks postpartum. The response rate was 68% (n = 2830).
Interventions:
None.
Measurements and results:
Sleep was measured using the Pittsburgh Sleep Quality Index (PSQI), and depressive symptoms using the Edinburgh Postnatal Depression Scale (EPDS). The prevalence of sleep problems, defined as PSQI > 5, was 57.7%, and the prevalence of depression, defined as EPDS ≥ 10, was 16.5%. The mean self-reported nightly sleep duration was 6.5 hours and sleep efficiency 73%. Depression, previous sleep problems, being primiparous, not exclusively breastfeeding, or having a younger or male infant were factors associated with poor postpartum sleep quality. Poor sleep was also associated with depression when adjusted for other significant risk factors for depression, such as poor partner relationship, previous depression, depression during pregnancy and stressful life events. Sleep disturbances and subjective sleep quality were the aspects of sleep most strongly associated with depression.
Conclusions:
Poor sleep was associated with depression independently of other risk factors. Poor sleep may increase the risk of depression in some women, but as previously known risk factors were also associated, mothers diagnosed with postpartum depression are not merely reporting symptoms of chronic sleep deprivation.
Citation:
Dørheim SK; Bondevik GT; Eberhard-Gran M; Bjorvatn B. Sleep and depression in postpartum women: a population-based study. SLEEP 2009;32(7):847-855.
Keywords: Postpartum, sleep, depression, PSQI, EPDS
DEPRESSION AND INSOMNIA ARE COMORBID AND INTERRELATED CONDITIONS,1,2 AND INSOMNIA IS OFTEN A PRECURSOR OF, AS WELL AS A NEGATIVE prognostic factor for, depression.3,4 Postpartum women sleep less during the early weeks following delivery than during pregnancy and other periods of reproductive age.5,6 At the same time, these women have an increased risk of depression.7,8 However, little attention has been devoted to the altered sleep pattern during the postpartum period and a possible association with maternal depression.9,10
The International Classification of Sleep Disorders, 2nd edition, defines insomnia as the presence of a sleep problem despite adequate opportunities for sleep.11 Such opportunities for sleep may be hard to find during the first postpartum months, as several factors can influence sleep among new mothers, including physical changes, demands from the infant and social factors.12 However, it has been suggested that sleep deprivation during the postpartum period caused by such factors may develop into chronic insomnia.13 Some authors have also suggested that sleep deprivation in healthy mothers could produce daytime sleepiness, cognitive deficits, fatigue, and irritability, consistent with mood symptoms reported postpartum,14 and they have postulated that the mothers were “sleepy, not weepy.”15
On the other hand, postpartum depression may aggravate an already impaired sleep quality, as experiencing difficulties with sleep is a symptom of depression.16 It is important to identify maternal depression because of its negative effects upon both the mother and child. Without help or treatment, the consequences may be prolonged and expensive for the women, for their families and in terms of the demands made on healthcare resources. In cases of severe depression, especially with psychotic symptoms, there is a risk of suicide.17 Depression in the mother may also affect the child's cognitive, emotional, and social development.18 Women suffering from depression are less likely to breastfeed,19 and, in developing countries, maternal depression is associated with poor infant growth.20
Prevalence estimates of depression in the postpartum period range from nearly 0% to 60%,21 depending on the study population and methodology. However, an overall estimate of 13% is commonly cited.22 One of the strongest determinants of postpartum depression is a previous history of depression.22–24 There is also a familial and genetic component.25 In addition to sleep disturbances, other factors may increase the risk of postpartum depression. Psychological distress, depression, and stressful life events during the previous year are all associated with an increased risk of the condition, as is a poor relationship with a partner.19,22–24
Associations between poor maternal sleep quality and depressive symptoms have been reported in questionnaire studies in smaller, selected samples of first-time mothers.26,27
Studying sleep diaries from primiparous women during the first postpartum month, Swain et al. found a correlation between the time spent awake at night and dysphoric mood during the first postpartum week,28 whereas Wolfson et al. found no differences in sleep diaries between depressed and non-depressed mothers.29 Associations between poor infant sleep, maternal daytime tiredness, and depressive symptoms have been reported in population studies. However, they mainly focused on infant sleep and did not use validated tools for the measurement of maternal sleep. Further studies including a larger and more heterogeneous group of women have been recommended.26
This cross-sectional study therefore aimed to estimate the prevalence of and risk factors for poor maternal sleep and depression simultaneously among postpartum women in a large, unselected population, using validated and commonly used tools. We also aimed to examine whether known risk factors for depression remained associated with depression after adjusting for the expected impaired sleep quality in this period. In this way, we wished to challenge the notion that mothers diagnosed with postpartum depression are in reality just reporting the effects of chronic sleep deprivation.14,15 Finally, we wanted to examine whether poor sleep is an independent risk factor for postpartum depression, and, if so, to find out which aspects of postpartum sleep are associated with depression in this period.
METHOD
Study Population and Sampling
From October 2005 to September 2006, all women who gave birth to a live child at Stavanger University Hospital in Norway were invited to participate in a cross-sectional questionnaire study of sleep and depressive symptoms. The hospital recruits women from a population of 300,000, including both urban and rural areas, and it is the region's only facility for deliveries. Seven weeks after delivery, questionnaires were mailed to the women living within the hospital's catchment area. Women whose children had died at birth or before the questionnaire was mailed were excluded. Women who did not respond within 2.5 weeks received a reminder, and women who replied later than 20 weeks after delivery were excluded (3 women).
