Abstract
Annexins act as targets of calcium signals in eukaryotic cells, and recent results suggest that they play an important role in plant stress responses. We found that in Arabidopsis (Arabidopsis thaliana), AnnAt1 (for annexin 1) mRNA levels were up-regulated in leaves by most of the stress treatments applied. Plants overexpressing AnnAt1 protein were more drought tolerant and knockout plants were more drought sensitive than ecotype Columbia plants. We also observed that hydrogen peroxide accumulation in guard cells was reduced in overexpressing plants and increased in knockout plants both before and after treatment with abscisic acid. Oxidative protection resulting from AnnAt1 overexpression could be due to the low level of intrinsic peroxidase activity exhibited by this protein in vitro, previously linked to a conserved histidine residue found in a peroxidase-like motif. However, analyses of a mutant H40A AnnAt1 protein in a bacterial complementation test and in peroxidase activity assays indicate that this residue is not critical to the ability of AnnAt1 to confer oxidative protection. To further examine the mechanism(s) linking AnnAt1 expression to stress resistance, we analyzed the reactive S3 cluster to determine if it plays a role in AnnAt1 oligomerization and/or is the site for posttranslational modification. We found that the two cysteine residues in this cluster do not form intramolecular or intermolecular bonds but are highly susceptible to oxidation-driven S-glutathionylation, which decreases the Ca2+ affinity of AnnAt1 in vitro. Moreover, S-glutathionylation of AnnAt1 occurs in planta after abscisic acid treatment, which suggests that this modification could be important in regulating the cellular function of AnnAt1 during stress responses.
Annexins are a multigene, multifunctional family of Ca2+-dependent membrane-binding proteins found in both animal and plant cells, where they serve as important components of Ca2+ signaling pathways (for a recent review, see Mortimer et al., 2008). In plants, their role in Golgi-mediated secretion during growth has been highlighted (Blackbourn et al., 1992; Clark et al., 1992, 2005; Carroll et al., 1998). Given the central signaling role of Ca2+, it is not surprising that abiotic stresses induce Δ[Ca2+]cyt (Chandra and Low, 1997; Knight, 2000; Cessna et al., 2001; Gao et al., 2004) and that an emerging conclusion from recent studies is that certain annexins may be targets of this Δ[Ca2+]cyt induced by abiotic stress. There are three lines of evidence that support this conclusion: expression of annexins is differentially regulated by various abiotic stresses; altering the expression of certain annexins alters the tolerance of plants to abiotic stress in different experimental models; and annexins have enzymatic functions likely to involve them directly in regulating stress-activated signaling pathways.
One of the first reports indicating a role for annexin in stress responses documented the up-regulation of an alfalfa (Medicago sativa) annexin in response to osmotic stress, abscisic acid (ABA), and drought (Kovacs et al., 1998). Transcriptome studies in Arabidopsis (Arabidopsis thaliana) have also revealed changes in annexin gene expression in response to a variety of abiotic stresses (Kreps et al., 2002). Moreover, annexin genes are up-regulated by reactive oxygen species (ROS; Apel and Hirt, 2004), and annexin protein content in Arabidopsis and Mimosa pudica is increased by ABA treatment. ABA may be a general regulator of annexin expression in a wide variety of plant species (Hoshino et al., 2004; Lee et al., 2004).
Several reports implicate a role for annexins specifically in salt and drought tolerance. Gorantla et al. (2005) reported that an annexin is highly expressed in drought-stressed seedlings of the drought-resistant rice (Oryza sativa) ‘Nagina 22’; drought also affects annexin expression in loblolly pine (Pinus taeda; Watkinson et al., 2003). Expanding these findings, Cantero et al. (2006) carried out quantitative real-time reverse transcription (RT)-PCR assays of the differential expression of all eight annexin genes in Arabidopsis after different stress stimuli and found that expression of most of these genes is differentially regulated by exposure to drought and salt as well as to other abiotic stresses. Lee et al. (2004) used T-DNA mutants to show that Arabidopsis AnnAt1 (for annexin 1) and AnnAt4 but not AnnAt2 play an important role during germination under salt and osmotic stresses. They found that the impaired ability of the ΔannAt1 mutants to germinate under stress conditions was rescued by complementation with wild-type AnnAt1. More recently, Jami et al. (2008) showed that ectopic expression of an annexin from Brassica juncea confers drought and salt tolerance to transgenic tobacco (Nicotiana tabacum) plants.
AnnAt1 is so far the best characterized of all the plant annexins at least partly due to the fact that it is the most abundant Arabidopsis annexin at the mRNA level as judged by Arabidopsis EST databases. The first results suggesting that AnnAt1 functions during oxidative stress were obtained from studies expressing this Arabidopsis annexin in heterologous systems. For example, expression of AnnAt1 in the ΔoxyR mutant of Escherichia coli sensitive to hydrogen peroxide (H2O2) greatly increased the organism's survivability in the presence of H2O2 (Gidrol et al., 1996). This bacterial strain is disrupted in its ability to survive oxidative stress due to deletion of the ΔoxyR regulatory factor. They demonstrated that partially purified protein preparations of AnnAt1 showed very low levels of peroxidase activity and hypothesized that AnnAt1 complemented the ΔoxyR bacteria by reducing H2O2 levels, allowing the bacteria to survive oxidative stress. The follow-up data described additional experiments supporting the ability of this protein to protect against oxidative cellular damage in mammalian cells and demonstrating that the antioxidant properties of AnnAt1 extend to a wide variety of cell types (Janicke et al., 1998; Kush and Sabapathy, 2001).
The hypothesis that AnnAt1 may have a low level of peroxidase activity has received support from the data of Gorecka et al. (2005). They found that an overexpressed version of AnnAt1 comigrates with a peroxidase stain in a gel assay and that purified preparations of recombinant AnnAt1 protein display peroxidase activity, although at a level significantly lower than that of horseradish peroxidase. They also found that AnnAt1 protein obtained from expression in Nicotiana benthamiana had three times more activity than bacterially expressed AnnAt1, suggesting that some posttranslational modification or in vivo binding partner might make the observed peroxidase activity physiologically relevant. More recently, additional evidence that certain plant annexins can exhibit peroxidase activity in vitro was obtained for maize (Zea mays) annexins (Laohavisit et al., 2009).
AnnAt1 has two well-conserved type II Ca2+-binding sites in the first and fourth repeats as well as a 30-amino acid sequence in the first repeat that has homology to heme-binding motifs found in plant peroxidases (Clark and Roux, 1995; Gidrol et al., 1996). This peroxidase-like motif contains a critical conserved His residue needed for the heme-binding domain that overlaps with the Ca2+-binding site in the first repeat, raising the possibility that Ca2+ could regulate the peroxidase activity observed in AnnAt1. Taken together with biochemical studies, these results strongly favor the hypothesis that AnnAt1 has intrinsic peroxidase activity in vitro. However, it remains to be determined if this activity is physiologically relevant.
The production of H2O2 is induced by several different biotic and abiotic stresses and helps to mediate plant responses to these stresses (Vanderauwera et al., 2005). So the annexin-peroxidase connection raises the possibility that annexins could play a role in limiting the duration of a plant's exposure to ROS or preventing the accumulation of damaging levels of these agents during stress-induced oxidative burst in plants. The possibility that certain annexins might function as peroxidases is made even more intriguing by the presence of a predicted redox-reactive center. Crystal structure data obtained for a cotton (Gossypium hirsutum) annexin suggests the presence of an S3 cluster in the first domain that could function as a redox reactive center (Hofmann et al., 2003). Hofmann (2004) pointed out that the S3 cluster could serve to reduce H2O2 during plant responses to stress as well as play a redox role in other contexts. Some plant annexins, including AnnAt1, have the amino acids conserved in positions needed to create the putative S3 cluster. Recently, the crystal structure for AnnAt1 was obtained, and this structure confirmed the presence of the S3 cluster in AnnAt1 (Protein Data Bank no. At1g35720; 1YCN).
Even though the apparent in vitro inherent peroxidase activity of AnnAt1 is an attractive hypothesis to explain its function in stress responses, there are a number of alternative mechanisms by which AnnAt1 could provide protection to cells from ROS, such as activating peroxidases by interacting with them, acting as pH- or H2O2-dependent Ca2+ channels, inhibiting lipid peroxidation in membranes, altering membrane properties by association with lipid rafts, affecting ion transport activities indirectly, or even acting as an H2O2 sensor (Gorecka et al., 2005, 2007; Konopka-Postupolska, 2007; Jami et al., 2008; Mortimer et al., 2008; Laohavisit et al., 2009). In order to begin discerning the precise role of AnnAt1 in stress responses, here we provide to our knowledge the first study to incorporate phenotypic analyses of both mutant Arabidopsis lines that cannot express AnnAt1 (ΔannAt1) and those that overexpress AnnAt1 (OE). We also carefully analyze the biochemical properties of this protein that may lead to its ability to function during stress responses.
RESULTS
Stress-Induced Expression of AnnAt1
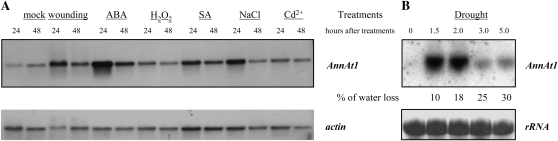
To assess if AnnAt1 can play a role in plant stress responses, the level of AnnAt1 mRNA in leaves treated with a wide spectrum of abiotic stressors was assessed. We observed up-regulation of AnnAt1 mRNA levels after practically all employed stimuli (Fig. 1A). The most pronounced and persistent induction took place after treatment with the stress hormone ABA, with the maximum transcript level attained after 24 h of stimulation. Even after 48 h of ABA treatment, the mRNA level remained elevated in comparison with the control. A similar kinetics of sustained induction was also observed after treatment with salicylic acid, although the transcript abundance after 24 h was substantially lower in comparison with ABA treatment. Wounding and NaCl treatment resulted in more transient mRNA elevation that declined within 48 h. Surprisingly, the induction after H2O2 was not as pronounced as the induction observed in response to the other treatments. This might be attributed to the mode of application: H2O2 is a rather unstable substance, and in diluted solution it undergoes rapid decomposition. Treatment with cadmium ions (heavy metal stress) resulted in a slight increase in AnnAt1 mRNA levels at 24 h. Interestingly, even in mock stimulation, when excised leaves were laid onto the water surface, some level of induction could be observed, especially after 48 h. This is most likely a consequence of wounding leaves using the experimental design employed in this trial. Finally, we examined the effects of drought treatments on AnnAt1 mRNA levels. Under the conditions used (detached leaves, short day), we observed a significant increase in the level of AnnAt1 mRNA, which occurred within 2 h of removing water (approximately 20% water loss), and then this level rapidly declined but remained higher in comparison with control levels. When plants were grown under long-day conditions, the effect was similar, although the kinetics of induction was delayed (reaching a maximum level after 4 h; 21% water loss; data not shown). The transcript level of bona fide drought-induced genes like Rab18 and RD29A after similar treatment increased after 11% and 17% water loss, respectively (Endo et al., 2008).
Figure 1.
Northern-blot analysis of AnnAt1 mRNA level after different treatments in Arabidopsis Col-0 plants. A, Detached, fully expanded leaves of 6-week-old plants were incubated on the surface of the following solutions (water [mock], 100 μm ABA, 150 mm NaCl, 1 mm salicylic acid [SA], 10 mm H2O2, and 100 mm Cd2+). To wound them, leaves were squeezed with grooved forceps five times per leaf. Total RNAs were isolated and hybridized with cDNA probes derived from the 3# untranslated region (764–1,116 nucleotides of the ORF) of AnnAt1 and actin 2 cDNA (1,062–1,283 nucleotides of the ORF). This experiment was performed twice and gave similar results. B, Detached, fully expanded leaves were subjected to drought (as described in “Materials and Methods”). Total RNAs were isolated and hybridized with AnnAt1 probe, as in A. As a loading control, 28S rRNA after staining with 0.2% (w/v) methylene blue is shown. The percentage of water loss at each time point is indicated at bottom. This experiment was performed twice and gave similar results.
Generation of Arabidopsis Plants with Different Levels of AnnAt1 Protein
Considering that AnnAt1 mRNA levels are induced by various abiotic stress treatments and especially during drought, we wanted to investigate the functional significance of AnnAt1 protein in the response/adaptation of plant cells to unfavorable environmental conditions. To assess this goal, we produced plants with different levels of AnnAt1 protein.
