Abstract
PACT is a double-stranded RNA-binding protein that also binds and activates the latent protein kinase, PKR, which plays a major role in cellular antiviral defense in mammals. For evaluating PACT's contribution to the innate immune system, Pact−/− mice have been generated; these mice exhibit notable developmental abnormalities including microtia, with craniofacial, ear, and hearing defects. Here we report that, in addition, Pact−/− mice had smaller body size and fertility defects, both of which were caused by defective pituitary functions. Pact−/− mice exhibited anterior pituitary lobe (AL) hypoplasia, which developed postnatally, when the second phase of pituitary expansion occurs. Among the 5 cell types in AL, the numbers of corticotrophs, gonadotrophs, and somatotrophs were equally decreased in Pact−/− mice with a greater impact on lactotrophs and a lesser impact on thyrotrophs. PACT mRNA and protein were highly expressed in the pituitary of wild-type (Wt) mice during the postnatal wave of AL proliferation, the same period in which the hypoplasia developed in Pact−/− mice. During this time, the pituitaries of Pact−/− mice did not exhibit significantly increased apoptosis compared with Wt mice but showed a decrease in cell proliferation. The inhibition of cell proliferation observed in vivo could be recapitulated in vitro in GH3 somato/lactotroph and LβT2 gonadotroph cell lines; knockdown of PACT expression with siRNA diminished the rate of proliferation of these cells. Our study revealed a physiologically significant role for PACT in cell proliferation and an essential role of a dsRNA-binding protein in mammalian pituitary expansion.
Keywords: adenohypophysis, development, fertility, hypoplasia, pituitary expansion
The human protein PACT (PRKRA) and its murine homolog RAX were first identified as protein activators of the latent protein kinase, PKR (1, 2). PACT, RAX, and PKR share the ability to bind double-stranded RNA (dsRNA), imparted by 2 dsRNA-binding motifs (dsRBMs) present in the amino-terminal half of each protein. Much of our understanding of PACT has been in the context of PKR activation, long been known for its critical role in the IFN-mediated cellular antiviral response (3–5). Upon binding viral dsRNA, PKR becomes activated by autophosphorylation and achieves its antiviral effect by subsequently blocking protein synthesis. A hallmark of PACT function is its ability to activate PKR in the absence of dsRNA, suggesting PACT has virus-independent cellular roles (6). The dsRBMs of PACT, domains 1 and 2, mediate strong interaction with the 2 dsRBMs of PKR, but the ability to activate PKR is imparted by a third domain of PACT that binds to the PKR kinase domain (7–9). Binding of domain 3 causes PKR autophosphorylation by disrupting an intramolecular interaction within PKR that is responsible for keeping PKR in an inactive conformation (10). In mammalian cells, PACT itself is phosphorylated by treatment with various chemical stresses or growth factor withdrawal, allowing it to associate with PKR with increased affinity (7, 9, 11, 12).
Besides the ability to bind dsRNA, dsRNA-binding proteins (DRBPs) function in a diverse array of critically important roles in the cell (reviewed in 13). In addition to regulating translation, many DRBPs have been implicated in development, RNA transport, processing, and stability. Often, distinct biochemical, cellular, and physiological effects are mediated by regions of the protein outside of the dsRBM motifs. In all cases, disruption of genes encoding DRBPs in mice has resulted in a major change in their phenotypes. Many times, disruption of specific dsRBPs can cause embryonic lethality, as in the case with those lacking RNA helicase A (14) and the RNA-editing enzyme ADAR1 (15). In contrast, ADAR2-deficient mice are viable, but develop seizures and die early (16). Deletion of spermatid perinuclear RNA-binding protein (Sprbp) in mice caused fertility defects, high mortality after birth, and reduced body size (17). Mice carrying a targeted disruption of Tarbp, which encodes the mouse orthologue of human TRBP, were sterile, significantly smaller than their littermates, and usually died at the time of weaning (18). These mice could survive to adulthood with a dietary supplement, but never grew to normal size and had defects in spermatogenesis.
The complete biological roles of PACT are still being defined. To study the role of PACT in physiology, we previously generated Pact−/− mice in which PACT protein expression was completely ablated by disrupting the single copy mouse gene (19). These mice had craniofacial defects, congenital abnormalities of outer and middle ears, were hearing impaired, and serve as an animal model for studying human microtia. Notably, these defects were not present in 2 models of PKR-deficient mice (20, 21), suggesting additional roles of PACT beyond those in innate immunity. The current study was designed to investigate the additional unique phenotypes present in Pact−/− mice: postnatal growth retardation and fertility defects. We show that at birth, Pact−/− animals have normal pituitary size but develop hypoplasia of the anterior lobe postnatally, leading to decreased hormone levels, fertility defects, and growth impairment.
Results
Mice Lacking PACT Have Postnatal Growth and Fertility Defects.
