Abstract
The prototypic formyl peptide N-formyl-Met-Leu-Phe (fMLF) is a major chemoattractant found in Escherichia coli culture supernatants and a potent agonist at human formyl peptide receptor (FPR) 1. Consistent with this, fMLF induces bactericidal functions in human neutrophils at nanomolar concentrations. However, it is a much less potent agonist for mouse FPR (mFPR) 1 and mouse neutrophils, requiring micromolar concentrations for cell activation. To determine whether other bacteria produce more potent agonists for mFPR1, we examined formyl peptides from Listeria monocytogenes and Staphylococcus aureus for their abilities to activate mouse neutrophils. A pentapeptide (N-formyl-Met-Ile-Val-Ile-Leu (fMIVIL)) from L. monocytogenes and a tetrapeptide (N-formyl-Met-Ile-Phe-Leu (fMIFL)) from S. aureus were found to induce mouse neutrophil chemotaxis at 1-10 nM and superoxide production at 10-100 nM, similar to the potency of fMLF on human neutrophils. Using transfected cell lines expressing mFPR1 and mFPR2, which are major forms of FPRs in mouse neutrophils, we found that mFPR1 is responsible for the high potency of fMIVIL and fMIFL. In comparison, activation of mFPR2 requires micromolar concentrations of the two peptides. Genetic deletion of mfpr1 resulted in abrogation of neutrophil superoxide production and degranulation in response to fMIVIL and fMIFL, further demonstrating that mFPR1 is the primary receptor for detection of these formyl peptides. In conclusion, the formyl peptides from L. monocytogenes and S. aureus are 100-fold more potent than fMLF in activating mouse neutrophils. The ability of mFPR1 to detect bacterially derived formyl peptides indicates that this important host defense mechanism is conserved in mice.
Keywords: Neutrophils, chemoattractant, bacteria, formyl peptides
Neutrophils migrate in response to stimulation with many types of chemoattractant substances, including bacterially derived N-formylmethionyl peptides and host-derived proteins (e.g., chemokines and the active complement component C5a) and lipids (e.g., leukotriene B4 and platelet-activating factor) (1). Although structurally diverse, these chemoattractants induce neutrophil chemotaxis by activating specific members of a family of structurally related G protein-coupled receptors (2). The ability of neutrophils to detect and respond to chemoattractants is highly important for host defense against invading bacteria. It also plays a critical role in the accumulation of neutrophils at uninfected inflammatory sites, where oxygen radicals and matrix-degrading enzymes from activated neutrophils can cause tissue damage. Among the neutrophil chemoattractants, N-formyl peptides such as the Escherichia coli-derived fMet-Leu-Phe (fMLF)3 are pathogen-associated molecular patterns and full agonists for activation of neutrophils. The ability of formyl peptides to activate all major bactericidal functions, including degranulation and superoxide generation, is in contrast to chemokines that are able to prime neutrophils, but are poor agonists for the induction of superoxide production. Because of these properties, fMLF has been widely used in studies of neutrophil activation.
Bacterial (and mitochondrial) protein synthesis is characterized by the use of N-formylmethionine, a feature explored by Schiffmann et al. (3) in the identification of synthetic peptides bearing N-formylmethionine as potent chemoattractants for human and rabbit neutrophils. Marasco et al. (4) subsequently determined that the prototypic chemotactic peptide fMLF is a major chemoattractant found in Escherichia coli culture supernatants. Later, molecular cloning studies led to the identification of the genes for the high-affinity formyl peptide receptors (FPRs) from humans (5, 6) and rabbits (7). Although these receptors mediate different neutrophil functions in response to different concentrations of fMLF (8), several groups have demonstrated in transfected cells that a single class of receptors (FPR1 in human) is responsible for fMLF-induced chemotaxis, degranulation, and superoxide production (9, 10, 11). The ability of fMLF to stimulate activation of heterotrimeric G proteins, small GTPases, protein kinase C, PI3Ks, and MAPKs contributes in part to the induced neutrophil functions. Structurally homologous receptors such as FPR-like 1 (FPRL1, also termed FPR2) have been identified in human neutrophils and monocytes (12, 13, 14, 15). These receptors, however, contribute very little to fMLF-induced neutrophil activation due to their low affinities for the prototypic formyl peptide (16).
In mice, the FPR family consists of eight genes, coding for seven putative proteins with predicted membrane disposition similar to that of human FPR1 (hFPR1) (17, 18, 19). mFPR1 is found in neutrophils and considered an orthologue of hFPR1. However, despite a sequence identity of 76% to hFPR1, mFPR1 displays relatively low affinity for fMLF, requiring agonist concentration of 100 nM or higher for activation of cellular functions such as calcium mobilization (17). Other neutrophil activities such as degranulation and superoxide production require fMLF concentrations of 5-10 μM in mice. Therefore, fMLF is 100-fold less potent for mouse neutrophils than for human neutrophils. The second mFPR, mFPR2, is encoded by mfpr-rs2 and shares 81% sequence identity with mFPR1 (58% with hFPR1). It responds even more weakly to fMLF, contributing to the second concentration optimum in mouse neutrophil chemotaxis assays (20). Based on studies using mfpr1-deficient neutrophils, none of the mFPR family members was able to mediate high-affinity interaction with fMLF in functional assays (20, 21). The absence of strong responses of mouse neutrophils to fMLF raises the question of whether formyl peptides are physiologically relevant agonists in mice.
To determine the functions of mFPRs in host defense, one of our laboratories generated mfpr1-deficient mice (21). Although these mice appeared healthy, they were unusually susceptible to infection with Listeria monocytogenes. Ex vivo study of neutrophils from these mice showed decreased responsiveness to fMLF. Therefore, the results from this study strongly support a role for mFPR1 in host defense against bacterial infection. Based on these findings, we hypothesized that the increased mortality of mfpr1-/- mice upon Listeria infection might result from failure of neutrophils in these mice to detect L. monocytogenes-derived chemotactic peptides. The current study investigates this possibility through functional characterization of a L. monocytogenes-derived formyl peptide exhibiting chemotactic activity at hFPR1 and FPRL1 (22). Also examined in this study is a Staphylococcus aureus-derived chemotactic peptide known to activate human leukocytes, but not tested on the cloned FPRs (23). Our findings demonstrate for the first time that chemotactic N-formyl peptides from certain strains of bacteria can serve as potent agonists for a mFPR.
MATERIALS AND METHODS
Materials
N-formyl-Met-Ile-Val-Ile-Leu (fMIVIL) and N-formyl-Met-Ile-Phe-Leu (fMIFL) were synthesized at the Protein Research Laboratory at the University of Illinois, purified to ≥90% homogeneity, and identified using mass spectrometry. fMLF (≥90% purity) was purchased from Sigma-Aldrich. W-peptide (WKYMVm) was synthesized at Macromolecular Resources. Other chemicals were purchased from Sigma-Aldrich.
Cell culture
The rat basophil leukemia cell line RBL-2H3 was transfected with an expression vector (pSSFV.neo) containing the mFPR1 cDNA, as previously described (24). An RBL-2H3 cell line expressing mFPR2 cDNA was constructed similarly, and functional expression of the receptor was confirmed in Ca2+ mobilization assay based on its response to WKYMVm. Stable transfectants were selected with G418 (500 μg/ml). Both cell lines were maintained in DMEM supplemented with 20% FBS and 250 μg/ml G418.