We obtained two putative AnnAt1 T-DNA insertional lines (ΔannAt1) from the Syngenta Arabidopsis Insertion Library (SAIL lines; Sessions et al., 2002) and one from GABI-Kat. (Rosso et al., 2003). Confirmation that the insertion site of these lines was at the predicted location was determined by PCR, using specific annexin gene primers and a left border primer from the T-DNA vector (Supplemental Fig. S1A). After self-pollination, homozygous plants were identified, and these lines were used to determine that the T-DNA insertion caused disruption of AnnAt1 mRNA levels. RT-PCR revealed that AnnAt1 mRNA was not present in these lines (Supplemental Fig. S1B). Western-blot analysis using AnnAt1-specific antibody showed that AnnAt1 protein was also not present in these lines (Supplemental Fig. S1C).
In order to obtain plants with elevated levels of AnnAt1, the AnnAt1 cDNA was subcloned into the pROK2 vector and introduced via agrotransformation into Arabidopsis ecotype Columbia (Col-0). A schematic representation of the T-DNA inserted region is shown in Supplemental Figure S2A. A total of 16 kanamycin-resistant F1 transgenic seedlings from independent transformation events were identified. The presence of transgene was confirmed for 12 lines with genomic PCR (Supplemental Fig. S2C), and five of them were further analyzed. Southern-blot analysis confirmed the presence of additional copy/copies stably integrated into the Arabidopsis genome (Supplemental Fig. S2B). To quantify the number of additional copies in individual OE lines, the genomic DNA was digested with EcoRI, which cuts only once within the T-DNA region, and probed with the full coding sequence of AnnAt1. This revealed several inserted loci of the transgenes in the Arabidopsis genome, indicating independent integration events in different transgenic lines. The number of transgene copies varied from one to five per line. A single band at about 5,000 bp, representing the endogenous gene of AnnAt1 (the size estimated on the basis of in silico digestion of the Arabidopsis genome with EcoRI is 4,863 bp), was detected in Arabidopsis Col-0. The hybridization results obtained for SacI digestion confirmed the presence of the transgene (data not shown). Interestingly, western-blot analysis showed that the lines representing the highest number of insertions (1 and 10) appear to have lower levels of annexin proteins (data not shown), probably due to posttranscriptional gene silencing. Nevertheless, in lines 5 and 12 (three and two additional copies, respectively), the level of AnnAt1 protein was elevated (Supplemental Fig. S2D), and those lines were used for further analyses.
Drought Tolerance of Plants Differing in AnnAt1 Protein Level
The dehydration experiments were performed on Col-0, ΔannAt1, and OE plants of the F4 generation. Two types of experiments were performed: first to check whether the changes in the level of endogenous AnnAt1 protein can affect a plant's tolerance to dehydration, and a second one to examine if they have an impact on the survivability and seed production after a prolonged period of dehydration.
In the first experiment, a total number of 50 plants per line were analyzed. Twenty-five seedlings were transferred into one pot and cultivated in the growing chamber for 4 weeks under short-day conditions. For the next 2 weeks, plants were kept under the same conditions except that water was completely withdrawn. The first symptoms of wilting appeared in the ΔannAt1 plants as early as 5 d after withdrawing water (Fig. 2A). It is worth noting that another symptom of plant stress response, phenylpropanoid accumulation (Dixon et al., 2002; Camera et al., 2004; Solecka, 2007), appearing as red spots on the abaxial side of leaves, was also much more enhanced in ΔannAt1 plants. At the same time, OE and Col-0 plants maintained turgor and stayed green. Prolonged stress resulted in complete loss of turgor in ΔannAt1 plants. At the point when the first symptoms of wilting appeared on Col-0, the OE plants still remained turgid and green.
Figure 2.
Tolerance to dehydration stress of Arabidopsis with different AnnAt1 levels. A, AnnAt1 expression confers increased tolerance to short-term drought. Twenty-five plants (Col-0, ΔannAt1-3, and 35S∷AnnAt1-12 [OE]) were grown in a single pot under short-day conditions. After 4 weeks, water was withdrawn. The photograph showing the differences in the reactions of plants to the short-lasting drought was taken after 5 d of water withdrawal. B, AnnAt1 expression confers increased tolerance to long-term drought. Arabidopsis plants (Col-0, ΔannAt1-1, and 35S∷AnnAt1-5 [OE]) were grown under short-day conditions. After 8 weeks, water was withdrawn for 3 weeks. The photograph showing the differences in each plant's ability to survive the period of total desiccation was taken 2 weeks after reinitiation of watering. These experiments were repeated twice and gave comparable results.
To assess the ability of Arabidopsis plants to regain vital function after severe drought stress (complete desiccation), a second set of experiments was performed. Five plants per line (OE/Col-0/ΔannAt1) were grown in separate pots in the growth chamber under short-day conditions. Four weeks after seedlings emerged, watering was drastically reduced to 5 mL per week per pot. After 3 weeks of treatment, the plants seemed to be completely dry and dead. At this time, watering was restored and a photograph was taken 2 weeks afterward (Fig. 2B). All OE plants were able to develop a new rosette and complete the life cycle after induction in long days. At the same time, no ΔannAt1 plants were able to survive and develop further after such a prolonged period of desiccation. These results provide evidence that drought tolerance positively correlates with the level of AnnAt1 protein. In the early stages of drought treatment, overexpression of AnnAt1 prevents loss of turgor and wilting. Over longer periods, increased levels of AnnAt1 protein facilitate the survival of dormant buds from which the drought-stressed plant can resurrect itself.
The Role of AnnAt1 in Oxidative Stress in Arabidopsis Cells
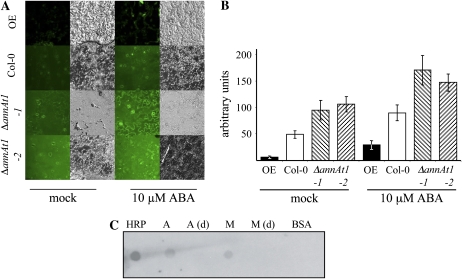
To gain more insight into the role of AnnAt1 in neutralizing ROS and providing tolerance to H2O2, we used 2,7-dichlorodihydrofluorescein diacetate (H2DCFDA), which is an oxidation-sensitive fluorescent dye that can be used as an indicator of oxidative stress. Epidermal peels from Col-0, OE, and ΔannAt1 plants were loaded with H2DCFDA and subsequently incubated in buffer supplemented or not with 10 μm ABA for 30 min. The H2O2 was detected as fluorescence in stomata cells of the epidermal peel upon incubation buffer (Fig. 3A).
Figure 3.
The role of AnnAt1 in oxidative stress in Arabidopsis cells. A, Confocal images of representative epidermal strips from Arabidopsis plants (Col-0, ΔannAt1-1, ΔannAt1-2, and OE-12) following treatment either with buffer [mock] or 10 μM ABA and staining with an H2O2-specific dye, H2DCFDA. Bright-field images of Col-0 and transgenic lines are also shown. This experiment was repeated four times with similar results, and all experiments were done double blind. B, Quantification of guard cell pigmentation from experiment A. The graph includes an n value of 30 cells from the epidermal strip. Error bars represent sd. C, In vitro peroxidase activity of recombinant AnnAt1 protein expressed in N. benthamiana. Approximately 10 μg of wild-type AnnAt1-His(6) (A) and H40A AnnAt1-His(6) (M) and 10 ng of horseradish peroxidase (HRP; positive control) and bovine serum albumin (BSA; negative control) were dot blotted onto PVDF and developed using enhanced chemiluminescence substrate without antibodies. As a control, heat denatured-samples (d) were spotted.
In these experiments, the accumulation of H2O2 was increased by ABA treatment in both Col-0 and plants differing in their endogenous AnnAt1 levels. However, the AnnAt1 OE line showed lower levels of H2O2 accumulation when compared with Col-0, while mutant annAt1 lines showed higher levels of H2O2 (Fig. 3).
AnnAt1 Can Reduce the Effect of Oxidative Stress in E. coli
We performed complementation of the ΔoxyR mutant phenotype of E. coli using AnnAt1 and mutated H40A AnnAt1 proteins. As a control, an isogenic E. coli MG1655 strain was used. Classical methods of assaying bacterial survivability were inapplicable to our experiments due to the already reported poor growth of the ΔoxyR strain that occurs in the defined minimal medium (Greenberg and Demple, 1988). Both measurement of optical density at 600 nm and serial dilution plating gave inconsistent results. Survivability of ΔoxyR in liquid culture was decreased, and the large amounts of cellular debris encountered interfered with optical density measurements. Moreover, during the early stages of colony formation, when bacterial density is low, aerobic conditions result in a nonspecific decrease in plating efficiency (Ma and Eaton, 1992). Only after several attempts were we able to establish an experimental protocol to successfully perform quantitative measurements of AnnAt1 effects on H2O2 survivability in the ΔoxyR strain.
We found that 6.25 and 12.5 mm H2O2 inhibits growth of the E. coli ΔoxyR. The diameters of cleared zones were 8.51 ± 4.9 mm and 11.22 ± 0.38 mm, respectively. AnnAt1 was able to complement the ΔoxyR mutation at both concentrations (no clearing zone), while the mutated protein H40A AnnAt1-His(6) complemented the ΔoxyR mutant only at the lower concentration (Table I). For the higher H2O2 concentrations, the cleared zone was visible for both MG1655 and ΔoxyR, but the latter always had a larger diameter in comparison with the control strain. However, neither AnnAt1 nor H40A AnnAt1 was able to complement the ΔoxyR mutant at the higher H2O2 concentrations. Similarly, neither AnnAt1 nor mutated H40A AnnAt1 influenced the response of isogenic strain MG1655 to H2O2 at any of the concentrations tested.
Table I.
AnnAt1 partially restores the growth of the E. coli oxidative stress mutant ΔoxyR in the presence of H2O2
These data represent one of two independent experiments. The experiments were performed as described in “Materials and Methods” in three replicates. Two isogenic strains, MG1655 (wild-type E. coli K-12) and ΔoxyR deletion mutant with the OxyR ORF replaced with the kanamycin cassette, were used. Bacteria were transformed with expression plasmid carrying the wild-type AnnAt1 and its mutated form with His-40 replaced with Ala (H40A AnnAt1). After solidification of bacteria suspension in top agar, 5 μL of H2O2 was applied. The diameter of the growth inhibition zone was determined after 18 h of incubation in 37°C.
| H2O2 Concentration | Diameter of Growth Inhibition Zonea
|
|||||
|---|---|---|---|---|---|---|
| Wild Type | Wild-Type AnnAt1 | Wild-Type H40A AnnAt1 | ΔoxyR | ΔoxyR AnnAt1 | ΔoxyR H40A AnnAt1 | |
| mm | mm | |||||
| 6.25 | 0 | 0 | 0 | 8.51 ± 4.93 | 0 | 0 |
| 12.50 | 0 | 0 | 0 | 11.22 ± 0.38 | 0 | 10.17 ± 1.32 |
| 25.00 | 4.95 ± 2.58 | 5.18 ± 0.34 | 8.34 ± 4.17 | 14.3 ± 1.82 | 13.42 ± 1.46 | 14.71 ± 1.18 |
| 50.00 | 8.38 ± 1.22 | 7.98 ± 2.5 | 9.21 ± 5.75 | 18.73 ± 0.43 | 18.36 ± 1.38 | 17.6 ± 1.15 |
The diameter of the growth inhibition zone is the mean of three replica plates. Values ± sd are shown.
