Abstract
The human brain demonstrates complex yet systematic patterns of neural activity at rest. We examined whether functional connectivity among those brain regions typically active during rest depends on ongoing and recent task demands and individual differences. We probed the temporal coordination among these regions during periods of language comprehension and during the rest periods that followed comprehension. Our findings show that the topography of this “rest network” varies with exogenous processing demands. The network encompassed more highly interconnected regions during rest than during listening, but also when listening to unsurprising vs. surprising information. Furthermore, connectivity patterns during rest varied as a function of recent listening experience. Individual variability in connectivity strength was associated with cognitive function: more attentive comprehenders demonstrated weaker connectivity during language comprehension, and a greater differentiation between connectivity during comprehension and rest. The regions we examined have generally been thought to form an invariant physiological and functional network whose activity reflects spontaneous cognitive processes. Our findings suggest that their function extends beyond the mediation of unconstrained thought, and that they play an important role in higher-level cognitive function.
Keywords: connectivity, default mode, individual differences, stochastic, fMRI
Neuroimaging studies characteristically associate brain regions with some cognitive function to the extent that blood oxygenation level-dependent (BOLD) contrast increases in those regions when that function is engaged, as compared with a resting state baseline condition. However, in some brain regions, task engagement has been repeatedly associated with a decreased BOLD response relative to a resting state, a pattern often referred to as deactivation. Task-deactivated regions are thought to consume more glucose during rest than during task performance; i.e., they are relatively more active during rest as determined by positron emission tomography (PET) (1). These regions include the inferior parietal lobules, posterior medial cortices, and the ventral and dorsal medial prefrontal cortices (2). These regions have also been characterized in terms of a specific functional network (sometimes referred to as a “default network”) based on the observation that at rest their BOLD responses are temporally synchronized (3, 4).
Given that human beings are often not engaged in any exogenously directed mental task, and may often simply be engaged in reflection or calm wakefulness, accounting for the functional role of these brain regions is fundamental to understanding mental activity: if neural activity in these regions is associated with a set of default human mental functions that are suspended when rest is interrupted by organized focal psychological processes, these regions could mediate a substantial portion of human mental life and experience. Two classes of explanation for the functional role of the default network have been put forward. One explanation is that activity in this network is intrinsic and spontaneous; it largely reflects unconstrained thought processes or basic physiological processes that are independent of mental activity. By contrast, the alternative explanation views activity in these regions as related to and constrained by ongoing and recent experiences.
On the internally oriented, or stochastic, explanation, the dominant mode of brain function is one of unconstrained activity in which the brain operates “on its own” (5). There are 2 variants of this explanation: some suggest that intrinsic activity reflects a physiological network, whereas others argue it reflects unconstrained and spontaneous cognitive processes, such as mind wandering or stimulus-unrelated thought. The former view is supported by research documenting the network in primates, during light sleep, and in vegetative states (6). The latter view is supported by work showing that greater activity in these regions (i.e., less deactivation) is associated with a tendency to report task-unrelated thoughts (7, 8) and with longer response times during task performance (9). Studies exploring the relation between activity in these regions and performance on an immediately following task have documented increased network activity within 2–6 sec before committing an error (10, 11) but also before subsequent insight solutions (12).
On the more externally oriented or deterministic approach, the temporally coherent pattern of neural activity seen in these regions during rest does not necessarily indicate that these regions mediate intrinsically oriented activity in absence of stimulation. Instead, this activity could be associated with processes that occur following completion of a previous behavior or cognitive activity. Examples of such postprocessing activity include memory consolidation, replay of recent experiences, and procedural learning (13), and such consolidations can occur quite rapidly (14). Several studies, though not specifically examining regions associated with the default network, support the notion that neural activity during wakeful rest is affected by prior task: using fMRI it has been shown that interhemispheric motor-cortex connectivity during rest varies depending on whether the rest period precedes or follows motor task performance (15). The impact of a preceding task may be extended in time: prior work examining rest connectivity before and after performance of a 5-min language task suggests such tasks may induce sustained changes in rest connectivity over several minutes (16). In addition, PET studies have revealed that in several cortical areas, regional cerebral blood flow during rest periods is modulated depending on whether a rest period precedes or follows performance of long task epochs (17, 18). Finally, machine learning techniques demonstrate that it is possible to accurately predict specific features of a prior task by analyzing EEG data collected within 1–4 sec posttask, indicating that posttask rest periods are strongly constrained by prior experience (19).
