Abstract
Yersinia enterocolitica is widespread in nature, but only a few bioserotypes are involved in human infections. Pigs are considered to be the major reservoirs of pathogenic strains. It is essential to have an accurate and rapid method for the detection of pathogenic yersiniae. To achieve this objective, 19-base synthetic oligonucleotide primers were used in a polymerase chain reaction (PCR) to detect the ail gene (which is conserved only in pathogenic strains) in strains of Y. enterocolitica and related species originating from pigs or pork products. Digoxigenin-labeled probes derived from the ail, inv, and yst genes were also evaluated on these strains. The PCR amplified a 273-bp fragment of the ail gene involved in eukaryotic cell invasion and serum resistance. The PCR detected template DNA only in strains of Y. enterocolitica traditionally classified as human pathogens but not in biotype 1A strains and related species. Other members of the family Enterobacteriaceae were also negative for the target gene. The digoxigenin-labeled ail probe gave identical results to the PCR. By use of this nonisotopic method, inv-homologous DNA was detected only among yersiniae, except for Y. ruckeri. Although all pathogenic serotypes of Y. enterocolitica were positive for the heat-stable enterotoxin yst gene, two strains of biotype 1A, one Y. intermedia strain, and six other species of the Enterobacteriaceae were also positive. Our results support the notion that pigs constitute an important reservoir of pathogenic Y. enterocolitica and that the inv-homologous sequence is Yersinia specific.
Full text
PDF


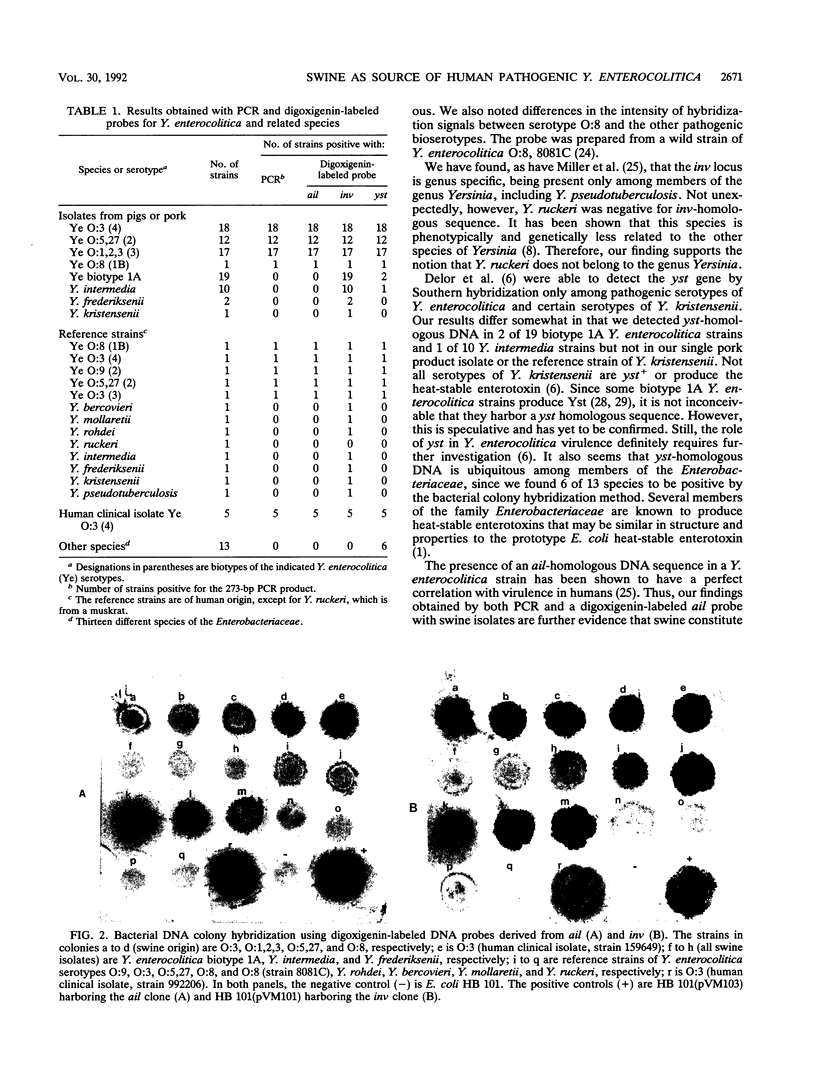


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betley M. J., Miller V. L., Mekalanos J. J. Genetics of bacterial enterotoxins. Annu Rev Microbiol. 1986;40:577–605. doi: 10.1146/annurev.mi.40.100186.003045. [DOI] [PubMed] [Google Scholar]
- Blumberg H. M., Kiehlbauch J. A., Wachsmuth I. K. Molecular epidemiology of Yersinia enterocolitica O:3 infections: use of chromosomal DNA restriction fragment length polymorphisms of rRNA genes. J Clin Microbiol. 1991 Nov;29(11):2368–2374. doi: 10.1128/jcm.29.11.2368-2374.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Border P. M., Howard J. J., Plastow G. S., Siggens K. W. Detection of Listeria species and Listeria monocytogenes using polymerase chain reaction. Lett Appl Microbiol. 1990 Sep;11(3):158–162. doi: 10.1111/j.1472-765x.1990.tb00149.x. [DOI] [PubMed] [Google Scholar]
- Cornelis G. R., Biot T., Lambert de Rouvroit C., Michiels T., Mulder B., Sluiters C., Sory M. P., Van Bouchaute M., Vanooteghem J. C. The Yersinia yop regulon. Mol Microbiol. 1989 Oct;3(10):1455–1459. doi: 10.1111/j.1365-2958.1989.tb00129.x. [DOI] [PubMed] [Google Scholar]
- Cornelis G., Laroche Y., Balligand G., Sory M. P., Wauters G. Yersinia enterocolitica, a primary model for bacterial invasiveness. Rev Infect Dis. 1987 Jan-Feb;9(1):64–87. doi: 10.1093/clinids/9.1.64. [DOI] [PubMed] [Google Scholar]
- Delor I., Kaeckenbeeck A., Wauters G., Cornelis G. R. Nucleotide sequence of yst, the Yersinia enterocolitica gene encoding the heat-stable enterotoxin, and prevalence of the gene among pathogenic and nonpathogenic yersiniae. Infect Immun. 1990 Sep;58(9):2983–2988. doi: 10.1128/iai.58.9.2983-2988.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneer H. G., Boychuk I. Species-specific detection of Listeria monocytogenes by DNA amplification. Appl Environ Microbiol. 1991 Feb;57(2):606–609. doi: 10.1128/aem.57.2.606-609.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkow S. Bacterial entry into eukaryotic cells. Cell. 1991 Jun 28;65(7):1099–1102. doi: 10.1016/0092-8674(91)90003-h. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fenwick S. G., Murray A. Detection of pathogenic Yersinia enterocolitica by polymerase chain reaction. Lancet. 1991 Feb 23;337(8739):496–497. doi: 10.1016/0140-6736(91)93436-d. [DOI] [PubMed] [Google Scholar]
