Abstract
In addition to stem cells providing a better understanding about the biology and origins of gliomas, new therapeutic approaches have been developed based on the use of stem cells as delivery vehicles. The unique ability of stem cells to track down tumor cells makes them a very appealing therapeutic modality. This review introduces neural and mesenchymal stem cells, discusses the advances that have been made in the utilization of these stem cells as therapies and in diagnostic imaging (to track the advancement of the stem cells towards the tumor cells), and concludes by addressing various challenges and concerns regarding these therapies.
Keywords: brain tumor, glioma, mesenchymal stem cell, neural stem cell, stem cell
Despite the large amount of research that has been carried out investigating the biology and treatment of gliomas, the median survival is still 1 year or less for glioblastoma multiforme (GBM) and approximately 3 years for anaplastic astrocytoma [1-5]. High-grade gliomas have the ability to infiltrate local structures and migrate long distances, even to the contralateral hemisphere, leading to disease recurrence despite aggressive resection [6-10]. Even after seemingly curative resection of the tumor, there are often microsatellites of tumor cells scattered throughout normal brain tissue that have the potential to continue proliferating and cause tumor recurrence in other areas of the brain [8,10,11]. Moreover, tumor infiltration of eloquent areas of the brain often limits the extent of tumor resection [5,9,10,12-14]. In the 1930s, Walter Dandy reported recurrence of contralateral gliomas even after hemispher ectomy [15]. Radiotherapy [16,17 and chemotherapy [3,18,19] have had limited success in treating gliomas [2,3]. Both are limited by their toxicity to normal brain tissue that could lead to further brain damage and decreased quality of life for patients [20-22]. Moreover, the effect of chemo therapy is often suboptimal because there is limited drug penetration across the blood—brain barrier [23-25]. Lastly, gliomas evade the immune system by local suppression of immune cells within the tumor microenvironment, thus limiting the ability of the immune system to attack the tumor cells [26,27].
Treatment of gliomas has also been difficult because their origin is still not fully understood (Figure 1) [28]. A significant number of similarities have been found between normal neural stem cells (NSCs) and neoplastic cells of neuroectodermal origin in terms of migratory capacity, self-renewal potential, molecular signature and their ability to integrate themselves into normal tissue [29,30]. Many now believe that the virulence of brain tumors is maintained by a subpopulation of ‘stem-like’ cells, which are referred to as brain tumor stem cells (BTSCs) [31-34]. There is mounting evidence suggesting that BTSCs are responsible for the highly invasive [35] and resistant [36] potential of many human brain tumors. This unique group of cells has the ability to migrate long distances in the brain parenchyma [37]. Moreover, they exhibit profound resistance to both chemotherapy and radiotherapy [38]. Some studies have even shown that GBMs bordering the lateral ventricle, an area that contains the subventricular zone NSC niche, may carry a worse prognosis for patients [39]. It is still unclear whether the origin of these BTSCs emanates from normal progenitor/stem cells that have become neoplastic or from the dedifferentiation of mature neural cells that have undergone mutations [28]. There is evidence that both etiologies may be possible [40].
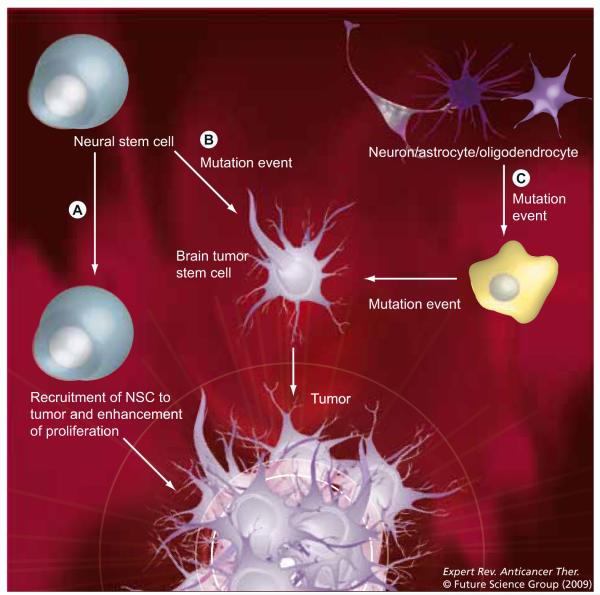
Figure 1. Three models on the origins of CNS malignancies.
(A) Normal adult NSCs are recruited to the site of tumor and help to regenerate tumor stromal cells. (B) NSCs undergo malignant transformation into a brain tumor stem cell. (C) Fully committed adult brain cells accumulate mutations over time to transform into brain tumor stem cells.
NSC: Neural stem cell.
The resemblance of the properties and attributes of stem cells to those of BTSCs has initiated interest in how stem cells can be armed to track and eradicate tumors [29,41,42]. In this review, we will discuss the tropism of stem cells for tumors, review the advances in neural and mesenchymal stem cell (MSC) therapies for gliomas, and finally outline the concerns and challenges in making stem cells into a treatment modality in humans.