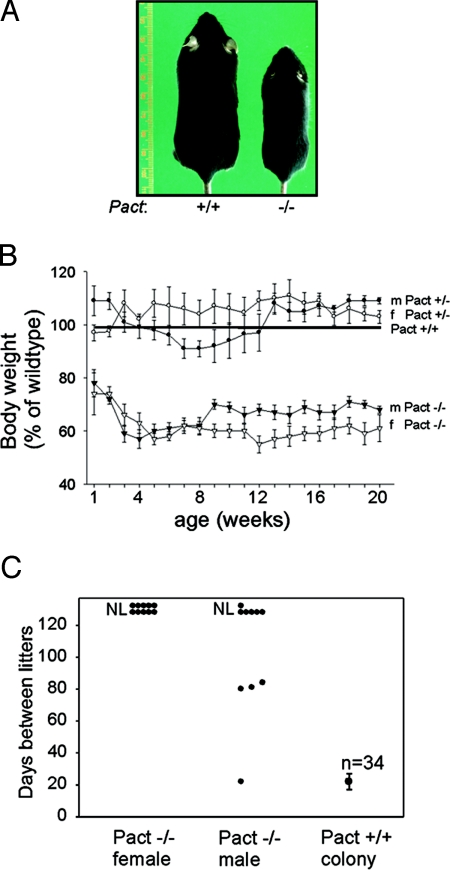
Previously, we generated Pact−/− mice and showed that these mice had developmental defects of the craniofacial area and the external/internal ears, which led to hearing impairment (19). Adult mice had a severe reduction in the size of the outer ear and a large reduction in overall body size versus wild-type (Wt) mice (Fig. 1A). At birth, there was no noticeable difference in body size or weight of Pact−/− mice when compared to Wt mice or Pact+/−mice. However, by 1 week after birth, the Pact−/− mice clearly showed a significant difference in both body size and weight (Fig. 1B). The difference in weight did not change over the whole life span of the Pact−/− mice, which typically weighed approximately 40% less than Wt mice. In the current study, Pact−/− mice were tested for fertility by placing them with proven breeders for 128 days. Pact−/− females were completely sterile and never gave birth to any animals, compared to Wt females which consistently produced litters every 21–24 days (Fig. 1C). Most Pact−/− males did not sire any litters, and the majority of the successful breeders took at least 3 times longer to impregnate females. Vaginal plug studies showed that 50% of Pact−/− mice were plugged by proven breeder males over a 30-day period, indicating that at least some of the females demonstrate estrus behavior. However, examination of the reproductive tracts of plugged Pact−/− mice at 10 or 21 days postcoitus showed no implantation sites or embryos. Plugged Wt mice delivered pups at 21 days after breeding, while plugged Pact−/− mice were not pregnant after 30 days.
Fig. 1.
Growth retardation and fertility defects in Pact−/− mice. (A) Pact+/+ female (Left) and a Pact−/− female littermate (Right) at 90 days after birth. (B) Comparison of body weights of male and female Pact+/− and Pact−/− mice expressed as a percentage of male or female Pact+/+, respectively (set at 100). Error bars, S.E., and n varies from 6 to 28 mice per time point. (C) Fertility defects of Pact−/− mice. Individual male or female Pact−/− mice were placed with proven C57/BL6 breeders for a total of 128 days and cages were monitored for litters. All proven breeders previously sired or gave birth successfully to 1 litter. Ten male Pact−/− mice and 10 female Pact−/− mice were tested. NL, no litters by 128 days.
Pact−/− Mice Have Ovarian and Mammary Gland Defects.
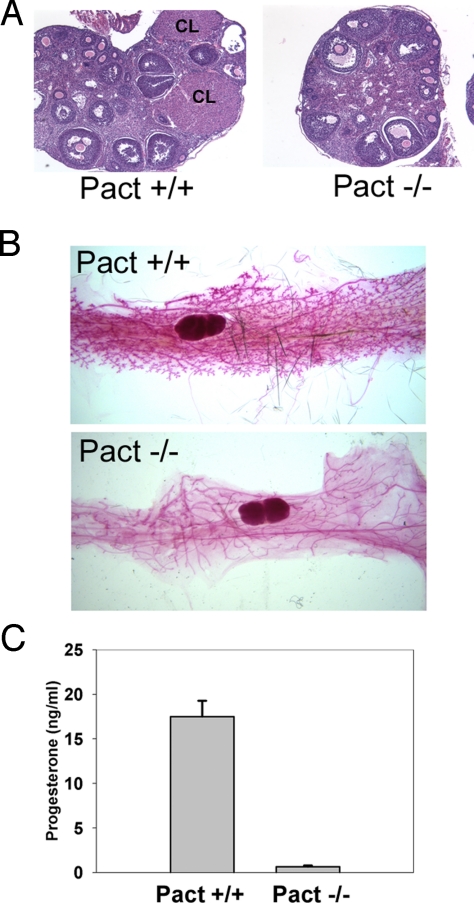
Analysis of the ovaries of postpubertal Pact−/− mice revealed that while they were small, they had normal preantral follicular development and maturation. However, the ovaries were defective in the formation of the corpus luteum (CL). We sectioned through entire ovaries of Wt and Pact−/− mice and consistently found that Wt ovaries had several CL per section while CL were mostly absent in Pact−/− ovaries (Fig. 2A), although some sections had 1. This suggested that Pact−/− mice were not ovulating to the extent of Wt mice or that they could not form CL efficiently, both processes being mediated by pituitary hormones. When whole mount mammary glands were examined, there was defective tertiary branching and very few alveolar buds in the Pact−/− animals (Fig. 2B), likely due to low serum progesterone and/or prolactin levels (22, 23). In rodents, prolactin is the major luteotrophic hormone, released by the pituitary, that maintains the structural and functional integrity of the corpus luteum for several days after mating (24). Progesterone, produced by the CL, acts on the uterus to prepare it for implantation (25). Direct measurement of serum progesterone showed severely reduced levels in Pact−/− mice (Fig. 2C). Collectively, the sharp decrease in progesterone levels, lack of CL, and mammary gland branching defects were consistent with low levels of prolactin secreted by the pituitary.
Fig. 2.
Low progesterone levels accompanied by ovarian and mammary gland defects in Pact −/− mice. (A) Reduced numbers of corpora lutea in the ovaries of Pact−/− mice. Panels show representative sections of H&E-stained ovaries. CL, corpus luteum. (B) Defective tertiary branching and alveoli formation in mammary glands of Pact−/− mice. Whole mammary gland mounts of Pact+/+ and Pact−/− are shown. (C) Serum progesterone levels in female Pact+/+ and Pact−/− mice, measured by RIA. Pact+/+, n = 3; Pact−/−, n = 3.
Pact−/− Mice Have Anterior Lobe-Specific Pituitary Hypoplasia.
