Abstract
Objective: The age-related change is important part of degenerative disc disease. However, no appropriate animal model or objective evaluation index is available. This study aimed to investigate the features of intervertebral disc degeneration in aging process of rats. Methods: 22-month-old Sprague-Dawley (SD) rats were used as spontaneously occurring intervertebral disc degeneration models and 6-month-old rats as young controls. Expression of collagen types II and X was measured by immunohistochemistry. Degenerations of intervertebral discs were scored according to Miyamoto’s method. Numbers and areas of afferent vascular buds were measured. The thicknesses of non-calcified and calcified layers were measured and statistically analyzed. Results: There were less collagen type II expression and more collagen type X expression in the calcified layer of the cartilage endplates and nucleus pulposus in the rats of the aged group than in the young control. There were fewer and smaller afferent vascular buds in the rats of the aged group than in the young control group. The ratio of the non-calcified to the calcified layers in the rats of the aged group significantly decreased, compared with that of the young control group (P<0.01). Conclusion: Rats can spontaneously establish intervertebral disc age-related degeneration. The expression of collagen types II and X, numbers and areas of afferent vascular buds, the ratio of the non-calcified to the calcified layers, and water and glycosaminoglycan contents in the nucleus pulposus are sensitive indexes of intervertebral disc degeneration.
Keywords: Intervertebral disc, Degeneration, Afferent vascular bud, Calcified layer, Aged
INTRODUCTION
Intervertebral disc degeneration is the pathological process, in which the cells and matrix of disc tissue underwent endplate and nucleus changes, such as the abnormal composition of collagen, degradation of proteoglycans, and decrease of water content in the nucleus pulposus (Lipson and Muri, 1981), resulting in the abnormality of disc physiological functions. It is the initial and basic pathological process of disc or spinal disease, which is the main factor of back-leg pain (Luoma et al., 2000). The prevalence rate of back-leg pain from disc degeneration was as high as 60% to 80% (Diwan et al., 2000). The studies on the cause of disc degeneration found that mechanical factors (Hansson and Holm, 1991), gene profiling (Annunen et al., 1999; Ala-Kokko, 2002; Virtanen et al., 2007), and smoking (Kaila-Kangas et al., 2003) were considered; especially, aging was a close correlation (Miller et al., 1988; Boos et al., 2002).
In recent years, with the continuous deepening of study (Zhang et al., 2005; 2008), age-related degenerative disc research has become important for disc disease (Antoniou et al., 1996; Götz et al., 1997). However, there is a lack of appropriate animal models and objective evaluation index, delaying the research on aged intervertebral disc degeneration. In the present study, rats were fed to 22 months old to induce aging-related intervertebral disc degeneration. Their morphological features and collagen expression were examined and compared with rats fed to 6 months old. The indicators were evaluated in order to provide the relevant experimental data for basic and clinical researches.
MATERIALS AND METHODS
Animals
Female Sprague-Dawley (SD) rats (specific-pathogen free (SPF) grade) were provided and maintained at the Experimental Animal Center of Xi’an Jiaotong University, Shanxi, China. They were housed under constant conditions at a temperature of (23±1) °C, a humidity of (40±5)%, and a 12 h light/12 h dark cycle, with free access to pellet food and tap water. The animal experiments were performed according to the European Community guidelines for care and use of animals, and approved by the Ethic Committee for Animal Use of Xi’an Jiaotong University, China.
Study protocol
A total of 21 SD rats were included in the study. They were normally fed. At the 6th month, 9 of them were sacrificed to be used as young controls. At the 22th month, the remaining 12 were sacrificed and used as the aged group. The discs from 1 to 5 of the lumbar spine were collected and fixed in formalin. Tissues were embedded in wax and sectioned (3 μm thick). Sections were stained with hematoxylin and eosin (H&E) or 0.1% (w/v) toluidine blue.