The difference between these 2 views of the default network hinges on whether the network's functional organization (i.e., its topography) transcends the nature of both ongoing and recent activity. For example, on the intrinsic account, network organization during rest should be independent of the nature of the mental experience that preceded the resting state, as this network is reflective of a system that carries out a different set of functions. Furthermore, this approach predicts that functional connectivity (FC) within the network should be invariant across different ongoing tasks. Consistent with this idea, several studies have reported no difference in network connectivity for task vs. rest (20, 21). However, recent work has documented that the spatial distribution of this network, defined via independent component analysis (ICA), can vary with particular task demands, stimulus salience, or depending on whether individuals are at rest (22–25). Linking these spatial variations to cognitive function has proved difficult because these studies have used relatively demanding tasks, such as N-back memory tasks, Stroop attention tasks, or solving moral dilemmas. These structured tasks likely constrain freedom for spontaneous thought, thus forming an upper bound on the network's potential impact on cognitive processing.
Therefore, our first goal was to examine (i) how FC within the network relates to ongoing task context and (ii) whether FC during wakeful rest is constrained by prior task context. Though we use the term task to refer to activities in which participants were engaged, we did not direct participants' behavior or elicit any response; in practice, we asked participants to passively listen to auditory materials that varied in content, in absence of any guidance or demand for particular response. This afforded ample freedom for spontaneous mental activity. These 2 questions address whether exogenous constraints affect connectivity, but do not address whether variations in connectivity relate to endogenous cognitive processes that carry real-world outcomes. Our second goal was therefore to establish the relation between FC in the network and higher-level cognitive processing; specifically, we determined how connectivity within the network during language comprehension relates to the effectiveness of comprehension. To summarize, we determined (i) whether FC during rest is sensitive to features of a preceding passive-listening task, (ii) whether FC during passive listening is sensitive to informational content, and (iii) whether variations in FC across individuals, if existent, are significant for cognitive function.
To determine the extent to which activity in the default network is constrained by ongoing and recent demands, we examined FC within this network during periods of passive listening to linguistic material, and during wakeful rest periods that followed these listening periods. This linguistic material consisted of auditory sentences that appeared as the concluding sentences of short stories. In 2 conditions, these sentences were identical but were made to convey more or less information by manipulating the story context that preceded them: the more-informative condition was one where a sentence segment was more surprising given prior context, and the less-informative condition was one where the exact same segment was less surprising given prior context. We could therefore establish whether FC in the network varies when individuals are listening to the same utterance, as a function of its informativeness (a third condition consisted of sentences that were not thematically related). Similarly, we could establish if FC during wakeful rest periods that follow comprehension varies as a function of the immediately preceding language content. If FC during rest were to vary in such a manner, this would indicate that the network's inherent topography (i.e., its defining functional characteristic) is constrained by recent experience. This conclusion would be further supported if FC within the network differed for periods of rest and language comprehension. Finally, to determine the relation between such fluctuations in connectivity and behavior we examined whether differences in FC during language comprehension were associated with interindividual differences in the ability to subsequently remember the presented materials.
Results
Our first analysis was a validity check that identified regions demonstrating reliable connectivity during rest with 2 seed regions typically identified in studies of the default rest network: the left precuneus and the left angular gyrus (median Z-normalized correlation value across participants >3). The findings confirmed that during wakeful rest, regions demonstrating strong connectivity with both seed regions included the inferior parietal lobules, posterior superior temporal gyrus (STG), dorsomedial prefrontal regions (dmPFC), supplementary motor areas (SMA), and posterior midline regions, including the posterior cingulate gyrus (PCgG). The subsequent analyses addressed the study's theoretical questions regarding the context sensitivity of FC, and were based on a 2 (task: rest vs. listening) × 3 (content: more informative, less informative, nonnarrative) ANOVA that examined FC values in the 6 conditions of interest. Note that in the ANOVA, “Rest” refers to rest periods that followed each story presentation (beginning 10 sec after story presentation and extending for 16 sec from that point), and these were partitioned as a function of language content that preceded them. All analyses were conducted against the 2 seed regions, and reliable modulations of FC were defined as those that held in analyses conducted against both seed regions (see Experimental Procedures). In the results, FC strength is presented as Z-normalized correlation values so that values above a Z score of 1.65 index a statistically reliable correlation.
Differences in Connectivity During Listening and Rest.