Measure of Sleep
The Pittsburgh Sleep Quality Index (PSQI)30 was used to measure global sleep quality. The PSQI is a widely used self-rating questionnaire that assesses clinical and subjective sleep complaints during the previous month. Nineteen individual items generate 7 component scores (range 0–3, with higher scores indicating worse sleep): subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction. The sum of the scores for the 7 components yields one global sleep quality score (maximum score 21). A cut-off value of 5 has shown a sensitivity of 90% and a specificity of 87% for discrimination between “good” and “poor” sleepers. It has been translated into Norwegian and validated.31
Four questions taken from a Norwegian population study asked about any history of sleep problems outside perinatal periods, each coded yes/no (previously having experienced difficulties falling asleep, multiple awakenings at night, early morning awakenings, and sleep problems affecting daytime function).32 Women who answered yes to one or more of these questions were classified as having had “previous sleep problems,”
Measure of Mental Health
The Edinburgh Postnatal Depression Scale (EPDS)33 was used to measure depressive symptoms. The EPDS is a 10-item self-rating questionnaire developed to screen for depression in the postpartum period. It asks about symptoms present during the last 7 days. Each question has 4 alternative answers, scored 0–3, giving a maximum score of 30. Item 7 on the scale asks whether the woman has been “so unhappy that I have had difficulty sleeping.” In the statistical analyses for associated factors in the present study the EPDS scores were dichotomized as high ( ≥ 10) or low score ( < 10). This cut-off was found to have good psychometric properties in a Norwegian validation study.34 Women scoring ≥ 10 on the EPDS were classified as depressed.
Variables Obtained from Birth Records
Demographic characteristics (age, marital status, address) and obstetric history (parity, previous stillbirths and miscarriages, previous caesarean sections) were obtained from the hospital's birth records. These records also provided information about the present mode and time of delivery and characteristics of the infants, such as sex, twins/triplets, gestational age, birth weight, and Apgar scores.
Other Variables
The women were asked about their highest completed education and their main occupation (employed, self-employed, housewife, student, unemployed, or receiving disability/ rehabilitation benefit). Most women in Norway are entitled to 44 weeks of maternity leave with full pay after delivery, based on their income, whereas women without independent income receive a cash grant immediately after delivery. Students are given funding enabling them to postpone their studies for 6 months. Most women would therefore be at home with the baby at the time of the study. We also asked about breastfeeding practice (exclusively, with supplement, or not breastfeeding) and where the baby slept at night (cosleeping, separate bed, separate room, other). Information about any history of depression was obtained using a scale consisting of 5 questions (concerning sadness, appetite changes, lack of energy, self-blame, and concentration) constructed to measure lifetime history of major depression based on the DSM-IV criteria.35 When a woman reported having experienced ≥ 3 of these symptoms simultaneously for more than 2 weeks, she was asked to specify when this had occurred: during pregnancy, after the current delivery, and/or previously. A question about depression among close family members was coded yes, no, or don't know. We also asked about the experience of 10 specific stressful life events during the last year (rated emotionally “not so difficult, difficult, or very difficult”), as described by Eberhard-Gran et al.19 Finally, women who had a partner were asked to rate their satisfaction with the relationship (very content, content, some discontent, or very discontent).
Data Analysis
The questionnaires were optically scanned, and later manually checked for seemingly abnormal figures that appeared during data cleaning. If any of the data contributing to the PSQI total score were missing (110/2830, 3.9%), these respondents were excluded from the analysis of the corresponding subscore(s). However, if the sum of the remaining scores was > 5, these respondents were allocated to the group of high scorers. Similarly, if only one subscore was missing, and the sum of the remaining observations was 2 or less, these women were counted as low scorers. Only 39 women (1.4%) could not be categorized as either high or low scorers. For missing data in the EPDS (n = 49, 1.7%), sample means for these questions were used when the woman had completed at least 8 of the 10 questions contributing to the score (n = 44).
The distributions of the data were checked for normality. The scales' internal consistencies were calculated using Cronbach α. For numerical data, medians, means, standard deviations (SD) and 95th percentiles were calculated. Differences in means were tested using the Student independent t-test. Proportions are presented for categorical data. The correlation between PSQI score and the age of the infant was examined by linear regression. Effect sizes for the differences in mean PSQI scores between depressed and non-depressed women were calculated with Hedges bias correction. An actigraphy study of a subsample of this population found worse sleep among primiparas.36 Receiver operating characteristic (ROC) curves with coordinate estimates were therefore calculated for primiparas and for multiparas to suggest separate cut-off levels on the PSQI for an increased risk of depression. Differences between women scoring above or below cut-off values of the PSQI and the EPDS were tested by logistic regression analyses. The variables significantly (P < 0.05) associated with a PSQI value > 5 or with an EPDS value ≥ 10 in univariate logistic regression analyses were included in forward multiple logistic regression models (based upon likelihood ratios) along with age. This resulted in adjusted odds ratios (OR) with 95% confidence intervals (CI). To adjust for potential colinearity between the PSQI and the EPDS, these analyses were repeated without item 7 on the PSQI (daytime function) and without item 7 on the EPDS (sleep), without changing their respective cut-off levels. SPSS 15.0 for Windows was used for all statistical analyses except for the calculation of effect sizes, for which HyperStat Online's effect size calculator was used.37 Statistical significance was set to a P-value lower than 0.01 in the final models due to the large sample size and number of calculations.
Ethical Considerations
A letter of invitation was sent to the women along with the questionnaire, explaining the purpose of the study and that participation was voluntary. After giving the subjects a full description of the study, written informed consent was obtained. The study was approved by the National Data Inspectorate and the Regional Committee for Medical Research Ethics in Western Norway.
RESULTS
A total of 2830 women participated in the study (68% of all eligible women). Table 1 describes participation rates for specific sub-groups of mothers. The mean age of the mothers at the time of reply was 30 years (SD 4.7; range 18–48). The median age of the infants at the time of reply was 7.7 weeks, the mean age was 8.4 weeks (SD 1.5; range 6.6–19.6), and the 95 percentile was 11.2 weeks.
Table 1.