In Vitro Determination of the Redox Status of the Intramolecular Disulfide Bonds of AnnAt1
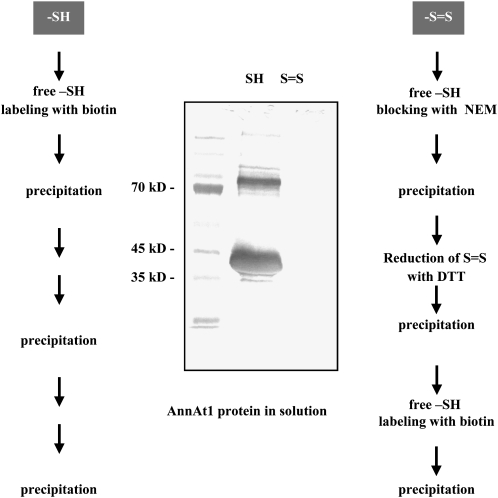
To study the biological redox status of Cys residues of AnnAt1 in vitro, we exploited the ability of biotin to label free sulfhydryl (SH) groups in proteins under special conditions, as described in “Materials and Methods.” We found that both monomer and dimer bands of AnnAt1 were labeled with 3-N-maleimido-propionyl biocytin (MPB) before chemical reduction (Fig. 4). This result provides evidence that both AnnAt1 Cys residues remain reduced (i.e. do not form an intramolecular disulfide bond) under normal conditions. Moreover, the presence of free SH groups in dimers strongly suggests that dimerization of AnnAt1 is not achieved via intermolecular disulfide bond formation. Thus, under biological conditions, they remain free either for posttranslational modifications or oxidation leading to disulfide bond formation.
Figure 4.
Determination of the in vitro redox status of Cys groups in AnnAt1-His(6) protein. A schematic diagram showing the step-by-step procedure for this experiment is shown. Ten micrograms of AnnAt1-His(6) protein was analyzed. N-ethylmaleimide (NEM) was used to block free SH groups. Dithiothreitol (DTT) was the chemical reducing agent used. Protein SHs were labeled with MPB. After electrophoresis and blotting, one of the replicate filters was probed with avidin conjugated with horseradish peroxidase. To confirm equal loading, the second filter was immunoprobed with anti-annexin antibody (data not shown). This experiment was performed three times and gave comparable results.
Mechanisms of Annexin Oligomerization (Intermolecular Disulfide Bonds)
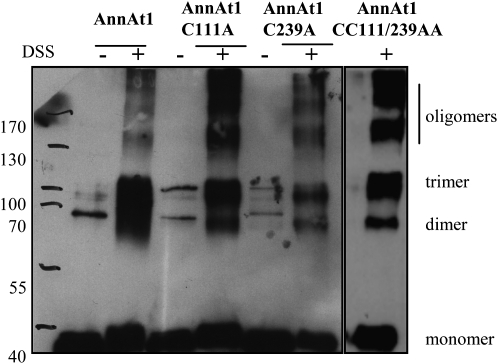
To test whether AnnAt1 oligomer formation could be mediated by the formation of intermolecular disulfide bonds, we generated mutated forms of AnnAt1 protein, in which one or both Cys residues were replaced with Ala. These amino acid substitutions did not modify protein migration through PAGE under nondenaturing conditions, which suggests that such a mutation did not have a significant impact on the protein tertiary structure. Then we compared the ability of wild-type and mutated AnnAt1 proteins to form oligomers. For this experiment, proteins were isolated from bacterial cultures and were never in contact with any reducing agent. As it can be seen in Figure 5, cross-linking with disuccinimidyl suberate (DSS) revealed the presence of exactly the same pattern of dimers, trimers, and higher oligomers in the case of all mutated forms and wild-type proteins. It was also true for double mutant A111C/A239C, which did not posses any Cys residue.
Figure 5.
The role of Cys residues in the formation of AnnAt1 oligomers. Ten micrograms of purified recombinant proteins, wild type or mutated AnnAt1-His(6) (with one or both Cys residues replaced by Ala), was subjected to chemical cross-linking with DSS. Control samples were subjected to the same procedure except that instead of incubation with DSS the equivalent amount of solvent, DMSO, was added. After electrophoresis and blotting, the AnnAt1 protein was visualized with the enhanced chemiluminescence system. This experiment was performed three times and gave comparable results.
Glutathionylation of AnnAt1 Protein and the Possible Biological Consequences of This Modification
In Vitro S-Glutathionylation and Its Consequences for Protein Function
As discussed above, AnnAt1 possesses two potentially active SH groups, and we wanted to test if these groups could undergo other oxidation-driven modifications. Specifically, we tested if the two Cys residues in AnnAt1 could undergo glutathionylation and, if so, what would be the biological consequences of this process.
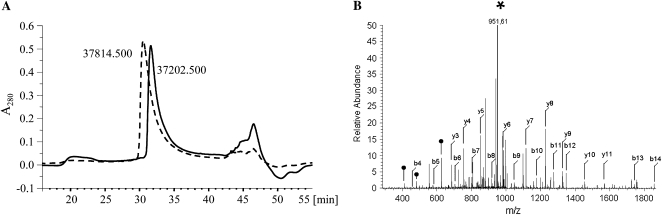
The reaction with oxidized glutathione increased the hydrophilicity of AnnAt1, which was manifested with shortening of its retention time on the C18 reverse-phase column (Fig. 6A). The molecular masses of collected samples determined by Micromass quadrupole time-of-flight mass spectrometer were 37,814 and 37,202 D for glutathionylated and nonmodified protein, respectively. This result suggests the incorporation of 2 mol of reduced glutathione (GSH) per mol of AnnAt1 (the molecular mass of AnnAt1 changes of about 612 D that are near the exact mass of two glutathione molecules), indicating that both Cys-111 and Cys-239 cross-react with oxidant. Depending on the time of AnnAt1 incubation with oxidized glutathione (GSSG), an additional peak was observed (data not shown), representing partially oxidized annexin protein with one glutathione substitution (Mr = 37,443). Molecular sequencing of this additional peak with liquid chromatography-tandem mass spectrometry (LC-MS/MS) revealed that it represents a mixture of AnnAt1 proteins, glutathionylated in Cys-111 or Cys-239. Additional fractions have also been observed with unknown compositions and dimerization products with Mr = 74,104 and 74,408 (data not shown).
Figure 6.
In vitro and in planta S-glutathionylation of AnnAt1 protein. A, HPLC C18 reverse-phase trace of 280 nm of nonmodified AnnAt1-His(6) protein (solid line) and AnnAt1-His(6) protein after incubation with oxidized glutathione (dashed line). The shift of the main peak protein testifies that incubation results in an increase in protein hydrophilicity. The molecular masses of collected samples determined by quadrupole time-of-flight mass spectrometer were 37,202 D for nonmodified protein and 37,814 D for glutathionylated protein, which suggests the incorporation of 2 mol of GSH per mol of AnnAt1. This experiment was performed three times and gave comparable results. B, Fragmentation spectrum of the Cys-111 glutathionylated peptide (WTSSNQVLMEVACTR), derived from AnnAt1 isolated from N. benthamiana. Major peaks on the spectrum can be annotated, accordingly, with the theoretical collision-induced dissociation fragmentation chart of the analyzed peptide (Supplemental Table S3), to b- and y-series daughter ions resulting from peptide fragmentation. Almost all theoretical daughter ions can be found on the spectrum, unequivocally confirming addition of the glutathione moiety. The most abundant peak on the spectrum (*) corresponds to cysteinylglycine-annexin-modified peptide. Apparently, the γ-peptide bond in the glutathione is the most labile in the whole structure and can be assumed as a neutral loss marker of glutathione-modified peptides. All major peaks on the spectrum are assigned to b- and y-series daughter ions. Peaks that originated from subsequent fragmentation of the glutathione moiety from the glutathione-y3 daughter ion are also indicated (•).
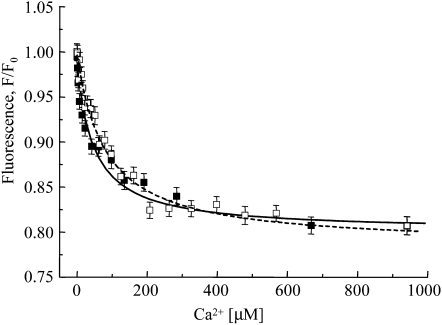
As a measure of the biological activity of AnnAt1 that might be changed by glutathionylation, we calculated the calcium-binding activity of glutathionylated AnnAt1, employing a fluorescent titration method that is based on the measurement of Trp fluorescence. This approach is especially well suited for work with annexins, which are known to undergo structural changes upon calcium binding and expose Trp residue/residues to the solvent. The calcium-binding affinity of glutathionylated and nonmodified proteins was analyzed. Additionally, nontreated protein was analyzed as a control. The results obtained for nontreated protein and HPLC-purified nonmodified proteins were similar, which provided evidence that the purification procedure did not affect the protein. The theoretical curve of Ca2+ titration of the protein fluorescence signal is given in Figure 7. The binding constants for one calcium-binding site was estimated at Ka = 2.0 ± 0.2 × 104 m−1. Thus, glutathionylation resulted in a decrease in the Ca2+ affinity of about 50%.
Figure 7.
S-Glutathionylation of AnnAt1-His(6) affects its Ca2+-binding affinity. Ca2+ titration of the relative fluorescence signal of AnnAt1-His(6) reduced (black squares, AnnAt1r, solid line) and its glutathione derivative (white squares, GS=AnnAt1, dashed line). Protein concentrations were 0.55 μm. Error bars represent sd. The calcium-binding constant Ka calculated for reduced protein was 2.0 ± 0.2 × 104 m−1. S-Glutathionylation results in a 50% decrease in the Ka, or 1.0 ± 0.2 × 104 m−1. This experiment was performed twice and gave comparable results.
In Vivo S-Glutathionylation of AnnAt1 Protein
In order to determine if AnnAt1 protein is subject to S-glutathionylation in vivo, AnnAt1 protein was isolated from leaves of ABA-treated plants (transient expression system) and purified with Strep-Tactin. Samples were frozen and kept at −80°C until analysis using standard proteomic protocols under nonreducing conditions was performed. As a control, a parallel sample subjected to chemical reduction with dithiothreitol and alkylation with iodoacetamide was analyzed. LC-MS/MS sequencing was performed for peptides obtained by tryptic digestion of AnnAt1 protein. The presence of AnnAt1 was confirmed in every sample, yielding on average 70% total protein coverage and very high protein MASCOT scores.
Peptides of interest might exist in the following forms: intact peptides without any Cys modification, peptides with carbamidomethyl Cys (resulting from alkylation), and peptides with glutathionylated Cys. Carbamidomethylated peptides were expected to be present in the control samples, while intact or glutathionylated peptides might be found in samples analyzed without a chemical reduction step. Intact (unmodified) Cys residues were not found. In chemically reduced samples, both Cys residues were converted to carbamidomethyls. In contrast, MASCOT searches performed on samples without reduction and alkylation revealed two species of glutathionylated peptides containing Cys-111 and Cys-239. Due to relatively low MASCOT scores, raw spectra were investigated. The molecular masses of identified parent ions that after deconvolution might be interpreted as peptides containing glutathionylated Cys residues equaled 2,028.86 and 2,457.17 D (for peptide with Cys-111 and Cys-239, respectively), which corresponds to the theoretical masses of each peptide with a glutathione molecule (Supplemental Table S1).
To unequivocally confirm the presence of such modification and eliminate the possibility of false-positive assignment, manual analysis of the spectra was carried out. Figure 6B shows an example of a fragmentation spectrum for the peptide WTSSNQVLMEVACTR containing Cys-111. All major peaks on the spectrum can be assigned to b- and y-series daughter ions, in accordance with the theoretical masses of daughter ions indicated in Supplemental Table S2. Fragmentation begins with the breakage of the γ-peptide bond of GSH, which appears to be the most labile linkage within the whole structure and thus can be used as a neutral loss marker of glutathione-modified peptides. Accordingly, the most abundant peak of the spectrum (doubly charged: 951.61 D) was identified as cysteinyl-Gly-modified annexin peptide. Moreover, other peaks resulting from further specific fragmentation of glutathione were also identified. Taken together, these observations provide evidence for a covalent bond between glutathione and the WTSSNQVLMEVACTR peptide via a free thiol group of Cys-111. The same analysis was performed for the second Cys-containing peptide from AnnAt1 protein (STIQCLTRPELYFVDVLR), and similar results were obtained (data not shown). In conclusion, we detected S-glutathionylated AnnAt1 protein in samples isolated from leaves 15 min (only Cys-111) and 30 min (both Cys-111 and Cys-239) after ABA treatment.
DISCUSSION
As sessile organisms, plants cannot avoid adverse environmental conditions (such as soil salinity, heavy metal contamination, and drought) that have a negative impact on their growth. Instead, they have evolved general strategies that enable them to grow and accomplish their life cycle in nonoptimal environments. Because the plasma membrane is a key interface between plant cells and their environment, membrane proteins are of special interest for characterizing plant cell stress responses. Due to their ability to bind calcium and negatively charged membrane phospholipids in a reversible manner, annexins could serve as a potential link between calcium signaling and membrane signaling during stress responses.