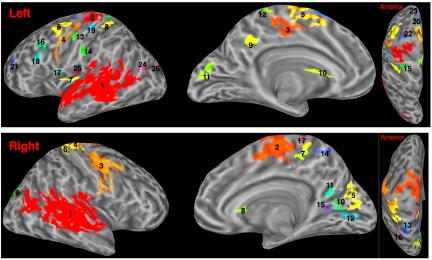
A large number of regions, bilaterally, demonstrated reliable differences in FC depending on whether individuals were listening to the stories or were wakefully resting after listening. Fig. 1 presents these clusters [clusters are marked by different colors; supporting information (SI) Table S1 reports centers of mass in Talairach space]. Clusters encompassed regions typically associated with default network (e.g., precuneus, PCgG, aspects of the superior temporal sulcus [STS]) but also regions including the precentral gyrus (PreCG) bilaterally. We treated each of these clusters as a functional region of interest (fROI) and established the mean connectivity value in each fROI during rest and during listening (values for all fROIs are provided in Fig. S1). All fROIs but 2 (left no. 21, right no. 11), demonstrated reliably stronger FC during rest than during listening.
Fig. 1.
Areas showing differences in functional connectivity for listening and rest. Centers of mass and FC values reported in Table S1 and Fig. S1.
Differences in Functional Connectivity as Function of Language Content.
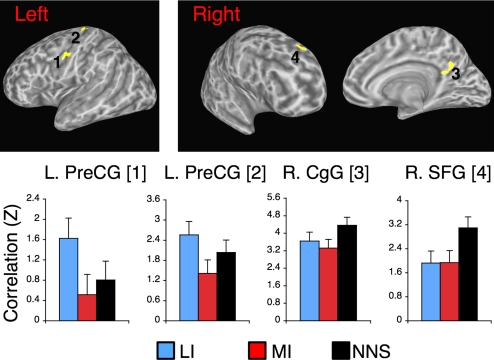
The existence of differences in FC between listening and rest demonstrates one type of influence of context on FC. We next examined whether the processing of utterance content also mediates the degree of FC with seed regions in the network. This whole-brain analysis probed for the effect of content independent of task (i.e., collapsing over the listening period and the rest periods that followed them; a main effect of Content in the voxel-wise ANOVA). This analysis identified 4 areas in which FC differed as function of language content (Fig. 2; see Table S2 for centers of mass in Talairach space). As the figure shows, the nature of modulation was such that FC tended to be weaker while listening to more-informative contents relative to the other 2 conditions.
Fig. 2.
Regions where functional connectivity with seed regions varied as a function of language content. Here, listening periods and the rest periods that followed them are considered jointly. FC values with precuneus seed region are shown for brevity. Standard error bars indicate the SE of the mean across participants. Because of the within-participant design, SE bars are uninformative with respect to differences between conditions. LI, less informative content; MI, more informative content; NNs, nonnarrative content.
Differential Effects of Content Type on FC During Listening and Rest.
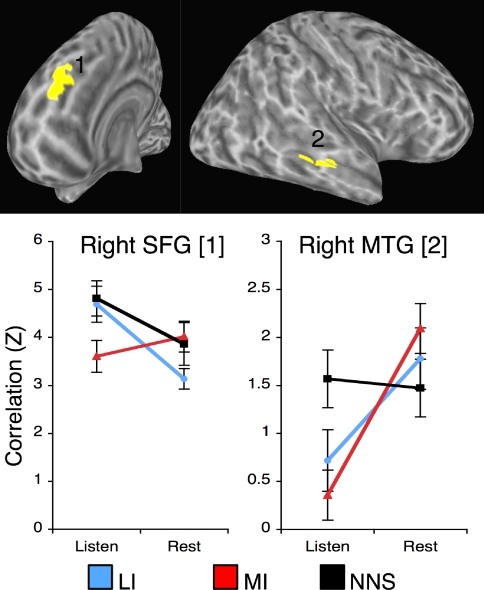
A whole-brain analysis identified 2 areas in which the impact of language content on connectivity differed for listening and rest. Fig. 3 presents the nature of the interaction in each area. For instance, a cluster in the right middle temporal gyrus (MTG) showed a strong differentiation between FC during listening and rest, but this differentiation held for the 2 conditions where proper stories were presented, but not for the one where unrelated sentences were presented. The data highlight how certain regions may be differentially recruited into broader networks that are observed to be active during listening or rest.
Fig. 3.
Regions where functional connectivity varied as function of both content and task. FC values with precuneus seed region are shown for brevity. Talairach coordinates: SFG [8, 48, 32], MTG [52, −28, −5]. Identical FC patterns were found for angular G. seed region. LI. less informative content; MI, more informative content; NNS, nonnarrative contents.
Regions in Which Functional Connectivity During Rest Varied as Function of Preceding Content.
A whole-brain analysis identified 6 functional regions where FC during rest varied as a function of the immediately preceding language content. These were located bilaterally in the vicinity of PreCG, and in the right PCgG and right dmPFC (Fig. S2 and Table S2).