Immunohistochemistry of collagen types II and X
Expression of collagen types II and X was detected by immunohistochemistry method. Sections were stained with rabbit monoclonal antibodies to rat collagen types II and X (Molecular Experiments Center of Erlangen-Nuremberg University, Erlangen-Nuremberg, Germany). Sections were digested with 2 mg/ml hyaluronidase (Sigma, Chicago, USA) for 30 min at room temperature, followed by digestion with pronase or protease. For detection of collagen type II, sections were digested with 1 mg/ml pronase (Sigma, Chicago, USA) at room temperature for 30 min, followed by overnight incubation with antibodies to rat collagen type II at 4 °C. For detection of collagen type X, sections were digested with 0.02 mg/ml protease (Sigma, Chicago, USA) at room temperature for 30 min, followed by overnight incubation with antibodies to rat collagen type X at 4 °C. Then sections were labeled with alkaline phosphatase-biotin conjugated rabbit IgG at room temperature for 30 min, and developed with 3-hydroxy-2-naphtylocid 2,4-dimethylanilid to produce a red color. Positive signals were characterized with red particles. Pictures were taken from these slides, and analyzed by Color Image Analysis System (Medical and Biological Corporation of Beihang University, Beijing, China).
Score of degenerating disc according to Miyamoto’s method
Slides of the discs were carefully observed and scored according to Miyamoto’s method (Miyamoto et al., 1991) as shown in Table 1.
Table 1.
Scoring of vertebral disc degeneration by Miyamoto’s method*
| Score | Morphology of the vertebral disc |
| 1 | Normal vertebral disc with properly arranged fibrous rings and nucleus pulposus was observed |
| 2 | Lameller structure of the fibrous rings disappeared. Hyperplasia of the cartilage endplates was observed |
| 3 | The nucleus pulposus crimpled or disappeared |
| 4 | Fractures were observed in the fibrous rings |
| 5 | Intervertebral disc herniation or osteophytes were observed |
Miyamoto et al., 1991
Measurements of afferent vascular bud
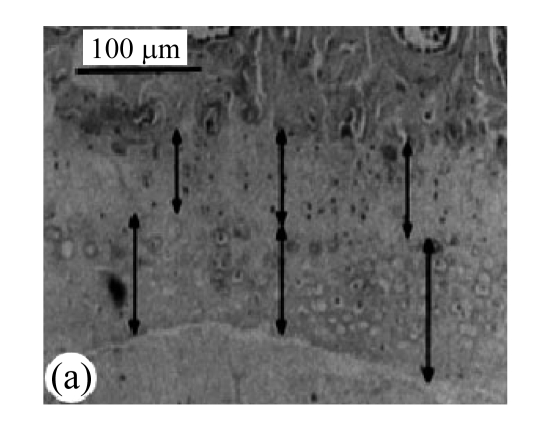
Slides of the H&E-stained vertebral bodies of the cartilage endplates were observed under 10× microscopes. As shown in Fig.1a, the numbers of the afferent vascular buds in the cartilage endplates were calculated. Vascular area was analyzed by square method (Turgut et al., 2003).
Fig.1.
Block diagram of calculation of afferent vascular bud relative area and cartilage endplate (H&E staining)
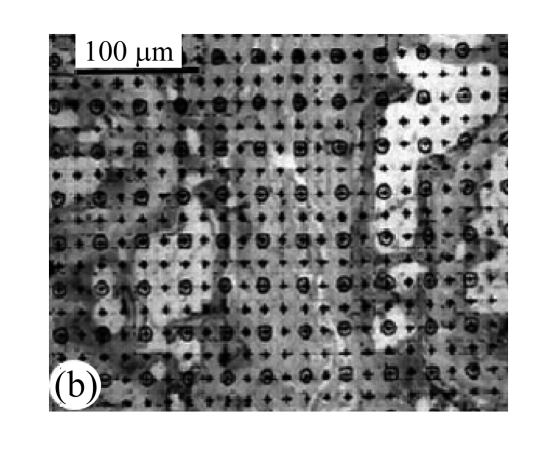
(a) Vascular area was analyzed by square method and the percentage of vascular area to the whole was calculated; (b) Tidemark was used as separatrix, the thicknesses of calcified layer and non-calcified layer in front 1/3, middle point, and post 1/3 of median sagittal plane in nucleus pulposus were measured, and average values were calculated
Measurements of the thicknesses of calcified layer and non-calcified layer of cartilage endplates
H&E-stained slides of the cartilage endplates were observed under 10× microscopes. Tidemark was used as separatrix. As shown in Fig.1b, the thicknesses of calcified and non-calcified layers in front 1/3, middle point, and post 1/3 of median sagittal plane in the nucleus pulposus were measured and average values were calculated. The ratio of non-calcified layer to calcified layer was calculated.