Selected Population Demographics and Response Rates
| Target population |
Included |
OR (95% CI) | Response | |||
|---|---|---|---|---|---|---|
| n | % | n | % | % | ||
| All delivered women | 4191 | 100 | 2830 | 100 | 67.5 | |
| Relationship status | ||||||
| Partnered | 3980 | 95.0 | 2716 | 96.0 | 1.0 | 68.2 |
| Single | 211 | 5.0 | 114 | 4.0 | 1.8 (1.4–2.4)** | 54.5 |
| Age | ||||||
| ≥ 24 years | 3651 | 87.1 | 2523 | 89.2 | 1.0 | 69.1 |
| < 24 years | 540 | 12.9 | 307 | 10.8 | 1.7 (1.4–2.0)** | 56.9 |
| Length of gestation | ||||||
| ≥ 37 weeks | 3936 | 93.9 | 2685 | 94.9 | 1.0 | 68.2 |
| < 37 weeks | 255 | 6.1 | 145 | 5.1 | 1.6 (1.3–2.1)** | 57.2 |
| Number of children | ||||||
| 1-2 children | 3285 | 78.4 | 2265 | 80.0 | 1.0 | 68.9 |
| 3 or more children | 906 | 21.6 | 565 | 20.0 | 1.3 (1.2–1.6)** | 62.6 |
| Mode of delivery | ||||||
| Vaginal | 3669 | 87.5 | 2490 | 88.0 | 1.0 | 67.9 |
| Operative* | 522 | 12.5 | 340 | 12.0 | 1.1 (0.9–1.4) | 65.3 |
| Infants | ||||||
| Single baby | 4109 | 98.0 | 2774 | 98.0 | 1.0 | 67.5 |
| Twins | 82 | 2.0 | 56 | 2.0 | 1.0 (0.6–1.5) | 68.3 |
Both elective and emergency, similar response rates;
P < 0.001, binary logistic regression
Global Sleep Quality, PSQI
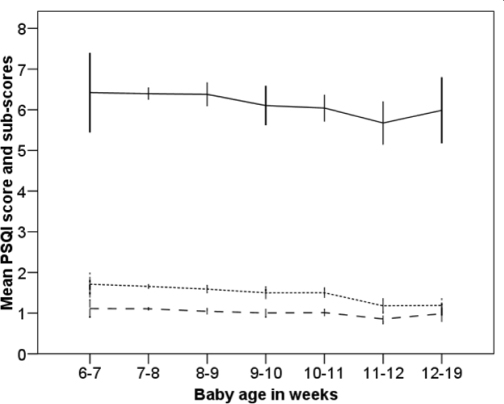
The mean score for the PSQI was 6.3 (SD 3.1), with 57.7% scoring above the cut-off value ( > 5). The Cronbach α for the scale was 0.71. This increased to 0.73 when omitting the PSQI subscore 6 (sleep medication). The mean total and subscores on the PSQI are shown in Table 2, along with differences by depressive status. The table also shows numeric values for some of the variables contributing to the PSQI scores (sleep onset latency, total hours' sleep at night and sleep efficiency). Linear regression showed that the total PSQI score decreased only slightly according to the age of the infant (R2 = 0.03, P < 0.001) (Figure 1). The decline in the mean PSQI score was mainly accounted for by a reduced subscore 4 (i.e., better sleep efficiency) among the 187 women (7% of the total sample) who responded in the 11th to the 19th postpartum week. The numerical change in sleep efficiency between early and late responders was 6% (from 72% in week 6-7 to 78% in week 11–19). Fifty-eight women (2.0%) had used sleep medication the previous month, 24 (0.8%) at least weekly.
Table 2.
PSQI Scores and Self-Reported Sleep Variables by Depressive Status
| Missing | All women | EPDS < 10 | EPDS ≥ 10 | Effect size** | |
|---|---|---|---|---|---|
| (n = 2830) | n = 2359 a | n = 466a | |||
| Mean (SD) | Mean (SD) | Mean (SD) | |||
| PSQI total (0-21) | 110 | 6.3 (3.1) | 5.7 (2.7) | 9.2 (3.1) | 1.2 |
| PSQI subscores (0-3): | |||||
| 1: Sleep quality | 8 | 1.0 (0.7) | 0.9 (0.6) | 1.6 (0.8) | 1.0 |
| 2: Sleep latency | 41 | 0.7 (0.9) | 0.6 (0.8) | 1.2 (1.0) | 0.7 |
| 3: Sleep duration | 18 | 1.1 (0.8) | 1.0 (0.8) | 1.5 (0.9) | -0.6 |
| 4: Habitual sleep efficiency | 29 | 1.6 (1.1) | 1.5 (1.1) | 2.0 (1.0) | 0.4 |
| 5: Sleep disturbances | 30 | 1.0 (0.5) | 0.9 (0.5) | 1.3 (0.6) | 0.9 |
| 6: Use of sleep medication | 6 | 0.03 (0.3) | 0.02 (0.2) | 0.11 (0.5) | 0.3 |
| 7: Daytime dysfunction | 20 | 1.0 (0.7) | 0.9 (0.6) | 1.7 (0.8) | 1.3 |
| Sleep variables, by PSQI | |||||
| Sleep onset latency, minutes | 30 | 19 (33) | 17 (31) | 26 (38) | 0.3 |
| Sleep at night, hours | 18 | 6.5 (1.3) | 6.6 (1.2) | 5.8 (1.3) | -0.6 |
| Sleep efficiency (%) | 29 | 73 (14) | 74 (14) | 68 (15) | -0.5 |
All P values < 0.001;
Five women had missing EPDS scores;
PSQI – Pittsburgh Sleep Quality Index; EPDS – Edinburgh Postnatal Depression Scale; SD – Standard deviation
Figure 1.
Changes in mean PSQI score and subscores by postpartum week.
—PSQI total score*
------- Sleep efficiency, subscore 4*
_ _ _ Sleep duration, subscore 3*
PSQI - Pittsburgh Sleep Quality Index.
Reduced scores indicate better global sleep quality, better sleep efficiency, and longer sleep duration.
Error bars: 95% confidence interval.
*Significant change (P < 0.01, linear regression). The PSQI subscores 1: Subjective sleep quality, 2: Sleep onset latency, 5: Sleep disturbances, 6: Sleep medication and 7: Daytime dysfunction did not show significant changes by postpartum week, and are not shown.