Differential Connectivity for Good and Poor Comprehenders.
A memory test that followed the fMRI scan indicated that participants exhibited marked individual differences in their memory for the materials. To determine whether these differences were associated with FC in the default network, we focused on those fROIs that demonstrated differential FC for listening and rest (Fig. 1). For each fROI we determined if there was an association between participants' FC values when listening to stories and their performance on the subsequent memory test. Of the 43 fROIs identified, 39 demonstrated a negative correlation between participants' memory accuracy and the degree of FC with the seed region. This indicates that, on the whole, reduced synchronization with default network seed regions was beneficial for comprehension. This negative correlation was statistically reliable for 11 such fROIs (false discovery rate corrected), which included the paracentral lobule (PCL), PCgG, precuneus, and lingual gyrus (see Fig. S3 for scatter plots). In most regions, participants showing low accuracy on the subsequent memory test demonstrated stronger correlations (Z-normalized correlation values often >3). In contrast, participants showing good memory accuracy sometimes demonstrated activity that was anticorrelated with the seed regions (indexed by negative Z values).
Differential Modulation of Connectivity for good and poor Comprehenders.
Finally, we examined whether comprehension performance was also related to the differentiation between FC during listening and rest. For each of the functional regions that differentiated listening from rest (Fig. 1), we calculated the difference (delta) between FC during rest and during listening for each participant [delta-FC = FC(rest) − FC(listening)]. We then correlated delta-FC with participants' memory accuracy. Five regions demonstrated a reliable relation between delta-FC and memory accuracy (Fig. S4). In these regions, which included the PCgG, the PCL, and middle frontal gyrus, better comprehenders demonstrated a stronger differentiation between FC during listening and rest.
Discussion
We examined the functional connectivity (FC) of regions within the default network during periods of language comprehension and during rest periods that immediately followed them to determine the degree of FC modulation within the network and its relation to cognitive function. We focused on 3 issues: (i) whether the functional organization of this network during rest, operationalized as FC, is sensitive to antecedent task context, (ii) whether FC is sensitive to the exogenous features of ongoing context (e.g., rest vs. listening, or listening to different language content), and (iii) whether the degree of FC during comprehension and its modulation (vs. rest) is related to the effectiveness of comprehension. The answer to all 3 questions was affirmative: connectivity patterns were dynamic, systematically linked to both recent and ongoing demands, and related to comprehension effectiveness.
Modulation of Functional Connectivity as Function of Recent Experience.
We found that connectivity during nontask rest periods varied as a function of recent experience. In 3 regions, FC during rest was weaker after listening to more-informative (surprising) content than after listening to less-informative (nonsurprising) or nonnarrative content. These included the right PCgG and the medial aspect of the right SFG, regions often associated with the default network, and that showed strongly correlated activity with both seed regions in the current study. In the other regions, FC was lower for more-informative content than for one of the other experimental conditions, but not both.
These findings are consistent with a number of theoretical models. It is possible that these regions play a role in posttask functions such as rehearsal, elaboration, or encoding to memory (13). These regions may also be involved in active disengagement from a previous task, a process that could vary with the features of that task. Conversely, the differential connectivity displayed by these regions as function of recent past may reflect a sort of “cognitive inertia” in which the now-finished listening prompts differential internal processes that permeate into the nonlistening rest period. Our data cannot determine among these possibilities, and this is indeed an important issue for future studies in this domain. A final possibility is that such modulation of FC reflects a physiological carryover effect from sluggish BOLD responses that have their onset during the listening stage. However, statistical analyses and number of design features and data points argue against it. First, when analyzing FC during rest we considered acquisition periods that began 10 sec after the termination of each listening period and extended 16 sec from that point on. Our own analyses using a peak-fitting algorithm (26) indicated that the BOLD response associated with the last sentence returned to baseline between 10 and 14 sec (see Experimental Procedures), which is consistent with prior work (e.g., ref. 27) demonstrating that task-related BOLD response tends to decay within 10–15 sec after stimulation, and shows low response variance between 10 and 15 sec poststimulus. Finally, in the current study we were able to robustly discriminate between FC during listening and rest, indicating that FC during rest was not a “ballistic” extension of FC during listening.
Though our findings are consistent with such various accounts of the impact of a recent task on FC during subsequent rest, they are not consistent with the view that this is a network that solely mediates spontaneous intrinsic thought. If activity in this network reflected solely the generation of task-unrelated thoughts/stream of consciousness (8) or mind wandering (7), one would predict no association between its connectivity during rest and experiences in the recent past. Though this network is clearly associated with spontaneous activity in absence of stimulation (3), our findings offer a more nuanced view of its functional role during rest, that does not rely solely on the notion of spontaneous, task-stochastic process. As mentioned in the introduction, recent EEG work (19) supports exactly this point in showing that prior mental states can be accurately identified using machine learning techniques when using only those data collected during posttask periods.