Measurements of water content and glycosaminoglycan content of nucleus pulposus
Water content and glycosaminoglycan content of the nucleus pulposus were measured by 1,9-dimethylmethylene blue method as described previously (Farndale et al., 1982). Weights of samples were measured before and after being dried at 60 °C for 12 h. Then samples were digested with papain at 60 °C for 12 h. Samples and standards were incubated with 1,9-dimethylmethylene. Optical density at 525 nm (OD525) was measured.
Statistical analysis
Data were analyzed by using the statistics software package (SPSS for Windows, version 14.0, Chicago, USA). One-way analysis of variance (ANOVA) test was performed, and Post Hoc multiple comparisons were done with least significant difference (LSD). Results were presented as mean±standard deviation (SD); a P value of less than 0.05 was regarded as statistically significant.
RESULTS
Clinical characteristics
All sacrificed rats were healthy during life time. No accidental death or diseases were observed when they were fed in the Experimental Animal Center of Xi’an Jiaotong University. No pathological findings were observed in their dead bodies.
Morphological changes
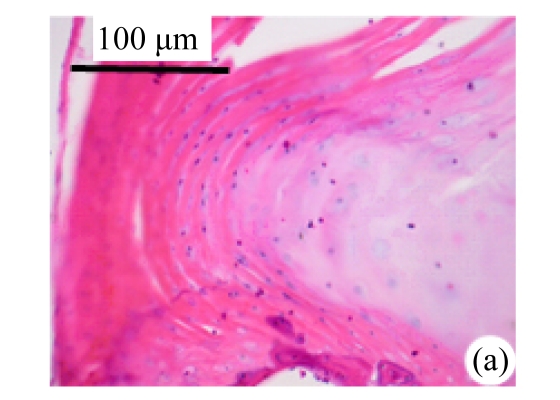
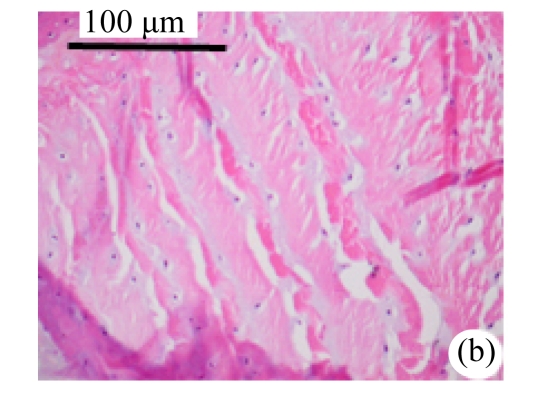
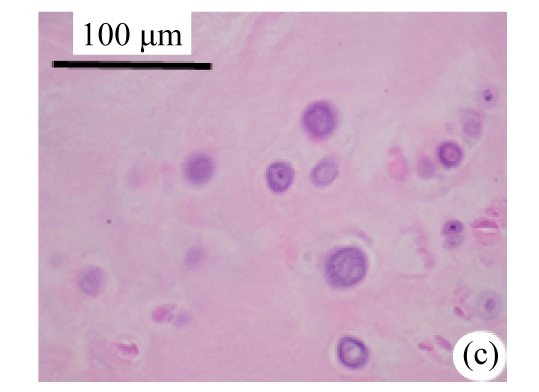
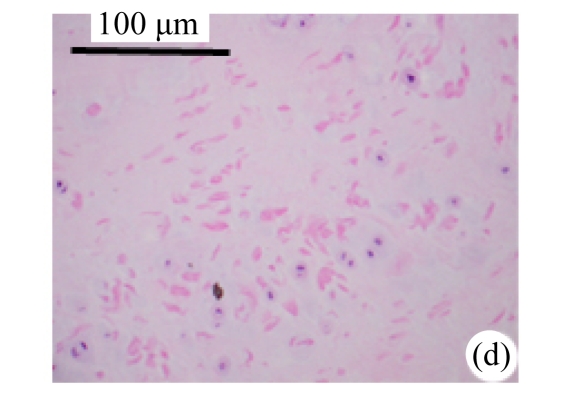