ROS are produced continuously during normal cellular metabolism. However, during the response to many stress conditions that disrupt cellular homeostasis, the production of ROS is significantly enhanced, exceeding the rate of their breakdown, due to the increased activity of specific enzymatic systems (e.g. NADPH oxidase) that catalyze such reactions (Keller et al., 1998; Desikan et al., 2001, 2004; Laloi et al., 2004; Foyer and Noctor, 2005; Torres and Dangl, 2005). Thus, although ROS is an important component of signaling during abiotic and biotic stress, the overproduction of ROS leads to oxidative damage to cells and cellular membranes. Results from a study on AnnBj1, the B. juncea homolog of AnnAt1, shows that ectopic expression of AnnBj1 in tobacco plants provides both abiotic and biotic stress tolerance and provides evidence that this close relative of AnnAt1 can regulate the level and the extent of ROS accumulation and lipid peroxidation during stress responses (Jami et al., 2008). In this study, we focused on characterizing this activity in AnnAt1 and determining the molecular basis of this activity. We also documented opposite drought-related phenotypes for loss-of-function and gain-of-function AnnAt1 mutants consistent with AnnAt1 function during drought stress in planta, and all of these analyses were performed in Arabidopsis.
Expression of AnnAt1 Is Up-Regulated by a Variety of Stress Treatments
As a first approach, we checked if AnnAt1 expression can be modified in response to a variety of different abiotic stresses. In contrast to previous reports (Kovacs et al., 1998; Cantero et al., 2006), we tested for expression changes in fully expanded leaves of 6- to 8-week-old plants. We found that AnnAt1 mRNA levels were significantly elevated after virtually all applied treatments, reaching the highest level and being the most sustained after treatment with ABA and during drought (Fig. 1).
The promoters of stress-inducible genes were shown to contain two major classes of cis-acting elements, ABRE (for ABA-responsive element) and DRE (for dehydration-responsive element; Yamaguchi-Shinozaki and Shinozaki, 1994, 2005), that function in ABA-dependent and ABA-independent gene expression, respectively. Analysis of the AnnAt1 promoter (performed with the software www.athamap.de; Galuschka et al., 2007) revealed the presence of a number of cis-regulatory elements (Supplemental Table S3). Among them, two of higher interest are a “classical” ABRE, PyACGT(tt)GG/TC (position approximately −156), and a putative dehydration-responsive element from the DRE family, TACCGACAT (position approximately −50). Further analysis of the AnnAt1 promoter with a newly developed quantitative computational approach (MotifFinder) revealed that its promoter structure is typical for ABA-regulated genes (i.e. it contains a series of modified ABREs, all with P values less than 10−5, indicating that they are significant for the ABA-regulated response; Suzuki et al., 2005). We found that other stresses, like heavy metal treatment or wounding, also led to AnnAt1 mRNA elevation. Taken together, we can conclude that AnnAt1 expression is up-regulated by a wide range of stresses and may function in stress-induced, ABA-dependent as well as ABA-independent pathways.
The Protective Role of AnnAt1 in Drought Stress
To verify if AnnAt1 is indeed relevant to resistance against stress, we generated Arabidopsis plants expressing different levels of this protein. For these purposes, independent stabilized lines overexpressing the AnnAt1 open reading frame (ORF) under the constitutive cauliflower mosaic virus 35S promoter (OE) were created and loss-of-function (ΔannAt1) Arabidopsis plants from seed collections were obtained (Supplemental Fig. S1). Since most of the treatments that result in AnnAt1 mRNA accumulation (e.g. chilling, heat shock, dehydration, and osmotic stress) lead to water deficit in cells, we decided to apply experimental drought treatments to mature plants with increased or decreased levels of endogenous AnnAt1 protein. We found that at the mature plant stage, the level of AnnAt1 positively correlates with a plant's resistance to water deficit. Furthermore, AnnAt1-overproducing plants were capable of returning to full health after drying up almost completely and were then still able to flower and produce viable seeds after such severe drought conditions. We did not observe significant differences in physiological parameters (quantum yield of PSII, leaf temperature) between Arabidopsis plants with manipulated AnnAt1 levels and Col-0 wild-type plants under control conditions or during drought stress (Supplemental Table S4). Although Jami et al. (2008) showed that ectopic expression of AnnBj1 from B. juncea in tobacco leads to abiotic stress tolerance at the seedling stage, our results showing both increased drought sensitivity in loss-of function mutants and drought tolerance in gain-of-function mutants in Arabidopsis provide direct evidence that AnnAt1 from Arabidopsis plays a role in the plant cell's adaptive responses to stress conditions.
To our knowledge, our results are the first to document the positive effect of annexin overproduction on the stress response of mature plants. The fact that AnnAt1-overproducing plants retain growth and productivity potential in the conditions of severe drought is of particular importance. Furthermore, in our experiments, we either completely withheld water or drastically reduced watering over a prolonged time, which is similar to the gradual real-life drought stress that crop plants encounter in nature. Contemporary agriculture suffers from soil salinity, contamination with heavy metals, and climate instability that results in water inaccessibility. Severe drought stress results in significant reduction of assimilation per leaf area that leads to diminished harvested biomass. When the drought period occurs during a particular phase of development, it can cause a complete reduction of the yield due to inhibition of pollination and fruit setting (Saini, 1997). Thus, the discovery of genetic features to transgenically confer drought tolerance on agriculturally valuable crops is a key goal of plant science today. In this regard, annexins seem to be especially promising candidates, since they exert protective effects on vegetative tissues and also support flowering and fruit setting; thus, they could be applied to both potatoes (Solanum tuberosum) and kernel crops, like maize or grains.
There are numerous mechanisms by which AnnAt1 might provide protection against oxidative stress, and the mechanisms for AnnAt1-mediated protection may be different in different tissues. In vegetative tissues, one possibility is that AnnAt1 might protect membranes from irreversible damage resulting from lipolysis and oxidation of lipids as well as membrane proteins (Jami et al., 2008). In reproductive tissue, the presence of annexins in the rapidly growing tip region of pollen tubes (Blackbourn et al., 1992) and at sites of intensive cell wall synthesis, like the embryo sac (Okamoto et al., 2004), is in line with their postulated role in Ca2+-mediated exocytosis (Thiel et al., 1994; Carroll et al., 1998; for review, see Konopka-Postupolska, 2007). Thus, it cannot be excluded that overexpressed AnnAt1 protein could support microgametogenesis and/or macrogametogenesis under stress conditions.
AnnAt1 Is a Unique Member of the Arabidopsis Annexin Gene Family
Plant annexins are a bona fide multigene family, and several members appear to be expressed at the same time and in the same place. There are some indications that in a single cell, different annexins can act at the same time, participating in a synergistic way to support a single cellular process, although their mode of action is slightly different. The most detailed and compelling example of such cooperation between annexins 1, 2, and 6 emerged from analysis of annexin function in mammalian smooth muscle cells (Babiychuk and Draeger, 2000; Draeger et al., 2005). However, it is not clear if a similar step-by-step mechanism also operates in plant cells or other less specialized animal cell types.
The first evidence that a cooperation scenario could also be operating in plants comes from the results of Lee et al. (2004), who suggested that AnnAt1 and AnnAt4 play distinct although complementary roles in the same stress-induced pathways. Another line of evidence was presented by Cantero et al. (2006), who found that mRNA levels of particular annexins were cooperatively but distinctly regulated after exposure of 7-d-old seedlings to salt, cold, and drought. For example, after seedling dehydration, transcript levels of AnnAt1, AnnAt3, AnnAt6, and AnnAt8 were all up-regulated at least 13-fold; in contrast, AnnAt4 was induced exclusively by salt stress. Our results provide direct evidence that, despite some redundancy within the family, the function of different annexins could be unique. Structural differences among the family members, of course, would contribute to functional specialization. Analysis of primary structure reveals that AnnAt1 has a unique structural property (e.g. has very low pI [approximately 5]).
Another important factor that can explain the lack of complementation in a multigene family is the potential for spatial and temporal separation of isoforms. The question of spatial distribution of annexins in plant tissues remains open, since the data are rather limited; nevertheless, there are some indications that the two most homologous Arabidopsis annexins, AnnAt1 and AnnAt2, are differentially expressed in a temporal and spatial manner (Clark et al., 2001).
ROS-Modulating Activity of AnnAt1 and Its Ability to Protect against Oxidative Stress
Stresses are known to induce an oxidative burst in cells and result in the production of ROS. There are several pathways, both constitutive and induced, that can lead to the accumulation of ROS, such as photosynthesis, photorespiration, and mitochondrial respiration or NAD(P)H oxidases, amine oxidases, and cell wall-bound peroxidases. The enhanced production of ROS can pose a threat to cells, but it is also thought that ROS act as secondary messengers involved in stress-response signaling (Mittler, 2002; Mittler et al., 2004). Regulation of ROS accumulation via calcium signaling has been well documented (Price et al., 1994; Keller et al., 1998; Torres et al., 1998; Pei et al., 2000), and our results suggest that AnnAt1 is able to regulate the ROS levels under normal conditions in nontreated cells and to influence the magnitude of the oxidative burst after ABA induction. However, the molecular mechanism of this regulation remains to be established. Such an effect could be attributed either to the direct ROS-neutralizing enzymatic activity of AnnAt1 protein (catalase, peroxidase, or dismutase) or its ability to modulate endogenous ROS-producing and ROS-catalyzing systems.
Previous reports dealing with this problem provide only partial clarifications because they were based either on nonquantitative approaches (Gidrol et al., 1996; Janicke et al., 1998; Kush and Sabapathy, 2001) or on in vitro studies (Gorecka et al., 2005). AnnAt1 complemented the lack of OxyR transcription factor and restored the growth of mutant E. coli in the presence of high concentrations of H2O2 in the medium (Gidrol et al., 1996). Since there is no similarity in the primary structure of AnnAt1 and OxyR, it was highly unlikely that this effect could be achieved via functional complementation of transcription factor action. Therefore, it was hypothesized that AnnAt1 can function as one of the downstream elements that are under the control of OxyR (i.e. as a factor catalyzing ROS neutralization). In support of this hypothesis, a putative catalase motif with a conserved His (His-40) responsible for heme coordination was identified in the N-terminal region of the AnnAt1 molecule, and partially purified AnnAt1 displayed low levels of peroxidase activity in vitro (Gidrol et al., 1996).
More recently, Gorecka et al. (2005) reported that recombinant AnnAt1 protein isolated from bacterial or plant sources indeed possessed inherent peroxidase activity and that mutation of the critical His into Ala (H40A) abolished this activity. However, the reported activity of wild-type AnnAt1 protein was extremely low (106-fold lower then horseradish peroxidase), and the level of activity differed significantly depending on the source of protein (4.88 ± 1.1 a.u. and 1.69 ± 0.25 a.u. for AnnAt1 recombinant protein isolated from plant versus bacterial expression systems, respectively). In contrast to the results of Gorecka et al. (2005), we found that the mutated AnnAt1 protein (H40A AnnAt1) purified from the plant expression system in N. benthamiana does retain a comparable level of in vitro peroxidase activity (Fig. 3B). Thus, our results suggest that the low level of in vitro peroxidase activity of AnnAt1 is not dependent on this conserved His residue.
These results prompted us to check if mutated AnnAt1 protein could also complement the ΔoxyR mutation in E. coli. In our experiments, log-phase bacteria were grown within the top agar to ensure them stable conditions with limited oxygen availability. In such conditions, AnnAt1 restored the growth of ΔoxyR at concentrations of H2O2 up to 12.5 mm, but we were not able to confirm the complementation of the oxyR mutation by AnnAt1 in the range of H2O2 concentrations that was reported by Gidrol et al. (1996). One explanation for this inconsistency between our results and those obtained by Gidrol et al. (1996) may be the differences between the bacterial strains employed in the studies. The ΔoxyR mutation used in the previous experiments was on a different genetic background, RK4936 (13 mutations; E. coli resources at Yale [http://cgsc2.biology.yale.edu]), which is much more modified in comparison with the bona fide wide-type strain MG1655 that was used in our experiments. Taken together, these data indicate that the ability of AnnAt1 to increase the survivability of mutant bacteria in enhanced H2O2 concentrations depends on the experimental conditions (i.e. the phases of culture growth and medium in which challenge was performed). H40A AnnAt1 provided protection against 6.5 mm but not 12.5 mm H2O2, even though it exhibited peroxidase activity in in vitro experiments. This result raised the question of whether the H40A mutated protein is able to retain the wild-type physiological secondary structure.