The ovarian defect was not intrinsic to this tissue because the Pact−/− mice could be superovulated, which suggested a defect in the hypothalamic-pituitary axis. We mimicked FSH and LH release from the pituitary by injecting the mice with pregnant mare serum gonadotrophin (PMSG) and human chorionic gonadotrophin (hCG), which is well-established method to determine whether fertility defects are intrinisic or extrinsic to the ovary. After injection of superovulatory hormones, Pact−/− mice produced 39 oocytes while Wt mice produced 30 oocytes using the same regimen. Thus, Pact−/− mice can ovulate successfully if given the proper signals from the pituitary. Direct measurement of serum FSH showed reduced levels in uninjected Pact−/− mice compared with Pact+/+ mice [supporting information (SI) Fig. S1A], while the same comparison showed that serum LH levels were significantly reduced in the Pact−/− animals (Fig. S1B). It appears that levels of FSH are above a threshold that would allow Pact−/− mice to exhibit normal preantral follicular development and maturation, but the LH levels are not high enough to allow proper ovulation, paralleling the ovarian phenotype (Fig. 2A).
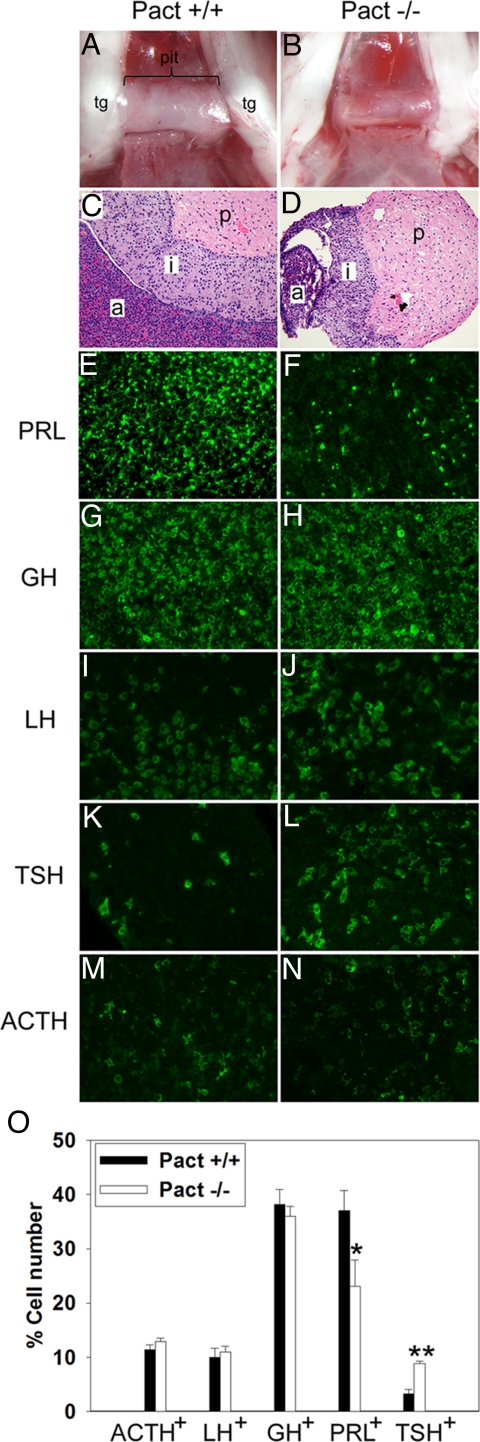
Examination of pituitaries of Pact−/− mice showed a striking hypoplasia of the pituitary in adult mice (Fig. 3 A and B). Notably, the hypoplasia occurred only in the anterior lobe, but not the posterior or intermediate lobes of the pituitary (Fig. 3 C and D). In Pact−/− animals, the posterior or intermediate lobe size to body size ratios were similar to those of Wt animals, but the anterior lobe ratio to body size was sharply decreased. This indicated that there is a specific anterior lobe hypoplasia that cannot be attributed solely to small body size.
Fig. 3.
Severe hypoplasia of the anterior pituitary lobe in adult Pact−/− mice. Adult pituitaries from female littermates Pact+/+ (A and C) or Pact−/− mice (B and D) were examined at P90. (A) and (B) are light micrographs showing the pituitary (pit), which lies perpendicular between the 2 trigeminal nerves (tg) on the base of the skull. C and D are H&E-stained sections of pituitary. p, posterior lobe; i, intermediate lobe; a, anterior lobe. When normalized to body size, Pact−/− mice have normal sized posterior and intermediate lobes, but a severely reduced anterior lobe. All anterior pituitary lobe cell lineages are present in Pact−/− mice. Immunohistochemistry of pituitaries from Pact+/+ (E, G, I, K, and M) and Pact−/− (F, H, J, L, and N) mice. (E and F) Prolactin (lactotrophs); (G and H) Growth Hormone (somatotrophs). (I and J) Luteinizing Hormone, (gonadotrophs); (K and L) Thyroid-stimulating Hormone, (thyrotrophs); (M and N) Adrenocorticotophic hormone (corticotrophs). (O) Hypoplasia in adult Pact−/− mice is more pronounced in the lactotroph population, and the percentage of thyrotrophs is increased. The percentage of cells expressing each hormone per DAPI-stained cell is shown. Hormone-positive/DAPI-positive averages were determined from counting multiple sections from each mouse; error bars, SEM. * = P < 0.05, ** = P < 0.005, Pact+/+ vs. Pact−/− mice. The number of animals represented for each hormone are indicated for Wt and ko, respectively: ACTH: n = 5, 6; LH: n = 4, 5; GH: n = 6, 6; PRL: n = 6, 7; and TSH: n = 5, 5. An average of 8,300 cells were counted for each hormone per genotype.
The anterior lobe is a highly differentiated tissue comprised of 5 types of cells: lactotrophs, somatotrophs, gonadotrophs, thyrotrophs, and corticotrophs; each cell type produces a particular hormone in response to stimulatory or inhibitory signals from the hypothalamus. To determine whether the hypoplasia was a result of the lack of a specific lineage or cell type within the anterior lobe, immunohistochemistry was performed using specific antisera for hormones produced by each cell type (Fig. 3 E–N). In the Pact−/− pituitary, cells of all lineages were present, suggesting that the anterior lobe hypoplasia in Pact−/− mice was not confined to any 1 lineage. To quantify all of the lineages in the hypoplastic Pact−/− pituitary, we counted the number of cells expressing the particular hormones. In Pact−/− pituitaries, the hypoplasia equally decreased corticotrophs, gonadotrophs, and somatotrophs, but the decrease was more severe in the number of lactotrophs (Fig. 3O). The diminished percentage of lactotrophs paralleled an increase in the percentage of thyrotrophs.