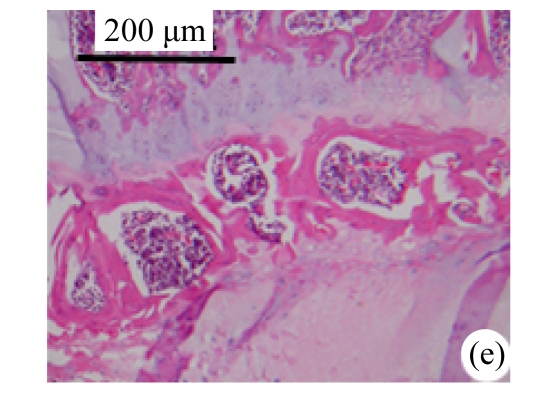
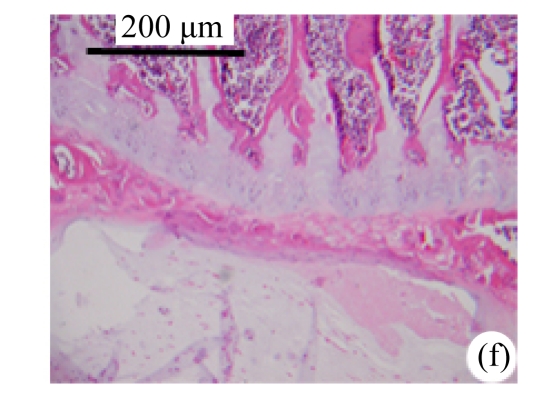
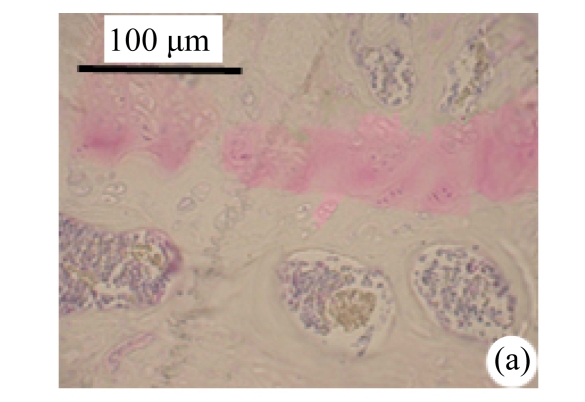
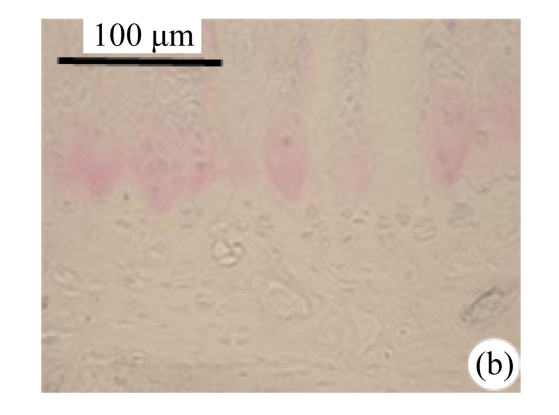
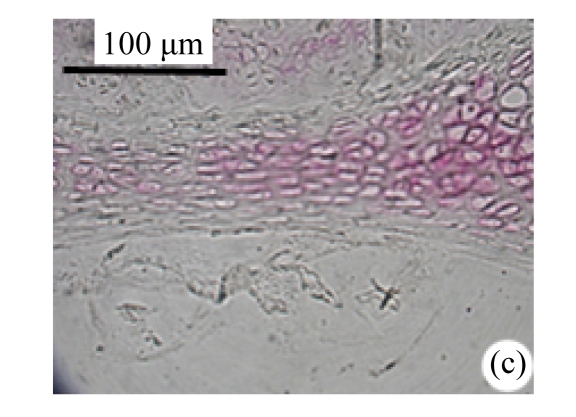
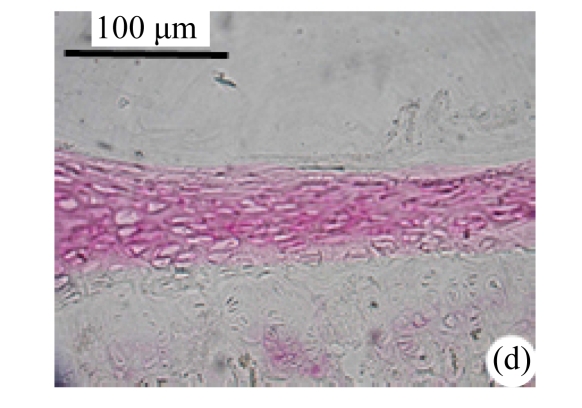
Abundant changes were observed in H&E-stained slides of rats in both the young and aged groups (Fig.2). Discs of rats in the aged group showed thinner cartilage endplates and fewer cells and front tidemarks than the discs of young control rats. The rats of the aged group had fewer and smaller afferent buds and fewer cells in the fibrous rings and nucleus pulposus than young control rats. The structure of nucleus pulposus was normal in the aged group. No fibrosis was observed in the nucleus pulposus in aged rats. As shown in 0.1% (w/v) toluidine blue-stained slides, there was weaker staining in the calcified layer of the cartilage endplates in aged rats than in young controls.
Fig.2.
Change of intervertebral disc histomorphology between young controls and aged rats
The annular fibrosus arranged regularly and more cells were seen in young controls (a) than in aged rats (b). Dead cells in the nucleus gelatinosus were not observed in young controls (c), but were obvious in aged rats (d). Obviously more vascular buds were seen in young controls than in aged rats. Non-clarify layers of young controls (e) were significantly thicker than those of the aged group (f)
Expression of collagen types II and X
As shown in Fig.3, there was less collagen type II expression in the calcified layer of the cartilage endplates and nucleus pulposus in aged rats than in young controls. There was more collagen type X expression in the calcified and non-calcified layers of the cartilage endplates of aged rats than in those of young controls. But there was no collagen type X expression in the fibrous rings and nucleus pulposus in both groups.
Fig.3.
Expression of intervertebral disc collagen types II and X between the young and aged groups
Expression of collagen type II in the cartilage endplate-calcified layer increased in young rats (a), but decreased or lost in the aged group (b). Expression of collagen type X in the non-calcified layer of the cartilage endplate in young rats (c) was obviously smaller than that of the aged group (d)
Expression of collagen types II and X was analyzed by Image Analysis System. As shown in Table 2, collagen type II expression in the cartilage endplates of aged rats significantly decreased compared with that of young controls (188.41±11.7 vs 208.41±13.3, P<0.01). Collagen type X in the cartilage endplates of aged rats significantly increased compared with that of young controls (135.20±33.9 vs 88.41±23.7, P<0.01).
Table 2.
