Abstract
Fruit ripening is a developmental process that is associated with increased susceptibility to the necrotrophic pathogen Botrytis cinerea. Histochemical observations demonstrate that unripe tomato (Solanum lycopersicum) fruit activate pathogen defense responses, but these responses are attenuated in ripe fruit infected by B. cinerea. Tomato fruit ripening is regulated independently and cooperatively by ethylene and transcription factors, including NON-RIPENING (NOR) and RIPENING-INHIBITOR (RIN). Mutations in NOR or RIN or interference with ethylene perception prevent fruit from ripening and, thereby, would be expected to influence susceptibility. We show, however, that the susceptibility of ripe fruit is dependent on NOR but not on RIN and only partially on ethylene perception, leading to the conclusion that not all of the pathways and events that constitute ripening render fruit susceptible. Additionally, on unripe fruit, B. cinerea induces the expression of genes also expressed as uninfected fruit ripen. Among the ripening-associated genes induced by B. cinerea are LePG (for polygalacturonase) and LeExp1 (for expansin), which encode cell wall-modifying proteins and have been shown to facilitate susceptibility. LePG and LeExp1 are induced only in susceptible rin fruit and not in resistant nor fruit. Thus, to infect fruit, B. cinerea relies on some of the processes and events that occur during ripening, and the fungus induces these pathways in unripe fruit, suggesting that the pathogen itself can initiate the induction of susceptibility by exploiting endogenous developmental programs. These results demonstrate the developmental plasticity of plant responses to the fungus and indicate how known regulators of fruit ripening participate in regulating ripening-associated pathogen susceptibility.
Plant interactions with the biosphere include encounters with diverse microorganisms. A plant becomes infected when it does not limit colonization by pathogenic microorganisms that eventually interfere with the essential activities of the plant cells. Events in the plant host, in addition to processes initiated by the pathogen during the infection, result in resistance or facilitate susceptibility.
The resistance of a plant toward a pathogen depends on the inherited characteristics of the two organisms (Agrios, 2005). However, not all organs or tissues respond equally to invading microorganisms, and susceptibility can depend on development in the organ. For example, in many pathosystems, such as powdery mildew (Erysiphe necator) on grape (Vitis vinifera) and stripe rust (Puccinia graminis f. sp. tritici) on wheat (Triticum aestivum), young leaves are more susceptible to infections than older ones (Doster and Schnathorst, 1985; Lalancette and Hickey, 1985; Qayoum and Line, 1985; Reuveni et al., 1986; Roumen et al., 1992). The transition that fruit undergo during ripening is another example of a developmental process that is coincident with increased susceptibility.
Ripe fleshy fruit are more susceptible to disease and decomposition than unripe green fruit (Prusky, 1996). The increased susceptibility of ripe fruit to opportunistic pathogens is likely to facilitate the dispersal of mature seed (Gillaspy et al., 1993; Prusky, 1996). Fruit ripening is a complex, developmentally regulated network of processes and events encompassing alterations in gene expression and chemical and physiological changes. The textural, metabolic, organoleptic, and nutritional properties of ripening tomato (Solanum lycopersicum) fruit have been investigated, and the concurrent increase in susceptibility is mentioned frequently (Giovannoni, 2001, 2004, 2007b; Alba et al., 2005). The increased susceptibility of ripe fruit to pathogens may be an inherent outcome of ripening, or, alternatively, the susceptibility of fruit may require only some of the events and processes of ripening. Among the processes that occur during ripening, the disassembly of cell walls as fruit soften is crucial for susceptibility (Cantu et al., 2008a). Ripening in tomato fruit is regulated by ethylene and transcription factors, including NON-RIPENING (NOR or NAC-NOR), RIPENING-INHIBITOR (RIN; Vrebalov et al., 2002), and COLORLESS NON-RIPENING (Manning et al., 2006). Mutations in the genes for these transcription factors result in fruit that fail to complete normal ripening (Vrebalov et al., 2002; Manning et al., 2006; Giovannoni, 2007b). Ethylene is perceived by His kinase ethylene receptors (Chang and Shockey, 1999), and a mutation in the receptor, LeETR3 (NEVER-RIPE [Nr]), interferes with ethylene perception, significantly delaying fruit ripening (Wilkinson et al., 1995). How susceptibility and other processes that constitute the ripening syndrome are regulated independently and cooperatively by these transcription factors and the production and perception of ethylene is not fully understood (Cara and Giovannoni, 2008).
The interaction of tomato fruit with the ascomycete Botrytis cinerea (teleomorph Botryotinia fuckeliana) is a model pathosystem for investigating necrotrophic microorganisms (Powell et al., 2000; Company and Gonzalez-Bosch, 2003; Flors et al., 2007; Cantu et al., 2008a, 2008b). B. cinerea is an opportunistic pathogen with a broad host range and is notoriously aggressive on fleshy fruit. Dissection of B. cinerea pathogenicity and virulence has been assisted by the development of molecular and genetic tools, including the availability of sequenced and annotated genomes of the strains B05.10 and T4 (ten Have et al., 1998; van Kan, 2006). During B. cinerea infections of plant tissues, cell wall-degrading enzymes are secreted by the fungus. Although some of these enzymes are required for virulence (ten Have et al., 1998, 2001; Kars et al., 2005), the fungus fails to infect ripe fruit in the absence of endogenous fruit cell wall disassembly (Cantu et al., 2008a).
Plant vegetative tissue responses to infection with B. cinerea have been studied extensively in Arabidopsis (Arabidopsis thaliana) and, to a lesser extent, in tomato (Glazebrook, 2005). A complex cross talk of signaling pathways is known to regulate pathogen virulence and the resistance of vegetative tissues to B. cinerea. Ethylene and jasmonic acid limit infections of Arabidopsis and tomato leaves (Ferrari et al., 2003; Mengiste et al., 2003; AbuQamar et al., 2006; Zheng et al., 2006), but the role of salicylic acid is ambiguous (Ferrari et al., 2003). However, since ethylene promotes ripening, it may contribute to fruit susceptibility, in contrast to its role in infected leaves. The congruence of the hormonal regulation of fruit ripening and fruit susceptibility suggests that processes that provide resistance or allow infections in fruit may differ from those in vegetative tissues.
The roles of the regulators of ripening in the susceptibility of fruit to pathogens have not been explored, although historically it has been assumed that, in the absence of ripening, fruit susceptibility would be limited. To test this assumption, we examined the responses of unripe green and ripe tomato fruit to B. cinerea and the impact of NOR, RIN, and ethylene-regulated developmental pathways on susceptibility and responses of fruit to the fungus. Our results indicate that whether the fungus is able to invade and grow on fruit depends on the activation of some, but not all, of the pathways that constitute fruit ripening.
RESULTS
Infections of Mature Green and Red Ripe Tomato Fruit
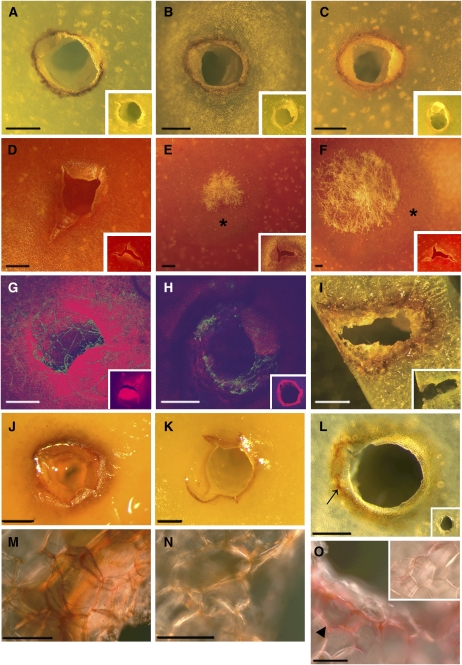
When mature green (MG) unripe tomato (‘Ailsa Craig’ [AC]) fruit were inoculated with an aqueous suspension of 5 × 103 conidia of B. cinerea, growth of the fungus at 1 d post inoculation (1 dpi) was limited to the puncture sites and soft rot symptoms did not develop in most cases (approximately 80%; Fig. 1, A–C, H, and I). In contrast, when red ripe (RR) fruit were inoculated, tissue rotting and fungal growth were evident at 1 dpi and became extensive throughout the surrounding pericarp tissue (Fig. 1, D–G). At 3 dpi, growth of the hyphae, observed using a GFP-expressing strain of B. cinerea, extended 2 to 3 mm into the RR fruit tissue (Fig. 1G), but fungal growth was limited to the surface of the inoculated site in MG fruit (Fig. 1H). On MG fruit, a necrotic response was observed at the sites of infection as early as 1 dpi (Fig. 1, A–C and I) but not in wounded, uninoculated sites (Fig. 1, insets in A–C and I) and rarely in inoculation sites on RR fruit (Fig. 1, D–F). In MG fruit, B. cinerea caused the accumulation of hydrogen peroxide around the inoculation sites three to four cell layers deep, beyond the cells that displayed the necrotic response (Fig. 1, A, J, and M). Wounding alone resulted in a less intense oxidative burst that was detectable only on the surface of the site and barely visible within the pericarp cells (Fig. 1, K and N). B. cinerea induced lignin (Fig. 1L) and suberin (Fig. 1O) accumulation at the sites of inoculation but not in wounded, uninoculated sites of MG fruit.
Figure 1.
B. cinerea on inoculated tomato fruit. A to C, Infection sites on the fruit surface of MG fruit at 1 (A), 2 (B), and 3 (C) dpi. A dark ring of necrosis is visible within 1 dpi. No ring is seen in water-inoculated sites (insets). D to F, Infection sites at 1 (D), 2 (E), and 3 (F) dpi of RR fruit. No necrotic ring is seen, and mycelium growth is slight at 1 dpi but expanding at 2 and 3 dpi. Water-soaked macerated tissue (asterisks) beyond the mycelia is apparent at 2 and 3 dpi. G and H, RR (G) and MG (H) fruit inoculated with B. cinerea B05.10 expressing GFP at 3 dpi. On RR fruit (G), mycelia have spread throughout the pericarp; on MG fruit (H), mycelia spread is limited to the inoculation site surface. I, Transverse section through a MG inoculation site at 3 dpi (as in C) demonstrates that the dark necrotic ring surrounds the entire inoculation wound site. J, K, M, and N, 3,3′-Diaminobenzidine staining of hydrogen peroxide viewed from the surface of the inoculated (J) and wounded (K) fruit demonstrates that within 1 dpi, B. cinerea induces hydrogen peroxide accumulation at the site of inoculation. M and N show micrographs of cross-sections of fruit pericarp tissue surrounding the inoculation (M) and wounding (N) sites. L and O, Phloroglucinol (L) and safranin (O) staining demonstrate that lignin and suberin accumulate in response to B. cinerea (arrow and arrowhead indicate phloroglucinol and safranin reactive material, respectively). Bars in A to L = 0.5 mm; bars in M to O = 0.2 mm. Note that RNA for microarray analyses was collected from MG and RR lesions at 1 dpi (as in A, D, J, M, L, and O). Insets, when present, show a water-inoculated site at the same posttreatment time as the larger image.
Regulation of Fruit Ripening and Susceptibility
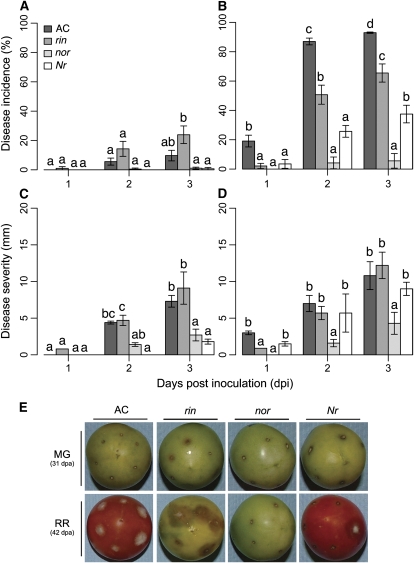
To understand how regulators of fruit ripening influence the susceptibility to B. cinerea, evaluations of infections of fruit from the tomato ripening mutants rin, nor, and Nr in the AC background were made. Fruit at two ripening stages, 31 DPA (dpa; equivalent to wild-type MG AC fruit) and 42 dpa (equivalent to wild-type RR AC fruit) were inoculated, and lesion development (Fig. 2) and accumulated fungal biomass (Fig. 3) were measured. As expected, the disease incidence, disease severity (Fig. 2), and fungal biomass accumulation (Fig. 3) were greater on wild-type RR AC fruit than on MG fruit.