Isolation of mouse neutrophils
CD1 mice (Charles River Laboratories) were used in initial experiments. In some experiments, mFPR1 knockout (KO) mice (LN 169) (21) on the background of C57BL/6 (Taconic Farms) were used in conjunction with wild-type (WT) Taconic C57BL/6 mice of same age and gender. Mice 8-10 wk of age were sacrificed with CO2 inhalation, followed by cervical dislocation. All experiments involving the use of mice were conducted according to protocols approved by the Institutional Animal Care and Use Committee at University of Illinois, Chicago. Femurs and tibias were isolated and then rinsed in HBSS-prep (Ca2+/Mg2+ with 20 mM HEPES, 0.5% BSA, 1% glucose). The bones were cleared of all remaining tissue and then flushed with HBSS-prep using a 25G 5/8” needle. After bone marrow was flushed, marrow was passed through a 70-μm cell strainer to remove aggregated cells. Cells were pelleted at 1300 rpm for 10 min and then resuspended in 50 ml of HBSS after treatment with ACK buffer (ammonium chloride potassium: 8.29 g/L NH4Cl, 1.00 g/L KHCO3, 0.0372 g/L disodium EDTA 2H2O, membrane filtered (pH 7.4) ± 0.2, osmolality 290 ± 5% mOsm/Kg H2O) to lyse RBC. Cells were pelleted again and resuspended in 3 ml of HBSS. Cells were layered over a discontinuous gradient consisting of 5 ml of 1.077 Nycoprep underlayed with 3 ml of 72% Percoll, then centrifuged at 2400 rpm for 20 min at room temperature with no acceleration and no brake. Neutrophils were isolated from the Nycoprep/Percoll interface and washed twice with HBSS. After the final wash, cells were resuspended in appropriate assay buffer until use.
Calcium mobilization assay
Stable transfectants of RBL cells were grown to 90% confluence in black/clear-bottom 96-well assay plates. Before assay, the cells were washed with 0.5% BSA in HBSS (with Ca2+ and Mg2+) and incubated in the same buffer. Mouse bone marrow neutrophils were suspended at 2.5 × 106 cells/ml in HBSS containing 0.5% BSA, and 80 μl of the cell suspension was added to each well. All Ca2+ mobilization assays were conducted with the use of a FLIPR Calcium Plus Kit (Molecular Devices). Cells were loaded for 1 h at 37°C with FLIPR calcium sensitive dye, according to the manufacturer’s protocols. The addition of agonists was robotically controlled, and samples were read on a FlexStation (Molecular Devices). Cells were excited at 485 nm, and Ca2+ fluorescence was detected with an emission wavelength of 525 nm.
Measurement of superoxide production
Mouse bone marrow neutrophils were isolated, as described above. Superoxide generation was measured using an isoluminol enhanced chemiluminescent method (25). Neutrophils were assayed in HBSS containing 0.5% BSA with a cell density of 5 × 106 cells/ml. Cells were incubated at 37°C for 5 min in the presence of 50 μl of isoluminol, 40 U/ml HRP, and 5 μM cytochalasin B. After preincubation, 200 μl of the above mixture was added to each well of a white 96-well flat-bottom plate. Superoxide production was measured in a Wallac Victor2 1420 Multilabel Counter. Basal chemiluminescence was measured every minute for a total of 5 min, after which neutrophils were stimulated with agonists at variable concentrations and chemiluminescence was measured every minute for 15 min for each concentration. Samples containing 250 U of superoxide dismutase were run in parallel as controls.
Chemotaxis assay
Mouse bone marrow neutrophils were isolated, as described above. Neutrophils were resuspended in HBSS containing 0.5% BSA at 5 × 106 cells/ml. Neutrophils were prewarmed at 37°C for 20 min. A 24-well flat-bottom Ultra low attachment surface plate (Costar) was precoated with HBSS containing 0.5% BSA for 15 min at 37°C. The buffer was removed from the plate, and agonists of different concentrations were added to each well (300 μl/well). After each agonist was added to the wells, a Millicell sterilized culture plate insert with 3 μm pore size and 12 mm diameter was added to each well, followed by immediate addition of 200 μl of neutrophils. Plates were incubated at 37°C for 30 min, and chemotactic movement was halted by removal of the insert. Cells that migrated to the bottom chamber were counted. To distinguish between chemotactic movement and chemokinetic movement, the number of cells that migrated in the presence of ligand in top and bottom chambers was subtracted from samples with ligand present only in the bottom chambers. Chemotaxis index was calculated as described previously (24).
RNA analysis
Mouse bone marrow-derived neutrophils were lysed using TRIzol (Invitrogen). Addition of chloroform and subsequent centrifugation produces organic and aqueous phases. The aqueous phase containing RNA was added to isopropyl alcohol, and RNA was precipitated. Isolated RNA was treated with DNase I. Two micrograms of the DNase I-treated RNA was used for the reverse-transcriptase reaction. RNA was incubated with oligo(dT) at 70°C for 10 min, and then incubated with SuperScript II reverse transcriptase. Using the reverse-transcriptase product, PCR was conducted with the following primers to determine levels of mRNA in mouse neutrophils: for mFPR1, forward (5′-CAGAATTCCAGCCATGGACACCAACATGTCTC-3′) and reverse (5′-GCGAATTCTTTACATTGCATTTAAAGTG-3′); for mfpr-rs1, forward (5′-GGCAACTCTGTTGAGGAAAG-3′) and reverse (5′-GGCTCTCGGTAGACGAGA-3′); for mFPR2, forward (5′-GTCAAGATCAACAGAAGAAACC-3′) and reverse (5′-GGGCTCTCTCAAGACTATAAGG-3′). Cloned DNA fragments of mfpr1, mfpr-rs1, and mfpr-rs2 were used in parallel PCR as controls. All PCR conditions were 94°C for 5 min, followed by 30 cycles of 94°C for 30 s, 55°C for 1 min, and 72°C for 1 min, with a final extension at 72°C for 7 min. All samples were analyzed on 1% agarose gels.
Degranulation assay
To measure β-glucuronidase release, purified mouse neutrophils were preincubated with 10 μM cytochalasin B in HBSS containing 20 mM HEPES (pH 7.4) and 0.1% BSA (HBSS-HB) for 15 min on ice, followed by 15 min at 37°C. The cells were then stimulated for 10 min with indicated amounts of agonists at 37°C. The reaction was terminated by placing samples on ice, and supernatant was immediately separated from cell pellet by centrifugation. The amount of β-glucuronidase was quantified by incubating 20 μl of supernatant with 20 μl of 10 mM 4-methylumbelliferyl β-d-glucuronide hydrate in 0.1 M sodium acetate (pH 4.0) and 0.1% Triton X-100 at 37°C for 15 min. The reaction was terminated by adding 300 μl of Stop Solution (pH 10.4) containing 50 mM glycine and 5 mM EDTA. Fluorescence was measured immediately on a FlexStation (Molecular Devices) with excitation wavelength at 365 nm and emission wavelength at 460 nm. Total cellular β-glucuronidase was determined with cell lysate in 0.1% Triton X-100. Data are presented as percentage of total β-glucuronidase released.
MAPK assay
Cells were cultured for 24 h in six-well plates and serum starved for 4 h before stimulation. The reaction was terminated by placing plates on ice and adding 250 μl of ice-cold SDS-PAGE loading buffer (15% (v/v) glycerol, 125 mM Tris-Cl (pH 6.8), 5 mM EDTA, 2% (w/v) SDS, 0.1% bromphenol blue, and 1% 2-ME). Samples were transferred to microcentrifuge tubes and sonicated twice for 15 s each. After boiling, samples were analyzed by SDS-PAGE and Western blotting using anti-ERK1/2 and anti-phospho-ERK1/2 Abs (Cell Signaling Technology) at a 1/1000 dilution. After washing, the membrane was incubated with an HRP-conjugated anti-rabbit Ab (Bio-Rad) (1/3000 dilution). The resulting immunocomplex was visualized using SuperSignal West Pico Chemiluminescence kit (Pierce), according to manufacturer’s instructions.