The forward multiple logistic regression analysis showed that depression was the factor most strongly (highest OR) associated with sleep problems during this period (Table 3). In addition, previous sleep problems, being primiparous, breastfeeding with supplement, or having a male infant remained factors associated with postpartum sleep problems (PSQI > 5). There was also a small difference in sleep for mothers with younger babies (mean difference in infant age of 0.1 weeks, SD 0.06, P < 0.01) and duration of pregnancy (mean difference of 0.2 weeks, SD 0.07, P < 0.001). Better maternal sleep was associated with the baby sleeping in a different room. When omitting EPDS question 7 (about sleep) and PSQI subscore 7 (daytime function) from the forward multiple analysis, but keeping the same cut-off on the scales, OR for depression was reduced from 6.4 to 4.1 (95% CI 3.2–5.4, P < 0.001), and OR for previous sleep problems from 3.1 to 2.3 (95% CI 1.9–2.8, P < 0.001), whereas the other variables showed only minor changes with unchanged significance.
Table 3.
Variables Associated with Poor Global Sleep Quality (PSQI > 5) in 2791a Postpartum Mothers, Results from Forward Multiple Logistic Regression Analysis
| Totalb | PSQI ≤ 5 | PSQI > 5 | OR | Adj. ORc | ||||
|---|---|---|---|---|---|---|---|---|
| n = 2791 | n = 1181 | n = 1610 | (95% CI) | (95%CI) | ||||
| n % |
n % |
n % |
||||||
| Depressive symptoms | ||||||||
| EPDS < 10 | 2325 | 83.4 | 1128 | 95.6 | 1197 | 74.5 | 1.0 | 1.0 |
| EPDS ≥ 10 | 462 | 16.6 | 52 | 4.4 | 410 | 25.5 | 7.4 (5.5–10.0)** | 6.4 (4.6–8.9)** |
| Previous sleep problems | ||||||||
| No | 1936 | 73.8 | 978 | 86.2 | 958 | 64.4 | 1.0 | 1.0 |
| Yes, 1-4 | 687 | 26.2 | 157 | 13.8 | 530 | 35.6 | 3.4 (2.8–4.2)** | 3.1 (2.5–3.8)** |
| Parity | ||||||||
| Multipara | 1570 | 56.3 | 729 | 61.7 | 841 | 52.2 | 1.0 | 1.0 |
| Primipara | 1221 | 43.7 | 452 | 38.3 | 769 | 47.8 | 1.5 (1.3–1.7)** | 1.7 (1.4–2.0)** |
| Feeding method | ||||||||
| Breastfeeding only | 2165 | 77.7 | 966 | 81.9 | 1199 | 74.5 | 1.0 | 1.0 |
| Breastfeeding and supplement | 376 | 13.5 | 118 | 10.0 | 258 | 16.0 | 1.8 (1.4–2.2)** | 1.5 (1.2–2.0)* |
| Not breastfeeding | 247 | 8.9 | 95 | 8.1 | 152 | 9.4 | 1.3 (1.0–1.7) | 1.1 (0.8–1.5) |
| Sex of newborn | ||||||||
| Female | 1343 | 48.1 | 615 | 52.1 | 728 | 45.2 | 1.0 | 1.0 |
| Male | 1448 | 51.9 | 566 | 47.9 | 882 | 54.8 | 1.3 (1.1–1.5)** | 1.3 (1.1–1.5)* |
| Place sleeping at night | ||||||||
| Infant in mother's bed | 445 | 16.0 | 166 | 14.1 | 279 | 17.4 | 1.0 | 1.0 |
| separate bed | 2217 | 79.7 | 958 | 81.5 | 1259 | 78.4 | 0.8 (0.5–1.1) | 0.6 (0.4–1.0) |
| separate room | 120 | 4.3 | 52 | 4.4 | 68 | 4.2 | 0.8 (0.6–1.0) | 0.7 (0.6–0.9)* |
P < 0.001
P < 0.01 PSQI – Pittsburgh Sleep Quality Index. EPDS – Edinburgh Postnatal Depression Scale.
39 women could not be classified as high or low scorers on the PSQI, due to missing data.
181 women had missing values for one or more of the variables included in the final model. “Earlier sleep problems” was missing for 168 women, for the other variables, between 0-9 observations were missing.
Adjusted for all the variables in the table, maternal age, infant age (P < 0.01) and duration of pregnancy (P < 0.001). The following variables (which were all individually associated with PSQI > 5, P < 0.05) were entered in the forward logistic regression, but not included in the final model because of non-significance: marital status (married/cohabiting or not), level of education, employment status (full-time income or not), earlier depression, depression during pregnancy, stress factors previous year, delivery method (caesarean section or not) and twins.
Depression
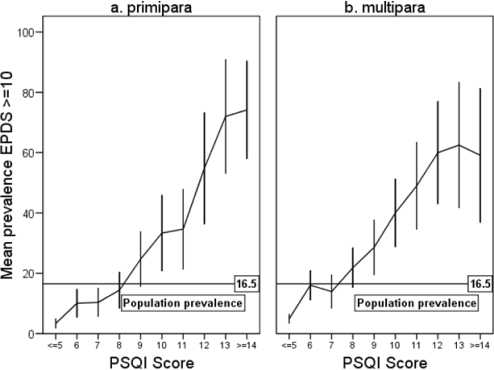
The mean EPDS score was 5.3 (SD 4.5). Cronbach α was 0.86. The prevalence of depression, defined as EPDS ≥ 10, was 16.5%. Figure 2 shows the proportions (95% CI) of women with depressive symptoms by total score on the PSQI, stratified by parity. The figure illustrates that PSQI scores above 7 for multiparas (sensitivity 0.73, specificity 0.67), or 8 for primiparas (sensitivity 0.74, specificity 0.72) implied a risk of depressive symptoms above the mean 16.5% population prevalence.
Figure 2.
Prevalence of depression (EPDS ≥ 10) by PSQI scores and parity.
EPDS – Edinburgh Postnatal Depression Scale.
PSQI – Pittsburgh Sleep Quality Index (Range 0-21).
Error bars – 95% confidence interval.