When we checked the electrophoretic mobility of native mutated protein on a nondenaturing gel, we found that it differs significantly from the nonmutated form (data not shown). We also found that the circular dichroism spectrum of mutated protein differs from that of the wild type, indicating an increased content of β-sheet (Supplemental Fig. S3). According to the crystal structure of AnnAt1, obtained with Geno3D, His-40 is situated in a nonhelical linker between the third and fourth helices of the first endonexin repeat, and in this region the N-terminal linker could be attached to the globular head of the molecule. This suggests that the replacement of His by Ala modifies the secondary structure of annexin and that a lack of protection results from the disturbance of overall structure.
A similar observation was reported by Hofmann et al. (2000) for p34 annexin from Capsicum annuum. On the basis of the crystal structure of p34, they reported that the N-terminal domain is attached to the core via H bonding between His-48 and backbone carbonyl CO-13. They also showed that the artificial elongation of the N-terminal tail makes the fusion protein more stable than the wild type. In summary, the replacement of a positively charged, large His by a small, hydrophobic Ala seems to impair the protein function by altering the overall structure of the protein but has only a minor effect on specific activity. The evolutionary conservation of this His between plant annexins (Hofmann et al., 2000; Konopka-Postupolska, 2007) confirms that this amino acid is important for the structure and/or function of these proteins. The His residue is the amino acid that has a side chain that can be ionized, resulting in a charge change within the physiological range of pH. Thus, it can function in metal ligand coordination (e.g. in membrane metal transporters), as “charge-relay” systems in the enzyme catalytic center, and as a proton sensor (Wiebe et al., 2001). Interestingly, AnnAt1 was suggested to function as a proton sensor in plant cells (Gorecka et al., 2007).
Analyses of S3 Cluster Participation in AnnAt1 Oligomerization
As discussed earlier, AnnAt1 has inherent peroxidase activity, albeit at a very low level. This low peroxidase activity may actually be indicative of ROS-mediated effects on AnnAt1 function, possibly through the regulation of AnnAt1 oligomerization. In fact, AnnAt1 is able to form oligomers, and the oligomerization state of AnnAt1 is affected by changes in redox state (Gorecka et al., 2005). The function of plant annexin oligomerization remains unclear; however, Hofmann et al. (2003) proposed that it can constitute a structural basis of an oxidative stress response. In order to better characterize the ROS-neutralizing activity of AnnAt1, we performed an analysis of the biological redox status of the AnnAt1 Cys residues in vitro. Cys is known to be one of the most reactive amino acid residues and can be subjected to oxidation and the formation of disulfide bonds within the molecule or between different molecules. It was previously reported that the S3 cluster (Cys-Met-Cys), originally identified by Hofmann (2003) as a conserved motif in cotton annexin Gh1, is also present in AnnAt1 from Arabidopsis (Cys-111 and Cys-239). Analysis of the AnnGh1 crystal structure revealed that although both Cys residues are positioned in the tertiary structure in close proximity, such that the formation of disulfide bonds is sterically possible, both side chains exist in the reduced form. In contrast, the complementary Cys-132 and Cys-261 from the animal annexin A2 monomer form an intramolecular disulfide bond, as indicated by crystallographic data (Burger et al., 1996). Thus, we examined the potential role of the S3 cluster in AnnAt1 oligomerization.
In our experiments, we were able to confirm that the two Cys residues do not form disulfide bonds via intramolecular or intermolecular bonds or get oxidized to sulfenic acid in vitro. Thus, in contrast to animal annexin A2, our results provide evidence that redox-driven oligomerization of AnnAt1 is achieved not via covalent S-S bonding but occurs via a different type of interaction. We hypothesize that oligomerization could be mediated by electrostatic interactions. Although the Cys residues in AnnAt1 appear to be hidden within the structure and thus seem to be unavailable for such modifications, another possible role for the Cys residues in the S3 cluster found in certain annexins is to serve as target sites for modification by ROS. Although the full S3 cluster is not a conserved feature of most plant annexins because the Met residue has often diverged, the two Cys residues in the S3 cluster appear to be highly conserved. All eight Arabidopsis annexins have these two Cys residues, and homology modeling shows that these two residues are among the most highly conserved in plant annexins. Thus, our findings on AnnAt1 Cys residues may have implications for plant annexin function in general.
Analyses of Posttranslational Modifications to Arabidopsis AnnAt1 by ROS
Summarizing, the observed protective effect of AnnAt1 during oxidative stress crosses evolutionary boundaries (i.e. it can be observed both in eukaryotic and prokaryotic cells). Direct H2O2-neutralizing activity of AnnAt1 is so weak that it would need to be activated by a posttranslational modification or a binding partner to be relevant at the physiological level. An alternative hypothesis is that the AnnAt1-driven protective effect results from Ca2+-dependent modulation of endogenous ROS-producing and/or ROS-neutralizing systems, such as the glutathione redox couple (GSH/GSSG) that has been shown to function as a key player in the maintenance of cellular redox homeostasis in bacteria as well as in animal and plant cells (Dixon et al., 2005).
The generation of ROS leads to redox regulation of signaling proteins by posttranslational modification via oxidation of certain amino acids. Included among the oxidative changes induced by ROS are cysteinyl modifications such as protein S-glutathionylation and S-nitrosylation (O'Brian and Chu, 2005). These modifications, like protein phosphorylation, can alter the activity of targeted proteins in a fast and reversible manner (Anselmo and Cobb, 2004; Lindermayr et al., 2005). In plants, S-glutathionylation and S-nitrosylation are induced by abiotic stress through the mediation of ROS (Gould et al., 2003; Dixon et al., 2005). A recent proteomic study in Arabidopsis has identified AnnAt1 as an S-nitrosylated protein, indicating that the Cys residues in AnnAt1 are reactive and susceptible to biological oxidation (Lindermayr et al., 2005). Our in vitro data showing that Arabidopsis AnnAt1 can be S-glutathionylated on two Cys residues provides important additional data showing that these residues are reactive. The reactivity of these Cys residues may be an indication that AnnAt1 is one of the proteins in plant cells involved in H2O2 perception (Hancock et al., 2006). Importantly, we found that the Cys residues in AnnAt1 are S-glutathionylated in vivo in response to ABA treatment, which provides evidence that this posttranslational modification of AnnAt1 is physiologically relevant during stress responses.
A mixed disulfide bridge between the main cellular antioxidant, glutathione, and different proteins (so called S-glutathionylation) is a ubiquitous and reversible protein modification. S-Glutathionylation was previously regarded as a simple mechanism for protecting proteins against oxidative damage, but more recently, S-glutathionylation has been recognized as a widespread posttranslational modification that is part of the adaptive stress responses of cells to a changing environment and can act as a regulator of signal transduction cascades (Fratelli et al., 2004). Thus, S-glutathionylation can support dual functions: protecting proteins from irreversible oxidation and participation in redox signaling. Glutathionylation has been shown to both inhibit protein function (e.g. enolase or 6-phosphogluconolactase) and activate protein function (e.g. apoptosis signal-regulated kinase 1; Nadeau et al., 2007). It was also able to regulate the overall cell response (i.e. cytoskeleton rearrangement) to external stimulation, as was shown for NIH 3T3 cells (Wang et al., 2003). In animal cells, oxidative stress inhibits MEKK1 activity by inducing site-specific glutathionylation within the ATP-binding domain of this protein kinase (Cross and Templeton, 2004). Interestingly, animal annexin A2 was found to be regulated by reversible binding with glutathione on Cys-8 and Cys-132, and this modification decreases its ability to interact with liposomes and F-actin (Caplan et al., 2004). Thus, similar to phosphorylation and other posttranslational modifications, glutathionylation can result in changes in protein conformation and activity (Filomeni et al., 2005).
ROS are universal signals in eukaryotic cells, and it appears that ROS signaling is intimately linked with calcium signals. This interaction between ROS and Ca2+ allows for cross talk within and between signal transduction pathways (Mori and Schroeder, 2004). For example, glutathionylation regulates the activity of a number of Ca2+-binding proteins in animal cells, including S100 and the ryanodine Ca2+ channel (Aracena et al., 2003; Goch et al., 2005). In plant cells, glutathione is an important component of the cellular redox system, which induces Ca2+ influx and regulates gene expression changes (Gomez et al., 2004; Meyer, 2008), and plays a critical role during stress responses (Ogawa, 2005). Thus, we tested the effects of glutathionylation on Ca2+ binding of AnnAt1 and found that this modification resulted in a 50% decrease in the Ca2+ affinity of AnnAt1. Although this change is modest, it is likely to be physiologically relevant, since AnnAt1 binds to membranes in a Ca2+-dependent manner and may have altered activities in the presence of calcium. Because ROS can alter its Ca2+ affinity, and thus its Ca2+-dependent functions, AnnAt1 would be an excellent candidate to mediate cross talk between Ca2+ and ROS during plant abiotic stress responses.
CONCLUSION
Our study has identified AnnAt1 as an important component of ABA-induced responses of plants to environmental stresses. Functional analyses performed with plants differing in their endogenous level of AnnAt1 protein revealed that overexpression has a protective effect on plant survivability under drought conditions, while lack of expression increases stress sensitivity. Our results also suggest that these stress phenotypes may be due to the ability of AnnAt1 to modulate the time course and/or the extent of oxidative stress. The precise mechanism of this process is still not clear, although we showed that AnnAt1 is a subject of oxidation-driven posttranslational regulation (S-glutathionylation) that can influence its “canonical” function. Thus, it is tempting to speculate that AnnAt1 can modulate the oxidative burst by buffering the endogenous systems that protect against oxidative damage. This hypothesis seems to be even more plausible, because the protective effect of AnnAt1 expression can cross evolutionary boundaries and is also observable in prokaryotic cells. Additional studies will be required in order to gain a more detailed understanding of AnnAt1 function during stress responses.
MATERIALS AND METHODS
Plants with Different Levels of AnnAt1
Functional loss-of-function plants (T-DNA insertional lines) were obtained form the Arabidopsis Seeds Banks (http://signal.salk.edu). The gain-of-functions mutants were obtained by introducing to Arabidopsis (Arabidopsis thaliana) Col-0 the additional copy/copies of AnnAt1 full coding sequence under the 35S cauliflower mosaic virus promoter (35S∷AnnAt1) by agrotransformation. Details of the procedures are described in the Supplemental Materials and Methods S1.
Plant Treatments
Arabidopsis seeds of Col-0, knockout (ΔannAt1), and overexpressor (OE) plants were surface sterilized by incubation with (1) 70% (v/v) ethanol for 2 min and (2) 7% (v/v) HOCl for 10 min in an Eppendorf tube and subsequently washed six times with 1 mL of sterile, deionized water. After 2 d of synchronization at 4°C, seeds were plated onto 1× Murashige and Skoog medium with 1% (w/v) Suc with or without 50 μg mL−1 kanamycin (OE or Col-0 and ΔannAt1, respectively). Seeds were maintained in the growth chamber (Percival AR-66 L; light intensity of 275 μmol m−2 s−1, humidity of approximately 80%, temperature of 20°C) under short-day conditions (8 h of light/16 h of darkness) for 1 week before transferring the seedlings to soil in separate pots (except where different treatment is indicated). For collecting seeds, after 4 weeks in short days, plants were transferred into long-day conditions (18 h of light/6 h of darkness) to induce flowering.
Collecting of Plant Material for AnnAt1 mRNA Analysis in Different Abiotic Stresses for Northern Blotting
For northern-blot analysis, two plants per time point were used. Fully expanded leaves from 6-week-old plants were detached and laid onto the surface of the following solutions: water (mock treatment), 100 μm ABA, freshly prepared 10 mm H2O2, 1 mm salicylic acid, 150 mm NaCl, and 100 mm CdCl2. For wounding, before detaching and floating on water, leaves were squeezed five times with grooved forceps. Incubation was performed in the growth chambers under the conditions noted above for 24 and 48 h. For drought stress treatment, fully expanded leaves from two 8-week-old plants grown under standard short-day conditions were detached and laid in petri dishes. To avoid rapid dehydration, the dishes were transferred into a plastic container with cover and incubated on the bench. To estimate the water loss, the leaves were weighed immediately after detaching and at indicated time points.