PACT Is Essential for Postnatal Development of the Anterior Lobe.
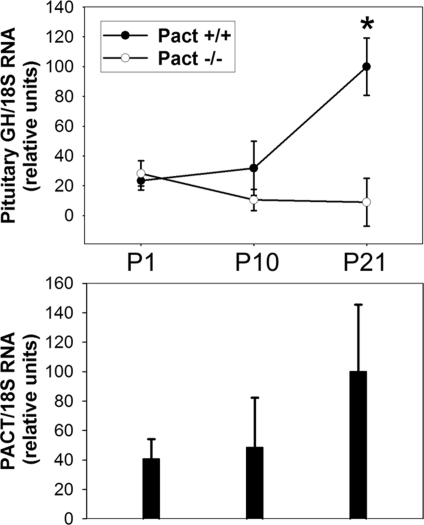
During rat and mouse pituitary development, anterior lobe (AL) cells normally undergo both a pre- and postnatal wave of proliferation (26, 27). Sections of postnatal day 1 (P1) Pact−/− pup heads revealed that the AL hypoplasia is not present at birth, since both Wt and Pact−/− pituitaries were almost identical in size (Fig. S2A and B). The hypoplasia started to develop after birth, which we demonstrated using real time RT-PCR to quantify the reduced numbers of anterior lobe cells in the Pact−/− mice. We quantified growth hormone (GH) mRNA as a marker for the number of cells within the pituitary. GH mRNA from pooled brain and pituitary collected at P1, P10, and P21, was normalized to the amount of 18S ribosomal RNA. On postnatal day 1, there were similar numbers of anterior lobe cells in Wt and Pact−/− pituitaries. However by day P21, the knockout mice had significantly less GH/18S mRNA levels than Wt mice (Fig. 4, upper panel), indicative of a reduced number of anterior pituitary cells. This reduction persisted until adulthood. The same tissue preparations revealed that PACT mRNA was highly expressed in Wt mice during the postnatal wave of AL proliferation. At postnatal day 10, PACT mRNA levels were increased from those on the day of birth, and doubled by day P21 (Fig. 4, lower panel). PACT protein was also highly expressed in isolated pituitary at day P21 during pituitary expansion, and remained high in adult animals (Fig. S3). The absence of PACT in the Pact deficient mice paralleled the reduction in the size of the anterior pituitary lobe, which was visibly noticeable by day P21 (Fig. S2C and D).
Fig. 4.
Anterior pituitary hypoplasia, as measured by GH mRNA expression, occurs by day P21 in Pact−/− mice. (Top) Quantitative RT-PCR of GH mRNA at P1, P10, and P21 from brain and pituitary. There are fewer GH producing cells in Pact−/− mice by day P21. 18S RNA levels were measured to normalize for body size differences between Wt and Pact−/− animals. Relative values were calculated such that all samples were compared to the P21 Pact +/+ mean, which was set to 100. Pact+/+, n = 3 for each age; Pact−/−, n = 3 for each age. * = P < 0.05, P21 Pact+/+ vs. P21 Pact−/− mice. (Bottom) Pact mRNA levels in brain and pituitary extracts from Pact+/+ mice shown Top at P1, P10, and P21. Real-time RT-PCR was used to quantify Pact mRNA levels. Relative values were calculated such that all samples were compared to the P21 mean, which was set to 100. Similar extracts from the Pact−/− mice showed no detectable PCR product.
Postnatal Proliferation of Pituitary AL Depends On PACT.
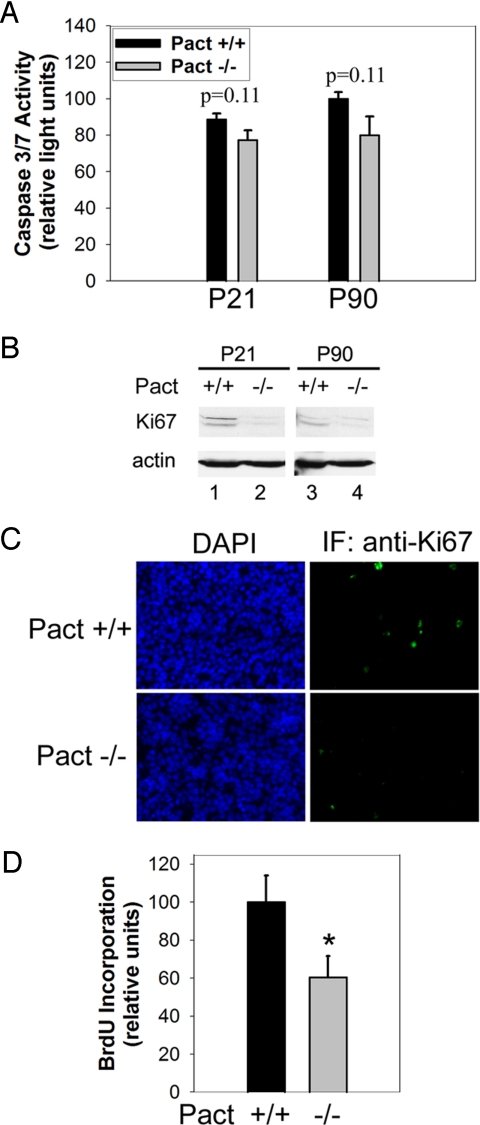
We determined whether the hypoplasia in Pact−/− mice was the result of increased apoptosis by measuring levels of caspase 3/7 activity from pooled pituitary extracts. Not only was there no increase, but there was a modest decrease in caspase 3/7 activity in Pact−/− mice compared with Wt littermates at day P21 or P90 (Fig. 5A). To investigate if Pact−/− hypoplasia was due to decreased cell proliferation in the knockout animals, we measured the levels of Ki67 and incorporation of bromodeoxyuridine (BrdU), markers for proliferating cells. Ki67 is expressed in actively dividing cells, but not quiescent cells (28), whereas BrdU is incorporated into new DNA during the S-phase of the cell cycle (29). Western blotting of whole pituitary extracts revealed decreased Ki67 levels in P21 and P90 Pact−/− mice compared to Wt littermates (Fig. 5B). Moreover, Ki67 immunohistochemistry of anterior pituitary lobe sections showed a decreased number of cells staining positive for Ki67 in Pact−/− animals at day P21 (Fig. 5C). Further analysis of pituitaries from BrdU-injected mice showed that Pact−/− do not incorporate BrdU to the extent of their Wt littermates, suggesting a regulatory role of PACT in proliferation of cells in the anterior lobe (Fig. S4). Quantification of BrdU labeling showed that anterior lobes from Pact−/− mice had 40% fewer BrdU-labeled cells than Wt control pituitaries (Fig. 5D). In both sets of mice, proliferating cells were scattered throughout the anterior lobe instead of being concentrated in discrete regions. We conclude that the presence of PACT was necessary for the normal proliferation of the anterior pituitary lobe after birth.