Characteristics of young and aged rats
| Index | Aged group (n=12) | Young controls (n=9) | t | P |
| Value of Miyamoto’s classes | 0.85±0.36 | 0.81±0.23 | 0.291 | 1.1300 |
| Quantity of vascular bud | 13.96±4.55 | 19.76±6.95 | 2.313 | 0.0300 |
| Relative area of vascular bud (%) | 24.07±8.80 | 41.91±12.03 | 3.933 | 0.0009 |
| Ratio of non-calcified layer/calcified layer | 0.82±0.23 | 1.21±0.38 | 2.925 | 0.0080 |
| Collagen type X (non-calcified layer) | 135.20±33.9 | 88.41±23.7 | 3.533 | 0.0030 |
| Collagen type II (calcified layer) | 188.41±11.7 | 208.41±13.3 | 3.658 | 0.0018 |
| Collagen type II (nucleus pulposus) | 338.41±31.7 | 408.41±53.3 | 3.765 | 0.0014 |
| Water/hydrous nucleus pulposus | 0.7300±0.0279 | 0.8356±0.0357 | 7.605 | 0.0007 |
| Glycosaminoglycan/dry nucleus pulpous | 0.1683±0.0189 | 0.3689±0.0440 | 14.231 | 0.0003 |
Scores of disc degeneration
Degenerations of discs were scored according to Miyamoto’s method (Table 2). No significant difference of the scores between aged and young rats (0.85±0.36 vs 0.81±0.23) was noted.
Numbers and areas of afferent buds
As shown in Table 2, there were fewer and smaller afferent vascular buds in the cartilage endplates in aged rats than in young rats (P<0.01).
Non-calcified layer/calcified layer ratio
As shown in Table 2, non-calcified layer/calcified layer ratio in aged rats significantly decreased compared with young rats (0.82±0.23 vs 1.21±0.38, P<0.01).
Water and glycosaminoglycan contents of the nucleus pulposus
As shown in Table 2, the ratio of water content to wet weight of the nucleus pulposus in aged rats was 13% lower than that in the young control group (P<0.01). The ratio of glycosaminoglycan content to dry weight of the nucleus pulposus in aged rats was 55% lower than that in the young control group (P<0.01).
DISCUSSION
Disc degeneration is an age-related physiological process, which occurs in the age of 20 years or even earlier, is accelerated by environmental factors such as stress, and even causes the protrusion. In order to study the disc degeneration solely by age, rats were selected as the experimental animals, because rats crawl, instead of upright walking like humans. Its intervertebral disc endures less pressure than humans, so the animal model can accurately represent changes of intervertebral discs in the aging process. The lifespan of rats is usually 2 to 3 years. 22-month-aged rats are just like humans in their fifties, the ages when intervertebral disc degeneration diseases usually occur.
It has been reported that non-calcified layer of cartilage endplate was closely related with nutrition supply of the nucleus polpusus (Urban et al., 2004). Our results show that afferent vascular buds could be observed in non-calcified layer of the cartilage endplate. There were fewer and smaller afferent vascular buds in the aged group compared with the young control group. The permeability of bone-cartilage endplate-intervertebral disc was decided by numbers of afferent vascular buds (Katz et al., 1986). Our results show that nutrition supply of intervertebral disc was impaired in the process of aging. Boos et al.(1997) reported that difference of collagen type X may be associated with development of the degeneration of intervertebral disc. Calcium had been demonstrated to increase production of collagen type X in vitro cultured chondrocytes (He et al., 2004; Reginato et al., 1993), which suggests the association between collagen type X and calcified matrix. Our results show that collagen type X was only observed in the calcified and non-calcified layers of the cartilage endplates, suggesting that the cartilage endplate was being calcified. The cartilage endplate in the aged group had higher calcification, resulting in dysfunction of the channel of disc nutrition.
Collagen type II locates in the inner layer of the fibrosis rings, especially the nucleus pulposus, which can keep water and endure and transfer pressure. It has been demonstrated that collagen II decreased while collagen I increased when intervertebral disc degenerates (Ahsan et al., 2001). As shown in our results, there was less collagen II expression in the calcified layer of the cartilage endplate and nucleus pulposus of aged rats compared with the young control group. It could be demonstrated that intervertebral disc degenerates more severely in aged rats than in young controls.