RESULTS
Staphylococcus- and Listeria-derived formyl peptides are potent chemoattractants for mouse neutrophils
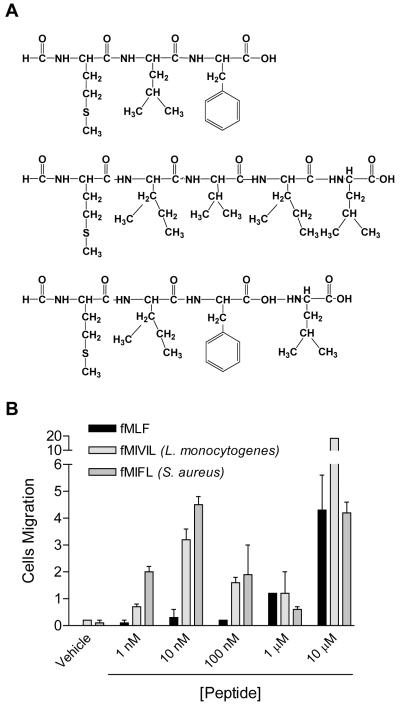
Previous studies have shown that fMLF is a major chemotactic peptide from E. coli that activates human and rabbit neutrophils in nanomolar concentrations (4). However, activation of mouse neutrophils requires micromolar concentrations of fMLF (17). We sought to determine whether formyl peptides from other bacterial strains are potent chemotactic peptides for mouse neutrophils. The Staphylococcus- and Listeria-derived peptides (Fig. 1A) were chosen based on their chemotactic properties on human neutrophils (22, 23). fMIFL is a S. aureus-derived tetrapeptide, and fMIVIL is one of the several L. monocytogenes-derived pentapeptides known for their interaction with the H2-M3 murine class 1-b MHC molecule (26). When tested in ex vivo chemotaxis assays, both fMIFL and fMIVIL induced chemotaxis of mouse neutrophils in low nanomolar concentrations, whereas fMLF did not stimulate significant neutrophil migration at 100 nM (Fig. 1B). It was notable that both fMIFL- and fMIVIL-induced neutrophil chemotaxis exhibited a typical bell-shaped dose-response curve in the concentration range from 1 to 1000 nM, with optimum values at 10 nM. A second peak appeared at a much higher concentration (10 μM), and most likely was mediated through another receptor (20).
Figure 1. Composition of the chemotactic peptides used in the study and the chemotaxis response they induced in mouse neutrophils.
A, Amino acid sequence of the formyl peptides from E. coli (fMLF), L. monocytogenes (fMIVIL), and S. aureus (fMIFL). B, Chemotaxis of mouse neutrophils induced by the formyl peptides. Neutrophils isolated from CD1 mice were stimulated with indicated concentrations of fMLF, fMIVIL and fMIFL. Chemotaxis assay was conducted using Transwell method as described in Materials and Methods. Chemotaxis index (means ± SD) shown in the figure is representative of two separate experiments done in duplicate.
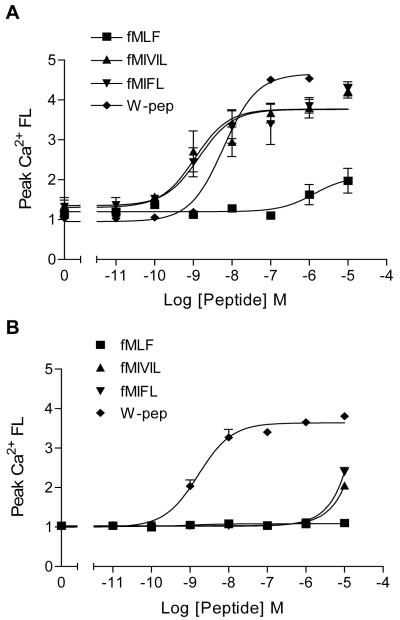
Leukocyte chemoattractant receptors are G protein-coupled receptors that respond to agonist stimulation with the activation of multiple signaling pathways, leading to the generation of second messengers such as diacylglycerol and inositol(1,4,5)-trisphosphate. The resulting mobilization of Ca2+ from intracellular stores and Ca2+ influx are often used as indicators of agonist-induced cell activation. A comparison of fMLF, fMIFL, and fMIVIL revealed major differences of these peptides in the induction of Ca2+ flux in mouse neutrophils. At an agonist concentration of 10 nM, fMIFL and fMIVIL, but not fMLF, stimulated robust and sustained Ca2+ mobilization (Fig. 2A). The dose-response curve of Ca2+ mobilization was determined using these peptides (Fig. 2B). The fMLF-induced Ca2+ mobilization was not evident at agonist concentrations below 100 nM. In comparison, maximal Ca2+ flux was observed with 100 nM fMIFL and fMIVIL. At agonist concentrations of 10-100 μM, an additional increase in Ca2+ flux was observed with these peptides, suggesting the involvement of more than one receptor in this functional assay.
Figure 2. Induction of calcium mobilization in neutrophils stimulated with Listeria monocytogenes and Staphylococcus aureus-derived N-formylpeptides.
A, Time-dependent mobilization of Ca2+ in mouse bone marrow neutrophils stimulated with fMLF, fMIVIL and fMIFL. Peptides (10 nM) were added at the time indicated by an arrow. Tracings shown are relative Ca2+ fluorescence (FL), expressed as the ratio of the reading at 525 nM over baseline, and are representative of three separate experiments that gave similar results. B, Dose response curve of fMLF, fMIVIL, and fMIFL in stimulating Ca2+ mobilization in mouse neutrophils. Ca2+ assay was conducted similarly as described above. Peak values of Ca2+ mobilization at given agonist concentrations are shown as means ± SEM, based on three separate experiments, each conducted in duplicate.
Superoxide generation is an important bactericidal function of neutrophils. We compared the abilities of the three formyl peptides to induce superoxide generation in mouse neutrophils, and found a threshold for responsiveness above 1 μM fMLF. In comparison, fMIVIL and fMIFL could trigger superoxide generation at concentrations as low as 10 nM (Fig. 3), representing a left shift of the dose-response curve of approximately two orders of magnitude. At 5 μM, fMLF-induced superoxide production was about one-half of that induced by fMIVIL and fMIFL, suggesting that fMLF is not only less potent, but probably also less efficacious in the induction of this neutrophil function.
Figure 3. Neutrophil superoxide production induced by the Listeria monocytogenes and Staphylococcus aureus-derived N-formylpeptides.
A, Time-dependent superoxide production in fMLF, fMIVIL and fMIFL (1 μM each) stimulated mouse neutrophils. Superoxide dismutase (SOD, 250 units), added to some samples 10 min before stimulation, effectively inhibited the detection of superoxide based on isoluminol-enhanced chemiluminescent as detailed in Materials and Methods. Representative tracings from one of the two experiments are shown. Note difference in Y-axis scale between the samples. B, Dose response of mouse neutrophils to the peptides in superoxide production assay, conducted similarly as described above. Data shown are cumulative superoxide production, determined as integrated areas under curve in the first 5 min after agonist stimulation, and are representative of two separate experiments done in duplicate.