Fig. 5.
Levels of proliferation, but not apoptosis, are significantly reduced in pituitaries of postnatal day 21 Pact−/− mice versus Pact+/+ mice. (A) Caspase 3/7 assay of pituitaries from postnatal day 21 and 90 from Pact+/+ and Pact−/− mice. Protein content of each extract was determined, and caspase activity measured from the same set amount of protein. The amount of caspase activity in P90 wt mice was set to 100, and the values for the other mice are presented relative to that value. The nominal background level of caspase activity of buffer alone was subtracted from all values. n = 5 for all mice per time point. P < 0.11, P21 Pact+/+ vs. P21 Pact−/− mice or P90 Pact+/+ vs. P90 Pact−/− mice. (B) Proliferating-antigen Ki67 western blot of extracts from pituitaries from Pact+/+ and Pact−/− mice. Western blotting for mouse actin serves as a protein loading control. (C) Immunohistochemistry using pituitary sections from P21 Pact+/+ and Pact−/− mice. (Left) DAPI staining, showing all nuclei. (Right) Ki67-expressing cells revealed by green immunofluorescence. (D) Quantification of BrdU incorporation in the anterior pituitary lobe as a measure of proliferation in P21 Pact+/+ and Pact−/− mice. The percentage of cells incorporating BrdU per DAPI-stained cell was determined. The amount of BrdU incorporation in Pact +/+ mice was set to 100, and the value for Pact−/− is presented relative to that value. *, P < 0.05, P21 Pact+/+ vs. P21 Pact−/− mice. At least 4,800 cells were counted for each genotype.
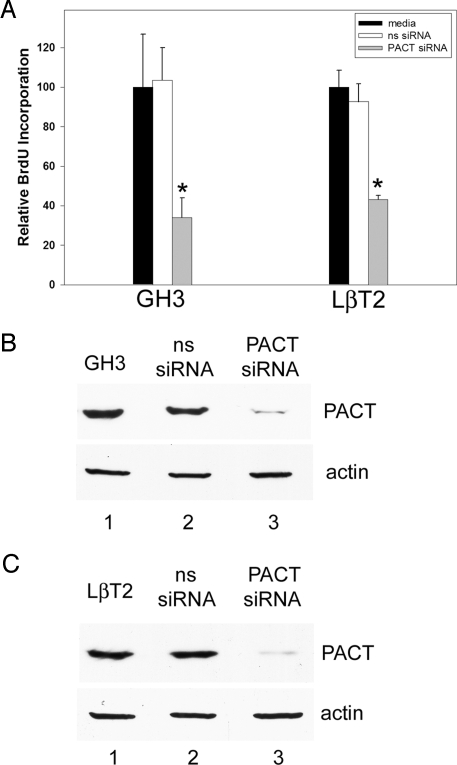
To further examine the role of PACT in proliferation of different lineages of the pituitary, we silenced its expression in rat somato/lactotroph GH3 cells and mouse LβT2 gonadotroph cells. PACT is highly expressed in both cell lines as well as in another gonadotroph mouse line, αT3. The GH3 cell line exhibits characteristics of somatotrophs and lactotrophs, expressing both GH and prolactin (PRL) (30). The LβT2 cell line secretes luteinizing hormone (LH) in response to GnRH (31, 32). Proliferation of GH3 and LβT2 cells was inhibited, as measured by BrdU incorporation (Fig. 6A and Fig S5), after small interfering RNA (siRNA)-mediated knockdown of PACT (Fig. 6 B and C). Non-targeting pools of siRNA did not alter proliferation of either cell line, demonstrating the decrease in proliferation in Pact siRNA knockdowns was specifically due to the absence of PACT.
Fig. 6.
Knockdown of PACT with siRNA in somatolactotrophs and in gonadotroph cell lines decreases cell proliferation. After 72 h of adding siRNA to PACT to actively dividing cells, BrdU was added for 1 h, and proliferation measured by immunostaining for the amount of BrdU incorporation. (A) Reduced BrdU incorporation after PACT knockdown in GH3 somatolactotrophs and LβT2 gonadotrophs. BrdU-positive/DAPI-positive averages were determined from counting multiple fields from each treatment. The amount of BrdU incorporation in media treatments were set to 100, and the values for the other treatments are presented relative to those values. Error bars, SEM. * = P < 0.05, ns siRNA vs. PACT siRNA. (B and C) PACT western blot of extracts from cells in (A). Western blotting for mouse actin serves as a protein loading control.