Figure 2.
Susceptibility of tomato ripening mutants to B. cinerea. A and B, Disease incidence (percentage of inoculation sites with soft rot symptoms) of MG fruit (A; 31 dpa) and RR fruit (B; 42 dpa). C and D, Disease severity (e.g. diameters of expanding soft rot lesions) for inoculated MG (C) and RR (D) fruit. Different letters indicate significant differences between genotypes at a given time point (P ≤ 0.01; error bars indicate se; n = 5). E, Representative inoculated fruit (3 dpi) for each genotype and ripening stage.
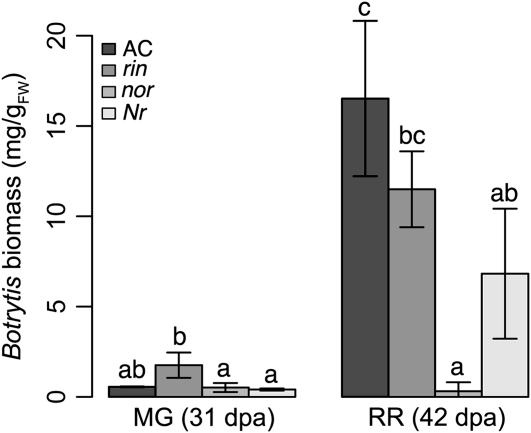
Figure 3.
B. cinerea biomass accumulation during infection of tomato ripening mutants. B. cinerea biomass accumulation at 3 dpi in infected MG fruit (31 dpa) and RR fruit (42 dpa) measured using test strips coated with the monoclonal antibody BC12.CA4 (EnviroLogix). Letters correspond to significant differences between genotypes (P ≤ 0.01; error bars indicate sd; n = 3). FW, Fresh weight.
Since they do not ripen normally, rin and nor mutant fruit fail to soften and do not develop red color (Tigchelaar et al., 1973; Thompson et al., 1999). At 31 and 42 dpa, nor fruit were significantly less susceptible to B. cinerea compared with wild-type MG or RR fruit (Fig. 2, B, D, and E, and Fig. 3). The severity of the symptoms of disease (Fig. 2, C and D) and the accumulation of fungal biomass (Fig. 3) in infected rin fruit at 31 and 42 dpa were indistinguishable or greater than those of inoculated wild-type MG or RR fruit. Thus, the ripening pathways that allow B. cinerea to grow on ripe fruit are controlled by NOR and only slightly, or not at all, by RIN.
Perception of Ethylene and Fruit Susceptibility
Ethylene regulates climacteric tomato fruit ripening, and ripening fruit produce ethylene (Table I; Alexander and Grierson, 2002). No significant increase in ethylene evolution from MG and RR wild-type fruit inoculated with B. cinerea was observed at 1 dpi (Table I), although significant accumulation was observed at 3 dpi. Uninoculated rin and nor fruit produced less ethylene at all ripening stages compared with uninoculated wild-type fruit (Table I; Herner and Sink, 1973; McGlasson et al., 1975a, 1975b; Thompson et al., 1999).
Table I.
Ethylene production (nL g−1 fresh weight h−1) in healthy (H), wounded (W), and inoculated (I) MG fruit at 31 dpa and RR fruit at 42 dpa from wild-type (AC) and ripening mutant (rin, nor, and Nr) plants
Different letters correspond to significant differences when different dpi of the same combination of genotype, treatment, and stage are compared (P ≤ 0.05).
| Plant | Treatment | MG (31 dpa) | RR (42 dpa) | ||||
|---|---|---|---|---|---|---|---|
| 1 dpi | 2 dpi | 3 dpi | 1 dpi | 2 dpi | 3 dpi | ||
| AC | H | 0.86 a | 1.47 a | 2.39 a | 13.51 a | 10.88 a | 10.48 a |
| W | 1.16 a | 2.39 a | 5.54 a | 7.59 a | 11.05 a | 9.84 a | |
| I | 0.50 a | 2.20 a | 6.98 b | 15.20 a | 15.63 a | 25.47 b | |
| rin | H | 0.51 a | 0.33 a | 0.34 a | 0.35 a | 0.24 a | 0.15 a |
| W | 0.82 a | 0.41 a | 0.32 a | 0.18 a | 0.18 a | 0.13 a | |
| I | 0.90 a | 2.77 a | 4.97 a | 0.98 a | 6.40 a,b | 9.75 b | |
| nor | H | 0.50 a | 0.57 a | 0.51 a | 0.73 a | 0.71 a | 0.76 a |
| W | 0.54 a | 0.50 a | 0.45 a | 1.33 a | 0.60 a | 0.96 a | |
| I | 0.67 a | 1.65 a | 1.98 a | 0.65 a | 1.97 a | 2.91 a | |
| Nr | H | 0.65 a | 0.82 a | 1.42 a | 18.34 a | 17.1 a | 15.92 a |
| W | 0.63 a | 0.59 a | 0.35 a | 13.52 a | 13.06 a | 13.97 a | |
| I | 1.26 a | 1.68 a | 7.87 a | 19.62 a | 24.14 a | 44.76 b |
Ethylene production in infected wild-type, rin, nor, and Nr mutant fruit was measured at the two ripening stages evaluated for susceptibility (Table I). Inoculation of 31-dpa rin fruit led to a 15-fold increase in ethylene production at 3 dpi compared with 1 dpi; no increase at 3 dpi was observed following inoculations of nor fruit at the equivalent stage, and ethylene production increased only 3-fold by 3 dpi in inoculated wild-type MG fruit. Although Nr fruit were fully resistant to B. cinerea at 31 dpa and partially resistant at 42 dpa (Fig. 2, A and C, and Fig. 3), inoculations at both stages resulted in an increased production of ethylene, similar to the increase observed in infected wild-type AC fruit (Table I). The mutation in the ethylene receptor LeETR3 (Nr) slows the typical ripening-associated fruit changes and reduces but does not eliminate sensitivity to ethylene, possibly due to the compensating accumulation of other receptors (Rick and Butler, 1956; Tieman et al., 2000; Alba et al., 2005). In the AC background, the Nr mutation reduces but does not eliminate ethylene sensitivity, resulting in a weak phenotype that may explain the incomplete resistance of Nr fruit at 42 dpi (Lanahan et al., 1994; Yen et al., 1995).
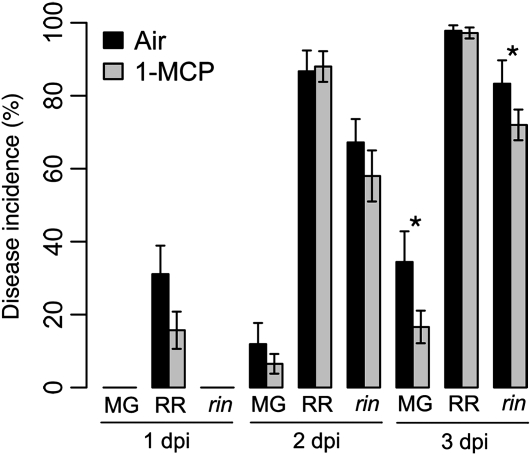
To further test the role that ethylene perception plays in the ripening-associated increased susceptibility of fruit to B. cinerea, infections of MG and RR wild-type and 42-dpa rin mutant fruit were assessed following treatment of the fruit with the ethylene receptor inhibitor 1-methylcyclopropene (1-MCP; Fig. 4). Treatment of wild-type MG AC fruit with 1-MCP for 18 h was sufficient to block further ripening of concurrently harvested and treated fruit. Treatment of wild-type MG fruit with 1-MCP caused a significant 50% reduction in susceptibility when compared with untreated controls at 3 dpi. Treatment of rin fruit with 1-MCP resulted in a small (12%), although significant, reduction in susceptibility compared with untreated rin fruit. 1-MCP treatment did not lead to increased resistance of wild-type RR fruit. Because disease symptom development and the accumulation of fungal biomass are reduced in the partially ethylene-insensitive Nr fruit and because the ethylene-responsive nor fruit are resistant at 31 and 42 dpa, the susceptibility of these fruit after 1-MCP treatment was not assessed.
Figure 4.
Effect of 1-MCP on fruit susceptibility. Disease incidence (percentage of inoculation sites with soft rot symptoms) for MG and RR AC fruit and rin fruit (42 dpa; i.e. RR equivalent). Immediately prior to inoculation and within 2 h of harvest, fruit were treated for 18 h with air or 15 nL L−1 1-MCP. Asterisks indicate significant differences within each fruit stage and at each dpi (P ≤ 0.05; error bars indicate sd; n = 3).
Transcriptional Profiling of Fruit Responses to B. cinerea
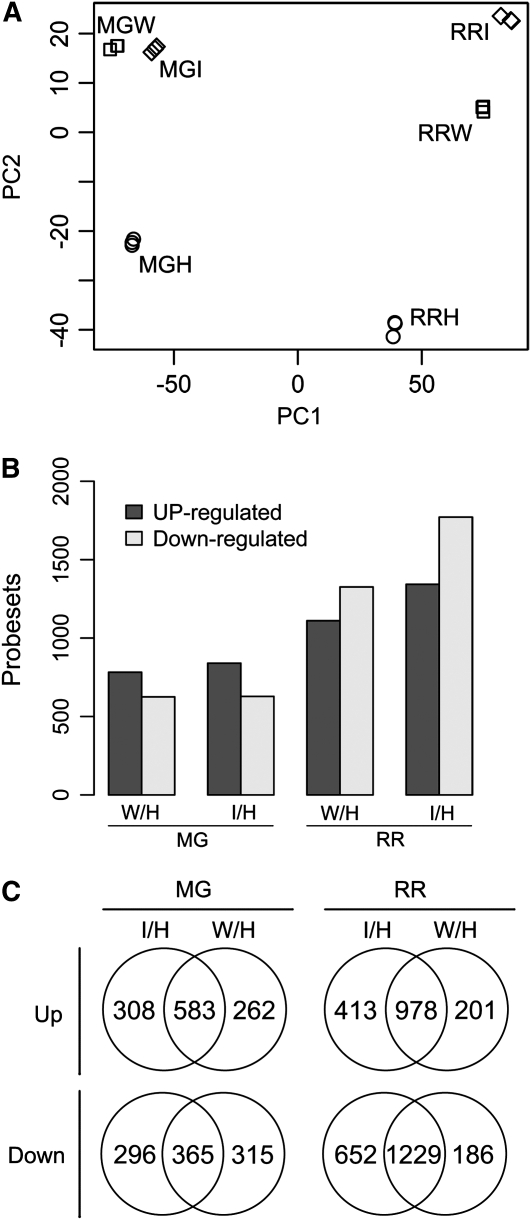
To characterize fruit gene expression in response to B. cinerea during ripening, tomato transcriptome analysis was carried out using RNA prepared from wild-type AC MG and RR fruit at 1 dpi, showing symptoms as in Figure 1 (A and D). RNA was isolated from the infection sites and the immediately adjacent tissue and from healthy and wounded but not inoculated MG and RR fruit. Three biological replicates were prepared for each ripening stage and each treatment. To identify the sources of variation, principal component analysis using the robust multiarray average (RMA)-normalized expression data for all probe sets confirmed that the data were consistent within biological replicates, since the hybridizations of replicates clustered tightly (Fig. 5A).
Figure 5.
Summary of microarray data sets. A, Principal component analysis of RMA-normalized microarray data demonstrates tight clustering of biological replicate data. MGH, MGI, and MGW (MG healthy, inoculated, and wounded fruit) and RRH, RRI, and RRW (RR healthy, inoculated, and wounded fruit) are distinguished by PC1 (78%) and PC2 (9%). B, Bar graphs show the number of probe sets that are either up- or down-regulated in MG and RR fruit when comparisons of expression in wounded or inoculated fruit are made with the healthy controls (ANOVA; adjusted P < 0.01). C, Venn diagrams of the data in B, showing the overlap in probe sets that are up- or down-regulated when inoculated and wounded samples are compared with the healthy control samples.