mFPR1 is responsible for the potent agonistic activities of fMIFL and fMIVIL in transfected cells
The FPR family consists of three members in humans (27, 28). In mice, seven homologues of hFPRs have been identified (17, 18, 19). mRNA distribution studies have suggested that the mouse receptors encoded by mfpr1 (mFPR1), mfpr-rs1 (mLXA4R), and mfpr-rs2 (mFPR2) are mainly expressed in leukocytes (19, 20, 29, 30). To determine which receptor mediates the agonistic activities of fMIFL and fMIVIL, we first examined the relative abundance of these receptors in mouse neutrophils by RT-PCR. As shown in Fig. 4A, the transcripts of mfpr1 and mfpr-rs2 were abundant in mouse neutrophils, but the transcript of mfpr-rs1 was not detectable. To investigate the functional responses of these gene products to fMIFL and fMIVIL, we prepared stable cell lines expressing mFPR1 and mFPR2 in the rat basophilic leukemia cell line RBL-2H3. mFPR1-expressing RBL cells have previously been shown to recognize and respond to WKYMVm (24), a synthetic peptide identified from screening a peptide library (31) and known to activate both hFPR1 and FPRL1 (32). Therefore, we used WKYMVm as a positive control. A similar RBL cell line expressing mFPR2 was established. Both cell lines responded to WKYMVm (100 nM) in Ca2+ mobilization assays (Fig. 4B). However, when challenged with 100 nM fMIVIL (Fig. 4C) or fMIFL (Fig. 4D), only the mFPR1-RBL cells responded with Ca2+ mobilization, indicating differences between the two receptors in the detection of these peptides. Dose-dependent Ca2+ mobilization was determined in the two RBL cell lines (Fig. 5). In mFPR1-RBL cells, fMIVIL and fMIFL exhibited nearly identical efficacy and potency with EC50 of 10 nM (Fig. 5A). WKYMVm was slightly less potent, but appeared to be more efficacious, and fMLF induced Ca2+ flux only when used at concentrations above 1 μM (Fig. 5A). In assays using the mFPR2-RBL cells, neither fMIVIL nor fMIFL induced Ca2+ mobilization unless used at concentrations above 1 μM (Fig. 5B). Based on these results, the potency of fMIVIL and fMIFL is at least three orders of magnitude higher for mFPR1 than for mFPR2. Collectively, these results demonstrate preferential activation of mFPR1 by fMIVIL and fMIFL.
Figure 4. Listeria monocytogenes and Staphylococcus aureus N-formyl peptides are selective agonists for mFPR1.
A, RT-PCR detection of the transcripts of mouse formyl peptide receptors in neutrophils (PMN). The sizes of the individual transcripts, after PCR amplification, are indicated by the cDNA controls included in a parallel experiment. Data are representative of three separate experiments. B-D, Agonist-induced Ca2+ mobilization in RBL-mFPR1 cells (left panels) and RBL-mFPR2 cells (right panels) that were stimulated with WKYMVm (W-pep, B), fMIVIL (C), and fMIFL (D). All 3 peptides were used at 100 nM in assays using RBL-mFPR1. In assays using RBL-mFPR2, WKYMVm was used at 100 nM, and fMIVIL and fMIFL were each used at 1 μM. ATP, used in (B) to demonstrate equal loading of the fluorescent dye, was used at 1 μM. One representative set of tracings, from assays done on the same day, is presented out of a total of three separate experiments.
Figure 5. Dose-dependent induction of Ca2+ mobilization in transfected RBL cells stimulated with Listeria monocytogenes and Staphylococcus aureus -derived N-formyl peptides.
A, RBL-mFPR1 (RBL cells transfected with a plasmid encoding mFPR1) and B, RBL-mFPR2. Each peptide was used at the indicated concentrations. W-pep, WKYMVm. Peak values of Ca2+ mobilization at the indicated agonist concentrations are shown as means ± SEM, based on two separate experiments, each conducted in triplicate. The dose curves of W-pep reflect means ± SD based on two experiments, each done in duplicate.
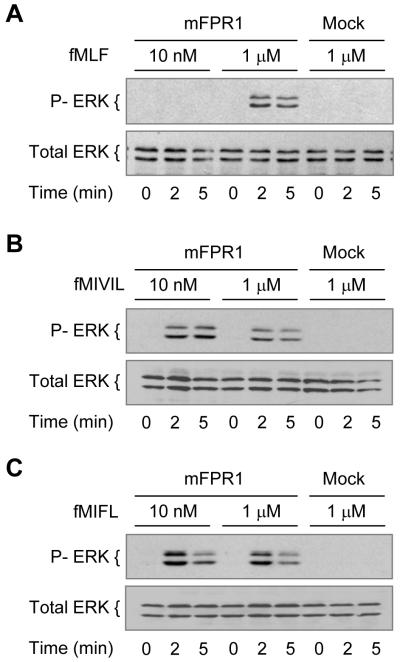
Stimulation of human neutrophils with fMLF results in rapid phosphorylation of the MAPKs ERK1/2, which have apparent molecular masses of 44 and 42 kDa (p44 and p42, respectively) (33, 34). In transfected RBL cells that express mFPR1, WKYMVm, but not fMLF, was able to induce ERK1/2 phosphorylation at 50 nM (24). In the present study, we found that fMLF could induce ERK1/2 phosphorylation at 1 μM, but not at 10 nM, in mFPR1-RBL cells (Fig. 6A). In comparison, fMIVIL and fMIFL could induce ERK1/2 phosphorylation to a similar extent when used at 10 nM, and increasing the concentration of these peptides to 1 μM did not enhance the phosphorylation of ERK1/2 (Fig. 6, B and C). Based on the appearance and intensity of the phosphorylated ERK1 and ERK2, the Staphylococcus-derived fMIFL is more potent than fMIVIL in stimulating this function. At the concentrations tested, none of the peptides was able to induce detectable phosphorylation of ERK1/2 in mock-transfected RBL cells (Fig. 6) or in the mFPR2-RBL cell line (data not shown).
Figure 6. ERK1/2 phosphorylation in transfected RBL cells stimulated with Listeria monocytogenes and Staphylococcus aureus-derived N-formylpeptides.
RBL-mFPR1 and RBL cells were serum starved for four hours and stimulated with fMLF (A), fMIVIL (B), and fMIFL (C) at indicated concentrations. After various time intervals, cells were harvested and the phosphorylated ERK1/2 were determined by Western Blotting using an anti-phospho-ERK Ab. Equal loading of the proteins was determined with an anti-ERK1/2 Ab. Data shown are representative of two independent experiments.
Neutrophils from mfpr1-/- mice display compromised response to fMIFL and fMIVIL
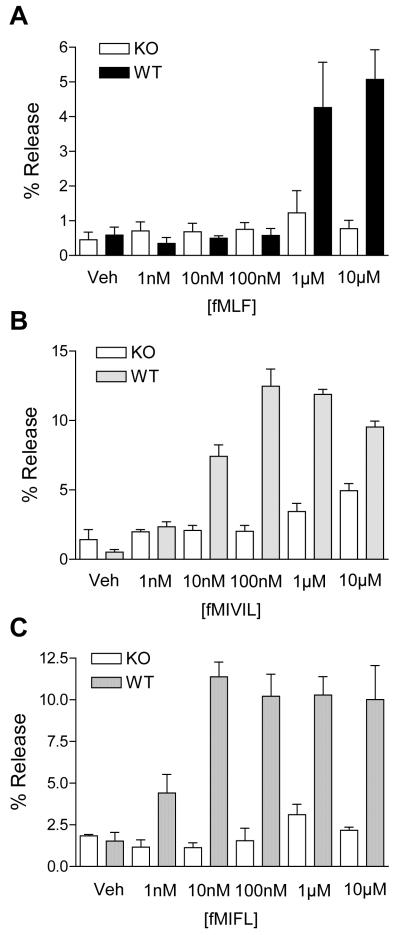
In a previous publication, we have shown that genetic loss of mFPR1 rendered mice more susceptible to infection with L. monocytogenes, resulting in increased mortality compared with WT controls (21). We speculated that the compromised immunity to L. mnocytogenes in mfpr1-/- mice might result from the inability of the neutrophils to effectively respond to Listeria-derived formyl peptides. To examine this possibility, we conducted ex vivo superoxide production assays with both WT and mfpr1-/- neutrophils. As shown in Fig. 7A, neutrophils prepared from mfpr1-/- mice (KO) and WT controls responded similarly to phorbol ester stimulation in the superoxide production assay, indicating that the loss of mFPR1 does not affect the assembly of a functional NADPH oxidase complex. When the cells were challenged with fMLF, fMIFL, and fMIVIL, drastically different results were obtained from the WT and mfpr1-/- neutrophils. In WT neutrophils, both fMIFL and fMIVIL induced potent superoxide production (Fig. 7), whereas fMLF was less potent (notice the difference in y-axis scale). In mfpr1-/- neutrophils, very little superoxide was generated with any of the three peptides (Fig. 7, B-D). These results indicate that the absence of mFPR1 directly contributed to the inability of the mfpr1-/- neutrophils to effectively mount a bactericidal function when stimulated with these peptides.