Table 4 shows factors significantly associated with depression (EPDS ≥ 10) in the forward multiple logistic regression analysis. Poor sleep quality was also associated with depression when adjusted for other significant risk factors for postpartum depression: poor partner relationship, previous depression, depression during pregnancy, and stressful life events. Daytime function, sleep disturbances, subjective sleep quality, and sleep onset latency were the PSQI subscores associated with a high depressive score. Repeating the forward multiple analysis without EPDS question 7 (sleep) and PSQI subscore 7 (daytime function) and keeping the same cut-off in the EPDS for depression, we found that the size of the odd ratios of subjective sleep quality (OR 2.9, 95% CI 2.4–3.5, P < 0.001) and sleep disturbances (OR 3.1, 95% CI 2.4–4.1, P < 0.001) decreased, but still remained significantly associated with depression. The association between depression and sleep onset latency became non-significant. The other factors remained significantly associated with depression, with odds ratios similar to those shown for each factor in Table 4.
Table 4.
Variables Associated with Depression (EPDS ≥ 10) Among 2825a Postpartum Women, Results from Multiple Logistic Regression Analysis
| Total | EPDS < 10 | EPDS ≥ 10 | OR | Adj. OR c | ||||
|---|---|---|---|---|---|---|---|---|
| n = 2825 |
n (2359 b) |
n (466 b) |
(95% CI) | (95%CI) | ||||
| PSQI global score | n | % | n | % | n | % | ||
| PSQI ≤ 5 | 1180 | 42.3 | 1128 | 48.5 | 52 | 11.3 | 1.0 | |
| PSQI > 5 | 1607 | 57.7 | 1197 | 51.5 | 410 | 88.7 | 7.4 (5.5–10.0)** | |
| PSQI subscores (0-3): | ||||||||
| 1: Subjective sleep quality | 1.6 (1.3–2.0) ** | |||||||
| 2: Sleep onset latencyd | 1.4 (1.2–1.6) ** | |||||||
| 5: Sleep disturbances | 2.3 (1.8–3.1) ** | |||||||
| 7: Daytime functiond | 3.1 (2.5–3.8) ** | |||||||
| Relationship to partner | ||||||||
| Very content | 1935 | 68.8 | 1721 | 73.2 | 214 | 46.3 | 1.0 | 1.0 |
| Content | 636 | 22.6 | 491 | 20.9 | 145 | 31.4 | 2.4 (1.9–3.0) ** | 1.8 (1.3–2.4) ** |
| Some/very discontent | 173 | 6.2 | 86 | 3.7 | 87 | 18.8 | 8.1 (5.8–11.3) ** | 4.0 (2.6–6.1) ** |
| Do not have a partner | 68 | 2.4 | 52 | 2.2 | 16 | 3.5 | 2.5 (1.4–4.4) ** | 1.2 (0.6–2.7) |
| Depression during pregnancy | ||||||||
| No | 2599 | 93.0 | 2235 | 95.7 | 364 | 79.3 | 1.0 | 1.0 |
| Yes | 196 | 7.0 | 101 | 4.3 | 95 | 20.7 | 5.8 (4.3–7.9) ** | 2.4 (1.6–3.6) ** |
| Previous depression | ||||||||
| No | 2048 | 73.3 | 1800 | 77.0 | 248 | 54.0 | 1.0 | 1.0 |
| Yes | 747 | 26.7 | 536 | 23.0 | 211 | 46.0 | 2.9 (2.3–3.5) ** | 1.7 (1.3–2.3) ** |
| Stress factors last year, rating | ||||||||
| 0 | 1324 | 49.2 | 1207 | 53.8 | 117 | 26.1 | 1.0 | 1.0 |
| 1-5 | 1091 | 40.5 | 877 | 39.1 | 214 | 47.8 | 2.5 (2.0–3.2) ** | 1.4 (1.1–1.9) * |
| > 5 | 276 | 10.3 | 159 | 7.1 | 117 | 26.1 | 7.6 (5.6–10.3) ** | 2.2 (1.4–3.3) ** |
P < 0.001
P < 0.01. PSQI – Pittsburgh Sleep Quality Index. EPDS – Edinburgh Postnatal Depression Scale.
5 women had missing EPDS scores.
237 women had missing data for one or more of the variables, and were not included in the final model. “Stress factors” were missing for 134 women; for the other variables, between 0-43 observations were missing.
Adjusted for all the variables in the table and maternal age.
Not significant when adjusting for overlapping constructs in the PSQI and the EPDS, see text. The following variables (which were all individually associated with depressive symptoms, P < 0.05) were entered in the forward logistic regression, but not included in the final model because of non-significance: level of education, employment status, depression in close family, earlier sleep problems, marital status, feeding method, and gender of baby.
DISCUSSION
Two months after delivery, nearly 60% of the postpartum women experienced poor global sleep quality, whereas 16.5% had depressive symptoms. Sleep disturbances and poor subjective sleep quality were associated with depression independently of other significant risk factors for the disease. Previous sleep problems and being a first-time mother were associated with poor global sleep quality, but not with depression.
The first three months after delivery are characterized by a continuous change in sleep parameters.38 Hence, postpartum women may have difficulties evaluating the previous month's sleep, as required by the PSQI. However, the psychometric properties of the scale were acceptable and similar to previous validations.30,31 There was a significant, although small decline in PSQI scores with increasing age of the infant, mainly due to improved sleep efficiency from the 11th postpartum week (Figure 1). The other subscores remained stable throughout the period. The PSQI therefore seems to be valid for use also during the postpartum period.
The prevalence of EPDS ≥ 10 was higher than previously reported from Norway.19,39 This may reflect a possible increase in psychiatric symptoms among young women in Norway.40 However, mothers who were single, younger, had more than two children or a premature baby were less likely to participate, and this may have influenced the prevalence estimates slightly.
Both depressed and non-depressed women reported lower sleep efficiency than the normal 85% or more41 and had a shorter sleep duration than previously reported from Norway.31,32 The PSQI scores were lower than found among first-time mothers two to three weeks postpartum,27 and women with depression (EPDS ≥ 10) had lower PSQI scores than the depressed group in the original validation.30 However, not all women with elevated EPDS scores will be severely depressed, due to false positives and the inclusion of women with minor depression.42
Sleep and Depression
Depression was associated with poor global sleep quality also when adjusted for known psychosocial stressors and individual disposition to depression. The duration of sleep and sleep efficiency were not associated with depression, whereas poorer subjective reports of sleep quality and sleep disturbances were. Huang et al. also found sleep disturbances and daytime dysfunction to be the PSQI subscores most strongly related to depressive symptoms three weeks after delivery.27 Similarly, Dennis et al. reported an association between daytime tiredness and maternal depression.43 However, the association between daytime dysfunction and depression may be explained by colinearity between the two scales, as the association weakened, but remained significant, when overlapping questions were omitted from the analyses.