Understanding the degree to which past cognitive activity modulates ongoing activity in the network, and how this influence wanes over time, are important questions for future work. The domain studied here, focusing on comprehension of short auditory contents followed by rest intervals is one that lends itself to context-dependent creative thought. Circumstances that do not allow for reconsideration of recent information may not afford such processes. To illustrate, research on auditory speech comprehension shows that subsequent recall of content is poorer when the content is presented consecutively than when content is delivered via self-paced listening that affords opportunities for intermediate consolidation (28).
Modulation of Functional Connectivity as a Function of Ongoing Context.
We found 2 types of evidence for context-induced modulation of FC: Certain regions showed differential FC (with seed regions) as function of linguistic content, and yet others showed differential FC during listening compared to rest. The vast majority of areas that differentiated listening from rest also demonstrated greater FC during rest. These included large sections of the lateral temporal cortex (STS, STG, and MTG). This finding is consistent with the role of these regions in language comprehension, indicating that task-relevant regions may demonstrate weaker synchronization with default network regions during task performance.
A different type of sensitivity to ongoing context was seen in regions where FC varied as a function of linguistic content. The most striking modulation was found in 2 clusters in the left PreCG. These regions demonstrated relatively strong FC during listening to less-informative contents, but weaker and nonreliable FC when listening to more-informative contents. It is important to keep in mind that in these 2 experimental conditions participants heard the exact same sentences, whose meaning was manipulated by contextual manipulations, i.e., by changing details given in prior sentences (see Experimental Procedures). Thus, the reduced FC in these regions while listening to more-informative content reflects internally driven meaning-integration processes rather than any difference in sensory input per se. To summarize, both of these analyses indicated that context does impose considerable changes on the degree of connectivity within the network.
Our finding of stronger FC within this network during rest is consistent with recent findings in the literature (22, 24). It accords well with work by Fransson (24) demonstrating substantially weaker FC within the network during a demanding working memory task than during rest. In that study, decreased FC with respect to a precuneus seed region was reported bilaterally in regions including the precuneus, MFG, SFG (medial aspects), and MTG, which is in good accordance with our findings for rest vs. listening. We note, however, that the relation between the network's FC during task and rest periods could itself depend on task properties. For example, some studies have reported no difference in connectivity within the network for task vs. rest (20, 21), whereas others reported stronger “default-mode” ICA components during task than during rest in certain nodes within this network (25, 29). For this reason, it has been difficult to relate task-modulated connectivity to cognitive function. To understand the functional significance of these changes we therefore examined their relation to behavior.
Relation Between Functional Connectivity and Individual Differences.
To understand whether reduced FC was associated with comprehension performance we analyzed those functional regions identified a priori as demonstrating differential FC during listening and rest. In a number of these regions better comprehenders demonstrated weaker FC than poorer comprehenders. Specifically, in the majority of these regions good comprehenders demonstrated almost no FC with the seed region, whereas poorer comprehenders demonstrated high and reliable FC. Thus, stronger connectivity within the network during cognitive processing is associated with less successful comprehension. In addition, we identified 5 regions where the magnitude of FC modulation was related to cognitive performance. In these, a stronger difference between FC during rest and listening was related to better subsequent memory for language content. This latter finding indicates that the source of these interindividual differences does not originate in the type of very low-frequency activity previously associated with worst task performance (30). Beyond this, we cannot say why it is that behavior was associated with FC strength in the network, as the strength of FC and its variation could reflect a modulating effect from regions outside the network.
To our knowledge, few studies have examined the relation between connectivity in the default network and individual differences in task performance. These studies (21, 31) examined connectivity between the posterior cingulate and anterior midline areas, and reported a positive correlation between cognitive ability and the connectivity of these areas. These anterior midline areas did not show modulation of FC between listening and rest in our current study and hence were not analyzed as an a priori functional ROI. Furthermore, of the 43 ROIs we identified, none demonstrated a reliable positive correlation between participants' cognitive performance and connectivity.
Our findings are compatible with a body of work indicating that greater deactivation in the default network is associated with greater “cognitive effort”: greater deactivation has been linked to exogenous factors such as greater task difficulty (32) or endogenous factors associated with better task performance (33). However, these aforementioned studies examined the relation between activation/deactivation and behavior, whereas our work addresses the relation between functional connectivity and behavior. Whether deactivation and FC in the network are associated, and if so under what circumstances, is an important question for future research (cf. ref. 20 for related findings).