Figure 7. Superoxide production in mouse neutrophils stimulated with Listeria monocytogenes and Staphylococcus aureus N-formylpeptides.
Bone marrow neutrophils were isolated from mfpr1-/- and C57BL/6 (WT) mice, and stimulated with various agonists as described in Materials and Methods. A, mfpr1-/- and WT neutrophils were stimulated with 200 ng/ml phorbol myristate ester, and superoxide production was determined as a function of time. Data shown are representative of two experiments. B-D, Neutrophils from mfpr1-/- and WT mice were stimulated with fMLF (B), fMIVIL (C) and fMIFL (D) at indicated concentrations. Data are means ± SEM of integrated area under curve during the first 10 min after agonist stimulation, and are representative of two separate experiments, each done in triplicate.
We also investigated whether the loss of mFPR1 could affect other bactericidal functions of neutrophils. Release of β-glucuronidase from chemoattractant-stimulated neutrophils is an indication of degranulation of primary granules, a process that contributes to killing of bacteria following phagocytosis (35). Neutrophils from mfpr1-/- mice and WT controls were stimulated with fMLF, fMIVIL, or fMIFL, and the release of β-glucuronidase was determined. Both fMIVIL and fMIFL induced significant enzyme release that peaked at 100 and 10 nM, respectively (Fig. 8, B and C). Induction of enzyme release by fMLF was not significant at concentrations below 1 μM (Fig. 8A). At the maximal concentration used (10 μM), fMLF caused release of 5% of cell-associated enzyme, about one-half of the maximal release induced by fMIVIL and fMIFL at lower concentrations. In mfpr1-/- neutrophils, none of the three peptides was able to induce β-glucuronidase release at 100 nM, and only a small amount of enzyme was released at peptide concentrations above 1 μM. Taken together, these results indicate that mFPR1 is largely responsible for the fMIVIL- and fMIFL-induced superoxide generation and degranulation in mouse neutrophils.
Figure 8. β-glucuronidase release from mouse neutrophils stimulated with Listeria monocytogenes and Staphylococcus aureus N-formylpeptides.
Bone marrow neutrophils from wild type (WT) and mfpr1-/- (KO) mice were pre-incubated with 10 μM cytochalasin B for 15 min on ice and then 15 min at 37°C. The cells were stimulated with fMLF (A), fMIVIL (B), and fMIFL (C) at the indicated concentrations for 10 min. The release of β-glucuronidase was quantified in a fluorescent assay as described in Materials and Methods, and expressed as percent of the total cellular β-glucuronidase which was determined with cell lysate. The results are expressed as means ± SEM, based on three independent experiments done in duplicate.
DISCUSSION
The tripeptide fMLF has been the prototypic formyl peptide studied since the discovery of neutrophil chemotactic activity in synthetic N-formyl peptides 3 decades ago (3). The biological relevance of fMLF was established by its isolation as the most potent chemotactic peptide from E. coli culture supernatants (4). The high-affinity binding of fMLF to human and rabbit neutrophils, its ability to activate major bactericidal functions of neutrophils, and its ready availability as a small synthetic peptide have made it one of the most frequently used chemoattractants in leukocyte biology research. There are, however, a number of instances in which neutrophils apparently fail to respond to fMLF. For example, neutrophils from cow, pig, dog, and cat do not respond to fMLF (reviewed in Ref. 36). Activation of mouse neutrophil functions requires fMLF in the micromolar concentration range (17, 20). These discrepancies have suggested that neutrophils from different species may recognize different and preferred chemotactic peptides. In fact, although fMLF is a major chemoattractant isolated from E. coli culture supernatants, there is evidence for the presence of chemotactic peptides of different composition in E. coli (37), Streptococcus sanguis (38), S. aureus (23, 39), L. monocytogenes (22, 26), and the mitochondria of mammalian cells (22, 40, 41). These peptides, however, have not been characterized for their potency in activating FPRs outside human and rabbit phagocytes.
Results from the current study demonstrate that, in mice, mFPR1 is a functional receptor able to respond to two distinct formyl peptides from S. aureus and L. monocytogenes. We have shown that genetic deletion of mfpr1 results in the loss of bactericidal activities in neutrophils stimulated with the two peptides. Our findings argue against the notion that mFPRs might not be physiologically relevant, and, when considered together with early observations that the mfpr1 KO mice had increased mortality after L. monocytogenes infection (21), suggest an important role of mFPR1 in host defense. The two peptides examined in this study are longer than fMLF and differ from fMLF at the second position (Ile vs Leu), and they are 100-fold more potent in stimulating neutrophil chemotaxis, superoxide generation, and degranulation, compared with fMLF. Because tritium-labeled fMLF is no longer available commercially, we were unable to conduct binding assays at this time to determine the binding affinity with these peptides. Based on the potency in functional assays, we predict that these two peptides interact with mFPR1 with affinities much higher than that of fMLF. Using transfected RBL-2H3 cells expressing either mFPR1 or mFPR2, we determined that the high potency of fMIVIL and fMIFL in neutrophil activation was mediated through mFPR1, but not mFPR2. At agonist concentrations above 1 μM, there was another increase in the response to the two peptides in neutrophil chemotaxis and Ca2+ mobilization assays (Figs. 1 and 2), which might be attributed to mFPR2, as in the case of fMLF (20). Also, although most of the superoxide generation activity was lost in mfpr1-/- neutrophils, there was residual degranulation at micromolar concentrations of fMIVIL and fMIFL, which could be mediated through mFPR2. Indeed, in mFPR2-RBL cells, both fMIVIL and fMIFL stimulated Ca2+ mobilization at concentrations above 1 μM (Fig. 5B). These findings indicate that mFPR1 responds to the two peptides more strongly than mFPR2 and is primarily responsible for the induced bactericidal functions in mouse neutrophils.