The primary principal component (PC1) accounted for 78% of the variation and differentiated between the MG and RR ripening stages, suggesting that most of the variation was associated with ripening. PC1 also differentiated between wounded and inoculated MG samples. A second principal component (PC2) accounted for 9% of the variation and differentiated between treatments (healthy versus wounded or infected) within each ripening stage. The same clustering and distribution were observed when the data were normalized with MAS5.0 (Affymetrix) or the PM-only model-based expression method of dChip (Li and Wong, 2001; Supplemental Fig. S1). Subsequent analysis was carried out on RMA-normalized data, as this method has superior sensitivity and specificity, particularly for differentially expressed genes (Irizarry et al., 2003; Harr and Schlotterer, 2006).
Two-way ANOVA with a 2 × 3 factorial design using R/maanova (Wu et al., 2003) identified significant (adjusted P < 0.01) differences among the treatments and ripening stages. Hybridization of 8,626 probe sets of the 10,038 features on the Affymetrix Tomato GeneChip array differed among all samples. A compilation of the fold changes of the hybridization data for each probe set (annotation date July 17, 2008, by Affymetrix) with P values is given in Supplemental Table S1. A 1.5-fold change cutoff was applied to select probe sets for further comparison. Figure 5B shows the numbers of probe sets whose expression was significantly up- or down-regulated when MG or RR fruit were either inoculated or wounded, compared with healthy MG or RR fruit, respectively.
In MG fruit, although inoculation with B. cinerea or wounding without inoculation produced similar numbers of expression changes, infection, but not wounding, up-regulated 308 of 891 (35%) probe sets and down-regulated 296 of 661 (45%) probe sets, suggesting that more than one-third of the expression changes are attributable to infection by B. cinerea and not to wounding (Fig. 5C). In RR fruit, B. cinerea infection caused the down-regulation of 652 of 1,881 (35%) probe sets and up-regulation of 413 of 1,391 (30%) probe sets (Fig. 5C). Thus, within 1 dpi in both MG and RR fruit, about one-third of the transcriptome changes are in response to B. cinerea and are not observed when fruit are simply wounded.
B. cinerea-Inducible Gene Expression Changes
In order to identify expression changes due specifically to the fruit-B. cinerea interaction and not as a consequence of wounding or normal healthy fruit development, we selected probe sets whose hybridization intensities significantly increased or decreased in response to B. cinerea compared with both wounded and healthy material (Fig. 6). The transcriptional changes that were a result of normal fruit ripening during the experiment were distinguished using comparisons of uninfected fruit.
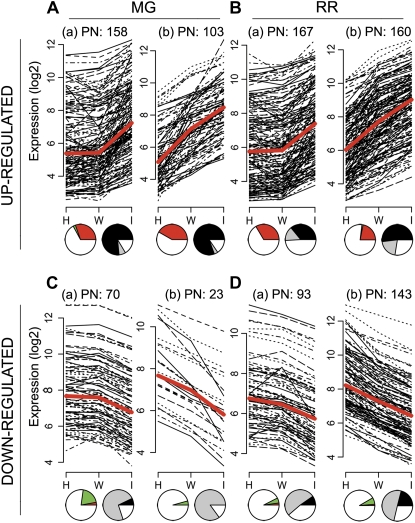
Figure 6.
Analysis of probe sets up- and down-regulated by B. cinerea infection. Changes in expression levels (log2) are shown for up-regulated (A and B) and down-regulated (C and D) probe sets hybridizing to RNA from MG (A and C) and RR (B and D) fruit. The (a) sets are the probe sets that are not differentially expressed when healthy (H) and wounded (W) RNA samples are analyzed but are up- or down-regulated when infected (I) samples are compared with either healthy or wounded samples. The (b) sets are the probe sets whose expression is significantly increased when wounded samples are compared with healthy samples and is further significantly increased when infected samples are compared with wounded samples. Each line in the plots represents a specific probe set; the heavy red line represents the median expression change (H versus W versus I). The pie charts below each panel indicate how the expression of the probe sets in each collection was changed by infection at the other ripening stage or by ripening. The left pie chart indicates the percentages of probe sets that were up-regulated (red), down-regulated (green), or not differentially expressed (white) when fruit of the other ripening stage were inoculated. The right pie charts depict the percentages of probe sets of each collection that were up-regulated (black), down-regulated (gray), or not differentially expressed (white) when RNA samples from healthy RR versus healthy MG fruit are compared. The number of probe sets (PN) in each collection is shown above each panel.
Comparisons of the probe sets altered by infection uniquely in MG or RR fruit identified genes that are ripening stage-specific responses to infection. In MG fruit, 75% of the probe sets whose expression was changed specifically by B. cinerea infection were not altered when RR fruit were inoculated; in RR fruit, 81% of the probe sets that were specifically up- or down-regulated by infection were not significantly altered in infected MG fruit (Fig. 6).
Comparing probe sets altered by infection in both MG and RR fruit identified genes whose induction by infection did not depend on the fruit ripening stage. A total of 36% of the 261 probe sets induced by B. cinerea in MG fruit also were induced in RR fruit, and 12% of the 93 probe sets down-regulated by B. cinerea in MG fruit also were down-regulated by infection in RR fruit. In RR fruit, the expression of 327 probe sets was induced by B. cinerea, and 28% of these were also significantly up-regulated when MG fruit were challenged with B. cinerea conidia. Only 6% of the 236 probe sets down-regulated by B. cinerea in RR fruit also were down-regulated in MG fruit. Very few (1%) of the probe sets showed an opposite expression pattern at the other ripening stage (Fig. 6).
Transcriptional changes observed in both MG and RR fruit in response to B. cinerea suggested pathways activated by B. cinerea regardless of the ripening stage of the fruit. In both ripening stages, oxylipin signaling is activated by the fungus (Table II). Allene oxide synthase (AOS; AJ271093) expression was increased 3- and 30-fold by infection of MG and RR fruit, respectively. AOS converts lipoxygenase-derived fatty acid hydroperoxides to unstable allene epoxides that are precursors of jasmonic acid, a phytohormone involved in abiotic and biotic stress responses in vegetative tissues, including B. cinerea-infected leaves (Glazebrook, 2005). Activation of the octadecanoid defense-signaling pathway is supported by the up-regulation of the pathogen-inducible oxygenases DOX1 (3.8-fold [MG] and 6.3-fold [RR]; AY344539) and loxD (10.1-fold [MG] and 10.7-fold [RR]; U37840; Heitz et al., 1997; Tirajoh et al., 2005) and the antifungal divinyl ether synthase LeDES (93-fold [MG] and 2.9-fold [RR]; AF317515; Itoh and Howe, 2001). Wounding MG and RR fruit did not induce AOS, DOX1, or loxD expression, although loxD expression was 4.5-fold greater in healthy RR fruit than in healthy MG fruit.
Table II.
Fold changes of selected genes
Fold changes are expressed as log2 of the ratios of the expression of the probe sets. Asterisks identify statistically significant fold changes (P < 0.01; 1.5-fold change cutoff is applied). I, Inoculated; H, healthy; W, wounded.
| Probe Set | Accession No. | Gene | MG
|
RR
|
RRH/MGH | ||||
|---|---|---|---|---|---|---|---|---|---|
| I/H | W/H | I/W | I/H | W/H | I/W | ||||
| Common responses of MG and RR fruit to B. cinerea | |||||||||
| Les.13.1.S1_at | AJ271093 | AOS | 1.75* | 0.57 | 1.18* | 5.19* | 2.91* | 2.28* | 0.51 |
| Les.5915.1.S1_at | AY344539 | DOX1 | 1.94* | −0.08 | 2.02* | 2.66* | −0.47 | 3.13* | 0.59* |
| Les.3632.1.S1_at | U37840 | loxD | 3.34* | 2.26* | 1.08* | 3.43* | 2.45* | 0.98* | 2.17* |
| Les.3493.1.S1_at | AY013255 | PLDβ1 | 1.84* | 0.98* | 0.86* | 1.03* | −1.40* | 2.43* | 1.98* |
| Les.129.1.S1_at | AF317515 | LeDES | 6.54* | 0.10 | 6.44* | 1.56* | −2.49* | 4.05* | 5.53* |
| Les.3608.1.S1_at | X71593 | CEVI-1 | 6.93* | 4.79* | 2.14* | 3.18* | −0.04 | 3.22* | 2.28* |
| Les.2832.1.S1_at | X94943 | CEVI16 | 3.12* | 0.63* | 2.49* | 1.76* | −1.36* | 3.12* | 0.34 |
| Les.4307.1.S1_at | AY257487 | PR5 | 4.60* | 2.13* | 2.47* | 1.91* | 0.56 | 1.35* | 3.74* |
| Les.248.1.S1_a_at | AJ277563 | pi1 | 1.31* | −0.72* | 2.03* | 0.83* | −1.15* | 1.98* | 1.17* |
| Les.254.1.S1_at | AY081905 | THT1-3 | 5.33* | 4.22* | 1.11* | 4.70* | 2.58* | 2.13* | 0.37 |
| Les.4038.1.S1_at | AY081908 | THT1-4 | 1.58* | 0.05 | 1.52* | 2.51* | −0.18 | 2.69* | 0.55 |
| Les.3687.1.S1_at | AY081906 | THT7-1 | 1.62* | 0.79* | 0.83* | 2.15* | 1.04* | 1.10* | −0.60* |
| Les.3686.1.S1_at | AY081907 | THT7-8 | 1.25* | 0.20 | 1.05* | 1.13* | 0.03 | 1.11* | 0.33* |
| Les.3667.1.S1_at | U13054 | Cel1 | −1.09* | −0.49* | −0.60* | −1.30* | −0.94* | −0.36 | −1.27* |
| MG-specific responses to B. cinerea | |||||||||
| Les.2591.1.S1_at | M80608 | LOC543987 | 1.90* | −2.50* | 4.40* | 0.22 | −0.81* | 1.04* | 2.29* |
| Les.3653.1.S1_at | X74905 | TomQ'a | 0.86* | −0.70* | 1.56* | −1.64* | −2.44* | 0.80* | −2.55* |
| Les.3406.1.S1_at | Z15140 | LOC544148 | 1.70* | 1.08* | 2.78* | 0.16 | 0.50 | 0.66* | 1.81* |
| Les.122.1.S1_at | Z15141 | LOC544149 | 1.73* | 0.25* | 1.48* | −0.11 | 0.05 | −0.16 | 2.17* |
| Les.441.1.S1_s_at | X94944 | cevi19 | 5.19* | 0.43 | 4.76* | −1.66* | −3.19* | 1.53* | 2.70* |
| Les.2137.1.S1_at | AY359965 | Eix1 | 1.47* | −1.20* | 2.67* | −0.58* | −0.63* | 0.05 | 4.07* |
| Les.3712.1.S1_at | AF154003 | Pirin | 3.42* | 1.67* | 1.75* | 0.46 | −1.77* | 2.23* | 3.26* |
| Les.127.1.S1_at | AJ006376 | SBT3 | 2.01* | 1.35* | 0.66* | −1.52* | −4.73* | 3.21* | −0.13 |
| Les.72.1.S1_at | AF048747 | FPS1 | 1.62* | 0.49 | 1.13* | −0.66* | −1.48* | 0.82* | 1.70* |
| LesAffx.67147.1.S1 | BG131971 | IPI | 1.23* | 0.41 | 0.82* | −0.18 | −1.03* | 0.85* | −0.83* |