From Intrinsic Thought to a Model of Constrained Spontaneity.
On the basis of our findings, we propose that regions associated with the “default network” may mediate associative thought that is triggered by properties of the recent past, or ongoing context, but is not strongly determined by it. Apart from the data reported here, there is some support for this viewpoint. First, in an independent analysis of these data (34), we showed that memory for more informative content is associated with increased activity in default network regions, i.e., a return to baseline. This suggests that increased activity in this network is particularly related to the type of elaborative thinking that mediates encoding of complex information to memory. Such an explanation is consistent with the claim that this network mediates construction of elaborative associations that are triggered by input stimuli (35). Furthermore, a number of studies have implicated these regions in constructing representations of both past events and possible future events (36) and social cognition (37). Thus, the emerging picture is that rather than mediating a solely intrinsic and spontaneous function, regions of the default network mediate elaboration and association of inputs. Language comprehension perhaps epitomizes the type of situation that calls on the ability to construct these sorts of complex and rich internal representations, sometimes referred to as mental models (38) or mental spaces (39). Constructing enriched models of the past, the future, or of ongoing linguistic input may therefore use the same network.
The account we outline here pertains to the function of this network during circumstances where activity and rest are intertwined, which are abundant in everyday life. The fact that this network's connectivity is context bound in the way identified in the current study should not be interpreted as indicating that features of ongoing and recent context are the only factors determining the degree of ordered activity in the network: the nonrandom activity it shows in vegetative states and primates (6) makes this point clearly. Similarly, synchronized low-frequency connectivity patterns within this network during long rest periods point to a physiological generator, perhaps subcortically (40). Our data therefore do not directly bear on what functional features, if any, are mediated by the default network during such restful states. Conversely, the relevance of functional theories derived from studies of rest to the kind of situations we are studying here is not immediate. The changes in connectivity we identify occur at frequencies higher than those driving connectivity during prolonged rest states, and likely index different functions than the spontaneous ones that occur in those states. As such, our results are in accordance with data implicating this network with brain-behavior regularities that occur at higher frequencies. For example, individuals who are practiced in dismissing structured thought (through Zen mediation) show faster recovery of activity in default network regions immediately following task trials (41), indicating that posttask activity in the network may be under regulatory control. Other work shows that in this network, patterns of increasing activity that precede error trials are “reset” immediately after an error is made (10). Finally, transient deactivations in the network are tightly linked in time to events that demand very weak conceptual processing, with greater deactivation for more difficult conditions (42). All these findings indicate that activity in this network can quickly vary in tandem with behavioral changes.
More generally, the relevance of data acquired during rest in humans or primates to the understanding of brain-behavior relations is an ongoing domain of inquiry and debate (43). This debate is particularly relevant for the study of high-level cognitive functions such as language, because certain networks identified in primates, e.g., mirror neuron regions (44), are implicated in high-level functions in humans, such as syntactic working memory, speech perception, or even social cognition. As such, the fact that the default network is often associated with high-level functions such as social cognition and thinking of the past and future is consistent with the idea that its activity in awake humans extends beyond basic physiology. In this sense, our account can complement a version of the intrinsic view that speaks to the network's functional role during long rest periods, but without making the inductive leap to stating that this mode of operation maintains during wakeful states. A useful conceptual middle ground between these 2 complementary approaches may be that the network mediates creative thought, which is a mode of thought that bridges the freedom to generate content with some degree of task dependence (45).
Quantifying the relative degree of spontaneity or context-dependence of this network's activity is a major goal of future work. This is likely to be a nontrivial endeavor, as even seemingly random patterns can reflect complex regularities (46). In any case, our findings suggest reconsidering the notion that the default network mediates purely spontaneous cognitive functions, as they indicate that its mode of connectivity is sensitive to both ongoing and recent demands. Furthermore, they indicate that to understand its modes of connectivity it would be useful to consider how ongoing and recent task demands as well as individual abilities jointly determine the nature of synchronized activity within this network.