One of the peptides tested in this study, fMIFL, was originally identified from S. aureus culture supernatants and shown to stimulate chemotaxis in human monocytes (23). This peptide was not previously tested on the individual FPRs cloned from humans, mice, and other species. Our results demonstrate that, with amino acid substitution at the second position (fMet-Ile vs fMet-Leu) and addition of a Leu to the C terminus, the S. aureus-derived formyl peptide acquires significant potency toward mFPR1. This finding suggests that extending the length of the formyl peptide might be critical to an improved potency of the peptide at mFPR1, which is intriguing because peptide length does not seem to be critical to binding and activation of hFPR1. The binding pocket of hFPR1 can accommodate 5 aa, and the formyl peptide most likely assumes a position with the N-formyl group placed deep into the binding pocket (42). Therefore, for peptides longer than 5 aa, the additional residues at the carboxyl end, or the conjugated FITC moiety, may be exposed to the outside of the binding pocket without reducing the potency of the ligand (42). The discrepancy in formyl peptide length and potency between hFPR1 and mFPR1 may be attributed to the structures of the respective ligand-binding pockets, because sequence comparison has identified potentially important differences in amino acid sequence. The hFPR1 contains Arg/Lys at positions 84/85 that have been implicated in high-affinity fMLF binding (43, 44). mFPR1, however, lacks these positively charged residues at the corresponding sites (Ser/Met at positions 84/85). Further investigation of the ligand-binding pocket of hFPR1 also revealed that not only Arg84/Lys85 residues at the carboxyl end of transmembrane helix II were important, but an aspartic acid in helix VII (Asp284) interacting with Lys85 was also crucial for high-affinity fMLF binding (44). The charge interaction between Asp284 and Lys85 is conserved in the rabbit FPR1, which binds fMLF with high affinity (7). In mFPR1, the negatively charged residue at the equivalent position of Asp284 is replaced with a positively charged residue (Lys). Therefore, the charge interaction important for human and rabbit FPR1 to bind fMLF is absent in mFPR1 based on primary sequence comparison. Because of these differences, formyl peptides with additional 1 or 2 aa such as the tetrapeptide (fMIFL) from S. aureus and the pentapeptide (fMIVIL) from L. monocytogenes may be more capable of activating mFPR1 than fMLF due to increased length for a distinct and possibly deeper binding pocket. In contrast, the tripeptide fMLF may be able to fit into a smaller binding pocket because of the presence of the charged residues elevating from the hydrophobic plasma membrane in hFPR1.
Our findings not only indicate that mFPR1 is a physiologically relevant pattern recognition receptor for the detection of bacterially derived formyl peptides, but also raise new possibilities about the evolution of the FPR gene family. mFPR1 has been considered the orthologue of hFPR1 with 76% amino acid identity. Although this is the closest mouse receptor to hFPR1, the sequence difference in the ligand-binding region highlights an important similarity to FPRL1, which features Ser/Met at positions 84/85 and an Asn at the position equivalent to Asp284 in hFPR1 (reviewed in Refs. 27 and 45). Consistent with this finding, FPRL1, which is a low-affinity receptor for fMLF, can respond well to fMIVIL and several other L. monocytogenes and mitochondia-derived peptides (22). The similarity between mFPR1 and FPRL1 further extends to their use of cyclic ADP-ribose for Ca2+ mobilization and chemotaxis (46, 47, 48). In contrast, hFPR1 is not functionally coupled to CD38 and cyclic ADP-ribose for Ca2+ mobilization and chemotaxis (47). mFPR2, shown to be a low-affinity receptor for formyl peptides (20), shares similar structural features with mFPR1 and lacks positively charged residues at the end of transmembrane helix II and a counter charge in transmembrane helix VII. Like mFPR1, mFPR2 is dependent on ADP-ribose for Ca2+ mobilization and chemotaxis (47). These features indicate that hFPR1 may have evolved independently from the mouse genome. In conclusion, mFPR1 shares several features with FPRL1 despite being closest in primary sequence to hFPR1. The observation that fMIVIL and fMIFL can activate mFPR1 with higher potency than fMLF should provide a tool for analysis of the relationship between evolution and function of the FPR family genes in humans and mice.
The identification of the S. aureus- and L. monocytogenes-derived peptides as highly potent agonists for mFPR1 strongly supports the notion that FPRs are pattern recognition receptors capable of detecting the presence of bacteria and activating the bactericidal functions of neutrophils. Results from the current study complement previous findings that deletion of the mfpr1 gene from mice renders these mice more susceptible to L. monocytogenes infection (21), indicating a role for mFPR1 in host defense against bacterial infection. Interestingly, one of the peptides tested in this study, fMIVIL, was originally identified as a L. monocytogenes peptide that is presented to CTLs by the H2-M3 MHC class Ib molecule in mice (49, 50). H2-M3 is structurally different from G protein-coupled chemoattractant receptors such as FPRs, but it recognizes N-formyl peptides (51, 52) and is known to play a nonredundant role in host defense against L. monocytogenes (53, 54). Our identification of fMIVIL as a potent agonist for mFPR1 raises the possibility that there may be cross-talk between H2-M3 and the G protein-coupled mFPR1. Another intriguing question is why the mFPRs do not respond strongly to fMLF. The natural habitat and behavior of mice differ considerably from those of modern humans, and these rodents may be frequently exposed to E. coli infection. As a result, it is possible that mice may have developed other mechanisms of defense against E. coli infection. Also, although fMLF is a major chemotactic peptide in E. coli culture supernatants, it is unlikely the only chemotactic peptide derived from E. coli. Therefore, mouse neutrophils may respond better to these other formyl peptides. In humans, the emergence of hFPR1, with distinct features in its ligand-binding pocket, clearly offers an advantage to the detection of certain bacterial peptides such as fMLF. Combined with the presence of FPRL1, which recognizes a larger collection of bacteria- as well as host-derived agonists, including fMIVIL (22, 27), the repertoire of ligands recognized by the hFPRs is remarkably broad. The discovery of the S. aureus- and L. monocytogenes-derived peptides as potent agonists for mFPR1 suggests that a similar pattern recognition mechanism may exist in other species whose neutrophils do not respond well to fMLF (36). Animal models have proven to be useful tools in the study of neutrophil functions in vivo, and delineation of the differences between human physiology and mouse disease models is of importance to reach meaningful conclusions. The current study introduces two potent and physiologically relevant chemotactic peptides to researchers using mouse models, and may facilitate studies of host defense mechanisms.
ACKNOWLEDGEMENT
The authors would like to thank Dr. Zhen-Guo Wang for preparing the mFPR2-RBL cell line, and Dr. Nancy Freitag for helpful discussions.
Abbreviations
- fMLF
fMet-Leu-Phe
- FPR
formyl peptide receptor
- fMIVIL
fMet-Ile-Val-Ile-Leu
- fMIFL
fMet-Ile-Phe-Leu
- W-peptide
WKYMVm (D-Met)
- GPCR
G protein-coupled receptor
- ERK
extracellular-signal regulated kinase
Footnotes
Supported in part by National Institutes of Health Grants AI033503, AI040176 and HL077806. E.L.S. is supported by a National Research Service Award / National Institutes of Health Institutional T32 Training Grant, “Lung Physiology and Pathophysiology” (T32 HL007829).
DISCLOSURE
The authors have no financial conflict of interest.