Discussion
We previously established a crucial role of the dsRNA-binding protein PACT in proper development of craniofacial areas, the ears, and hearing (19). Here, we report additional and more pervasive developmental defects of Pact−/− mice and trace their origin to the central defect in development of the anterior lobe of the pituitary. These mice were smaller in size and females had developmental defects of ovaries and mammary glands, which led to sterility. Ovaries of adult Pact−/− mice contained reduced numbers of corpus luteum; however, these mice could be superovulated, indicating a defect not intrinsic to the tissue, but in the hypothalamic-pituitary axis. These findings are reminiscent of the observed functional roles of other dsRNA-binding proteins, such as TRBP, Staufen, TENR and SPNR, in reproductive organs (17, 18, 33, 34). We demonstrated a specific hypoplasia of the pituitary anterior lobe, which resulted in a reduced number of cells comprising each lineage within the AL. We conclude that PACT deficiency caused a global defect in proliferation of anterior lobe cells, with especially decreased numbers of lactotrophs. Although the relative number of thyrotrophs was increased in the AL of the Pact−/− mouse (Fig. 3O), the absolute number might be the same as in a Wt mouse, indicating that the absence of PACT did not affect proliferation of these cells. All of the AL cell types were present in Pact−/− mice (Fig. 3), but because they were fewer in number, there was a decrease in pituitary mRNA production (Fig. 4A), leading to lower serum levels of pituitary hormones (Fig. S1). This decrease led to a lower level of gonadal hormones, resulting in mammary gland and ovarian defects (Fig. 2). Based on these results, we conclude that PACT deficiency causes hypopituitarism, suggesting PACT−/− mice could be an animal model for combined pituitary hormone deficiency (CPHD), a congenital human pituitary condition. It is unknown how the anterior pituitary defects relate to the microtia observed in these animals. In humans, microtia can occur as the only clinical abnormality or as part of a syndrome found together with the congenital defects of distant organs (35).
Before birth, precursor cells of the anterior pituitary lineages undergo dynamic regulation of proliferation and differentiation (reviewed in 27). Development of the anterior lobe is controlled by both intrinsic and extrinsic signals that regulate cascades of transcription factor gene expression. Modulation of specific signals permits terminal differentiation and the emergence of 5 cell types in a temporal and spatial fashion. In mice, lineage specification is largely completed by birth. However at birth, postnatal mice and rats undergo a second wave of pituitary proliferation, called expansion, which continues for 3–4 weeks (26). Although differentiated, postnatal anterior pituitary cells also have the adaptive and reversible ability to undergo mitosis under certain conditions. For example, in the pregnant female, estrogen mediates the gradual proliferation of lactotrophs. Recent studies have implicated Cdk4 in the postnatal proliferation of somato/lactotrophs leading to AL hypoplasia in Cdk4−/− animals, whereas the gonadotrophs remain unaffected (36).
Pact-deficient mice appear to have normal embryonic development of the pituitary; both the size of the pituitary and its level of GH mRNA were similar in newborn Wt and Pact−/− mice. However, the postnatal phase of AL expansion was defective in Pact−/− mice. Our experiments established that this was not due to enhanced cell death, but that PACT was required for proper cell proliferation. In vivo assays demonstrated that PACT had a direct effect on the proliferation of cells scattered throughout the anterior lobe. Compared to Wt animals on postnatal day 21, Pact-deficient animals had significant decreases in the numbers of cells labeling for BrdU and immunostaining for Ki67. These results were corroborated by those obtained in vitro using 2 cell lines of AL origin; we showed that ablation of PACT expression by siRNA resulted in decreased DNA replication and cell proliferation. PACT was highly expressed shortly after birth in the pituitary of Wt mice at the time of postnatal expansion. This correlation opens the possibility that PACT may affect cell proliferation in a cell and tissue-specific fashion, since Pact−/− mouse embryonic fibroblasts (MEFs) proliferate normally. Although PACT is expressed in both MEFs and most mouse tissues at low levels, some organs such as pituitary and brain express high levels of PACT, which may be required for its action. Indeed, PACT is also expressed at high levels in the reproductive organs and a part of the observed defect in their development might be directly attributable to the absence of PACT in the organs themselves.
Further cellular and biochemical studies will be needed to delineate the mechanism by which PACT facilitates cell proliferation; however, the known properties of PACT do not provide any obvious lead toward this goal. Because different domains of PACT can either activate or inhibit the protein kinase PKR (4, 7, 8, 37), it may be instructive to examine whether the observed action of PACT on cell proliferation is mediated by PKR. PACT is a dsRNA-binding protein as well, and this property may contribute to the observed effects. Finally, PACT is known to directly interact with other dsRNA-binding proteins and such an interaction with Dicer is required for the latter protein's optimum function in the miRNA processing pathway (38). Thus, it remains possible that the observed role of PACT in cell proliferation is connected to its role in miRNA biosynthesis.
In summary, PACT is a dsRNA-binding protein shown to be required for postnatal pituitary expansion in mice. In contrast to mice, human pituitary development is normally completed during the first trimester. The majority of combined pituitary hormone deficiency (CPHD) cases are known to be caused by mutations in transcription factors involved in this process. It is possible that PACT could regulate prenatal transcription factor gene expression whose effects on proliferation would not be manifested until after birth. In 1 example, mutations in the PROP1 transcription factor led to pituitary hypoplasia and reduced production of pituitary hormones that characterizes CPHD (39). The age of onset and the severity of the disease is variable in affected individuals. In addition, hormone deficiencies are progressive and can occur in childhood or later in life. It would be interesting to determine whether mutations in PACT or defects in PACT expression contribute to the onset or progression of this or related pituitary disorders.
Materials and Methods
Generation of Pact-deficient mice, cell lines, histology, mammary gland analysis, hormones, western blotting, and immunohistochemistry for Ki67 and pituitary hormones are described in detail in SI Methods. All animal experiments were approved by the CCF Institutional Animal Care and Ethics Committee.
Quantitative RT-PCR.
RNA was isolated from tissues using TRIzol reagent according to the manufacturer (Invitrogen). Reverse transcription was performed with random hexamers on DNase treated RNA with the SuperScript III Random Prime Synthesis Kit for RT-PCR (Invitrogen) according to the manufacturer. Real-time quantitative PCR was done using the primer sets for GH and PACT, listed in SI Methods. Real time quantitative PCR was done using an iCycler (Bio-Rad) with Sybr Green I (Applied Biosystems) as a fluorescent indicator.