Abiotic Stress Tolerance: Whole Plant Assays (Dehydration Tolerance, Regeneration Ability, Measurement of Chlorophyll Fluorescence, and Stomata Conductivity)
For experiment 1, 25 plants, representing different lines (Col-0, OE line 5, ΔannAt1 line 1), were grown in soil in one pot for 4 weeks in standard short-day conditions. Afterward, watering was withdrawn and the photograph was taken after 7 d of drought. This experiment was designed to ensure a wide representation of plants of a given phenotype.
For experiment 2, five plants representing different lines (Col-0, OE line 5, ΔannAt1 line 3) were grown in soil in separate pots for 4 weeks in standard short-day conditions. For the next 3 weeks, plants were watered once per week with 5 mL per pot and then watering was restored.
Gene Expression Analysis
Northern Hybridization
Leaves were collected at indicated time points and tissue was immediately frozen. Total RNA was extracted from the frozen leaves with the Trizol method according to the manufacturer's protocol (www.invitrogen.com). For northern blots, 20 μg of total glyoxylated RNA was separated on a 1% (w/v) agarose gel in 15 mm sodium phosphate, pH 6.5, and transferred to Hybond N (Amersham) filters. Hybridization was performed according to Church and Gilbert (1984) with two randomly radiolabeled probes: (1) 3# region (764–1,116 nucleotides) of AnnAt1 (At1g35720), and (2) 3# region (1,062–1,283 nucleotides) of actin 2 (At3g18780). After hybridization, the membrane was washed twice in 2× SSC, 0.1% (w/v) SDS at room temperature, followed by 30 min in 50°C. The membrane was exposed at −70°C to x-ray film for 24 or 48 h.
Detection of ROS and Confocal Microscopy
To qualitatively and quantitatively assess ROS generation, fluorescence microscopy was performed. Epidermal peels were removed from the abaxial surface of 4-week-old fully expanded leaves of Col-0 and AnnAt1 transgenic plants according to the method of Murata et al. (2001). The peels were incubated in 30 mm KCl and 10 mm MES-KOH, pH 7.0, for 1 h under continuous light of 300 μmol m−2 s−1 before treating with 50 μm H2DCFDA (Invitrogen/Molecular Probes) for another 20 min in the dark. H2DCFDA is a cell-permeable indicator for ROS. It is nonfluorescent until removal of the acetate groups by intracellular esterases, followed by oxidation that occurs within the cell. The excess dye was removed by three washes with the same fresh buffer and then exposed to 10 μm ABA (Sigma-Aldrich) for 30 min in the dark. Epidermal peels incubated in a buffer without ABA served as control samples. The fluorescence was visualized by the Leica Laser Scanning Confocal Microscope (Leica; SP2 AOBS) at an excitation of 488 nm and emission wavelength of 530 nm. For quantification of fluorescence, a uniform area from each image of a guard cell was selected, and NIH ImageJ was used to analyze the mean pigmentation value for that area (across all pixels selected).
Cloning and Purification of Wild-Type and Point-Mutated Versions of Annexin AnnAt1 and in Vitro Protein Analysis
In order to obtain full-length cDNA coding for AnnAt1 (At1g35720), RT-PCR was performed. Total RNA used as a template was isolated from fully expanded leaves of 8-week-old Arabidopsis plants with Trizol reagent. For expression in Escherichia coli, the full coding sequence of AnnAt1 was subcloned into pET28a+-expressing vector between NcoI and XhoI sites using AnnAt1 pET28a+ primers (for primer sequences, see Supplemental Table S5). To obtain mutated versions of the protein, namely H40A, C111A, C239A, and CC111/239AA, site-directed mutagenesis was performed. Wild-type and mutated AnnAt1-His(6) proteins were expressed in E. coli strain BL21 after induction with 0.25 mm isopropyl-β-d-thiogalactopyranoside for 24 h at 18°C. The whole purification procedure was performed under nondenaturing conditions according to the manufacturer's protocol (QIA Expressionist), and proteins were finally purified to homogeneity on a chromatography nickel-nitrilotriacetic acid agarose (Qiagen) minicolumn. The purity of obtained proteins was verified by SDS-PAGE followed by silver staining; their identities and the presence of mutations were confirmed with MS/MS in Mass Spectrometry Lab (Institute of Biochemistry and Biophysics PAS).
Complementation of the ΔoxyR Mutation in E. coli by AnnAt1
MG1655 [F−, Δ(araD-araB)567, ΔlacZ4787(∷rrnB-3), LAM−, rph-1, Δ(rhaD-rhaB)568, hsdR514] and isogenic ΔoxyR strains were kindly provided by Dr. G. Storz. ΔoxyR is a single-gene deletion (the OxyR ORF was replaced with a kanamycin cassette; oxyR749∷kan) mutant derived from MG1655 and is a part of the Keio collection of nonessential gene deletions (Baba et al., 2006). Mutant ΔoxyR, lacking a functional gene, is more sensitive to the oxidative stress compared with the isogenic strain.
Full cDNA of wild-type and H40A AnnAt1 (with His-40 replaced with Ala) plants was subcloned into pQE80L in an orientation resulting in N-His(6)-tagged protein. In this system, expression of the introduced gene is driven by an inducible coliphage T5 lac promoter. After verification of the cDNA correctness, the vector was introduced into MG1655 and ΔoxyR. The ability of AnnAt1 to restore the E. coli growth in the presence of elevated levels of H2O2 was investigated with a modified killing zone method.
The E. coli culture was suspended in Luria-Bertani top agar (0.15%, w/v) and overlaid on the Luria-Bertani plates supplemented with an appropriate antibiotic. After solidification, 5 μL of freshly prepared H2O2 was spotted directly onto the agar surface. Plates were incubated at 37°C for 18 h, and then the diameters of the growth inhibition zones were measured.
In Vitro Peroxidase Activity Assay
For obtaining of AnnAt1-His(6) and its mutated version A40H AnnAt1-His(6) in Nicotiana benthamiana leaves, a deconstructed virus strategy was employed (Gleba et al., 2004, Marillonnet et al., 2004). The full coding sequence of both proteins was cloned into the pICHI1990 vector. Plants were infected with a mixture of three Agrobacterium tumefaciens GV3303 strains carrying different parts of the expression system. Two weeks after infection, the produced protein was purified from leaves according to the Qiagen protocol under the native conditions. For in vitro peroxidase activity, approximately 10 μg of AnnAt1-His(6), 10 μg of A40H AnnAt1-His(6), 10 μg of bovine serum albumin (Sigma-Aldrich), and 10 ng of horseradish peroxidase (Sigma-Aldrich) were dot blotted onto a nitrocellulose membrane prewetted on a sheet of Whatman 3MM in 1× Tris-buffered saline (TBS; 20 mm Tris-HCl, pH 7.5, and 150 mm NaCl). The nitrocellulose membrane containing the completely absorbed protein was blocked for 1 h in 5% (w/v) skim milk in 1× TBS at room temperature. The membrane was washed for 15 min, followed by two changes for 5 min each in 1× TBS with gentle shaking. The membrane was incubated in SuperSignal West Pico Chemiluminescent Substrate (Pierce) without any antibodies and exposed to Hyperfilm-High Performance Chemiluminescence Film (Amersham).
In Vitro Glutathionylation of AnnAt1: Isolation and Characterization of Glutathionylated Protein
A 300-μL sample of 10 to 20 μm purified recombinant protein AnnAt1-His(6) was added to 300 μL of 6 m guanidium chloride (Fluka) and incubated with a 100-fold excess of GSSG (Sigma-Aldrich), pH 8.5, for 30 min at room temperature. Afterward, the protein was diluted 3-fold and purified by HPLC (Breeze System; Waters) with the reverse-phase analytical column C18 (ACE 5 C18-300). Purification was performed with a two-phase gradient; at the beginning, the guanidium chloride was rinsed out with buffer A (MilliQ water and 0.1% [v/v] trifluoroacetic acid) with a flow of 0.2 mL min−1, and then the modified forms of proteins were separated in increasing concentrations of buffer B (10% MilliQ water, 90% [v/v] acetonitrile, and 0.1% trifluoroacetic acid) of 0.5% min−1 and a flow of 1 mL min−1. The presence of the proteins in the outflow was monitored with a 220-nm UV absorbance monitor. The peaks, representing the protein fraction, were collected and subjected to further analysis. This experiment was performed three times with some modification to obtain better separation and gave comparable results.
The molecular masses of the intact proteins present in the collected samples were identified by electrospray ionization-MS using a Micromass Q-Tof Premier mass spectrometer in Mass Spectrometry Lab (IBB PAS). Two main peaks represent proteins with molecular masses of 37,814 and 37,202 D and correspond to proteins with two Cys residues substituted with glutathione molecules and nonmodified protein, respectively. The theoretical mass of AnnAt1-His(6) protein, calculated on the basis of primary sequence, is 37,026 D.
Measurement of Calcium Affinity with Fluorescence Titration
The measurement of calcium affinity was performed for the protein sample dissolved in 50 mm Tris buffer, pH 7.4. The fluorescence signal of AnnAt1-His(6) (λexc = 298 nm) decreases in the presence of Ca2+ ions. Its titration curve can be described according to Eftink (1997) using a simple model assuming that protein binds only one calcium ion. The theoretical curve of Ca2+ titration of the protein fluorescence signal, calculated with the best-fit parameters K (calcium-binding constant) and fB (relative change in fluorescence signal after binding the Ca2+ ion) was found by the NiceFit program.
Analysis of S-Glutathionylation of AnnAt1 Protein in Planta
For this experiment, the full coding sequence of AnnAt1 protein was tagged with Strep (WSHPQFEK) and His(6) tags and cloned into the binary vector pROK2. Transient expression in N. benthamiana was performed as described previously (Romeis et al., 2001). At 48 h after plasmid inoculation, leaves were either harvested (control) or infiltrated with 100 μm ABA and collected at the indicated time points (15, 30, 45, and 60 min). Samples were immediately frozen in liquid nitrogen and stored at −80°C until isolation.
Purification of AnnAt1 protein was performed via the StrepII tag according to the manufacturer's protocol (IBA). Leaves were ground in liquid nitrogen and thawed in 10 mL of Ex-strep buffer with 0.5% Triton X-100. After centrifugation, the supernatant was transferred into a new tube and incubated with 1 mL of Strep-Tactin Macroprep (IBA). After incubation, the resin was washed with 5 mL of W buffer (without Triton X-100), and protein was eluted by washing six times with 0.5 mL of 2.5 mm desthiobiotin.
LC-MS/MS Analysis of S-Glutathionylated Protein
The peptide mixture obtained from a standard tryptic digestion procedure (without reduction and alkylation) was applied to an RP-18 precolumn (Waters NanoAcquity, 20 mm × 180 mm) using water containing 0.1% trifluoroacetic acid as a mobile phase and then transferred to a HPLC RP-18 column (Waters NanoAcquity UPLC Column, 250 mm × 75 mm) using an acetonitrile gradient (0%–50% in 30 min) in the presence of 0.1% formic acid at a flow rate of 250 nL min−1. The column outlet was directly coupled to the ion source of the Ion Cyclotron Resonance spectrometer (Thermo Finnigan linear ion trap Fourier transform ion cyclotron resonance; Thermo Electron), working in the regime of data-dependent MS to MS/MS switch. The resulting mass spectra were used to search the nonredundant protein database of the National Center for Biotechnology Information (NCBInr version 20080624) using the MASCOT (matrixscience.com) search engine (eight-processor on-site license). Standard search parameters were as follows: taxonomy restriction, Arabidopsis; enzyme, semitrypsin; variable modifications, carbamidomethylation (C), oxidation (M); protein mass, unrestricted; peptide mass tolerance, 40 ppm; fragment mass tolerance, 0.8 D; maximum missed cleavages, one. A second search was also performed using the error-tolerant function of MASCOT. This mode allows iterating selected proteins through a list of chemical and posttranslational modifications present in the UniMod database (www.unimod.org). Potential modifications were validated by manual inspection of the MS/MS data. Hits were accepted if all strong peaks in the MS/MS spectrum were assigned either to y- or b-series ions and the spectrum contained y- or b-series signals corresponding to at least a stretch of three or more consecutive amino acids.