REFERENCES
- 1.Snyderman R, Uhing RJ. Phagocytic cells: Stimulus-response coupling mechanisms. In: Gallin JI, Goldstein IM, Snyderman R, editors. Inflammation: Basic Principles and Clinical Correlates. Raven Press; New York: 1992. pp. 421–439. [Google Scholar]
- 2.Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52:145–176. [PubMed] [Google Scholar]
- 3.Schiffmann E, Corcoran BA, Wahl SM. N-formylmethionyl peptides as chemoattractants for leucocytes. Proc. Natl. Acad. Sci. U.S.A. 1975;72:1059–1062. doi: 10.1073/pnas.72.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marasco WA, Phan SH, Krutzsch H, Showell HJ, Feltner DE, Nairn R, Becker EL, Ward PA. Purification and identification of formyl-methionyl-leucyl- phenylalanine as the major peptide neutrophil chemotactic factor produced by Escherichia coli. J. Biol. Chem. 1984;259:5430–5439. [PubMed] [Google Scholar]
- 5.Boulay F, Tardif M, Brouchon L, Vignais P. Synthesis and use of a novel N-formyl peptide derivative to isolate a human N-formyl peptide receptor cDNA. Biochem. Biophys. Res. Commun. 1990;168:1103–1109. doi: 10.1016/0006-291x(90)91143-g. [DOI] [PubMed] [Google Scholar]
- 6.Boulay F, Tardif M, Brouchon L, Vignais P. The human N-formylpeptide receptor: characterization of two cDNA isolates and evidence for a new subfamily of G-protein-coupled receptors. Biochemistry. 1990;29:11123–11133. doi: 10.1021/bi00502a016. [DOI] [PubMed] [Google Scholar]
- 7.Ye RD, Quehenberger O, Thomas KM, Navarro J, Cavanagh SL, Prossnitz ER, Cochrane CG. The rabbit neutrophil N-formyl peptide receptor. cDNA cloning, expression, and structure/function implications. J.Immunol. 1993;150:1383–1394. [PubMed] [Google Scholar]
- 8.Korchak HM, Wilkenfeld C, Rich AM, Radin AR, Vienne K, Rutherford LE. Stimulus response coupling in the human neutrophil. Differential requirements for receptor occupancy in neutrophil responses to a chemoattractant. J Biol Chem. 1984;259:7439–7445. [PubMed] [Google Scholar]
- 9.Prossnitz ER, Quehenberger O, Cochrane CG, Ye RD. Signal transducing properties of the N-formyl peptide receptor expressed in undifferentiated HL60 cells. J.Immunol. 1993;151:5704–5715. [PubMed] [Google Scholar]
- 10.Ali H, Richardson RM, Tomhave ED, Didsbury JR, Snyderman R. Differences in phosphorylation of formylpeptide and C5a chemoattractant receptors correlate with differences in desensitization. J Biol Chem. 1993;268:24247–24254. [PubMed] [Google Scholar]
- 11.He R, Nanamori M, Sang H, Yin H, Dinauer MC, Ye RD. Reconstitution of chemotactic peptide-induced nicotinamide adenine dinucleotide phosphate (reduced) oxidase activation in transgenic COS-phox cells. J Immunol. 2004;173:7462–7470. doi: 10.4049/jimmunol.173.12.7462. [DOI] [PubMed] [Google Scholar]
- 12.Ye RD, Cavanagh SL, Quehenberger O, Prossnitz ER, Cochrane CG. Isolation of a cDNA that encodes a novel granulocyte N-formyl peptide receptor. Biochem. Biophys. Res. Commun. 1992;184:582–589. doi: 10.1016/0006-291x(92)90629-y. [DOI] [PubMed] [Google Scholar]
- 13.Murphy PM, Ozcelik T, Kenney RT, Tiffany HL, McDermott D, Francke U. A structural homologue of the N-formyl peptide receptor: characterization and chromosome mapping of a peptide chemoattractant receptor family. J. Biol. Chem. 1992;267:7637–7643. [PubMed] [Google Scholar]
- 14.Bao L, Gerard NP, Eddy RL, Shows TB, Gerard C. Mapping genes for the human C5a receptor (C5AR), human FMLP receptor (FPR), and two FMLP receptor homologue orphan receptors (FPRH1,FPRH2) to chromosome 19. Genomics. 1992;13:437–440. doi: 10.1016/0888-7543(92)90265-t. [DOI] [PubMed] [Google Scholar]
- 15.Perez HD, Holmes R, Kelly E, McClary J, Chou Q, Andrews WH. Cloning of the gene coding for a human receptor for formyl peptides. Characterization of a promoter region and evidence for polymorphic expression. Biochemistry. 1992;31:11595–11599. doi: 10.1021/bi00161a044. [DOI] [PubMed] [Google Scholar]
- 16.Quehenberger O, Prossnitz ER, Cavanagh SL, Cochrane CG, Ye RD. Multiple domains of the N-formyl peptide receptor are required for high-affinity ligand binding. Construction and analysis of chimeric N-formyl peptide receptors. J.Biol.Chem. 1993;268:18167–18175. [PubMed] [Google Scholar]
- 17.Gao LJ, Murphy PM. Species and subtype variants of the N-formyl peptide chemotactic receptor reveal multiple important functional domains. J.Biol.Chem. 1993;268:25395–25401. [PubMed] [Google Scholar]
- 18.Gao JL, Chen H, Filie JD, Kozak CA, Murphy PM. Differential expansion of the N-formylpeptide receptor gene cluster in human and mouse. Genomics. 1998;51:270–276. doi: 10.1006/geno.1998.5376. [DOI] [PubMed] [Google Scholar]
- 19.Wang ZG, Ye RD. Characterization of two new members of the formyl peptide receptor gene family from 129S6 mice. Gene. 2002;299:57–63. doi: 10.1016/s0378-1119(02)01012-0. [DOI] [PubMed] [Google Scholar]
- 20.Hartt JK, Barish G, Murphy PM, Gao JL. N-formylpeptides induce two distinct concentration optima for mouse neutrophil chemotaxis by differential interaction with two N-formylpeptide receptor (FPR) subtypes. Molecular characterization of FPR2, a second mouse neutrophil FPR. J Exp Med. 1999;190:741–747. doi: 10.1084/jem.190.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao JL, Lee EJ, Murphy PM. Impaired antibacterial host defense in mice lacking the N-formylpeptide receptor. J Exp Med. 1999;189:657–662. doi: 10.1084/jem.189.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rabiet MJ, Huet E, Boulay F. Human mitochondria-derived N-formylated peptides are novel agonists equally active on FPR and FPRL1, while Listeria monocytogenes-derived peptides preferentially activate FPR. Eur J Immunol. 2005;35:2486–2495. doi: 10.1002/eji.200526338. [DOI] [PubMed] [Google Scholar]
- 23.Rot A, Henderson LE, Copeland TD, Leonard EJ. A series of six ligands for the human formyl peptide receptor: tetrapeptides with high chemotactic potency and efficacy. Proc. Natl. Acad. Sci. U.S.A. 1987;84:7967. doi: 10.1073/pnas.84.22.7967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He R, Tan L, Browning DD, Wang JM, Ye RD. The synthetic peptide Trp-Lys-Tyr-Met-Val-D-Met is a potent chemotactic agonist for mouse formyl peptide receptor. J Immunol. 2000;165:4598–4605. doi: 10.4049/jimmunol.165.8.4598. [DOI] [PubMed] [Google Scholar]
- 25.Wymann MP, von Tscharner V, Deranleau DA, Baggiolini M. Chemiluminescence detection of H2O2 produced by human neutrophils during the respiratory burst. Anal Biochem. 1987;165:371–378. doi: 10.1016/0003-2697(87)90284-3. [DOI] [PubMed] [Google Scholar]
- 26.Pamer EG, Wang CR, Flaherty L, Lindahl KF, Bevan MJ. H-2M3 presents a Listeria monocytogenes peptide to cytotoxic T lymphocytes. Cell. 1992;70:215–223. doi: 10.1016/0092-8674(92)90097-v. [DOI] [PubMed] [Google Scholar]
- 27.Le Y, Murphy PM, Wang JM. Formyl-peptide receptors revisited. Trends Immunol. 2002;23:541–548. doi: 10.1016/s1471-4906(02)02316-5. [DOI] [PubMed] [Google Scholar]
- 28.Ye RD, Boulay F. Structure and function of the leukocyte chemoattractant receptors. Adv. Pharmacol. 1997;39:221–289. doi: 10.1016/s1054-3589(08)60073-3. [DOI] [PubMed] [Google Scholar]