Associations between the amount of sleep and the perception of sleep quality may differ between depressed and non-depressed subjects.44 Mothers reporting good sleep quality despite an infant's sleep problem do not have a higher risk of depression,12 and improvements in infant sleep do not necessarily improve the EPDS score of the mother.45 We measured sleep using actigraphy and sleep diaries in a subsample from this study, and there were no differences in sleep (onset, duration, efficiency, number of awakenings, or subjective quality) between depressed and non-depressed women, despite several differences in the PSQI. Depressed mothers, however, reported lower daytime energy in their sleep diaries.36
Other Risk Factors for Poor Sleep
Previous sleep problems were associated with poor global sleep quality. Women with previous sleep problems may have more difficulty adapting to the required change of sleep pattern in the postpartum period.46 First-time mothers experience a greater change in sleep pattern after delivery than multiparous mothers,5 in line with the results from the present study. Breastfeeding mothers' global sleep quality was better than that of mothers who partially bottle-fed their infants, but not better than mothers who did not breastfeed at all. Blyton et al. documented better polysomnographic sleep among new mothers who breastfed.47 Longitudinal studies are needed to study whether partial breastfeeding leads to poorer maternal sleep, or whether this reflects a strategy to improve an already impaired sleep. Maternal sleep was better if the infant slept in a separate room compared to sharing a bed with the infant. Bed-sharing could reflect a strategy by the mother to calm a fussy infant in order to improve sleep.48 Our finding of worse sleep quality among mothers of younger and/or preterm babies corresponds with the documented development of infant nighttime sleep-wake patterns.49 Sex effects, with worse sleep registered among male infants, have also been found by others for sleep/wake patterns during the first two years of life.12,50
Other Risk Factors for Depression
Twenty-one percent of the depressed postpartum women reported having also been depressed during pregnancy, and 46% of them reported at least one previous depressive episode prior to conception. This underscores the recurring nature of depression51 and the higher risk of depression during the perinatal period for women with a history of psychiatric illness.22–24 Other strong risk factors for postpartum depression, such as poor partner relationship and stressful life events,22–24 also remained associated with depression in this study when adjusted for poor sleep. Mothers diagnosed with postpartum depression are therefore not merely reporting the symptoms of chronic sleep deprivation, as postulated earlier,14,15 and these other risk factors also need to be addressed in order to treat depressed mothers.
Depression after delivery is often not identified by the women and their helpers, whereas tiredness and lack of sleep are common complaints. Women who are tired may attribute this to poor sleep, but the tiredness could alternatively be caused by depression. Talking about sleep problems may provide an entry point for also discussing the mother's mental well-being. Some impairment of sleep should be expected, but when the PSQI score rises above 7 for multiparas or 8 for primiparas, there is an increasing risk of the woman being depressed (Figure 2).
Conclusion and Further Research
Due to the cross-sectional design, this study cannot determine causality. Others have suggested a bidirectional relationship between sleep and depression.1,52 Individual women may react differently to shorter sleep duration and lower sleep efficiency during the postpartum period. The sleep of women with a history of depression may be more sensitive to the psychobiological (hormonal, immunological, psychological, and social) changes associated with childbirth.53,54 Sleep could act as a moderator between these risk factors for depression and the precipitation of depression in women vulnerable to such sleep changes during the postpartum period. Longitudinal studies are needed to evaluate whether treatment of maternal sleep problems reduces depression and whether treatment of maternal depression improves sleep quality.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Bjorvatn has participated in research projects sponsored by Lundbeck AS and Sanofi-Aventis and has participated in speaking engagements for Sanofi-Aventis, Pfizer, Wyeth, and NycoMed. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We thank the women who participated in this study. We also thank Dr. Eli Smedvig and the other staff at the maternity unit at Stavanger University Hospital, as well as the public health nurses in Rogaland for providing women with information about the study. Special thanks to Dr. Leif Gjessing, Stavanger University Hospital, for providing data about the deliveries at the hospital. The study was funded by the Western Norway Regional Health Authority.
The study was carried out at Stavanger University Hospital and funded by the Western Norway Regional Health Authority. There was no off-label or investigational use of medications.