Caspase 3/7 Assay.
Pituitaries from Pact+/+ or Pact−/− mice were flash frozen in liquid nitrogen and extracts made as described in SI Methods. Caspase 3/7 activity was measured directly on these extracts using the Apo-ONETM Homogenous Caspase-3/7 assay protocol (Promega), using a 1:1 ratio of homogenous caspase 3/7 reagent to sample volume.
BrdU in Situ Detection.
To examine proliferation in the pituitary during postnatal development, individual mouse pups were injected with BrdU at 100 mg/g body weight and killed 2 h later. Pituitaries were embedded in OCT compound (Sakura Finetek) and frozen in liquid nitrogen. After cryosectioning and fixation, BrdU-labeled cells were detected with the BrdU In situ Detection Kit (BD Biosciences). For antigen retrieval, the tissue was boiled in Retrievigen solution and kept at 89 °C for 15 min. The signal was amplified using Tyramide signal amplification-fluorescein (NEN Life Science). Cells were counterstained using Vectashield with DAPI (Vector Labs).
siRNA and Cell Proliferation Assay.
Accell non-targeting mouse or rat siRNA pools and SMART pool siRNAs that specifically target mouse or rat Pact were obtained from Dharmacon Research and were introduced into GH3 or LβT2 cells using the manufacturer's protocol. After 72 h, cells were treated for 1 h with 5 μL/mL BrdU and then fixed. BrdU labeled cells were detected with the BrdU In situ Detection Kit as described above. Percentages of BrdU+ cells were quantified in random fields from cells treated with control, non-specific siRNAs, and targeted Pact siRNAs.
Supplementary Material
Acknowledgments.
We thank Kristen Lozada, Theresa Rowe, and Christine White for technical assistance; Linda Vargo (Cleveland Clinic Histology Core) and the Histology Core Facility of Case Western Reserve Unitversity (P30 CA43703) for sectioning; Terry Nett and Matt Allen (Colorado State) for follicle-stimulating/luteinizing hormone measurements; Judy Drazba and John Peterson for imaging assistance; Ken Korach and John Couse for initial consultation; and Jocelyn McDonald for comments on the manuscript. Research was supported by National Institutes of Health Grants #CA068782–21 and #CA062220–10S100005 (to G.C.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900735106/DCSupplemental.
References
- 1.Patel RC, Sen GC. PACT, a protein activator of the interferon-induced protein kinase, PKR. EMBO J. 1998;17:4379–4390. doi: 10.1093/emboj/17.15.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ito T, Yang M, May WS. RAX, a cellular activator for double-stranded RNA-dependent protein kinase during stress signaling. J Biol Chem. 1999;274:15427–15432. doi: 10.1074/jbc.274.22.15427. [DOI] [PubMed] [Google Scholar]
- 3.Williams BR. PKR, a sentinel kinase for cellular stress. Oncogene. 1999;18:6112–6120. doi: 10.1038/sj.onc.1203127. [DOI] [PubMed] [Google Scholar]
- 4.Peters GA, Khoo D, Mohr I, Sen GC. Inhibition of PACT-mediated activation of PKR by the herpes simplex virus type 1 Us11 protein. J Virol. 2002;76:11054–11064. doi: 10.1128/JVI.76.21.11054-11064.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li S, Min JY, Krug RM, Sen GC. Binding of the influenza A virus NS1 protein to PKR mediates the inhibition of its activation by either PACT or double-stranded RNA. Virology. 2006;349:13–21. doi: 10.1016/j.virol.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 6.D'Acquisto F, Ghosh S. PACT and PKR: Turning on NF-kappa B in the absence of virus. SciSTKE. 2001;89:1–4. doi: 10.1126/stke.2001.89.re1. [DOI] [PubMed] [Google Scholar]
- 7.Peters GA, Hartmann R, Qin J, Sen GC. Modular structure of PACT: Distinct domains for binding and activating PKR. Mol Cell Biol. 2001;21:1908–1920. doi: 10.1128/MCB.21.6.1908-1920.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang X, Hutchins B, Patel RC. The C-terminal, third conserved motif of the protein activator PACT plays an essential role in the activation of double-stranded-RNA-dependent protein kinase (PKR) Biochem J. 2002;366:175–186. doi: 10.1042/BJ20020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters GA, Li S, Sen GC. Phosphorylation of specific serine residues in the PKR activation domain of PACT is essential for its ability to mediate apoptosis. J Biol Chem. 2006;281:35129–35136. doi: 10.1074/jbc.M607714200. [DOI] [PubMed] [Google Scholar]
- 10.Li S, et al. Molecular basis for PKR activation by PACT or dsRNA. Proc Natl Acad Sci USA. 2006;103:10005–10010. doi: 10.1073/pnas.0602317103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel CV, Handy I, Goldsmith T, Patel RC. PACT, a stress-modulated cellular activator of interferon-induced double-stranded RNA-activated protein kinase, PKR. J Biol Chem. 2000;275:37993–37998. doi: 10.1074/jbc.M004762200. [DOI] [PubMed] [Google Scholar]
- 12.Chen G, Ma C, Bower KA, Ke Z, Luo J. Interaction between RAX and PKR modulates the effect of ethanol on protein synthesis and survival of neurons. J Biol Chem. 2006;281:15909–15915. doi: 10.1074/jbc.M600612200. [DOI] [PubMed] [Google Scholar]
- 13.Saunders LR, Barber GN. The dsRNA binding protein family: Critical roles, diverse cellular functions. FASEB J. 2003;17:961–983. doi: 10.1096/fj.02-0958rev. [DOI] [PubMed] [Google Scholar]