- Heery D. M., Gannon F., Powell R. A simple method for subcloning DNA fragments from gel slices. Trends Genet. 1990 Jun;6(6):173–173. doi: 10.1016/0168-9525(90)90158-3. [DOI] [PubMed] [Google Scholar]
- Hill W. E., Payne W. L., Aulisio C. C. Detection and enumeration of virulent Yersinia enterocolitica in food by DNA colony hybridization. Appl Environ Microbiol. 1983 Sep;46(3):636–641. doi: 10.1128/aem.46.3.636-641.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R. R., Falkow S. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature. 1985 Sep 19;317(6034):262–264. doi: 10.1038/317262a0. [DOI] [PubMed] [Google Scholar]
- Kapperud G., Dommarsnes K., Skurnik M., Hornes E. A synthetic oligonucleotide probe and a cloned polynucleotide probe based on the yopA gene for detection and enumeration of virulent Yersinia enterocolitica. Appl Environ Microbiol. 1990 Jan;56(1):17–23. doi: 10.1128/aem.56.1.17-23.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C., Swaminathan B., Cassaday P. K., Mayer L. W., Holloway B. P. Rapid confirmation of Listeria monocytogenes isolated from foods by a colony blot assay using a digoxigenin-labeled synthetic oligonucleotide probe. Appl Environ Microbiol. 1991 Jun;57(6):1609–1614. doi: 10.1128/aem.57.6.1609-1614.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwaga J. K., Iversen J. O. Laboratory investigation of virulence among strains of Yersinia enterocolitica and related species isolated from pigs and pork products. Can J Microbiol. 1992 Feb;38(2):92–97. doi: 10.1139/m92-015. [DOI] [PubMed] [Google Scholar]
- Lortie L. A., Harel J., Fairbrother J. M., Dubreuil J. D. Evaluation of three new techniques for the detection of STb-positive Escherichia coli strains. Mol Cell Probes. 1991 Aug;5(4):271–275. doi: 10.1016/0890-8508(91)90048-o. [DOI] [PubMed] [Google Scholar]
- Lowe T., Sharefkin J., Yang S. Q., Dieffenbach C. W. A computer program for selection of oligonucleotide primers for polymerase chain reactions. Nucleic Acids Res. 1990 Apr 11;18(7):1757–1761. doi: 10.1093/nar/18.7.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miliotis M. D., Morris J. G., Jr, Cianciosi S., Wright A. C., Wood P. K., Robins-Browne R. M. Identification of a conjunctivitis-associated gene locus from the virulence plasmid of Yersinia enterocolitica. Infect Immun. 1990 Aug;58(8):2470–2477. doi: 10.1128/iai.58.8.2470-2477.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Bliska J. B., Falkow S. Nucleotide sequence of the Yersinia enterocolitica ail gene and characterization of the Ail protein product. J Bacteriol. 1990 Feb;172(2):1062–1069. doi: 10.1128/jb.172.2.1062-1069.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Falkow S. Evidence for two genetic loci in Yersinia enterocolitica that can promote invasion of epithelial cells. Infect Immun. 1988 May;56(5):1242–1248. doi: 10.1128/iai.56.5.1242-1248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Farmer J. J., 3rd, Hill W. E., Falkow S. The ail locus is found uniquely in Yersinia enterocolitica serotypes commonly associated with disease. Infect Immun. 1989 Jan;57(1):121–131. doi: 10.1128/iai.57.1.121-131.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Finlay B. B., Falkow S. Factors essential for the penetration of mammalian cells by Yersinia. Curr Top Microbiol Immunol. 1988;138:15–39. [PubMed] [Google Scholar]