| Les.4735.1.S1_at | BT013429 | HMGS | 4.66* | −0.31 | 4.97* | 0.53 | −1.73* | 2.25* | 3.36* |
| Les.2490.1.S1_at | BT013122 | MDC | 2.04* | 0.65* | 1.39* | 0.26 | −1.14* | 1.41* | 1.08* |
| Les.274.1.S1_at | AF204784 | DDTFR10/A | 1.00* | 0.21 | 0.79* | −0.43 | −0.21 | −0.23 | 1.15* |
| Les.3662.1.S1_at | X59145 | ACS2 | 1.79* | 0.52 | 1.27* | 0.80* | 0.80* | 0.00 | 4.97* |
| Les.3661.1.S1_at | M63490 | ACS4 | 2.76* | 0.42 | 2.34* | −3.27* | −1.97* | −1.30* | 7.12* |
| Les.3461.1.S1_at | AJ272304 | Lin5 | 0.72* | −1.52* | 2.23* | −3.15* | −3.43* | 0.28 | 1.44* |
| Les.3654.1.S1_at | X04583 | LePG | 2.71* | −0.75* | 3.46* | −0.48 | −0.43 | −0.04 | 9.47* |
| Les.4449.1.S1_s_at | AY034075 | MAN4 | 2.45* | −0.93* | 3.38* | −3.01* | −2.48* | −0.53 | 2.30* |
| Les.4521.1.S1_at | AY534531 | TomAAT | 0.68* | −1.03* | 1.71* | −0.22 | −0.34* | 0.12 | 2.64* |
| RR-specific responses to B. cinerea | |||||||||
| Les.3505.1.S1_at | AF272366 | Ve1 | 0.02 | 0.00 | 0.01 | 1.09* | −0.08 | 1.17* | 0.82* |
| Les.3964.1.S1_at | AY157060 | WRKYIId-2 | −0.99* | −1.00* | 0.01 | 2.22* | 1.62* | 0.60* | 0.79* |
| Les.3574.1.S1_at | U89257 | LOC544043 | 0.17 | −0.70* | 0.87* | 1.69* | 0.84* | 0.85* | −1.20* |
| Les.3967.1.S1_at | AY157317 | LeFAD7 | −0.25 | 0.42 | −0.67* | −0.92* | −0.17 | −0.75* | −0.07 |
| Les.3481.1.S1_a_at | AF154425 | PLDa3 | −0.29 | 0.03 | −0.32 | −2.70* | −1.43* | −1.27* | −0.47 |
| Les.3014.1.S1_at | AY262025 | FESOD | −0.45 | 0.23 | −0.68* | −0.98* | −0.29* | −0.69* | −0.42 |
| Les.3750.1.S1_at | Y17225 | cdc2A-1 | −0.29 | 0.07 | −0.36 | −0.95* | −0.23 | −0.72* | −3.48* |
| Les.4024.1.S1_at | AJ459817 | psi14A | −0.22 | −0.23 | 0.01 | 2.75* | 0.53* | 2.22* | 1.57* |
| Les.2672.1.S1_s_at | AF305968 | psi14B | −0.14 | −0.09 | −0.05 | 2.62* | 1.37* | 1.24* | 2.69* |
| Les.2672.1.S1_x_at | AF305968 | psi14B | −0.58* | −0.31 | −0.26 | 2.55* | 1.44* | 1.11* | 2.60* |
| Les.4095.1.S1_at | AF022012 | IAA1 | −0.91* | −0.67* | −0.24 | 1.35* | 0.70* | 0.65* | −0.74* |
| Les.3707.1.S1_at | AF022013 | IAA2 | −0.15 | 0.00 | −0.15 | 2.33* | 0.69* | 1.64* | 0.76* |
| Les.50.1.S1_at | X79338 | rnalx | 0.27 | 0.10 | 0.17 | 1.83* | 0.96* | 0.87* | 0.98* |
| Les.3581.1.S1_at | U72396 | Le-HSP17.6 | −0.38 | −0.23 | −0.15 | 0.83* | 0.21 | 0.61* | −0.33 |
| Les.3739.1.S1_at | AB026983 | sHSP | −0.24 | 0.30 | −0.54 | 1.11* | −0.24 | 1.35* | −0.17 |
| Les.3134.1.S1_at | BG129203 | HSP90 | −0.15 | −0.09 | −0.06 | 1.88* | 1.07* | 0.80* | 0.39 |
| Les.3550.1.S1_at | AF096251 | Hsc70 | 0.33 | 0.17 | 0.17 | 0.65* | −0.14 | 0.79* | 0.43 |
| Les.3769.1.S1_at | AB013100 | ACS6 | −0.20 | −0.02 | −0.19 | 4.86* | 3.54* | 1.32* | −2.45* |
| Les.3472.1.S1_at | AF328784 | EIL1 | −0.26 | −0.28 | 0.02 | −0.89* | −0.30 | −0.59* | −0.43 |
| Les.3818.1.S1_at | AF502085 | EREB | 0.41 | −0.61* | 1.02* | 4.04* | 2.23* | 1.81* | −0.44 |
At both the MG and RR stages, B. cinerea caused the up-regulation of the phospholipase D PLDβ1 (3.6-fold [MG] and 2.0-fold [RR]; AY013255), which is rapidly and specifically activated in leaves by the fungal elicitor xylanase (Laxalt et al., 2001).
Inoculation of MG and RR fruit up-regulated three of four hydroxycinnamoyl-CoA:tyramine N-(hydroxycinnamoyl)transferases (THTs): THT1 to THT3 (AY081905) increased 40- and 26-fold in inoculated MG and RR fruit. THTs are involved in hydroxycinnamic acid amide biosynthesis, and in tomato leaves, THT expression is required for the biosynthesis of the antimicrobial metabolites p-coumaroyloctopamine and p-coumaroylnoradrenaline (von Roepenack-Lahaye et al., 2003). THT1 to THT3 are induced during the incompatible Pseudomonas syringae pv tomato interaction with tomato leaves (von Roepenack-Lahaye et al., 2003).
Among the responses to B. cinerea shared by MG and RR fruit, expression of Cel1 (U13054), in the β-1,4-endoglucanase family, decreased 2-fold, confirming reports that the Cel1 protein is reduced when tomato leaves or fruit are inoculated with B. cinerea (Real et al., 2004; Flors et al., 2007).
Although some of the alterations in gene expression in response to B. cinerea are similar in MG and RR fruit, most (75%–80%; Fig. 5) of the changes in response to inoculation were specifically activated in only one of the ripening stages (Table II). The expression of pathogen-responsive genes, such as chitinases (LOC544148 [Z15140] and LOC544149 [Z15141]) and a β-1,3-glucanase (LOC543987 [M80608]), increased when MG fruit were inoculated. In MG fruit, B. cinerea inoculation resulted in 2.8-fold up-regulation of Eix1 (AY359965), a cell surface glycoprotein receptor that in tobacco (Nicotiana tabacum) cells binds a fungal xylanase that induces ethylene (Ron and Avni, 2004). However, expression of nearly all of the genes with putative pathogen-responsive functions also increased during normal uninfected fruit ripening (see below); only the class III acidic β-1,3-glucanase, TomQ'a (X74905), increased 1.8-fold in infected MG fruit but did not increase in infected RR fruit. Expression of pathogen-responsive genes in the absence of microbial infection has been reported (e.g. during leaf senescence and fruit ripening; van Loon et al., 2006).
In RR, but not in MG, fruit infection with B. cinerea promoted the expression of the stress response and chaperone genes sHSP (AB026983), HSP90 (BG129203), and Hsc70 (AF096251) and a WRKY family transcription factor, WRKYIId-2 (AY157060). HSP90 is required for Mi resistance in tomato (Bhattarai et al., 2007), and Hsc70 is involved in tomato responses to Ralstonia solanacearum (Byth et al., 2001). Most of the other genes induced by infection of MG or RR fruit have unknown functions.
Fruit Ripening-Associated Transcriptional Changes
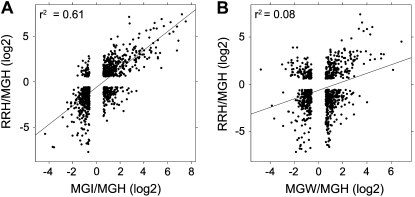
Comparisons of the transcriptomes of healthy MG and RR fruit identified genes whose expression changed as a consequence of ripening. A total of 1,901 probe sets were up-regulated and 1,800 down-regulated in healthy RR fruit compared with healthy MG fruit. Strikingly, 80% of the 261 transcriptional changes that result from B. cinerea infection of MG fruit also were observed as a consequence of ripening in healthy fruit (Fig. 6). The correlation between the expression changes due to infection and normal ripening was r2 = 0.61 (Fig. 7A). Wound-induced changes correlated less with those occurring as a consequence of ripening (r2 = 0.08; Fig. 7B), suggesting that the induction of ripening-related gene expression is a specific response of MG fruit to inoculation with B. cinerea conidia but is not a response to wounding. However, not all of the genes whose expression changed during normal uninfected fruit ripening were induced by B. cinerea in MG fruit; infection of MG fruit altered the expression of about 20% of the genes whose expression is associated with fruit ripening.
Figure 7.
Correlation between B. cinerea infection or wounding and ripening. A, Scatterplot showing the fold changes (log2) in expression caused by inoculation of MG fruit (MGI/MGH) compared with ripening of healthy MG fruit (RRH/MGH). B, Scatterplot showing the fold changes caused by wounding MG fruit (MGW/MGH) compared with ripening of healthy MG fruit. A 1.5-fold change threshold was applied. A linear trend line and the Pearson correlation coefficient (r2) are displayed. H, Healthy; I, inoculated; W, wounded.
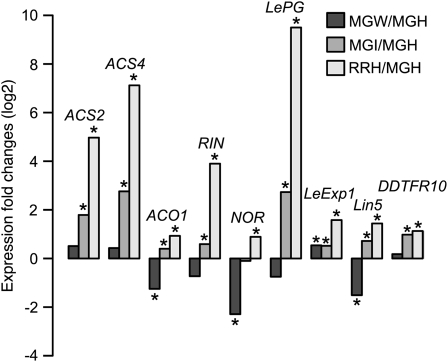
In MG fruit, B. cinerea infection caused 5- and 7-fold up-regulation of the two 1-aminocyclopropane-1-carboxylic acid synthases, ACS2 (X59145) and ACS4 (M63490; Fig. 8), key enzymes in the ripening-associated ethylene biosynthetic pathway. Expression of ACS2 and ACS4 increased 31- and 139-fold in healthy RR fruit compared with healthy MG fruit. Two other members of the ACS gene family, ACS3 (U17972) and ACS6 (AB013100), whose expression during ripening did not increase (Fig. 5; Nakatsuka et al., 1998), were not induced in MG fruit by B. cinerea, although ACS6 expression was induced 29-fold in infected RR fruit. Wounding did not cause significant changes in ACS2 and ACS4 expression in either MG or RR fruit. In infected MG fruit, expression of a 1-aminocyclopropane-1-carboxylic acid oxidase, ACO1 (X04792), expressed during ripening, increased 1.3-fold (P = 0.002). This change was significant, although it was less than 1.5-fold, and supported the conclusion that the ethylene biosynthetic pathway activated by fruit ripening was induced in MG fruit by B. cinerea. In contrast, wounding down-regulated ACO1 expression by 2.3-fold.
Figure 8.
Relative expression of selected tomato fruit ripening-related genes, based on microarray analysis. The relative expression of genes regulated by normal fruit ripening is compared with expression in healthy MG fruit (MGH). In each set, the left bar shows the relative change in expression of wounded MG fruit (MGW), the center bar shows the change of infected MG fruit (MGI), and the right bar shows the expression change in healthy RR fruit (RRH). Asterisks indicate significance within the comparison indicated by each bar (adjusted P < 0.01).
Infection did not change the expression in MG fruit of five of the six known ethylene receptors (LeETR1–LeETR15). LeETR3 (NR; U38666) and LeETR4 (AF118843) expression was 1.4-fold up-regulated by ripening in healthy fruit, as reported by Kevany et al. (2007). Wounding caused a 2.5-fold decrease in LeETR3 and a 2-fold decrease in LeETR4 expression in MG fruit. Expression of the other components of the ethylene signal transduction pathway, ERF1 (AY044236), EIL1 to EIL4 (AF328786, AF328785, AF328784, and AB108840), and EIN2 (AY566238), was unchanged by infection. Ethylene receptor protein accumulation and turnover, however, provide additional regulation of fruit ripening responses to ethylene (Kevany et al., 2007).