- 29.Takano T, Fiore S, Maddox JF, Brady HR, Petasis NA, Serhan CN. Aspirin-triggered 15-epi-lipoxin A4 (LXA4) and LXA4 stable analogues are potent inhibitors of acute inflammation: evidence for anti-inflammatory receptors. J Exp Med. 1997;185:1693–1704. doi: 10.1084/jem.185.9.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaughn MW, Proske RJ, Haviland DL. Identification, cloning, and functional characterization of a murine lipoxin A4 receptor homologue gene. J Immunol. 2002;169:3363–3369. doi: 10.4049/jimmunol.169.6.3363. [DOI] [PubMed] [Google Scholar]
- 31.Seo JK, Choi SY, Kim Y, Baek SH, Kim KT, Chae CB, Lambeth JD, Suh PG, Ryu SH. A peptide with unique receptor specificity: stimulation of phosphoinositide hydrolysis and induction of superoxide generation in human neutrophils. J Immunol. 1997;158:1895–1901. [PubMed] [Google Scholar]
- 32.Le Y, Gong W, Li B, Dunlop NM, Shen W, Su SB, Ye RD, Wang JM. Utilization of two seven-transmembrane, G protein-coupled receptors, formyl peptide receptor-like 1 and formyl peptide receptor, by the synthetic hexapeptide WKYMVm for human phagocyte activation. J Immunol. 1999;163:6777–6784. [PubMed] [Google Scholar]
- 33.Grinstein S, Furuya W. Chemoattractant-induced tyrosine phosphorylation and activation of microtubule-associated protein kinase in human neutrophils. J Biol Chem. 1992;267:18122–18125. [PubMed] [Google Scholar]
- 34.Torres M, Hall FL, O’Neill K. Stimulation of human neutrophils with formyl-methionyl-leucyl-phenylalanine induces tyrosine phosphorylation and activation of two distinct mitogen-activated protein-kinases. J Immunol. 1993;150:1563–1577. [PubMed] [Google Scholar]
- 35.Nauseef WM. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev. 2007;219:88–102. doi: 10.1111/j.1600-065X.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- 36.Styrt B. Species variation in neutrophil biochemistry and function. J Leukoc Biol. 1989;46:63–74. doi: 10.1002/jlb.46.1.63. [DOI] [PubMed] [Google Scholar]
- 37.Schiffmann E, Showell HV, Corcoran BA, Ward PA, Smith E, Becker EL. The isolation and partial characterization of neutrophil chemotactic factors from Escherichia coli. J. Immunol. 1975;114:1831–1837. [PubMed] [Google Scholar]
- 38.Miyake Y, Yasuhara T, Fukui K, Suginaka H, Nakajima T, Moriyama T. Purification and characterization of neutrophil chemotactic factors of Streptococcus sanguis. Biochim Biophys Acta. 1983;758:181–186. doi: 10.1016/0304-4165(83)90300-8. [DOI] [PubMed] [Google Scholar]
- 39.Rot A, Henderson LE, Sowder R, Leonard EJ. Staphylococcus aureus tetrapeptide with high chemotactic potency and efficacy for human leukocytes. J Leukoc Biol. 1989;45:114–120. doi: 10.1002/jlb.45.2.114. [DOI] [PubMed] [Google Scholar]
- 40.Carp H. Mitochondrial N-formylmethionyl proteins as chemoattractants for neutrophils. J.Exp.Med. 1982;155:264–275. doi: 10.1084/jem.155.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiang N, Fierro IM, Gronert K, Serhan CN. Activation of lipoxin A(4) receptors by aspirin-triggered lipoxins and select peptides evokes ligand-specific responses in inflammation. J Exp Med. 2000;191:1197–1208. doi: 10.1084/jem.191.7.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sklar LA, Fay SP, Seligmann BE, Freer RJ, Muthukumaraswamy N, Mueller H. Fluorescence analysis of the size of a binding pocket of a peptide receptor at natural abundance. Biochemistry. 1990;29:313–316. doi: 10.1021/bi00454a002. [DOI] [PubMed] [Google Scholar]
- 43.Quehenberger O, Pan ZK, Prossnitz ER, Cavanagh SL, Cochrane CG, Ye RD. Identification of an N-formyl peptide receptor ligand binding domain by a gain-of-function approach. Biochem Biophys Res Commun. 1997;238:377–381. doi: 10.1006/bbrc.1997.7298. [DOI] [PubMed] [Google Scholar]
- 44.Mills JS, Miettinen HM, Barnidge D, Vlases MJ, Wimer-Mackin S, Dratz EA, Sunner J, Jesaitis AJ. Identification of a ligand binding site in the human neutrophil formyl peptide receptor using a site-specific fluorescent photoaffinity label and mass spectrometry. J Biol Chem. 1998;273:10428–10435. doi: 10.1074/jbc.273.17.10428. [DOI] [PubMed] [Google Scholar]
- 45.Prossnitz ER, Ye RD. The N-formyl peptide receptor: a model for the study of chemoattractant receptor structure and function. Pharmacol Ther. 1997;74:73–102. doi: 10.1016/s0163-7258(96)00203-3. [DOI] [PubMed] [Google Scholar]
- 46.Partida-Sanchez S, Iribarren P, Moreno-Garcia ME, Gao JL, Murphy PM, Oppenheimer N, Wang JM, Lund FE. Chemotaxis and calcium responses of phagocytes to formyl peptide receptor ligands is differentially regulated by cyclic ADP ribose. J Immunol. 2004;172:1896–1906. doi: 10.4049/jimmunol.172.3.1896. [DOI] [PubMed] [Google Scholar]
- 47.Shi G, Partida-Sanchez S, Misra RS, Tighe M, Borchers MT, Lee JJ, Simon MI, Lund FE. Identification of an alternative G{alpha}q-dependent chemokine receptor signal transduction pathway in dendritic cells and granulocytes. J Exp Med. 2007;204:2705–2718. doi: 10.1084/jem.20071267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Partida-Sanchez S, Gasser A, Fliegert R, Siebrands CC, Dammermann W, Shi G, Mousseau BJ, Sumoza-Toledo A, Bhagat H, Walseth TF, Guse AH, Lund FE. Chemotaxis of mouse bone marrow neutrophils and dendritic cells is controlled by adp-ribose, the major product generated by the CD38 enzyme reaction. J Immunol. 2007;179:7827–7839. doi: 10.4049/jimmunol.179.11.7827. [DOI] [PubMed] [Google Scholar]
- 49.Gulden PH, Fischer P, 3rd, Sherman NE, Wang W, Engelhard VH, Shabanowitz J, Hunt DF, Pamer EG. A Listeria monocytogenes pentapeptide is presented to cytolytic T lymphocytes by the H2-M3 MHC class Ib molecule. Immunity. 1996;5:73–79. doi: 10.1016/s1074-7613(00)80311-8. [DOI] [PubMed] [Google Scholar]
- 50.Princiotta MF, Lenz LL, Bevan MJ, Staerz UD. H2-M3 restricted presentation of a Listeria-derived leader peptide. J Exp Med. 1998;187:1711–1719. doi: 10.1084/jem.187.10.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shawar SM, Cook RG, Rodgers JR, Rich RR. Specialized functions of MHC class I molecules. I. An N-formyl peptide receptor is required for construction of the class I antigen Mta. J Exp Med. 1990;171:897–912. doi: 10.1084/jem.171.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vyas JM, Shawar SM, Rodgers JR, Cook RG, Rich RR. Biochemical specificity of H-2M3a. Stereospecificity and space-filling requirements at position 1 maintain N-formyl peptide binding. J Immunol. 1992;149:3605–3611. [PubMed] [Google Scholar]
- 53.Lindahl KF, Dabhi VM, Hovik R, Smith GP, Wang CR. Presentation of N-formylated peptides by H2-M3. Biochem Soc Trans. 1995;23:669–674. doi: 10.1042/bst0230669. [DOI] [PubMed] [Google Scholar]
- 54.Xu H, Chun T, Choi HJ, Wang B, Wang CR. Impaired response to Listeria in H2-M3-deficient mice reveals a nonredundant role of MHC class Ib-specific T cells in host defense. J Exp Med. 2006;203:449–459. doi: 10.1084/jem.20051866. [DOI] [PMC free article] [PubMed] [Google Scholar]