Stem cell tropism for tumors
One of the remarkable properties of both NSCs and MSCs is their tropism for tumor cells [29,43]. These stem cells have the ability to migrate across the blood—brain barrier into the tumor when administered intra-arterially [29,44]. Stem cells have also been shown to migrate from the contralateral hemisphere into the tumor [29,43]. The tumor tropism of progenitor/stem cells is mediated by several receptor—ligand combinations [45]. The molecular basis of tumor tropism of stem cells in vivo is still poorly understood, but multiple in vitro studies have provided insight into the cytokines, growth factors and receptors involved in tumor tropism of stem cells [46]. These receptor—ligand interactions include SCF/c-Kit (in NSCs) [47-49], HGF/c-Met (in NSCs) [50], MCP-1/CCR2 (in NSCs) [51] and HMGB1/RAGE (in mesangioblasts) [52]. Kendall et al. observed that human HGF elicited the strongest chemotactic response from NSCs and that gliomatropism was critically dependent on c-Met and Ras—PI3K signaling [53]. EGF and its receptor have also been demonstrated to be involved in NSC migration [54], as well as the increased migration and invasion of glioma cells [55].
Malignant gliomas actively recruit mesenchymal progenitor cells by secreting angiogenic cytokines, such as VEGF [56], IL-8, TGF-ss1 and neurotrophin-3 [57]. In addition to its angiogenic properties, VEGF has also been shown to play a role in the tropism of NSCs for glioma cells [58].
Glioma extracellular matrix proteins have also been associated with the tropism of NSCs to gliomas [59] and MSCs to injured tissues [60]. Adhesion molecules, such as b1- and b2-integrins and L-selectin, may play a significant role in the mobilization and homing of MSCs to gliomas [61-63]. Matrix metalloproteases (MMPs) such as MMP2 and membrane type-1 matrix metalloprotease (MT1-MMP), as well as tissue inhibitor of metalloproteases-2 (TIMP-2) have been associated with MSC migration to injured tissues [59,60].
It is noteworthy to discuss the CXCR4—SDF-1α interaction, which plays an important role in inflammation, tumor tropism of stem cells and the pathology of gliomas [64-66]. The chemokine receptor CXCR4 has been found to be expressed in human and mouse NSCs, while its cognate ligand SDF-1α is expressed by reactive astrocytes and endothelium within regions of CNS injury and degeneration [64]. SDF-1α expressed by tumor-derived endothelium attracts NSCs to migrate to the tumor [65,67]. Inhibiting CXCR4—SDF-1α interactions prevented NSC migration towards gliomas [68]. With regard to its role in glioma pathology, the CXCR4—SDF-1α interaction increases glioma growth and cell migration as well as tumor angiogenesis [66,69]. Antagonists of CXCR4 have been shown to inhibit the growth of GBMs and medulloblastomas in experimental models [70]. The CXCR4—SDF-1α interaction may also play a role in metastatic malignancies to the brain [71]. It has been found that the CXCR4 receptor is expressed in breast cancer cells while SDF-1α is expressed on brain endothelium, thus providing a basis for the migration of circulating metastatic cells through the blood—brain barrier [71]. Inhibiting CXCR4 has been shown to prevent breast cancer metastasis to the brain in animal models [72].
Neural stem cells
The working definition of a stem cell includes the ability to self-renew and differentiate into several cell types [73,74]. Several immunocytochemically detectable markers have been proposed for NSCs, the most important being CD133 [75] and nestin [76,77]; but other less-used markers include mushashi1 [78-80] and Sox1/2 [81-83]. However, there are no markers that are sufficiently specific and sensitive to define a NSC and, thus, many rely on the operational definition of NSCs [84]:
Multipotency in which the neurospheres have the ability to produce mature cells in all three of the fundamental neural lineages (neurons, astrocytes and oligodendrocytes)
The ability to populate a developing region of the nervous system and/or an ablated/degenerated area of the nervous system with appropriate cell types
The ability to be serially transplanted
Self-renewal properties in which they are able to produce new NSCs
There are several potential sources of human NSCs [85], including the adult brain [86-90], fetal brains [91,92] and embryonic stem cells [93,94]. Within the adult brain, NSCs have been found in the sub ventricular zone (SVZ) [95-97], hippocampus [87-89], subcortical white matter [90], cerebellum [98] and olfactory bulbs [99]. Although adult NSCs and embryonic stem cell-derived neural progenitors exhibit many of the same properties, a few subtle differences in behavior and signaling pathways have been described [100]. For instance, embryonic stem cell-derived neural progenitors have a greater tendency to form neurons and have enhanced MAPK signaling, thus contributing to the high proliferation rate of these cells. Other sources of NSCs that have been explored include MSCs [101], adipose tissue stem cells [102] and skin stem cells [103], all of which have been demonstrated to have the potential to be reprogrammed into NSCs.
The progeny of NSCs are capable of integrating structurally and functionally into the surrounding host nervous system, thus having the potential to serve as treatments for nervous system repair [104-109]. The migratory capability of NSCs, along with their tropism for intracranial pathologies, make them attractive therapeutic agents for several neurological diseases, including stroke, Parkinson’s and Alzheimer’s disease, as well as brain tumors [79,92,110-113].