Cross-Linking of AnnAt1 Oligomers
For cross-linking of AnnAt1 oligomers in solution, DSS (Sigma-Aldrich), a homobifunctional cross-linking reagent with amine reactivity, was used. In this experiment, 10 μg of purified recombinant protein AnnAt1-His(6) (and its mutated versions C111A, C239A, and CC111/239AA) was incubated in 1× phosphate-buffered saline, pH 7.0, for 5 min at room temperature. Two microliters of freshly prepared DSS solution (in dimethyl sulfoxide [DMSO]) was added to the reaction mixture. For control, 2 μL of DMSO per sample was added. Reaction was stopped after 10 min by adding 100 mm Tris-Cl, pH 8.0. Finally, the samples were boiled in 1× solution for 5 min and separated by 10% (w/v) SDS-PAGE. Electrophoresis continued until the 35-kD band of marker migrated to the bottom line of plates. After transfer onto polyvinylidene difluoride (PVDF; Roche), annexin was detected with polyclonal antibody raised against AnnAt1 protein (Gorecka et al., 2005) and secondary anti-rabbit antibody conjugated with horseradish peroxidase (Sigma).
In Vitro Determination of the Cys Redox Status of AnnAt1
The principles of this analysis were given by Bayer et al. (1985, 1987) and Roffman et al. (1986). Biotin labeling was successfully used for the determination of the in vivo biological redox status of Cys residues in TGA1 (Després et al., 2003). A diagram showing this step-by-step procedure is depicted in Figure 4. The bacterially expressed protein AnnAt1-His(6), isolated under native conditions (in the absence of reducing agents), is believed to represent the in vivo biological redox status of Cys residues.
Two samples containing 10 μg of purified recombinant protein AnnAt1-His(6) in 50 mm Tris-Cl, pH 7.5, and 50 mm NaCl were processed in parallel. Proteins used in this experiment were isolated under native conditions and had never been in contact with reducing agents before the experiment. For detection of –SH groups, one sample was treated with 0.2 mm MPB (Fluka) for 1 h in darkness at room temperature. Proteins were then precipitated four times with acetone (15 min, −70°C) followed by centrifugation (15,000 rpm, 4°C). For the detection of disulfide bonds (oxidized Cys residues), the second sample in the first step was treated for 1 h in darkness at room temperature with 20 mm N-ethylmaleimide (Sigma-Aldrich) to block free SH groups. Then, disulfide bonds were chemically reduced with 20 mm dithiothreitol for 1 h at room temperature. In the third step, the newly emerged reduced free –SH groups were labeled with 0.2 mm MPB for 1 h in the dark at room temperature. To remove the regents, after each treatment the sample was precipitated with acetone. Cys-labeled proteins were then visualized after 12.5% SDS-PAGE and transfer onto PVDF (Roche) by staining with ExtrAvidin-Peroxidase (Sigma).
These results were confirmed by MS/MS sequencing of differentially labeled protein. The principles of this experiment were identical to those described above, with the exception that labeling of free and disulfide bond-forming Cys residues was achieved with iodoacetamide (Sigma-Aldrich) and S-methyl methanethiosulfonate (Fluka), respectively.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Generation and analysis of Arabidopsis ΔannAt1 plants.
Supplemental Figure S2. Generation and analysis of Arabidopsis 35S∷ AnnAt1 plants.
Supplemental Figure S3. Circular dichroism (CD) spectra of wild-type AnnAt1-His(6) and mutated H40A AnnAt1-His(6) proteins.
Supplemental Table S1. Theoretical molecular masses of Cys-containing peptides obtained after tryptic digestion of AnnAt1 protein.
Supplemental Table S2. Theoretical list of daughter ions resulting from fragmentation of the Cys-glutathionylated WTSSNQVLMEVACTR peptide.
Supplemental Table S3. A list of potential cis- and trans-acting elements in the AnnAt1 promoter.
Supplemental Table S4. The quantification of physiological parameters of Arabidopsis plants with different levels of AnnAt1 protein.
Supplemental Table S5. Primer information for the Arabidopsis genes used in this work.
Supplemental Materials and Methods S1.
Supplementary Material
Acknowledgments
We thank Jacek Oledzki, Agata Malinowska, and Michal Dadlez for participation in glutathionylation experiments; Malgorzata Wilkowicz for technical support in experiments with ΔoxyR survivability; Elliott Lhospital, Nathan Paczan, and Amy Rivera for participation in epidermal ROS staining experiments; and Craig Handley for help with western-blot analyses of annexin-overexpressing and knockout lines. We also thank Slawomir Pikula for kind donation of ΔannAt1-3 seeds, Guy Thompson for critical reading and discussion of the manuscript, and the Microscopy and Imaging Facility of the Institute for Cellular and Molecular Biology at the University of Texas at Austin for providing access to and assistance with the confocal microscope.
This work was supported by the Polish Ministry of Education and Science (grant nos. 2P06A 007 29 to D.K.-P. and PBZMNiSW–213/2006/5 to J.H.) and by the U.S. National Aeronautics and Space Administration (grant no. NAG2–1586 to S.R.).
The authors responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) are: Dorota Konopka-Postupolska (konopka@ibb.waw.pl) and Greg Clark (gbclark@uts.cc.utexas.edu).
The online version of this article contains Web-only data.
References
- Anselmo AN, Cobb MH (2004) Protein kinase function and glutathionylation. Biochem J 381 675–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55 373–399 [DOI] [PubMed] [Google Scholar]
- Aracena P, Sanchez G, Donoso P, Hamilton SL, Hildago C (2003) S-Glutathionylation decreases Mg2+ inhibition and S-nitrosylation enhances Ca2+ activation of RyR1 channels. J Biol Chem 278 42927–42935 [DOI] [PubMed] [Google Scholar]
- Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2 2006–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiychuk EB, Draeger A (2000) Annexins in cell membrane dynamics: Ca(2+)-regulated association of lipid microdomains. J Cell Biol 150 1113–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer EA, Safars M, Wilchek M (1987) Selective labeling of sulfhydryls and disulfides on blot transfers using avidin-biotin technology: studies on purified proteins and erythrocyte membranes. Anal Biochem 161 262–271 [DOI] [PubMed] [Google Scholar]
- Bayer EA, Zalis MG, Wilchek M (1985) 3-(N-Maleimido-propionyl)biocytin: a versatile thiol-specific biotinylating reagent. Anal Biochem 149 529–536 [DOI] [PubMed] [Google Scholar]
- Blackbourn HD, Barker PI, Huskisson NS, Battey NH (1992) Properties and partial protein-sequence of plant annexins. Plant Physiol 99 864–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger A, Berendes R, Liemann S, Benz J, Hofmann A, Göttig P, Huber R, Gerke V, Thiel C, Römisch J, et al (1996) The crystal structure and ion channel activity of human annexin II, a peripheral membrane protein. J Mol Biol 257 839–847 [DOI] [PubMed] [Google Scholar]
- Camera S, Gouzerh G, Dhondt S, Hoffmann L, Fritig B, Legrand M, Heitz T (2004) Metabolic reprogramming in plant innate immunity: the contributions of phenylpropanoid and oxylipin pathways. Immunol Rev 198 267–284 [DOI] [PubMed] [Google Scholar]
- Cantero A, Barthakur S, Bushart T, Morgan RO, Fernandez P, Chou S, Clark G, Roux SJ (2006) Expression profiling of the Arabidopsis annexin gene family during abiotic stress, germination and de-etiolation. Plant Physiol Biochem 44 13–24 [DOI] [PubMed] [Google Scholar]
- Caplan JF, Filipenko NR, Fitzpatrick SL, Waisman DM (2004) Regulation of annexin A2 by reversible glutathionylation. J Biol Chem 279 7740–7750 [DOI] [PubMed] [Google Scholar]
- Carroll AD, Moyen C, Van Kesteren P, Tooke F, Battey NH, Brownlee C (1998) Ca2+, annexins, and GTP modulate exocytosis from maize root cap protoplasts. Plant Cell 10 1267–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cessna SG, Messerli MA, Robinson KR, Low PS (2001) Measurement of stress-induced Ca2+ pulses in single aequorin-transformed tobacco cells. Cell Calcium 30 151–156 [DOI] [PubMed] [Google Scholar]
- Chandra S, Low PS (1997) Measurement of Ca2+ fluxes during elicitation of the oxidative burst in aequorin-transformed tobacco cells. J Biol Chem 272 28274–28280 [DOI] [PubMed] [Google Scholar]
- Church GM, Gilbert W (1984) Genomic sequencing. Proc Natl Acad Sci USA 81 1991–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark GB, Dauwalder M, Roux SJ (1992) Purification and immunolocalization of annexin-like protein in pea seedlings. Planta 187 1–9 [DOI] [PubMed] [Google Scholar]
- Clark GB, Lee DW, Dauwalder M, Roux SJ (2005) Immunolocalization and histochemical evidence for the association of two different Arabidopsis annexins with secretion during early seedling growth and development. Planta 220 621–631 [DOI] [PubMed] [Google Scholar]
- Clark GB, Roux SJ (1995) Annexins of plant cells. Plant Physiol 109 1133–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark GB, Sessions A, Eastburn DJ, Roux SJ (2001) Differential expression of members of the annexin multigene family in Arabidopsis. Plant Physiol 126 1072–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross JV, Templeton DJ (2004) Oxidative stress inhibits MEKK1 by site-specific glutathionylation in the ATP-binding domain. Biochem J 381 675–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R, Cheung MK, Bright J, Henson D, Hancock JT, Neill SJ (2004) ABA, hydrogen peroxide and nitric oxide signalling in stomatal guard cells. J Exp Bot 55 205–212 [DOI] [PubMed] [Google Scholar]
- Desikan R, Mackerness SAH, Hancock JT, Steven JN (2001) Regulation of the Arabidopsis transcriptome by oxidative stress. Plant Physiol 127 159–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Després C, Chubak C, Rochon A, Clark R, Bethune T, Desveaux D, Fober PR (2003) The Arabidopsis NPR1 disease resistance protein is a novel cofactor that confers redox regulation of DNA binding activity to the basic domain/leucine zipper transcription factor TGA1. Plant Cell 15 2181–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon DP, Skipsey M, Grundy NM, Edwards R (2005) Stress-induced protein S-glutathionylation in Arabidopsis. Plant Physiol 138 2233–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon RA, Achnine L, Kota P, Liu C-J, Reddy MS, Wang L (2002) The phenylpropanoid pathway and plant defence: a genomics perspective. Mol Plant Pathol 3 371–390 [DOI] [PubMed] [Google Scholar]
- Draeger A, Wray S, Babiychuk EB (2005) Domain architecture of the smooth-muscle plasma membrane: regulation by annexins. Biochem J 387 309–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eftink MR (1997) Fluorescence methods for studying equilibrium macromolecule ligand interactions. Methods Enzymol 278 221–257 [DOI] [PubMed] [Google Scholar]
- Endo A, Sawada Y, Takahashi H, Okamoto M, Ikegami K, Koiwai H, Seo M, Toyomasu T, Mitsuhashi W, Shinozaki K, et al (2008) Drought induction of Arabidopsis 9-cis-epoxycarotenoid dioxygenase occurs in vascular parenchyma cells. Plant Physiol 147 1984–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filomeni G, Rotilio G, Ciriolo MR (2005) Disulfide relays and phosphorylative cascades: partners in redox-mediated signaling pathways. Cell Death Differ 12 1555–1563 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17 1866–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratelli M, Gianazza E, Ghezzi P (2004) Redox proteomics: identification and functional role of glutathionylated proteins. Expert Rev Proteomics 1 365–376 [DOI] [PubMed] [Google Scholar]