Experimental Procedures
Technical details necessary for replicating the analysis workflow are given in Extended Experimental Procedures in SI Text. Anatomical and functional images were acquired from 23 participants (3-Tesla scanner, functional resolution = 3.8 mm3). Participants listened to 3 types of material: stories ending with surprising endings, stories with nonsurprising endings, and thematically unrelated sentences. Each auditory material was followed by 22 sec of rest. After the scan, memory for these contents was assessed. A regression-based analysis of these data was previously reported (34). For analysis of functional connectivity (FC), nuisance sources of variance were removed from the time series, which were then projected onto a surface representation of each participant's brain (using FreeSurfer) (47) and smoothed spatially to increase the signal-to-noise ratio. For each participant, individual-level FC maps were generated against 2 seed regions (left precuneus, left angular gyrus). FC maps were generated for each of the 6 cognitive states of interest (listening to the 3 types of content, and the rest periods that immediately followed). These were created by extracting and then concatenating the relevant acquisitions of interest from the main time series (see ref. 48). As rest periods, we considered acquisitions beginning 10 sec after the preceding story had ended and continuing 16 sec from that point on. Group-level maps were generated by first averaging the individual-level maps constructed for each of the 2 seed regions and then creating conjunction maps to find effects that were common to the analyses against both seed regions [the conjunction analysis accounted for the intrinsic correlation between the 2 seed regions (see Fig. S5) and controlled for a family-wise error rate of P < 0.05 using cluster thresholds]. A full description of these methods and the quantification of the relation between the strength of FC and subsequent memory for the materials is given in SI Text.
Supplementary Material
Acknowledgments.
We thank Anthony Dick, Charlie Gaylord, and Jeremy Skipper for their comments on drafts of this manuscript. The fMRI study was supported by National Institute of Deafness and other Communication Disorders Grant R01-DC03378 and by the James S. McDonnell Foundation under a grant to the Brain Network Recovery Group.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0903253106/DCSupplemental.
References
- 1.Gusnard DA, Raichle ME. Searching for a baseline: Functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2(10):685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 2.Shulman GL, et al. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 3.Fox MD, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fransson P. Spontaneous low-frequency BOLD signal fluctuations: An fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26(1):15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raichle ME, Gusnard DA. Intrinsic brain activity sets the stage for expression of motivated behavior. J Comp Neurol. 2005;493(1):167–176. doi: 10.1002/cne.20752. [DOI] [PubMed] [Google Scholar]
- 6.Boly M, et al. Intrinsic brain activity in altered states of consciousness: How conscious is the default mode of brain function? Ann NY Acad Sci. 2008;1129:119–129. doi: 10.1196/annals.1417.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mason MF, et al. Wandering minds: The default network and stimulus-independent thought. Science. 2007;315(5810):393. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKiernan KA, D'Angelo BR, Kaufman JN, Binder J. Interrupting the “stream of consciousness”: An fMRI investigation. Neuroimage. 2006;29(4):1185–1191. doi: 10.1016/j.neuroimage.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 10.Eichele T, et al. Prediction of human errors by maladaptive changes in event-related brain networks. Proc Natl Acad Sci USA. 2008;105(16):6173–6178. doi: 10.1073/pnas.0708965105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li CSR, Yan P, Bergquist KL, Sinha R. Greater activation of the “default” brain regions predicts stop signal errors. Neuroimage. 2007;38(3):640–648. doi: 10.1016/j.neuroimage.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kounios J, et al. The prepared mind: Neural activity prior to problem presentation predicts subsequent solution by sudden insight. Psychol Sci. 2006;17:882–890. doi: 10.1111/j.1467-9280.2006.01798.x. [DOI] [PubMed] [Google Scholar]
- 13.Miall RC, Robertson EM. Functional imaging: Is the resting brain resting? Curr Biol. 2006;16(23):998–1000. doi: 10.1016/j.cub.2006.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Floyer-Lea A, Matthews PM. Distinguishable brain activation networks for short- and long-term motor skill learning. J Neurophysiol. 2005;94(1):512–518. doi: 10.1152/jn.00717.2004. [DOI] [PubMed] [Google Scholar]
- 15.Peltier SJ, et al. Reductions in interhemispheric motor cortex functional connectivity after muscle fatigue. Brain Res. 2005;1057(1–2):10–16. doi: 10.1016/j.brainres.2005.06.078. [DOI] [PubMed] [Google Scholar]