As shown in our results, water and glycosaminoglycan contents of the nucleus pulposus of aged rats obviously decreased compared with young controls. These observations were in accordance with previous reports (Schmid et al., 1991; Feng and Song, 2004; Gruber et al., 2002). As reported previously, loss of water and glycosaminoglycans suggested degeneration of the nucleus pulposus (Cs-Szabo et al., 2002). In our results, the fibrosis of the nucleus pulposus, or intervertebral disc herniation was not observed in the rats of the aged group, which may be due to the discs of rats enduring less pressure than humans since they were reptiles. No difference was found on scores of intervertebral disc degeneration by Miyamoto’s method, which suggested that Miyamoto’s method was not a sensitive index for intervertebral disc degeneration.
In the present study, we demonstrated that rats could spontaneously establish an age-related degeneration of the intervertebral disc. We also measured expression of collagen types II and X, numbers and areas of afferent buds, non-calcified layer/calcified layer ratio, and water and glycosaminoglycan contents, which are all sensitive indexes for the intervertebral disc degeneration.
Footnotes
Project (No. 30400163) supported by the National Natural Science Foundation of China
References
- 1.Ahsan R, Tajima N, Chosa E, Sugamata M, Sumida M, Hamada M. Biochemical and morphological changes in herniated human intervertebral disc. J Orthop Sci. 2001;6(6):510–518. doi: 10.1007/s007760100006. [DOI] [PubMed] [Google Scholar]
- 2.Ala-Kokko L. Genetic risk factors for lumbar disc disease. Ann Med. 2002;34(1):42–47. doi: 10.1080/078538902317338634. [DOI] [PubMed] [Google Scholar]
- 3.Annunen S, Paassilta P, Lohiniva J, Perälä M, Pihlajamaa T, Karppinen J, Tervonen O, Kröger H, Lähde S, Vanharanta H, et al. An allele of COL9A2 associated with intervertebral disc disease. Science. 1999;285(5426):409–412. doi: 10.1126/science.285.5426.409. [DOI] [PubMed] [Google Scholar]
- 4.Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, Aebi M, Alini M. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. Journal of Clinical Investigation. 1996;98(4):996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boos N, Nerlich AG, Wiest I, von der Mark K, Aebi M. Immunolocalization of type X collagen in human lumbar intervertebral discs during ageing and degeneration. Histochem Cell Biol. 1997;108(6):471–480. doi: 10.1007/s004180050187. [DOI] [PubMed] [Google Scholar]
- 6.Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, Nerlich AG. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo award in basic science. Spine. 2002;27(23):2631–2644. doi: 10.1097/00007632-200212010-00002. [DOI] [PubMed] [Google Scholar]
- 7.Cs-Szabo G, Ragasa-San Juan D, Turumella V, Masuda K, Thonar EJ, An HS. Changes in mRNA and protein levels of proteoglycans of the anulus fibrosus and nucleus pulposus during intervertebral disc degeneration. Spine. 2002;27(20):2212–2219. doi: 10.1097/00007632-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 8.Diwan AD, Parvataneni HK, Khan SN, Sandhu HS, Girardi FP, Cammisa FPJr. Current concepts in intervertebral disc restoration. Orthop Clin North Am. 2000;31(3):453–464. doi: 10.1016/S0030-5898(05)70163-2. [DOI] [PubMed] [Google Scholar]
- 9.Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9(4):247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 10.Feng L, Song Y. Pathological development of researches on intervertebral disc degeneration. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2004;21(5):867–870. (in Chinese) [PubMed] [Google Scholar]
- 11.Götz W, Barnert S, Bertagnoli R, Miosge N, Kresse H, Herken R. Immunohistochemical localization of the small proteoglycans decorin and biglycan in human intervertebral discs. Cell Tissue Res. 1997;289(1):185–190. doi: 10.1007/s004410050864. [DOI] [PubMed] [Google Scholar]