- 14.Lee C-G, et al. RNA helicase A is essential for normal gastrulation. Proc Natl Acad Sci USA. 1998;95:13709–13713. doi: 10.1073/pnas.95.23.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Q, Khillan J, Gadue P, Nishikura K. Requirement of the RNA editing deaminase ADAR1 gene for embryonic erythropoiesis. Science. 2000;290:1765–1768. doi: 10.1126/science.290.5497.1765. [DOI] [PubMed] [Google Scholar]
- 16.Higuchi M, et al. Point mutation in an AMPA receptor gene rescues lethality in mice deficient in the RNA-editing enzyme ADAR2. Nature. 2000;406:78–81. doi: 10.1038/35017558. [DOI] [PubMed] [Google Scholar]
- 17.Pires-daSilva A, et al. Mice deficient for spermatid perinuclear RNA-binding protein show neurologic, spermatogenic, and sperm morphological abnormalities. Developmental Biology. 2001;233:319–328. doi: 10.1006/dbio.2001.0169. [DOI] [PubMed] [Google Scholar]
- 18.Zhong J, Peters AH, Lee K, Braun RE. A double-stranded RNA binding protein required for activation of repressed messages in mammalian germ cells. Nat Genet. 1999;22:171–174. doi: 10.1038/9684. [DOI] [PubMed] [Google Scholar]
- 19.Rowe TM, et al. A role of the double-stranded RNA-binding protein PACT in mouse ear development and hearing. Proc Natl Acad Sci USA. 2006;103:5823–5828. doi: 10.1073/pnas.0601287103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang YL, et al. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 1995;14:6095–6106. doi: 10.1002/j.1460-2075.1995.tb00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abraham N, et al. Characterization of transgenic mice with targeted disruption of the catalytic domain of the double-stranded RNA-dependent protein kinase, PKR. J Biol Chem. 1999;274:5953–5962. doi: 10.1074/jbc.274.9.5953. [DOI] [PubMed] [Google Scholar]
- 22.Horseman ND. Prolactin and mammary gland development. J Mammary Gland Biol Neoplasia. 1999;4:79–88. doi: 10.1023/a:1018708704335. [DOI] [PubMed] [Google Scholar]
- 23.Atwood CS, et al. Progesterone induces side-branching of the ductal epithelium in the mammary glands of peripubertal mice. J Endocrinol. 2000;167:39–52. doi: 10.1677/joe.0.1670039. [DOI] [PubMed] [Google Scholar]
- 24.Stocco C, Telleria C, Gibori G. The molecular control of corpus luteum formation, function, and regression. Endocr Rev. 2007;28:117–149. doi: 10.1210/er.2006-0022. [DOI] [PubMed] [Google Scholar]
- 25.Franco HL, Jeong JW, Tsai SY, Lydon JP, DeMayo FJ. In vivo analysis of progesterone receptor action in the uterus during embryo implantation. Semin Cell Dev Biol. 2008;19:178–186. doi: 10.1016/j.semcdb.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Smets G, et al. Ontogeny of hormone-secreting cells of the rat pituitary gland: an immunocytochemical study on dissociated cells. Histochem J. 1989;21:337–342. doi: 10.1007/BF01798496. [DOI] [PubMed] [Google Scholar]
- 27.Zhu X, et al. Genetic control of pituitary development and hypopituitarism. Curr Opin Genet Dev. 2005;15:332–340. doi: 10.1016/j.gde.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Gerdes JH, et al. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- 29.Packard DS, Jr, Menzies RA, Skalko RG. Incorporation of thymidine and its analogue, bromodeoxyuridine, into embryos and maternal tissues of the mouse. Differentiation. 1973;1:397–404. doi: 10.1111/j.1432-0436.1973.tb00137.x. [DOI] [PubMed] [Google Scholar]
- 30.Zeytin FN, et al. Establishment of clonal strains of rat pituitary tumor cells that secrete growth hormone. Endocrinology. 1968;114:2054–2059. doi: 10.1210/endo-82-2-342. [DOI] [PubMed] [Google Scholar]
- 31.Mellon PL, Windle JJ, Weiner RI. Immortalization of neuroendocrine cells by targeted oncogenesis. Recent Prog Horm Res. 1991;47:69–96. doi: 10.1016/b978-0-12-571147-0.50007-x. [DOI] [PubMed] [Google Scholar]
- 32.Turgeon JL, et al. Steroid and pulsatile gonadotropin-releasing hormone (GnRH) regulation of luteinizing hormone and GnRH receptor in a novel gonadotrope cell line. Mol Endocrinol. 1996;10:439–450. doi: 10.1210/mend.10.4.8721988. [DOI] [PubMed] [Google Scholar]
- 33.St Johnston D, Beuchle D, Nüsslein-Volhard C. Staufen, a gene required to localize maternal RNAs in the Drosophila egg. Cell. 1991;66:51–63. doi: 10.1016/0092-8674(91)90138-o. [DOI] [PubMed] [Google Scholar]
- 34.Connolly CM, Dearth AT, Braun RE. Disruption of murine Tenr results in teratospermia and male infertility. Dev Biol. 2005;278:13–21. doi: 10.1016/j.ydbio.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Alasti F, Van Camp G. Genetics of microtia and associated syndromes. J Med Genet. 2009 Mar 16; doi: 10.1136/jmg.2008.062158. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36.Jirawatnotai S, et al. Cdk4 is indispensable for postnatal proliferation of the anterior pituitary. J Biol Chem. 2004;279:51100–51106. doi: 10.1074/jbc.M409080200. [DOI] [PubMed] [Google Scholar]
- 37.Li S, Sen GC. PACT-mediated enhancement of reporter gene expression at the translational level. J Interferon Cytokine Res. 2003;23:689–697. doi: 10.1089/107999003772084806. [DOI] [PubMed] [Google Scholar]
- 38.Lee Y, et al. The role of PACT in the RNA silencing pathway. EMBO J. 2006;25:522–532. doi: 10.1038/sj.emboj.7600942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ward RD, et al. Role of PROP1 in pituitary gland growth. Mol Endocrinol. 2005;19:698–710. doi: 10.1210/me.2004-0341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.