Expression of the MADS box ripening-regulating transcription factor, RIN (AF448522), increased 16-fold during healthy fruit ripening (Fig. 8). In B. cinerea-infected MG fruit, RIN expression was increased 1.5-fold compared with uninfected MG fruit. The expression of another ripening regulator, NOR (BM410927), was 4-fold down-regulated when MG fruit were wounded and was not significantly changed when MG fruit were challenged with B. cinerea compared with uninoculated unwounded MG fruit. NOR expression increased 1.8-fold during normal healthy fruit ripening.
Expression of the gene encoding the cell wall-modifying enzyme polygalacturonase (LePG; X04583) showed the largest change among all ripening-associated probe sets; it increased 700-fold when expression levels in healthy RR fruit were compared with levels in healthy MG fruit. LePG transcript accumulation increased 6.5-fold when MG fruit were infected with B. cinerea but was unchanged when MG fruit were wounded. LePG expression decreased when RR fruit were infected.
Expansin (LeExp1; AF548376), another fruit ripening protein involved in cell wall polysaccharide disassembly, was up-regulated when MG fruit were either wounded or inoculated (1.4- or 1.5-fold, respectively). As expected, LeExp1 expression was up-regulated in healthy RR fruit by 3-fold compared with healthy MG fruit. In infected RR fruit, LeExp1 was down-regulated by 1.6-fold.
The ripening-associated genes DDTFR10 (AF204784), with unknown function, and Lin5 (AJ272304), an acid invertase, were induced by 2- and 1.6-fold, respectively, following inoculation of MG fruit, but their expression did not change when RR fruit were inoculated or wounded. Expression of these was induced by 2.2- and 2.7-fold in uninfected RR fruit compared with uninoculated MG fruit.
Expression of many of the ripening-associated genes (e.g. LePG, LeExp1, and ACS4) that were induced in MG fruit was down-regulated in RR fruit by B. cinerea. These changes are similar to those reported for gene expression in overripe RR tomato fruit (Brummell and Harpster, 2001; Alba et al., 2005).
B. cinerea Induced Transcript Changes in Ripening Mutants
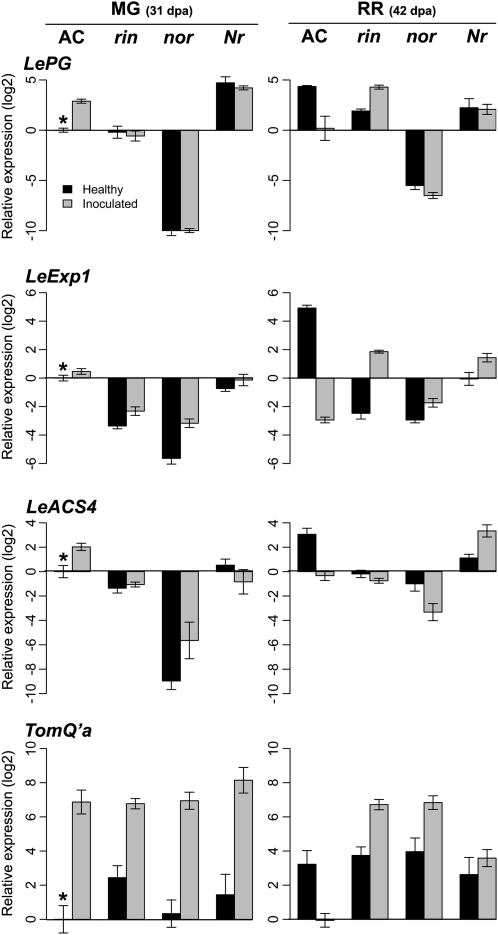
To determine whether the transcriptional changes in MG and RR fruit in response to B. cinerea depend on the regulation of ripening, the expression of LePG, LeExp1, ACS4, and TomQ'a in wild-type fruit and ripening mutants was characterized by quantitative reverse transcription (qRT)-PCR, using additional RNA samples from independent collections of infected and uninfected MG and RR fruit (Fig. 9). The expression of LePG, ACS4, and TomQ'a was not induced in wild-type MG and RR fruit by wounding, as observed in the microarray data. The expected increased expression of LePG, LeExp1, and ACS4 during uninfected wild-type fruit ripening was observed (Figs. 8 and 9). In healthy nor and rin fruit at 31 and 42 dpa, LeExp1 expression was lower than that in wild-type MG and RR fruit, respectively. In rin, LePG expression increased from 31 to 42 dpa but remained lower than in wild-type fruit at the RR stage. In nor fruit, LePG expression remained lower than in wild-type MG fruit even at 42 dpa.
Figure 9.
Relative expression of LePG, LeExp1, LeACS4, and TomQ'a in tomato ripening mutants in response to B. cinerea assessed by qRT-PCR. Gene expression at 3 dpi in infected MG fruit (31 dpa) and RR fruit (42 dpa) was measured using qRT-PCR. Values are relative to the expression of each of the genes in AC MG healthy fruit (asterisks).
The simultaneous expression of LePG and LeExp1 and the subsequent action of the encoded proteins on cell wall polysaccharide networks is critical for fruit susceptibility (Cantu et al., 2008a), and expression of both genes was induced by B. cinerea in wild-type MG fruit (Figs. 8 and 9). In wild-type RR fruit, both LePG and LeExp1 were down-regulated by B. cinerea infection, confirming the microarray data and supporting the conclusion that B. cinerea hastens the progression of RR fruit to the overripe stage, when LePG and LeExp1 expression is lower (Alba et al., 2005). Among the ripening mutants, only inoculation of rin fruit at the ripe equivalent stage (42 dpa) induced LePG and LeExp1 expression; this induction was not observed in inoculated nor fruit, suggesting that the rin and nor mutations control different B. cinerea-responsive fruit ripening pathways.
To determine whether the ripening-associated ethylene biosynthetic pathway is induced by B. cinerea, ACS4 expression was analyzed. As expected from the microarray data, ACS4 expression was induced in wild-type MG fruit by B. cinerea and by ripening; in wild-type RR fruit, ACS4 expression was reduced by infection (Fig. 9). In response to B. cinerea, ACS4 expression did not increase and was similar in both the susceptible rin and the resistant nor mutants at 31 and 42 dpa.
In wild-type MG and all ripening mutant fruit at both 31 and 42 dpa, B. cinerea induced the expression of TomQ'a (Fig. 9), a β-1,3-glucanase gene that has been associated with responses to pathogens (Domingo et al., 1994). TomQ'a expression decreased when wild-type RR fruit were inoculated, suggesting that the activation of responses to B. cinerea varies as a function of ripening.
DISCUSSION
Development alters susceptibility to pathogens, and tomato fruit ripening is an example of a dramatic developmental transition that has allowed analysis of changes in plant responses to pathogen challenge. In unripe tomato fruit, B. cinerea causes transcriptional and histochemical changes that support the conclusion that some of the responses by the fruit to a pathogen are resistance responses similar to those that have been observed by others in vegetative plant organs (Benito et al., 1998; Diaz et al., 2002; AbuQamar et al., 2006). Although unripe fruit are resistant to B. cinerea, the interaction with the fungus also results in transcriptional changes that are expressed as uninfected fruit ripen, which suggests that the fungus induces fruit ripening processes that ultimately render ripe fruit susceptible. However, B. cinerea does not activate all of the processes occurring in ripening fruit. Since B. cinerea is able to induce the expression of ripening genes in the rin mutant, whose fruit do not ripen but are susceptible, the pathogen may neither require nor activate all of the pathways that constitute fruit ripening. On the other hand, in ripe fruit, B. cinerea relies on at least some of the processes and events that have occurred during ripening in order to successfully infect the organ. The network of processes associated with ripening and those that are induced by B. cinerea demonstrate that, while resistance responses are elicited by the pathogen, developmental conditions in the host tissue determine the outcome of the fruit-pathogen interaction.
Most developmental processes are composed of networks of independent, related, and potentially redundant pathways and events. During ripening, developmental processes change the composition and structure of the fruit tissue that pathogens encounter. The ripening regulators RIN, NOR, and ethylene pleiotropically control many aspects of fruit ripening, including, it has been assumed, the increased susceptibility to B. cinerea (Giovannoni, 2001, 2007b). The results presented here demonstrate that mutants of the RIN and NOR transcription factors differ in their impact on the fruit ripening-associated acquisition of susceptibility. Prior to the MG stage, fruit from nor and rin mutants develop normally, but once mature, they fail to soften or synthesize lycopene and carotenoids and evolve ethylene only slightly and at late stages (McGlasson et al., 1975b). Our results and earlier observations (Lavy-Meir et al., 1989) show, however, that the increased susceptibility of ripe fruit to B. cinerea depends on NOR but not on RIN and only partially on the perception of ethylene by the fruit. Since rin mutant fruit and fruit treated with the ethylene perception inhibitor 1-MCP do not ripen and yet are susceptible to B. cinerea, the properties of ripe fruit that promote susceptibility are not controlled by RIN or ethylene perception. The pathways that are regulated independently and cooperatively by these transcriptional and hormonal ripening regulators are not known, but elucidation of their targets will complete our understanding of what constitutes fruit ripening.
Fruit ripening can be considered a form of regulated senescence (Giovannoni, 2007a), and B. cinerea, as a necrotrophic pathogen, promotes senescence. In Arabidopsis leaves, expression of senescence genes is up-regulated within 24 h after inoculation with B. cinerea (Swartzberg et al., 2007), and when senescence is accelerated, plants are more susceptible to B. cinerea (van Baarlen et al., 2007). The results reported here demonstrating that B. cinerea promotes fruit ripening also support the hypothesis that B. cinerea induces senescence events characteristic of the organ infected, which in this case includes ripening. B. cinerea produces abscisic acid (Marumo et al., 1982; Hirai et al., 1986) and ethylene (Cristescu et al., 2002); the former promotes senescence in leaves and the latter promotes ripening in fruit and senescence in vegetative tissues (Alexander and Grierson, 2002; Lim et al., 2007). Ethylene production by B. cinerea could account for the promotion of ripening in MG fruit, but inoculated green fruit do not produce detectable amounts of ethylene until 3 dpi, suggesting that ethylene may not be the immediate cause of the ripening promotion induced by the pathogen. In MG unripe fruit, B. cinerea secretes cell wall-degrading enzymes that target fruit wall polysaccharides (P. Shah, D. Cantu, A.L.T. Powell, R. Orlando, C. Bergmann, and G. Gutierrez, unpublished data), and products of the degradation of cell wall pectin in ripening tomatoes promote the production of ethylene and fruit ripening (Campbell and Labavitch, 1991; Melotto et al., 1994). Thus, B. cinerea itself may produce or cause the production of factors, in addition to ethylene, that indirectly induce fruit ripening. How B. cinerea activates ripening processes will require analysis of infections with fungal mutants that do not respond to, for example, simple sugars or do not produce ethylene. Whether the induction of ripening and the elicitation of defense responses in unripe fruit are induced by the signals from the fungus is a subject for future work. We have observed (D. Cantu, unpublished data) that application of killed B. cinerea mycelia induces defense-like responses similar to those observed when resistant unripe fruit are inoculated with viable spores; further characterization of the transcriptional responses of the fruit to nonviable mycelia is needed to see whether ripening responses also are promoted.
Ethylene may only partially facilitate infections of fruit, since susceptibility is not necessarily averted when ethylene perception is blocked. Nonripening Nr mutant fruit and unripe wild-type fruit treated with sufficient 1-MCP to stop normal ripening remain partially resistant to B. cinerea. Although they perceive ethylene, rin and nor mutant fruit fail to ripen (Herner and Sink, 1973; Tigchelaar et al., 1973; McGlasson et al., 1975a; Giovannoni, 2007b). Ethylene treatment of rin fruit induces the expression of some ripening-regulated genes (Lincoln and Fischer, 1988), and wounding rin and nor fruit induces ethylene production (Yokotani et al., 2004). However, the small reduction in the susceptibility of 1-MCP-treated rin fruit and the limited induction of ACS4 gene expression by B. cinerea infections of rin fruit suggest that ethylene perception may be only partially responsible for the nearly wild-type level of susceptibility observed in rin fruit. Additional ripening events not regulated by rin and not dependent on the perception of ethylene apparently promote susceptibility.