ABBREVIATIONS
- CI
Confidence interval
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders – 4th edition
- EPDS
Edinburgh Postnatal Depression Scale
- OR
Odds ratio
- PSQI
Pittsburgh Sleep Quality Index
- ROC
Receiver operating characteristic
- SD
Standard deviation
REFERENCES
- 1.Ohayon MM, Roth T. Place of chronic insomnia in the course of depressive and anxiety disorders. J Psychiatr Res. 2003;37:9–15. doi: 10.1016/s0022-3956(02)00052-3. [DOI] [PubMed] [Google Scholar]
- 2.Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007;3:S7–10. [PMC free article] [PubMed] [Google Scholar]
- 3.Neckelmann D, Mykletun A, Dahl AA. Chronic insomnia as a risk factor for developing anxiety and depression. Sleep. 2007;30:873–80. doi: 10.1093/sleep/30.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buysse DJ, Angst J, Gamma A, Ajdacic V, Eich D, Rossler W. Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep. 2008;31:473–80. doi: 10.1093/sleep/31.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee KA, Zaffke ME, McEnany G. Parity and sleep patterns during and after pregnancy. Obstet Gynecol. 2000;95:14–8. doi: 10.1016/s0029-7844(99)00486-x. [DOI] [PubMed] [Google Scholar]
- 6.Nishihara K, Horiuchi S, Eto H, Uchida S. Comparisons of sleep patterns between mothers in post-partum from 9 to 12 weeks and non-pregnant women. Psychiatry Clin Neurosci. 2001;55:227–8. doi: 10.1046/j.1440-1819.2001.00835.x. [DOI] [PubMed] [Google Scholar]
- 7.Munk-Olsen T, Laursen TM, Pedersen CB, Mors O, Mortensen PB. New parents and mental disorders: a population-based register study. JAMA. 2006;296:2582–9. doi: 10.1001/jama.296.21.2582. [DOI] [PubMed] [Google Scholar]
- 8.Eberhard-Gran M, Tambs K, Opjordsmoen S, Skrondal A, Eskild A. Depression during pregnancy and after delivery: a repeated measurement study. J Psychosom Obstet Gynaecol. 2004;25:15–21. doi: 10.1080/01674820410001737405. [DOI] [PubMed] [Google Scholar]
- 9.Ross LE, Murray BJ, Steiner M. Sleep and perinatal mood disorders: a critical review. J Psychiatry Neurosci. 2005;30:247–56. [PMC free article] [PubMed] [Google Scholar]
- 10.Lee KA. Alterations in sleep during pregnancy and postpartum: a review of 30 years of research. Sleep Med Rev. 1998;2:231–42. doi: 10.1016/s1087-0792(98)90010-7. [DOI] [PubMed] [Google Scholar]
- 11.AASM. Diagnostic and coding manual. 2nd ed. Westchester: IL: American Academy of Sleep Medicine; 2005. International classification of sleep disorders. [Google Scholar]
- 12.Bayer JK, Hiscock H, Hampton A, Wake M. Sleep problems in young infants and maternal mental and physical health. J Paediatr Child Health. 2007;43:66–73. doi: 10.1111/j.1440-1754.2007.01005.x. [DOI] [PubMed] [Google Scholar]
- 13.Silber MH. Clinical practice. Chronic insomnia. N Engl J Med. 2005;353:803–10. doi: 10.1056/NEJMcp043762. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong KL, Van Haeringen AR, Dadds MR, Cash R. Sleep deprivation or postnatal depression in later infancy: separating the chicken from the egg. J Paediatr Child Health. 1998;34:260–2. doi: 10.1046/j.1440-1754.1998.00213.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee KA, McEnany G, Zaffke ME. REM sleep and mood state in childbearing women: sleepy or weepy? Sleep. 2000;23:877–85. [PubMed] [Google Scholar]
- 16.APA. Diagnostic and statistical manual of mental disorders. fourth edition. Washington, DC: American Psychiatric Press; 1994. American Psychiatric Association. [Google Scholar]
- 17.Oates M. Perinatal psychiatric disorders: a leading cause of maternal morbidity and mortality. Br Med Bull. 2003;67:219–29. doi: 10.1093/bmb/ldg011. [DOI] [PubMed] [Google Scholar]
- 18.Murray L, Cooper PJ. Postpartum depression and child development. Psychol Med. 1997;27:253–60. doi: 10.1017/s0033291796004564. [DOI] [PubMed] [Google Scholar]
- 19.Eberhard-Gran M, Eskild A, Tambs K, Samuelsen SO, Opjordsmoen S. Depression in postpartum and non-postpartum women: prevalence and risk factors. Acta Psychiatr Scand. 2002;106:426–33. doi: 10.1034/j.1600-0447.2002.02408.x. [DOI] [PubMed] [Google Scholar]
- 20.Rahman A, Patel V, Maselko J, Kirkwood B. The neglected ‘m' in MCH programmes--why mental health of mothers is important for child nutrition. Trop Med Int Health. 2008;13:579–83. doi: 10.1111/j.1365-3156.2008.02036.x. [DOI] [PubMed] [Google Scholar]
- 21.Halbreich U, Karkun S. Cross-cultural and social diversity of prevalence of postpartum depression and depressive symptoms. J Affect Disord. 2006;91:97–111. doi: 10.1016/j.jad.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 22.O'Hara MW, Swain AM. Rates and risk of postpartum depression-a meta-analysis. Int Rev Psychiatry. 1996;8:37–54. [Google Scholar]
- 23.Brockington I. Postpartum psychiatric disorders. Lancet. 2004;363:303–10. doi: 10.1016/S0140-6736(03)15390-1. [DOI] [PubMed] [Google Scholar]
- 24.Robertson E, Grace S, Wallington T, Stewart DE. Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry. 2004;26:289–95. doi: 10.1016/j.genhosppsych.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Treloar SA, Martin NG, Bucholz KK, Madden PA, Heath AC. Genetic influences on post-natal depressive symptoms: findings from an Australian twin sample. Psychol Med. 1999;29:645–54. doi: 10.1017/s0033291799008387. [DOI] [PubMed] [Google Scholar]
- 26.Goyal D, Gay CL, Lee KA. Patterns of sleep disruption and depressive symptoms in new mothers. J Perinat Neonatal Nurs. 2007;21:123. doi: 10.1097/01.JPN.0000270629.58746.96. [DOI] [PubMed] [Google Scholar]
- 27.Huang CM, Carter PA, Guo JL. A comparison of sleep and daytime sleepiness in depressed and non-depressed mothers during the early postpartum period. J Nurs Res. 2004;12:287–96. doi: 10.1097/01.jnr.0000387513.75114.bb. [DOI] [PubMed] [Google Scholar]