- Nesbakken T., Kapperud G., Dommarsnes K., Skurnik M., Hornes E. Comparative study of a DNA hybridization method and two isolation procedures for detection of Yersinia enterocolitica O:3 in naturally contaminated pork products. Appl Environ Microbiol. 1991 Feb;57(2):389–394. doi: 10.1128/aem.57.2.389-394.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai C. H., Mors V. Production of enterotoxin by Yersinia enterocolitica. Infect Immun. 1978 Mar;19(3):908–911. doi: 10.1128/iai.19.3.908-911.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai C. H., Mors V., Toma S. Prevalence of enterotoxigenicity in human and nonhuman isolates of Yersinia enterocolitica. Infect Immun. 1978 Nov;22(2):334–338. doi: 10.1128/iai.22.2.334-338.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson D. E., Falkow S. Nonpathogenic isolates of Yersinia enterocolitica do not contain functional inv-homologous sequences. Infect Immun. 1990 Apr;58(4):1059–1064. doi: 10.1128/iai.58.4.1059-1064.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard D. R., Johnson W. M., Lior H., Tyler S. D., Rozee K. R. Detection of the aerolysin gene in Aeromonas hydrophila by the polymerase chain reaction. J Clin Microbiol. 1990 Nov;28(11):2477–2481. doi: 10.1128/jcm.28.11.2477-2481.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard D. R., Johnson W. M., Lior H., Tyler S. D., Rozee K. R. Rapid and specific detection of verotoxin genes in Escherichia coli by the polymerase chain reaction. J Clin Microbiol. 1990 Mar;28(3):540–545. doi: 10.1128/jcm.28.3.540-545.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins-Browne R. M., Miliotis M. D., Cianciosi S., Miller V. L., Falkow S., Morris J. G., Jr Evaluation of DNA colony hybridization and other techniques for detection of virulence in Yersinia species. J Clin Microbiol. 1989 Apr;27(4):644–650. doi: 10.1128/jcm.27.4.644-650.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tauxe R. V., Vandepitte J., Wauters G., Martin S. M., Goossens V., De Mol P., Van Noyen R., Thiers G. Yersinia enterocolitica infections and pork: the missing link. Lancet. 1987 May 16;1(8542):1129–1132. doi: 10.1016/s0140-6736(87)91683-7. [DOI] [PubMed] [Google Scholar]
- Thomas A., Smith H. R., Willshaw G. A., Rowe B. Non-radioactively labelled polynucleotide and oligonucleotide DNA probes, for selectively detecting Escherichia coli strains producing Vero cytotoxins VT1, VT2 and VT2 variant. Mol Cell Probes. 1991 Apr;5(2):129–135. doi: 10.1016/0890-8508(91)90007-7. [DOI] [PubMed] [Google Scholar]
- Valentine J. L., Arthur R. R., Mobley H. L., Dick J. D. Detection of Helicobacter pylori by using the polymerase chain reaction. J Clin Microbiol. 1991 Apr;29(4):689–695. doi: 10.1128/jcm.29.4.689-695.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernars K., Delfgou E., Soentoro P. S., Notermans S. Successful approach for detection of low numbers of enterotoxigenic Escherichia coli in minced meat by using the polymerase chain reaction. Appl Environ Microbiol. 1991 Jul;57(7):1914–1919. doi: 10.1128/aem.57.7.1914-1919.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wren B. W., Tabaqchali S. Detection of pathogenic Yersinia enterocolitica by the polymerase chain reaction. Lancet. 1990 Sep 15;336(8716):693–693. doi: 10.1016/0140-6736(90)92191-j. [DOI] [PubMed] [Google Scholar]