Responses involving phytohormones determine pathogen resistance in vegetative tissues. In leaves, defenses to B. cinerea utilize complex signaling networks involving ethylene, oxylipins, salicylic acid, and abscisic acid (Ferrari et al., 2003; Glazebrook, 2005; AbuQamar et al., 2006; Asselbergh and Höfte, 2007). While in both unripe and ripe fruit we have observed that B. cinerea induces the transcription of genes in pathways associated with these hormones, the concurrent promotion of fruit ripening by these hormones may be sufficient to offset their roles in defense responses. In ripe fruit, the accumulated consequences of ripening may attenuate the defense responses, thus leading to susceptibility. The observation that TomQ'a expression is induced in unripe wild-type fruit and in all ripening mutants at both 31 and 42 dpa but is down-regulated by B. cinerea in wild-type ripe fruit supports the conclusion that ripening affects the way fruit respond to the pathogen and what defenses are deployed. How fruit cells perceive and respond to fungal toxins or elicitors, such as cell wall fragments generated by fungal digestion of host cell walls, may also change during ripening (Côté and Hahn, 1994; Collado et al., 2000; An et al., 2005; van Kan, 2006; Staats et al., 2007). Although B. cinerea is a necrotroph and hypersensitive responses and cell death have been thought to increase its aggressiveness (Govrin and Levine, 2000; Dickman et al., 2001; van Baarlen et al., 2004, 2007), the timely and localized accumulation of antimicrobial substances, hydrogen peroxide, and lignin, all features of a hypersensitive response, effectively prevent expanding infections; the responses are evident in resistant unripe fruit but not in susceptible ripe fruit.
Most studies of plant-pathogen interactions have focused on responses that provide resistance, but the efficacy of the defense responses may depend on events in the host. Plant developmental processes that facilitate susceptibility are an integral part of the host-pathogen interaction and must be considered, as they can offset the defenses deployed or reduce their effectiveness. Tomato fruit ripening includes the progressive and extensive loss of firmness that is largely a result of metabolism of the cell wall polysaccharides (Brummell, 2006; Cantu et al., 2007, 2008a). The ripening-associated disassembly of hemicellulosic and pectic cell wall polysaccharides is known to be severely reduced in rin fruit and apparently is diminished in nor and 1-MCP-treated fruit, since they also have only limited softening (Seymour et al., 1987; Giovannoni et al., 1989; DellaPenna et al., 1990; Maclachlan and Brady, 1994). We have previously shown that fruit susceptibility to B. cinerea depends on LePG and LeExp1, two proteins that participate in the disassembly of the cell wall, which are expressed in ripe fruit even though B. cinerea secretes its own polysaccharide-hydrolyzing enzymes (van Kan, 2006; Cantu et al., 2008a). Here, we show that B. cinerea infections induce the expression of LePG and LeExp1 in unripe wild-type fruit and in susceptible 42-dpa rin fruit but not in resistant nor fruit. Infection of fruit, however, also results in the decreased expression of Cel1 (Gonzalez-Bosch et al., 1996; Flors et al., 2007), suggesting that specific aspects of ripe fruit cell wall disassembly are important for susceptibility. Changes in tomato fruit cuticle composition and structure that result from the Delayed Fruit Deterioration (DFD) mutation also contribute to changes in ripening-associated tomato fruit softening and B. cinerea susceptibility (Saladie et al., 2007).
We do not know whether it is the cell wall polysaccharide architectural integrity per se that limits or facilitates pathogen development. Plant cell walls are the sites of the early events during plant-pathogen interactions (Cantu et al., 2008b). Cell walls are not only physical barriers that limit pathogen access to cellular contents but are also involved in pathogen recognition and in the activation and deployment of plant responses (Vorwerk et al., 2004; Cantu et al., 2008b). Many plant proteins that function in pathogen recognition and response are embedded, secreted, or extend into the extracellular cell wall compartment (van Loon et al., 2006). The remodeling of the cell wall polysaccharide network during fruit ripening may influence how responses to pathogens using these proteins are implemented. When LePG and LeExp1 are expressed as wild-type fruit ripen or when they are induced by B. cinerea, for example, in infected rin fruit, changes in the cell wall may occur that reposition or lead to the loss of antipathogen proteins. Cell wall disassembly may also generate signals, including pectin-derived oligosaccharides (Cervone et al., 1989; Côté and Hahn, 1994; Vorwerk et al., 2004; Cantu et al., 2008b) that activate antipathogen responses, which are inoperative or unavailable before the onset of normal ripening. Alternatively, disassembly of the polysaccharide matrix may simply render the cell wall ineffective as a barrier. By knowing how ripening and the ripening-associated disassembly of the wall polysaccharide matrix alter the population of proteins in this apoplastic compartment and observing the consequences for susceptibility, it will be possible to clarify how the cell wall affects the growth of the fungus.
Our examination of fruit as they undergo ripening documents the developmental plasticity of interactions between the plant organ and the pathogen. This is demonstrated in unripe fruit, where plant responses provide resistance before ripening is complete, and in ripe fruit, where susceptibility is promoted by some, but not all, of the regulators of fruit ripening. The relevance of the resistance responses or the susceptibility facilitation depends on events in the plant tissue. The comprehensive characterization of the defenses of unripe fruit, those ripening functions activated upon inoculation, and the dissection of the ripening regulator-controlled pathways that promote fruit susceptibility to B. cinerea opens new possibilities to achieve high quality produce with diminished susceptibility to pathogens. Characterization of the B. cinerea-ripening fruit interaction provides a model of how developmental events in the host profoundly influence the outcome of a plant-pathogen interaction.
MATERIALS AND METHODS
Plant Material
The tomato (Solanum lycopersicum) ripening mutants rin, nor, and Nr and control lines, in the ‘Ailsa Craig’ background, were obtained from the C.M. Rick Tomato Genetics Resource Center (University of California at Davis). Plants were grown in typical greenhouse and field conditions in 2007 and 2008 in Davis, California. Fruit were tagged at 3 dpa and harvested at 31 dpa as MG and at 42 dpa as RR. Ripening stages were confirmed by the color, size, texture, and ethylene evolution of the fruit.
Fungal Culture and Fruit Inoculation
Fruit were inoculated as described by Cantu et al. (2008a). B. cinerea (B05.10) was provided by Jan van Kan (Wageningen University), and a GFP-expressing B. cinerea B05.10 strain was provided by Walter Mahaffee (U.S. Department of Agriculture). Conidia, collected from sporulating cultures grown on 1% potato dextrose agar (Difco), were counted and diluted to 500 conidia μL−1 for inoculations. On the day of harvest, fruit were disinfected by 10% (v/v) bleach followed by three water rinses. Fruit used as wounded or infected material were punctured (2 mm depth, 1 mm diameter) at seven sites; six sites were inoculated with 10 μL of conidia suspension, and the seventh was inoculated with 10 μL of sterile water. Fruit used as wounded material had 10 μL of water placed into the wounded sites. Healthy fruit were not wounded or inoculated. All fruit samples were incubated at 20°C in high humidity. Susceptibility was determined daily as disease incidence (percentage inoculation sites showing symptoms of tissue maceration or soft rot) and severity (diameters of the macerating lesions). The evaluation of susceptibility was repeated three to five times with 10 to 15 fruit per replicate. GFP-expressing B. cinerea was observed with a Bio-Rad MRC 1024 confocal laser microscope (excitation/emission, 488/522 nm). Detection of hydrogen peroxide was performed using 3,3′-diaminobenzidine (Sigma), as described by Thordal-Christensen et al. (1997). Two percent (w/v) phloroglucinol (Sigma) in concentrated HCl was used to detect lignin deposition. To visualize suberin accumulation, 0.01% (v/w) safranin (Sigma) in 50% ethanol was used as described in Lucena et al. (2003). Micrographs were taken using a MZFLIII stereomicroscope (Leica) and a DMR series microscope (Leica).
Measurement of B. cinerea Biomass
Fungal biomass was determined using QuickStix for B. cinerea (EnviroLogix; Meyer and Dewey, 2000) as described by Cantu et al. (2008a). Tissue (0.3 g) at 3 dpi was pooled (nine lesions per pool, three pools per line). The intensity of the monoclonal antibody reaction was determined using the QuickStix Reader (EnviroLogix) and converted into fungal biomass (μg g−1 fresh weight of fruit extracts) based on standard curves using known amounts of dry mycelium diluted into extracts from healthy fruit tissue.
Ethylene Measurement
Daily, three to five fruit were enclosed in air-tight 500-mL glass containers for 1 h at 20°C, and all ethylene measurements were replicated three times; each replicate had three sets of fruit of each treatment and each genotype. Head space gas (10 mL) was withdrawn and injected into a gas chromatograph (Carle) and interfaced to a Chromjet integrator (Spectra-Physics). The concentrations of ethylene were normalized based on standards, on the volume and weight of fruit, and expressed as nL g−1 fresh weight h−1.
1-MCP Treatment
Fruit were placed in an air-tight chamber containing 100 mg of Ethyl Block (15 nL L−1 1-MCP; Floralife) for 18 h at 20°C. As controls, similarly staged and harvested fruit were placed in an identical closed chamber without 1-MCP. Immediately after treatment, fruit were divided into three replication groups and inoculated and assessed (disease development, ethylene production) as above.
RNA Isolation
Total RNA was prepared from the combined fruit outer pericarp and epidermis within a 0.25-cm radius around and including the inoculation or wounded sites. Each biological replicate consisted of an independent pool of samples from at least five different fruit.
Three grams of tissue was ground in liquid nitrogen, and 3 mL of 100 mm Tris-HCl (pH 8.0), 25 mm EDTA, 75 mm NaCl, 1% SDS, and 10 mm β-mercaptoethanol were added. Three extractions with equal volumes of phenol:chloroform:isoamyl alcohol (25:24:1, v/v/v) and centrifugation for 15 min at 10,000g followed, and the nucleic acids were ethanol precipitated and resuspended in 1 mL of water before 250 μL of 8 m LiCl was added. After precipitation overnight and centrifugation (30 min at 12,000g at 4°C), the RNA pellet was treated with DNase (RNase-Free; Promega) followed by extraction with phenol:chloroform:isoamyl alcohol and two extractions with chloroform:isoamyl alcohol (24:1, v/v). The RNA concentration and purity were measured using a NanoDrop 1000 Spectrophotometer (Thermo Scientific). The RNA integrity was checked by agarose gel electrophoresis.
Target Preparation and Microarray Analysis
Ten micrograms of total RNA was processed for the microarray hybridizations using the Affymetrix GeneChip one-cycle target-labeling kit (Affymetrix). The resultant biotinylated complementary RNA was fragmented and hybridized to the GeneChip Tomato Genome Array (10,038 tomato probe sets for more than 9,200 tomato genes). The arrays were washed, stained, and scanned at the Microarray Core Facility (University of California at Davis School of Medicine). The hybridization data for the probes representing 10,209 probe sets of over 9,200 tomato transcripts on the Affymetrix Tomato GeneChips were imported into R (R Foundation for Statistical Computing; http://www.R-project.org). The raw data were background corrected, normalized, and summarized using the RMA (Irizarry et al., 2003) method in R/affy (http://www.bioconductor.org).
Correlations between replicates are shown in scatterplots in Supplemental Figure S2. The consistency of the replication was confirmed by hierarchical clustering based on Pearson's correlation coefficient (Supplemental Fig. S1A). Supplemental Figure S3 shows the correlation of the mean value of the hybridizations when infected MG and RR and wounded samples were each compared with the healthy samples from MG and RR fruit. The hybridization changes comparing healthy MG and RR fruit are shown in Supplemental Figure S3.