- 28.Swain AM, O'Hara MW, Starr KR, Gorman LL. A prospective study of sleep, mood, and cognitive function in postpartum and nonpostpartum women. Obstet Gynecol. 1997;90:381–6. doi: 10.1016/s0029-7844(97)89252-6. [DOI] [PubMed] [Google Scholar]
- 29.Wolfson AR, Crowley SJ, Anwer U, Bassett JL. Changes in sleep patterns and depressive symptoms in first-time mothers: last trimester to 1-year postpartum. Behav Sleep Med. 2003;1:54–67. doi: 10.1207/S15402010BSM0101_6. [DOI] [PubMed] [Google Scholar]
- 30.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 31.Pallesen S, Nordhus IH, Omvik S, Sivertsen B, Matthiesen SB, Bjorvatn B. Pittsburgh Sleep Quality Index. Tidsskrift for norsk psykologforening. 2005;42:714–7. [Google Scholar]
- 32.Ursin R, Bjorvatn B, Holsten F. Sleep duration, subjective sleep need, and sleep habits of 40- to 45-year-olds in the Hordaland Health Study. Sleep. 2005;28:1260–9. doi: 10.1093/sleep/28.10.1260. [DOI] [PubMed] [Google Scholar]
- 33.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–6. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 34.Eberhard-Gran M, Eskild A, Tambs K, Schei B, Opjordsmoen S. The Edinburgh Postnatal Depression Scale: validation in a Norwegian community sample. Nord J Psychiatry. 2001;55:113–7. doi: 10.1080/08039480151108525. [DOI] [PubMed] [Google Scholar]
- 35.Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. The lifetime history of major depression in women. Reliability of diagnosis and heritability. Arch Gen Psychiatry. 1993;50:863–70. doi: 10.1001/archpsyc.1993.01820230054003. [DOI] [PubMed] [Google Scholar]
- 36.Dorheim SK, Bondevik GT, Eberhard-Gran M, Bjorvatn B. Subjective and objective sleep among depressed and non-depressed postnatal women. Acta Psychiatr Scand. 2009;119:128–36. doi: 10.1111/j.1600-0447.2008.01272.x. [DOI] [PubMed] [Google Scholar]
- 37.Lane DM. Measuring Effect Size. In: Lane DM, editor. HyperStat Online Statistics Textbook. Houston, TX, USA: Rice Virtual Lab in Statistics; 2007. http://davidmlane.com/hyperstat/effect_size.html. [Google Scholar]
- 38.Kang MJ, Matsumoto K, Shinkoda H, Mishima M, Seo YJ. Longitudinal study for sleep-wake behaviours of mothers from pre-partum to post-partum using actigraph and sleep logs. Psychiatry Clin Neurosci. 2002;56:251–2. doi: 10.1046/j.1440-1819.2002.00992.x. [DOI] [PubMed] [Google Scholar]
- 39.Berle JO, Aarre TF, Mykletun A, Dahl AA, Holsten F. Screening for postnatal depression. Validation of the Norwegian version of the Edinburgh Postnatal Depression Scale, and assessment of risk factors for postnatal depression. J Affect Disord. 2003;76:151–6. doi: 10.1016/s0165-0327(02)00082-4. [DOI] [PubMed] [Google Scholar]
- 40.Statistics-Norway. Increase in young women seeing a psychologist Health Interview Survey 2005 2006. http://www.ssb.no/english/subjects/03/0/helseund_en/
- 41.Morin CM. New York, NY, USA: Guildford Press; 1993. Insomnia: Psychological assessment and management. [Google Scholar]
- 42.Eberhard-Gran M, Eskild A, Tambs K, Opjordsmoen S, Samuelsen SO. Review of validation studies of the Edinburgh Postnatal Depression Scale. Acta Psychiatr Scand. 2001;104:243–9. doi: 10.1034/j.1600-0447.2001.00187.x. [DOI] [PubMed] [Google Scholar]
- 43.Dennis CL, Ross L. Relationships among infant sleep patterns, maternal fatigue, and development of depressive symptomatology. Birth. 2005;32:187–93. doi: 10.1111/j.0730-7659.2005.00368.x. [DOI] [PubMed] [Google Scholar]
- 44.Edinger JD, Fins AI, Glenn DM, et al. Insomnia and the eye of the beholder: are there clinical markers of objective sleep disturbances among adults with and without insomnia complaints? J Consult Clin Psychol. 2000;68:586–93. [PubMed] [Google Scholar]
- 45.Hiscock H, Bayer J, Gold L, Hampton A, Ukoumunne OC, Wake M. Improving infant sleep and maternal mental health: a cluster randomised trial. Arch Dis Child. 2007;92:952–8. doi: 10.1136/adc.2006.099812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moline M, Broch L, Zak R. Sleep problems across the life cycle in women. Curr Treat Options Neurol. 2004;6:319–30. doi: 10.1007/s11940-004-0031-6. [DOI] [PubMed] [Google Scholar]
- 47.Blyton DM, Sullivan CE, Edwards N. Lactation is associated with an increase in slow-wave sleep in women. J Sleep Res. 2002;11:297–303. doi: 10.1046/j.1365-2869.2002.00315.x. [DOI] [PubMed] [Google Scholar]
- 48.Hauck FR, Signore C, Fein SB, Raju TN. Infant sleeping arrangements and practices during the first year of life. Pediatrics. 2008;122(Suppl 2):S113–20. doi: 10.1542/peds.2008-1315o. [DOI] [PubMed] [Google Scholar]
- 49.Anders TF, Keener M. Developmental course of nighttime sleep-wake patterns in full-term and premature infants during the first year of life. I. Sleep. 1985;8:173–92. doi: 10.1093/sleep/8.3.173. [DOI] [PubMed] [Google Scholar]
- 50.Spruyt K, Aitken RJ, So K, Charlton M, Adamson TM, Horne RS. Relationship between sleep/wake patterns, temperament and overall development in term infants over the first year of life. Early Hum Dev. 2007 doi: 10.1016/j.earlhumdev.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 51.Burt VK, Stein K. Epidemiology of depression throughout the female life cycle. J Clin Psychiatry. 2002;63(Suppl 7):9–15. [PubMed] [Google Scholar]
- 52.Krystal AD. Depression and insomnia in women. Clin Cornerstone. 2004;6(Suppl 1B):S19–28. doi: 10.1016/s1098-3597(04)80022-x. [DOI] [PubMed] [Google Scholar]
- 53.Coble PA, Reynolds CF, 3rd, Kupfer DJ, Houck PR, Day NL, Giles DE. Childbearing in women with and without a history of affective disorder. II. Electroencephalographic sleep. Compr Psychiatry. 1994;35:215–24. doi: 10.1016/0010-440x(94)90194-5. [DOI] [PubMed] [Google Scholar]
- 54.Riecher-Rössler A, Rohde A. Diagnostic classification of perinatal mood disorders. In: Riecher-Rössler A, Steiner M, editors. Perinatal stress, mood and anxiety disorders: from bench to bedside. Basel: Karger; 2005. pp. 6–27. [Google Scholar]