- 16.Waites AB, Stanislavsky A, Abbott DF, Jackson GD. Effect of prior cognitive state on resting state networks measured with functional connectivity. Hum Brain Mapp. 2005;24(1):59–68. doi: 10.1002/hbm.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sidtis JJ, Strother SC, Rottenberg DA. The effect of set on the resting state in functional imaging: A role for the striatum? Neuroimage. 2004;22(3):1407–1413. doi: 10.1016/j.neuroimage.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 18.Treyer V, Buck A, Schnider A. Effects of baseline task position on apparent activation in functional imaging of memory. Neuropsychologia. 2006;44(3):462–468. doi: 10.1016/j.neuropsychologia.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Murphy B, Dalponte M, Poesio M, Bruzzone L. Distinguishing concept categories from single-trial electrophysiological activity. In: Love BC, McRae K, Sloutsky VM, editors. Proceedings of the 30th Annual Meeting of the Cognitive Science Society; Austin, TX: Cognitive Science Society; 2008. pp. 403–408. [Google Scholar]
- 20.Greicius MD, Menon V. Default-mode activity during a passive sensory task: Uncoupled from deactivation but impacting activation. J Cogn Neurosci. 2004;16:1484–1492. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- 21.Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006;26(51):13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esposito F, et al. Independent component model of the default-mode brain function: Assessing the impact of active thinking. Brain Res Bull. 2006;70(4–6):263–269. doi: 10.1016/j.brainresbull.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Garrity AG, et al. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164(3):450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- 24.Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44:2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 25.Harrison BJ, et al. Consistency and functional specialization in the default mode brain network. Proc Natl Acad Sci USA. 2008;105(28):9781–9786. doi: 10.1073/pnas.0711791105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skipper JI, Goldin-Meadow S, Small SL. Gestures orchestrate brain networks for language understanding. Curr Biol. 2009;19:661–667. doi: 10.1016/j.cub.2009.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- 28.Titone D, Prentice KJ, Wingfield A. Resource allocation during spoken discourse processing: Effects of age and passage difficulty as revealed by self-paced listening. Mem Cognit. 2000;28(6):1029–1040. doi: 10.3758/bf03209351. [DOI] [PubMed] [Google Scholar]
- 29.Calhoun VD, Kiehl KA, Pearlson GD. Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Hum Brain Mapp. 2008;29:828–838. doi: 10.1002/hbm.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monto S, Palva S, Voipio J, Palva JM. Very slow EEG fluctuations predict the dynamics of stimulus detection and oscillation amplitudes in humans. J Neurosci. 2008;28(33):8268–8272. doi: 10.1523/JNEUROSCI.1910-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andrews-Hanna JR, et al. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56(5):924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15(3):394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- 33.Daselaar SM, Prince SE, Cabeza R. When less means more: Deactivations during encoding that predict subsequent memory. Neuroimage. 2004;23:921–927. doi: 10.1016/j.neuroimage.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 34.Hasson U, Nusbaum HC, Small SL. Brain networks subserving the extraction of sentence information and its encoding to memory. Cereb Cortex. 2007;17:2899–2913. doi: 10.1093/cercor/bhm016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bar M. The proactive brain: Using analogies and associations to generate predictions. Trends Cogn Sci. 2007;11(7):280–289. doi: 10.1016/j.tics.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: The prospective brain. Nat Rev Neurosci. 2007;8(9):657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- 37.Schilbach L, Eickhoff SB, Rotarska-Jagiela A, Fink GR, Vogeley K. Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Conscious Cogn. 2008;17(2):457–467. doi: 10.1016/j.concog.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 38.Johnson-Laird PN. Mental Models. Cambridge, MA: MIT Press; 1989. [Google Scholar]
- 39.Fauconnier G. Mental Spaces. Cambridge, UK: Cambridge Univ Press; 1994. [Google Scholar]
- 40.Uddin LQ, et al. Residual functional connectivity in the split-brain revealed with resting-state functional MRI. Neuroreport. 2008;19(7):703–709. doi: 10.1097/WNR.0b013e3282fb8203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pagnoni G, Cekic M, Guo Y. “Thinking about not-thinking”: Neural correlates of conceptual processing during Zen meditation. PLoS ONE. 2008;3(9):e3083. doi: 10.1371/journal.pone.0003083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh KD, Fawcett IP. Transient and linearly graded deactivation of the human default-mode network by a visual detection task. Neuroimage. 2008;41:100–112. doi: 10.1016/j.neuroimage.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 43.Morcom AM, Fletcher PC. Does the brain have a baseline? Why we should be resisting a rest. Neuroimage. 2007;37(4):1073–1082. doi: 10.1016/j.neuroimage.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 44.Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- 45.Christoff K, Gordon A, Smith R. The role of spontaneous thought in human cognition. In: Vartanian O, Mandel DR, editors. Neuroscience of Decision Making. Philadelphia: Psychology Press; 2008. [Google Scholar]
- 46.Costa M, Goldberger AL, Peng CK. Multiscale entropy analysis of complex physiologic time series. Phys Rev Lett. 2002;89(6) doi: 10.1103/PhysRevLett.89.068102. 068102(068101)–068102(068104) [DOI] [PubMed] [Google Scholar]
- 47.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis ll: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 48.Fair DA, et al. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage. 2007;35(1):396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.