Statistical analysis with ANOVA was done using the R/maanova package with 100 permutations (Wu et al., 2003). P values were adjusted to cope with the problem of multiple-test false discovery rates (Storey, 2002) using the R/qvalue package. Similar results were obtained using the robust multiple testing approach of Significant Analysis of Microarray (SAM; www-stat.stanford.edu/∼tibs/SAM/; Tusher et al., 2001). SAM analysis identified 7,801 significant hybridized probe sets with an estimated maximum false discovery rate (q value) of 0.01 based on 500 permutations. All of the significant probe sets identified by SAM were also identified by ANOVA; however, SAM did not include 825 probe sets called significant by R/maanova. SAM does not include a method for testing pair-wise differences; thus, pair-wise comparisons were done with the R/maanova package to identify the significantly changed probe set hybridizations (P < 0.01).
qRT-PCR
cDNA was synthesized using Moloney murine leukemia virus reverse transcriptase (Promega) from RNA obtained as described above. Three independent pools of fruit were used. The amplification was performed in a total reaction volume of 20 μL. Reactions included 2 μL of template, 10 μL of Fast SYBR Green Master Mix (Applied Biosystems), 1.8 μL of reverse primer (10 mm), 1.8 μL of forward primer (10 mm), and 4.4 μL of sterile molecular biology-grade water. All PCRs were performed with the same cycling conditions: 95°C for 10 min followed by 40 cycles of 95°C for 3 s and 60°C for 30 s. A melting curve for every target analyzed was included using the following conditions: 95°C for 15 s, 60°C for 1 min, and 95°C for 15 s. Tomato actin (LeACT; The Institute for Genomic Research no. TC116117), whose expression did not change in the microarray analysis (AFFX-Les-actin) and among the different amplified samples, was used as an internal control and processed in parallel. Amplification and data analysis were carried out on the ABI StepOne Plus Real-Time PCR System (Applied Biosystems) using, as a sample control, the data for each product obtained from healthy MG fruit. All templates and primer concentrations were optimized for the reactions initially using conventional RT-PCR. Primer sequences are shown in Supplemental Table S2.
Statistical Analysis
Data were analyzed by ANOVA followed by post hoc testing (Tukey's honestly significant difference) using R (R Foundation for Statistical Computing). For percentage values, statistical analysis was carried out after angular transformation.
The sequence data from this article have been deposited in the National Center for Biotechnology Information's Gene Expression Omnibus (GEO; Edgar et al., 2002) and are accessible through GEO (accession no. GSE14637; http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE14637).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Clustering of microarray raw hybridization data and RMA-, MAS5.0-, and dCHIP-normalized data.
Supplemental Figure S2. Correlations between biological replicates used in the microarray expression analysis.
Supplemental Figure S3. Effect of wounding, inoculation, and ripening on microarray data sets.
Supplemental Table S1. Fold changes of the hybridization data for each tomato probe set.
Supplemental Table S2. Genes and primer sequences for the qRT-PCR assays.
Supplementary Material
Acknowledgments
We thank Juan Pedro Sanchez, Dr. Olivier Cagnac, Dr. Rosa Rivero, and the Microarray Core Facility (University of California at Davis School of Medicine) for technical assistance.
This work was supported by the National Science Foundation (grant no. IOB 0544504). D.C. was supported by the Department of Plant Sciences, University of California at Davis, and B.B.-U. was supported by the Consejo Nacional de Ciencia y Tecnología (Ministerio de Ciencia y Tecnología, Costa Rica) and by the Vicerrectoría de Vida Estudiantil y Servicios Académicos (Instituto Tecnológico de Costa Rica).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Ann L.T. Powell (alpowell@ucdavis.edu).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- AbuQamar S, Chen X, Dhawan R, Bluhm B, Salmeron J, Lam S, Dietrich R, Mengiste T (2006) Expression profiling and mutant analysis reveals complex regulatory networks involved in Arabidopsis response to Botrytis infection. Plant J 48 28–44 [DOI] [PubMed] [Google Scholar]
- Agrios GN (2005) Plant Pathology, Ed 5. Elsevier Academic Press, Burlington, MA
- Alba R, Payton P, Fei ZJ, McQuinn R, Debbie P, Martin GB, Tanksley SD, Giovannoni JJ (2005) Transcriptome and selected metabolite analyses reveal multiple points of ethylene control during tomato fruit development. Plant Cell 17 2954–2965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander L, Grierson D (2002) Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. J Exp Bot 53 2039–2055 [DOI] [PubMed] [Google Scholar]
- An HJ, Lurie S, Greve LC, Rosenquist D, Kirmiz C, Labavitch JM, Lebrilla CB (2005) Determination of pathogen-related enzyme action by mass spectrometry analysis of pectin breakdown products of plant cell walls. Anal Biochem 338 71–82 [DOI] [PubMed] [Google Scholar]
- Asselbergh B, Höfte M (2007) Basal tomato defences to Botrytis cinerea include abscisic acid-dependent callose formation. Physiol Mol Plant Pathol 71 33–40 [Google Scholar]
- Benito EP, ten Have A, van't Klooster JW, van Kan JAL (1998) Fungal and plant gene expression during synchronized infection of tomato leaves by Botrytis cinerea. Eur J Plant Pathol 104 207–220 [Google Scholar]
- Bhattarai KK, Li Q, Liu Y, Dinesh-Kumar SP, Kaloshian I (2007) The Mi-1-mediated pest resistance requires Hsp90 and Sgt1. Plant Physiol 144 312–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummell DA (2006) Cell wall disassembly in ripening fruit. Funct Plant Biol 33 103–119 [DOI] [PubMed] [Google Scholar]
- Brummell DA, Harpster MH (2001) Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol Biol 47 311–340 [PubMed] [Google Scholar]
- Byth H-A, Kuun KG, Bornman L (2001) Virulence-dependent induction of Hsp70/Hsc70 in tomato by Ralstonia solanacearum. Plant Physiol Biochem 39 697–705 [Google Scholar]
- Campbell A, Labavitch J (1991) Induction and regulation of ethylene biosynthesis by pectic oligomers in cultured pear cells. Plant Physiol 97 699–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantu D, Vicente AR, Greve LC, Dewey FM, Bennett AB, Labavitch JM, Powell ALT (2008. a) The intersection between cell wall disassembly, ripening, and fruit susceptibility to Botrytis cinerea. Proc Natl Acad Sci USA 105 859–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantu D, Vicente AR, Greve LC, Labavitch JM, Powell ALT (2007) Genetic determinants of textural modifications in fruits and role of cell wall polysaccharides and defense proteins in the protection against pathogens. Fresh Produce 1 101–110 [Google Scholar]
- Cantu D, Vicente AR, Labavitch JM, Bennett AB, Powell ALT (2008. b) Strangers in the matrix: plant cell walls and pathogen susceptibility. Trends Plant Sci 13 610–617 [DOI] [PubMed] [Google Scholar]
- Cara B, Giovannoni JJ (2008) Molecular biology of ethylene during tomato fruit development and maturation. Plant Sci 175 106–113 [Google Scholar]
- Cervone F, De Lorenzo G, Pressey R, Darvill A, Albersheim P (1989) Host-pathogen interactions. XXXXIII. A plant protein converts a fungal pathogenesis factor into an elicitor of plant defense responses. Plant Physiol 90 542–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Shockey JA (1999) The ethylene-response pathway: signal perception to gene regulation. Curr Opin Plant Biol 2 352–358 [DOI] [PubMed] [Google Scholar]
- Collado IG, Aleu J, Hernández-Galán R, Durán-Patrón R (2000) Botrytis species: an intriguing source of metabolites with a wide range of biological activities. Structure, chemistry and bioactivity of metabolites isolated from Botrytis species. Curr Org Chem 4 1261–1286 [Google Scholar]
- Company P, Gonzalez-Bosch C (2003) Identification of a copper chaperone from tomato fruits infected with Botrytis cinerea by differential display. Biochem Biophys Res Commun 304 825–830 [DOI] [PubMed] [Google Scholar]
- Côté F, Hahn MG (1994) Oligosaccharins: structures and signal transduction. Plant Mol Biol 26 1379–1411 [DOI] [PubMed] [Google Scholar]
- Cristescu SM, De Martinis D, te Lintel Hekkert S, Parker DH, Harren FJM (2002) Ethylene production by Botrytis cinerea in vitro and in tomatoes. Appl Environ Microbiol 68 5342–5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DellaPenna D, Lashbrook CC, Toenjes K, Giovannoni JJ, Fischer RL, Bennett AB (1990) Polygalacturonase isozymes and pectin depolymerization in transgenic rin tomato fruit. Plant Physiol 94 1882–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz J, ten Have A, van Kan JAL (2002) The role of ethylene and wound signaling in resistance of tomato to Botrytis cinerea. Plant Physiol 129 1341–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman MB, Park YK, Oltersdorf T, Li W, Clemente T, French R (2001) Abrogation of disease development in plants expressing animal antiapoptotic genes. Proc Natl Acad Sci USA 98 6957–6962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo C, Conejero V, Vera P (1994) Genes encoding acidic and basic class III β-1,3-glucanases are expressed in tomato plants upon viroid infection. Plant Mol Biol 24 725–732 [DOI] [PubMed] [Google Scholar]
- Doster MA, Schnathorst WC (1985) Effect of leaf maturity and cultivar resistance on development of the powdery mildew fungus on grapevines. Phytopathology 75 318-321 [Google Scholar]
- Edgar R, Domrachev M, Lash AE (2002) Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30 207–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Plotnikova JM, De Lorenzo G, Ausubel FM (2003) Arabidopsis local resistance to Botrytis cinerea involves salicylic acid and camalexin and requires EDS4 and PAD2, but not SID2, EDS5 or PAD4. Plant J 35 193–205 [DOI] [PubMed] [Google Scholar]
- Flors V, Leyva Mde L, Vicedo B, Finiti I, Real MD, Garcia-Agustin P, Bennett AB, Gonzalez-Bosch C (2007) Absence of the endo-β-1,4-glucanases Cel1 and Cel2 reduces susceptibility to Botrytis cinerea in tomato. Plant J 52 1027–1040 [DOI] [PubMed] [Google Scholar]
- Gillaspy G, Ben-David H, Gruissem W (1993) Fruits: a developmental perspective. Plant Cell 5 1439–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni JJ (2001) Molecular biology of fruit maturation and ripening. Annu Rev Plant Physiol Plant Mol Biol 52 725–749 [DOI] [PubMed] [Google Scholar]
- Giovannoni JJ (2004) Genetic regulation of fruit development and ripening. Plant Cell (Suppl) 16 S170–S180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni JJ (2007. a) Fruit ripening and its manipulation. In S Gan, ed, Senescence Process of Plants. Blackwell Publishing, Oxford, pp 278–295
- Giovannoni JJ (2007. b) Fruit ripening mutants yield insights into ripening control. Curr Opin Plant Biol 10 283–289 [DOI] [PubMed] [Google Scholar]
- Giovannoni JJ, DellaPenna D, Bennett AB, Fischer RL (1989) Expression of a chimeric polygalacturonase gene in transgenic rin (ripening inhibitor) tomato fruit results in polyuronide degradation but not fruit softening. Plant Cell 1 53–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook J (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43 205–227 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Bosch C, Brummell DA, Bennett AB (1996) Differential expression of two endo-1,4-beta-glucanase genes in pericarp and locules of wild-type and mutant tomato fruit. Plant Physiol 111 1313–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govrin EM, Levine A (2000) The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr Biol 10 751–757 [DOI] [PubMed] [Google Scholar]