- 12.Gruber HE, Johnson T, Norton HJ, Hanley ENJr. The sand rat model for disc degeneration: radiologic characterization of age-related changes. Spine. 2002;27(3):230–234. doi: 10.1097/00007632-200202010-00004. [DOI] [PubMed] [Google Scholar]
- 13.Hansson T, Holm S. Clinical implications of vibration-induced changes in the lumbar spine. Orthop Clin North Am. 1991;22(2):247–253. [PubMed] [Google Scholar]
- 14.He HL, Wu ZH, Zhang JG, Wang YP, Zhou Y, Xu YQ, Yuan JG, Qiu GX. Primary study on collagen X gene expression in the apical disc of idiopathic scoliosis. Chinese Medical Journal. 2004;84(20):1681–1685. (in Chinese) [PubMed] [Google Scholar]
- 15.Kaila-Kangas L, Leino-Arjas P, Riihimäki H, Luukkonen R, Kirjonen J. Smoking and overweight as predictors of hospitalization for back disorders. Spine. 2003;28(16):1860–1868. doi: 10.1097/01.BRS.0000083284.47176.80. [DOI] [PubMed] [Google Scholar]
- 16.Katz MM, Hargens AR, Garfin SR. Intervertebral disc nutrition. Diffusion versus convection. Clin Orthop Relat Res. 1986;210:243–245. [PubMed] [Google Scholar]
- 17.Lipson SJ, Muri H. 1980 Volvo award in basic science. Proteoglycans in experimental intervertebral disc degeneration. Spine. 1981;6(3):194–210. doi: 10.1097/00007632-198105000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Luoma K, Riihimäki H, Luukkonen R, Raininko R, Viikari-Juntura E, Lamminen A. Low back pain in relation to lumbar disc degeneration. Spine. 2000;25(4):487–492. doi: 10.1097/00007632-200002150-00016. [DOI] [PubMed] [Google Scholar]
- 19.Miller JA, Schmatz C, Schultz AB. Lumbar disc degeneration: correlation with age, sex, and spine level in 600 autopsy specimens. Spine. 1988;13(2):173–178. doi: 10.1097/00007632-198802000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Miyamoto S, Yonenobu K, Ono K. Experimental cervical spondylosis in the mouse. Spine. 1991;16(10 Suppl.):S495–S500. doi: 10.1097/00007632-199110001-00008. [DOI] [PubMed] [Google Scholar]
- 21.Reginato AM, Tuan RS, Ono T, Jimenez SA, Jacenko O. Effects of calcium deficiency on chondrocyte hypertrophy and type X collagen expression in chick embryonic sternum. Dev Dynamics. 1993;198(4):284–295. doi: 10.1002/aja.1001980406. [DOI] [PubMed] [Google Scholar]
- 22.Schmid TM, Bonen DK, Luchene L, Linsenmayer TF. Late events in chondrocyte differentiation: hypertrophy, type X collagen synthesis and matrix calcification. In Vivo. 1991;5(5):533–540. [PubMed] [Google Scholar]
- 23.Turgut M, Uslu S, Uysal A, Yurtseven ME, Ustün H. Changes in vascularity of cartilage endplate of degenerated intervertebral discs in response to melatonin administration in rats. Neurosurg Rev. 2003;26(2):133–138. doi: 10.1007/s10143-003-0259-8. [DOI] [PubMed] [Google Scholar]
- 24.Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine. 2004;29(23):2700–2709. doi: 10.1097/01.brs.0000146499.97948.52. [DOI] [PubMed] [Google Scholar]
- 25.Virtanen IM, Karppinen J, Taimela S, Ott J, Barral S, Kaikkonen K, Heikkilä O, Mutanen P, Noponen N, Männikkö M, et al. Occupational and genetic risk factors associated with intervertebral disc disease. Spine. 2007;32(10):1129–1134. doi: 10.1097/01.brs.0000261473.03274.5c. [DOI] [PubMed] [Google Scholar]
- 26.Zhang YG, Guo X, Xu P, Kang LL, Li J. Bone mesenchymal stem cells transplanted into rabbit intervertebral discs can increase proteoglycans. Clin Orthop Relat Res. 2005;430:219–226. doi: 10.1097/01.blo.0000146534.31120.cf. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Sun Z, Liu J, Guo X. Advances in susceptibility genetics of intervertebral degenerative disc disease. Int J Biol Sci. 2008;4(5):283–290. doi: 10.7150/ijbs.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]