- Galuschka C, Schindler M, Bulow L, Hehl R (2007) AthaMap web tools for the analysis and identification of co-regulated genes. Nucleic Acids Res (Suppl 1) 35 D857–D862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao DJ, Knight MR, Trewavas AJ, Sattelmacher B, Plieth C (2004) Self-reporting Arabidopsis expressing pH and [Ca2+] indicators unveil ion dynamics in the cytoplasm and in the apoplast under abiotic stress. Plant Physiol 134 898–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidrol X, Sabelli PA, Fern YS, Kush AK (1996) Annexin-like protein from Arabidopsis thaliana rescues delta oxyR mutant of Escherichia coli from H2O2 stress. Proc Natl Acad Sci USA 93 11268–11273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleba Y, Klimyuk V, Marillonnet S (2004) Engineering viral expression vectors for plants: the ‘full virus’ and the ‘deconstructed virus’ strategies. Curr Opin Plant Biol 7 182–188 [DOI] [PubMed] [Google Scholar]
- Goch G, Vdovenko S, Kozlowska H, Bierzynski A (2005) Affinity of S100A1 protein for calcium increases dramatically upon glutathionylation. FEBS J 272 2557–2565 [DOI] [PubMed] [Google Scholar]
- Gomez LD, Noctor G, Knight MR, Foyer CH (2004) Regulation of calcium signalling and gene expression by glutathione. J Exp Bot 55 1851–1859 [DOI] [PubMed] [Google Scholar]
- Gorantla M, Babu PR, Lachagari VBR, Feltus FA, Paterson AH, Reddy AR (2005) Functional genomics of drought stress response in rice: transcript mapping of annotated unigenes of an indica rice (Oryza sativa L. cv. Nagina 22). Curr Sci 89 496–514 [Google Scholar]
- Gorecka KM, Konopka-Postupolska D, Hennig J, Buchet R, Pikula S (2005) Peroxidase activity of AnnAt1 from Arabidopsis thaliana. Biochem Biophys Res Commun 336 868–875 [DOI] [PubMed] [Google Scholar]
- Gorecka KM, Thouverey C, Buchet R, Pikula S (2007) Potential role of annexin AnnAt1 from Arabidopsis thaliana in pH-mediated cellular response to environmental stimuli. Plant Cell Physiol 48 792–803 [DOI] [PubMed] [Google Scholar]
- Gould KS, Lamotte O, Klinguer A, Pugin A, Wendehenne D (2003) Nitric oxide production in tobacco leaf cells: a generalized stress response? Plant Cell Environ 26 1851–1862 [Google Scholar]
- Greenberg JT, Demple B (1988) Overproduction of peroxide-scavenging enzymes in Escherichia coli suppresses spontaneous mutagenesis and sensitivity to redox-cycling agents in oxyR- mutants. EMBO J 7 2611–2617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock J, Desikan R, Harrison J, Bright J, Hooley R, Neill S (2006) Doing the unexpected: proteins involved in hydrogen peroxide perception. J Exp Bot 57 1711–1718 [DOI] [PubMed] [Google Scholar]
- Hofmann A (2004) Annexins in the plant kingdom: perspectives and potentials. Annexins 1 51–61 [Google Scholar]
- Hofmann A, Delmer DP, Wlodawer A (2003) The crystal structure of annexin Gh1 from Gossypium hirsutum reveals an unusual S-3 cluster: implications for cellulose synthase complex formation and oxidative stress response. Eur J Biochem 270 2557–2564 [DOI] [PubMed] [Google Scholar]
- Hofmann A, Proust J, Dorowski A, Schantz R, Huber R (2000) Annexin 24 from Capsicum annuum: x-ray structure and biochemical characterization. J Biol Chem 275 8072–8082 [DOI] [PubMed] [Google Scholar]
- Hoshino D, Hayashi A, Temmei Y, Kanzawa N, Tsuchiya T (2004) Biochemical and immunohistochemical characterization of Mimosa annexin. Planta 219 867–875 [DOI] [PubMed] [Google Scholar]
- Jami SK, Clark GB, Turlapati SA, Handley CA, Roux SJ, Kirti PB (2008) Ectopic expression of an annexin from Brassica juncea confers tolerance to abiotic and biotic stress treatments in transgenic tobacco. Plant Physiol Biochem 46 1019–1030 [DOI] [PubMed] [Google Scholar]
- Janicke RU, Porter AG, Kush A (1998) A novel Arabidopsis thaliana protein protects tumor cells from tumor necrosis factor-induced apoptosis. Biochim Biophys Acta 1402 70–78 [DOI] [PubMed] [Google Scholar]
- Keller T, Damude HG, Werner D, Doerner P, Dixon RA, Lamb C (1998) A plant homolog of the neutrophil NADPH oxidase gp91phox subunit gene encodes a plasma membrane protein with Ca2+ binding motifs. Plant Cell 10 255–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight H (2000) Calcium signaling during abiotic stress in plants. Int Rev Cytol 195 269–324 [DOI] [PubMed] [Google Scholar]
- Konopka-Postupolska D (2007) Annexins: putative linkers in dynamic membrane-cytoskeleton interactions in plants. Protoplasma 230 203–215 [DOI] [PubMed] [Google Scholar]
- Kovacs I, Ayaydin F, Oberschall A, Ipacs I, Bottka S, Dudits D, Toth EC (1998) Immunolocalization of a novel annexin-like protein encoded by a stress and abscisic acid responsive gene in alfalfa. Plant J 15 185–197 [DOI] [PubMed] [Google Scholar]
- Kreps JA, Wu YJ, Chang HS, Zhu T, Wang X, Harper JF (2002) Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant Physiol 130 2129–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kush A, Sabapathy K (2001) Oxy5, a novel protein from Arabidopsis thaliana, protects mammalian cells from oxidative stress. Int J Biochem Cell Biol 33 591–602 [DOI] [PubMed] [Google Scholar]
- Laloi C, Apel K, Danon A (2004) Reactive oxygen signalling: the latest news. Curr Opin Plant Biol 7 323–328 [DOI] [PubMed] [Google Scholar]
- Laohavisit A, Mortimer JC, Demidchik V, Coxon KM, Stancombe MA, Macpherson N, Brownlee C, Hofmann A, Webb AAR, Miedema H, et al (2009) Zea mays annexins modulate cytosolic free Ca2+ and generate a Ca2+-permeable conductance. Plant Cell 21 479–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Lee EJ, Yang EJ, Lee JE, Park AR, Song WH, Park OK (2004) Proteomic identification of annexins, calcium-dependent membrane binding proteins that mediate osmotic stress and abscisic acid signal transduction in Arabidopsis. Plant Cell 16 1378–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindermayr C, Saalbach G, Durner J (2005) Proteomic identification of S-nitrosylated proteins in Arabidopsis. Plant Physiol 137 921–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Eaton JW (1992) Multicellular oxidant defense in unicellular organisms. Proc Natl Acad Sci USA 89 7924–7928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marillonnet S, Giritch A, Gils M, Kandzia R, Klimyuk V, Gleba Y (2004) In planta engineering of viral RNA replicons: efficient assembly by recombination of DNA modules delivered by Agrobacterium. Proc Natl Acad Sci USA 101 6852–6857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AJ (2008) The integration of glutathione homeostasis and redox signaling. J Plant Physiol 165 1390–1403 [DOI] [PubMed] [Google Scholar]
- Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7 405–410 [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9 490–498 [DOI] [PubMed] [Google Scholar]
- Mori IC, Schroeder JI (2004) Reactive oxygen species activation of plant Ca2+ channels: a signaling mechanism in polar growth, hormone transduction, stress signaling, and hypothetically mechanotransduction. Plant Physiol 135 702–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer JC, Laohavisit A, Macpherson N, Webb A, Brownlee C, Battey NH, Davies JM (2008) Annexins: multifunctional components of growth and adaptation. J Exp Bot 59 533–544 [DOI] [PubMed] [Google Scholar]
- Murata Y, Pei ZM, Mori IC, Schroeder JI (2001) Abscisic acid activation of plasma membrane Ca2+ channels in guard cells requires cytosolic NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1-1 and abi2-1 protein phosphatase 2C mutants. Plant Cell 13 2513–2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau PJ, Charette SJ, Toledano MB, Landry J (2007) Disulfide bond-mediated multimerization of Ask1 and its reduction by thioredoxin-1 regulate H2O2-induced c-Jun NH2-terminal kinase activation and apoptosis. Mol Biol Cell 18 3903–3913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brian CA, Chu F (2005) Post-translational disulfide modifications in cell signaling: role of inter-protein, intra-protein, S-glutathionyl, and S-cysteaminyl disulfide modifications in signal transmission. Free Radic Res 39 471–480 [DOI] [PubMed] [Google Scholar]
- Ogawa K (2005) Glutathione-associated regulation of plant growth and stress responses. Antioxid Redox Signal 7 973–981 [DOI] [PubMed] [Google Scholar]
- Okamoto T, Higuchi K, Shinkawa T, Isobe T, Lorz H, Koshiba T, Kranz E (2004) Identification of major protein in maize embryo sac. Plant Cell Physiol 45 1406–1412 [DOI] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JI (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406 731–734 [DOI] [PubMed] [Google Scholar]
- Price AH, Taylor A, Ripley SJ, Griffiths A, Trewavas AJ, Knight MR (1994) Oxidative signals in tobacco increase cytosolic calcium. Plant Cell 6 1301–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roffman E, Meromsky L, Ben-Hur H, Bayer EA, Wilchek M (1986) Selective labeling of functional groups on membrane proteins or glycoproteins using reactive biotin derivatives and 125I-streptavidin. Biochem Biophys Res Commun 136 80–85 [DOI] [PubMed] [Google Scholar]
- Romeis T, Ludwig AA, Martin R, Jones JDG (2001) Calcium-dependent protein kinases play an essential role in a plant defence response. EMBO J 20 5556–5567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso MG, Li Y, Strizhov N, Reiss B, Dekker K, Weisshaar B (2003) An Arabidopsis thaliana T-DNA mutagenized population (GABI-Kat) for flanking sequence tag-based reverse genetics. Plant Mol Biol 53 247–259 [DOI] [PubMed] [Google Scholar]
- Saini HS (1997) Effects of water stress on male gametophyte development in plants. Sex Plant Reprod 10 67–73 [Google Scholar]
- Sessions A, Burke E, Presting G, Aux G, McElver J, Patton D, Dietrich B, Ho P, Bacwaden J, Ko C, et al (2002) A high throughput Arabidopsis reverse genetics system. Plant Cell 14 2985–2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solecka D (2007) Role of phenylpropanoid compounds in plant responses to different stress factors. Acta Physiol Plant 19 257–268 [Google Scholar]
- Suzuki M, Ketterling MG, McCarty DR (2005) Quantitative statistical analysis of cis-regulatory sequences in ABA/VP1- and CBF/DREB1-regulated genes of Arabidopsis. Plant Physiol 139 437–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel G, Rupnik M, Zorec R (1994) Raising cytosolic Ca2+ concentration increases membrane capacitance of maize coleoptile protoplasts: evidence for Ca2+ stimulated exocytosis. Planta 195 305–308 [Google Scholar]
- Torres MA, Dangl JL (2005) Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr Opin Plant Biol 8 397–403 [DOI] [PubMed] [Google Scholar]
- Torres MA, Onouchi H, Hamada S, Machida C, Hammond-Kossack KE, Jones JDG (1998) Six Arabidopsis thaliana homologues of the human respiratory burst oxidase (gp91phox). Plant J 14 365–370 [DOI] [PubMed] [Google Scholar]
- Vanderauwera S, Zimmermann P, Rombauts S, Vandenabeele S, Langebartels C, Gruissem W, Inze D, Van Breusegem F (2005) Genome-wide analysis of hydrogen peroxide-regulated gene expression in Arabidopsis reveals a high light-induced transcriptional cluster involved in anthocyanin biosynthesis. Plant Physiol 139 806–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Tekle E, Oubrahim H, Mieyal JJ, Stadtman ER, Chock PB (2003) Stable and controllable RNA interference: investigating the physiological function of glutathionylated actin. Proc Natl Acad Sci USA 100 5103–5106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkinson JI, Sioson AA, Vasquez-Robinet C, Shukla M, Kumar D, Ellis M, Heath LS, Ramakrishnan N, Chevone B, Watson LT, et al (2003) Photosynthetic acclimation is reflected in specific patterns of gene expression in drought-stressed loblolly pine. Plant Physiol 133 1702–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe CA, Dibattista ER, Fliegel L (2001) Functional role of polar amino acid residues in Na+/H+ exchangers. Biochem J 357 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki K (1994) A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell 6 251–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki K (2005) Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci 10 88–94 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.