- Harr B, Schlotterer C (2006) Comparison of algorithms for the analysis of Affymetrix microarray data as evaluated by co-expression of genes in known operons. Nucleic Acids Res 34 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitz T, Bergey DR, Ryan CA (1997) A gene encoding a chloroplast-targeted lipoxygenase in tomato leaves is transiently induced by wounding, systemin, and methyl jasmonate. Plant Physiol 114 1085–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herner RC, Sink KC (1973) Ethylene production and respiratory behavior of the rin tomato mutant. Plant Physiol 52 38–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai N, Okamoto M, Koshimizu K (1986) The 1′,4′-trans-diol of abscisic acid, a possible precursor of abscisic acid in Botrytis cinerea. Phytochemistry 25 1865–1868 [Google Scholar]
- Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP (2003) Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4 249–264 [DOI] [PubMed] [Google Scholar]
- Itoh A, Howe GA (2001) Molecular cloning of a divinyl ether synthase: identification as a CYP74 cytochrome P-450. J Biol Chem 276 3620–3627 [DOI] [PubMed] [Google Scholar]
- Kars I, Krooshof GH, Wagemakers L, Joosten R, Benen JAE, van Kan JAL (2005) Necrotizing activity of five Botrytis cinerea endopolygalacturonases produced in Pichia pastoris. Plant J 43 213–225 [DOI] [PubMed] [Google Scholar]
- Kevany BM, Tieman DM, Taylor MG, Dal Cin V, Klee HJ (2007) Ethylene receptor degradation controls the timing of ripening in tomato fruit. Plant J 51 458–467 [DOI] [PubMed] [Google Scholar]
- Lalancette N, Hickey KD (1985) Apple powdery mildew disease progress on sections of shoot growth: an analysis of leaf maturation and fungicide effects. Phytopathology 75 130–134 [Google Scholar]
- Lanahan MB, Yen HC, Giovannoni JJ, Klee HJ (1994) The Never Ripe mutation blocks ethylene perception in tomato. Plant Cell 6 521–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavy-Meir G, Barkai-Golan R, Kopeliovitch E (1989) Resistance of tomato ripening mutants and their hybrids. Plant Dis 73 976–978 [Google Scholar]
- Laxalt AM, ter Riet B, Verdonk JC, Parigi L, Tameling WIL, Vossen J, Haring M, Musgrave A, Munnik T (2001) Characterization of five tomato phospholipase D cDNAs: rapid and specific expression of LePLD beta 1 on elicitation with xylanase. Plant J 26 237–247 [DOI] [PubMed] [Google Scholar]
- Li C, Wong WH (2001) Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc Natl Acad Sci USA 98 31–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim PO, Kim HJ, Nam HG (2007) Leaf senescence. Annu Rev Plant Biol 58 115–136 [DOI] [PubMed] [Google Scholar]
- Lincoln JE, Fischer RL (1988) Regulation of gene expression by ethylene in wild-type and rin tomato (Lycopersicon esculentum) fruit. Plant Physiol 88 370–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucena M, Romero-Aranda R, Mercado J, Cuartero J, Valpuesta V, Quesada MA (2003) Structural and physiological changes in the roots of tomato plants over-expressing a basic peroxidase. Physiol Plant 118 422–429 [Google Scholar]
- Maclachlan G, Brady C (1994) Endo-1,4-β-glucanase, xyloglucanase, and xyloglucan endo-transglycosylase activities versus potential substrates in ripening tomatoes. Plant Physiol 105 965–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning K, Tor M, Poole M, Hong Y, Thompson AJ, King GJ, Giovannoni JJ, Seymour GB (2006) A naturally occurring epigenetic mutation in a gene encoding an SBP-box transcription factor inhibits tomato fruit ripening. Nat Genet 38 948–952 [DOI] [PubMed] [Google Scholar]
- Marumo S, Katayama M, Komori E, Ozaki Y, Natsume M, Kondo S (1982) Microbial production of abscisic acid by Botrytis cinerea. Agric Biol Chem 46 1967–1968 [Google Scholar]
- McGlasson WB, Dostal HC, Tigchelaar EC (1975. a) Comparison of propylene-induced responses of immature fruit of normal and rin mutant tomatoes. Plant Physiol 55 218–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlasson WB, Poovaiah BW, Dostal HC (1975. b) Ethylene production and respiration in aging leaf segments and in disks of fruit tissue of normal and mutant tomatoes. Plant Physiol 56 547–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melotto E, Greve LC, Labavitch JM (1994) Cell wall metabolism in ripening fruit. VII. Biologically active pectin oligomers in ripening tomato (Lycopersicon esculentum Mill.) fruits. Plant Physiol 106 575–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengiste T, Chen X, Salmeron J, Dietrich R (2003) The BOTRYTIS SUSCEPTIBLE1 gene encodes an R2R3MYB transcription factor protein that is required for biotic and abiotic stress responses in Arabidopsis. Plant Cell 15 2551–2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer UM, Dewey FM (2000) Efficacy of different immunogens for raising monoclonal antibodies to Botrytis cinerea. Mycol Res 104 979–987 [Google Scholar]
- Nakatsuka A, Murachi S, Okunishi H, Shiomi S, Nakano R, Kubo Y, Inaba A (1998) Differential expression and internal feedback regulation of 1-aminocyclopropane-1-carboxylate synthase, 1-aminocyclopropane-1-carboxylate oxidase, and ethylene receptor genes in tomato fruit during development and ripening. Plant Physiol 118 1295–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell ALT, van Kan J, ten Have A, Visser J, Greve LC, Bennett AB, Labavitch JM (2000) Transgenic expression of pear PGIP in tomato limits fungal colonization. Mol Plant Microbe Interact 13 942–950 [DOI] [PubMed] [Google Scholar]
- Prusky D (1996) Pathogen quiescence in postharvest diseases. Annu Rev Phytopathol 34 413–434 [DOI] [PubMed] [Google Scholar]
- Qayoum A, Line RF (1985) High temperature, adult plant resistance to stripe rust of wheat. Phytopathology 75 1121–1125 [Google Scholar]
- Real MD, Company P, García-Agustín P, Bennett AB, González-Bosch C (2004) Characterization of tomato endo-β-1,4-glucanase Cel1 protein in fruit during ripening and after fungal infection. Planta 220 80–86 [DOI] [PubMed] [Google Scholar]
- Reuveni M, Tuzun S, Cole JS, Siegel MR, Kuc J (1986) The effects of plant age and leaf position on the susceptibility of tobacco to blue mold caused by Peronospora tabacina. Phytopathology 76 455–458 [Google Scholar]
- Rick CM, Butler L (1956) Phytogenetics of the tomato. Adv Genet 8 267–382 [Google Scholar]
- Ron M, Avni A (2004) The receptor for the fungal elicitor ethylene-inducing xylanase is a member of a resistance-like gene family in tomato. Plant Cell 16 1604–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumen EC, Bonman JM, Parlevliet JE (1992) Leaf age related partial resistance to Pyricularia oryzae in tropical lowland rice cultivars as measured by the number of sporulating lesions. Phytopathology 82 1414–1417 [Google Scholar]
- Saladie M, Matas AJ, Isaacson T, Jenks MA, Goodwin SM, Niklas KJ, Xiaolin R, Labavitch JM, Shackel KA, Fernie AR, et al (2007) A reevaluation of the key factors that influence tomato fruit softening and integrity. Plant Physiol 144 1012–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour GB, Harding SE, Taylor AJ, Hobson GE, Tucker GA (1987) Polyuronide solubilization during ripening of normal and mutant tomato fruit. Phytochemistry 26 1871–1875 [Google Scholar]
- Staats M, van Baarlen P, Schouten A, van Kan JAL, Bakker FT (2007) Positive selection in phytotoxic protein-encoding genes of Botrytis species. Fungal Genet Biol 44 52–63 [DOI] [PubMed] [Google Scholar]
- Storey JD (2002) A direct approach to false discovery rates. J R Stat Soc Ser B Stat Methodol 64 479–498 [Google Scholar]
- Swartzberg D, Kirshner B, Rav-David D, Elad Y, Granot D (2007) Botrytis cinerea induces senescence and is inhibited by autoregulated expression of the IPT gene. Eur J Plant Pathol 120 289–297 [Google Scholar]
- ten Have A, Breuil WO, Wubben JP, Visser J, van Kan JAL (2001) Botrytis cinerea endopolygalacturonase genes are differentially expressed in various plant tissues. Fungal Genet Biol 33 97–105 [DOI] [PubMed] [Google Scholar]
- ten Have A, Mulder W, Visser J, van Kan JAL (1998) The endopolygalacturonase gene Bcpg1 is required for full virulence of Botrytis cinerea. Mol Plant Microbe Interact 11 1009–1016 [DOI] [PubMed] [Google Scholar]
- Thompson AJ, Tor M, Barry CS, Vrebalov J, Orfila C, Jarvis MC, Giovannoni JJ, Grierson D, Seymour GB (1999) Molecular and genetic characterization of a novel pleiotropic tomato-ripening mutant. Plant Physiol 120 383–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge D (1997) Subcellular localization of H2O2 in plants, H2O2 accumulation in papillae and hypersensitive response during barley-powdery mildew interaction. Plant J 11 1187–1194 [Google Scholar]
- Tieman DM, Taylor MG, Ciardi JA, Klee HJ (2000) The tomato ethylene receptors NR and LeETR4 are negative regulators of ethylene response and exhibit functional compensation within a multigene family. Proc Natl Acad Sci USA 97 5663–5668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigchelaar EC, Tomes M, Kerr M, Barman R (1973) A new fruit ripening mutant, non-ripening (nor). Rep Tomato Genet Coop 23 33 [Google Scholar]
- Tirajoh A, Aung TST, McKay AB, Plant AL (2005) Stress-responsive alpha-dioxygenase expression in tomato roots. J Exp Bot 56 713–723 [DOI] [PubMed] [Google Scholar]
- Tusher VG, Tibshirani R, Chu G (2001) Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 98 5116–5121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Baarlen P, Staats M, van Kan JAL (2004) Induction of programmed cell death in lily by the fungal pathogen Botrytis elliptica. Mol Plant Pathol 5 559–574 [DOI] [PubMed] [Google Scholar]
- van Baarlen P, Woltering EJ, Staats M, van Kan JAL (2007) Histochemical and genetic analysis of host and non-host interactions of Arabidopsis with three Botrytis species: an important role for cell death control. Mol Plant Pathol 8 41–54 [DOI] [PubMed] [Google Scholar]
- van Kan JAL (2006) Licensed to kill: the lifestyle of a necrotrophic plant pathogen. Trends Plant Sci 11 247–253 [DOI] [PubMed] [Google Scholar]
- van Loon LC, Rep M, Pieterse CMJ (2006) Significance of inducible defense-related proteins in infected plants. Annu Rev Phytopathol 44 135–162 [DOI] [PubMed] [Google Scholar]
- von Roepenack-Lahaye E, Newman MA, Schornack S, Hammond-Kosack KE, Lahaye T, Jones JDG, Daniels MJ, Dow JM (2003) p-Coumaroylnoradrenaline, a novel plant metabolite implicated in tomato defense against pathogens. J Biol Chem 278 43373–43383 [DOI] [PubMed] [Google Scholar]
- Vorwerk S, Somerville S, Somerville C (2004) The role of plant cell wall polysaccharide composition in disease resistance. Trends Plant Sci 9 203–209 [DOI] [PubMed] [Google Scholar]
- Vrebalov J, Ruezinsky D, Padmanabhan V, White R, Medrano D, Drake R, Schuch W, Giovannoni J (2002) A MADS-box gene necessary for fruit ripening at the tomato ripening-inhibitor (Rin) locus. Science 296 343–346 [DOI] [PubMed] [Google Scholar]
- Wilkinson JQ, Lanahan MB, Yen HS, Giovannoni JJ, Klee HJ (1995) An ethylene-inducible component of signal transduction encoded by Never-ripe. Science 270 1807–1809 [DOI] [PubMed] [Google Scholar]
- Wu H, Kerr MK, Cui XQ, Churchill GA (2003) MAANOVA: a software package for the analysis of spotted cDNA microarray experiments. In G Parmigiani, ES Garret, RA Irizarry, SL Zeger, eds, Analysis of Gene Expression Data: An Overview of Methods and Software. Springer, New York, pp 313–431
- Yen HC, Lee SY, Tanksley SD, Lanahan MB, Klee HJ, Giovannoni JJ (1995) The tomato Never-Ripe locus regulates ethylene-inducible gene-expression and is linked to a homolog of the Arabidopsis Etr1 gene. Plant Physiol 107 1343–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokotani N, Tamura S, Nakano R, Inaba A, McGlasson WB, Kubo Y (2004) Comparison of ethylene- and wound-induced responses in fruit of wild-type, rin and nor tomatoes. Postharvest Biol Technol 32 247–252 [Google Scholar]
- Zheng ZY, Abu Qamar S, Chen ZX, Mengiste T (2006) Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. Plant J 48 592–605 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.