Mesenchymal stem cells
Mesenchymal stem cells are another stem cell population with the ability to self-renew as well as give rise to multiple tissue types [114]. These primitive progenitors exist postnatally and are multipotent, with the ability to generate cartilage, bone, muscle, tendon, ligament and fat [114]. There is a paucity of developmental stage-specific markers for MSCs [115]. However, markers such as STRO-1 have been used in the isolation of MSCs. For isolation of MSCs, our laboratory uses flow cytometry to isolate for a cell population with the following cell marker characteristics: CD31- CD45-CD90+CD105+CD106+ [116-118].
Mesenchymal stem cells can be acquired from a variety of sources, including embryonic stem cells [119], bone marrow [120,121], peripheral blood [122], placenta [123], adipose tissue [124], skin [125 and umbilical cord blood [126-128]. Studies are being performedto determine the best sources of MSCs. Kern et al. observed that bone marrow-derived MSCs had a lower proliferation capacity in vitro than MSCs from adipose tissue or umbilical cord blood, making the latter two sources very appealing as sources of MSCs [129]. Moreover, a recent study by Kim et al. demonstrated that the IL-8-mediated glioma-tracking behavior of umbilical cord MSCs is much stronger than that of bone marrow-derived MSCs and that this phenomenon is due to overexpression of the IL-8 receptor and CXC chemokine receptors 1 and 2 on umbilical cord MSCs [130]. Due to this difference in receptor expression, umbilical cord-derived MSCs have a much greater capacity to migrate towards glioma cells than bone marrow-derived MSCs [130].
NSCs & MSCs as a therapy for brain tumors
Both NSCs and MSCs have potential use in therapy for brain tumors (see BOXES 1 & 2 for a summary). Studies have suggested that unmodified endogenous NSCs may have a natural ability to suppress tumor growth [131]. It has been demonstrated that endogenous NSCs exhibit significant tropism for gliomas and migrate from the SVZ to surround the tumor graft [131]. The same study found that the endogenous precursor cell accumulation around gliomas decreased with the age of the recipient; this correlated with increased tumor size and decreased survival times in aged mice [131]. Furthermore, when GBM cells were cocultured with unmodified NSCs, the rapid increase in tumor cell number was suppressed and there was increased GBM cell apoptosis [131]. This observation had also been documented in earlier studies by Weinstein et al. [132] and Staflin et al. [133].
Box 1.
Summary of neural stem cell therapies for gliomas.
| Chemotherapeutic vehicles | |
| • Cytosine deaminase | [23,110] |
| • HSV-thymidine kinase | [109,112] |
| • Deoxycytosine kinase (not yet used in NSCs) | [113] |
| • Carboxylesterase (not yet used in NSCs) | [114-116] |
| Viral delivery vehicles | |
| • Replication-conditional HSV-1 vectors | [111] |
| • Conditionally replicating adenovirus | [118,119] |
| Immunotherapeutic vehicles | |
| • IL-4 | [120,121] |
| • IL-12 | [122] |
| • IL-23 | [123] |
| • TRAIL | [127-132] |
| • IFN-β | [137] |
| Inhibition of glioma migration | |
| • PEX | [140] |
HSV: Herpes simplex virus; NSC: Neural stem cell; PEX: Hemopexin-like domain of MMP-2; TRAIL: TNF-related apoptosis-inducing ligand
Box 2.
Summary of mesenchymal stem cell therapies for gliomas.
| Chemotherapeutic vehicles | |
| • HSV-thymidine kinase | [172] |
| Viral delivery vehicles | |
| • Conditionally replicating adenovirus | [173] |
| Immunotherapeutic vehicles | |
| • IL-2 | [34,174,175] |
| • IL-18 | [176] |
| • IL-23 | [124] |
| • TRAIL | [177] |
| • IFN-α | [178] |
| • IFN-β | [36] |
HSV: Herpes simplex virus; TRAIL: TNF-related apoptosis-inducing ligand.
In 2000, Aboody et al. demonstrated that modified NSCs migrated great distances to tumor masses when introduced in various locations of the brain, including the ipsilateral and contralateral hemispheres, relative to the glioma [29]. NSCs werealso able to migrate to the intracranial glioma when introduced via the systemic circulation [29]. On histological studies, it was found that NSCs traveled toward and positioned themselves in direct juxtaposition to microsatellites of glioma cells that had migrated away from the tumor bulk and invaded normal brain tissue [29]. Finally, Aboody et al. demonstrated that treatment with NSCs genetically modified to deliver a drug-activating enzyme resulted in a reduction in the size of the tumor and increased host survival [29].
The ability of stem cells to efficiently cross the blood—brain barrier, home in on tumor cells and then secrete a therapeutic molecule provides a very enticing approach to treat tumors of the CNS. Compared with some of the other experimental therapies being studied, such as viral therapy, stem cells are showing potential to be much more powerful treatments because, in addition to being able to home in on tumor cells, they are fully capable of transcriptional, translational and post-translational expression of large amounts of genetic information, allowing them to secrete therapeutic substances into the tumor microenvironment [46].
NSCs & MSCs as chemotherapeutic vehicles
There are several ways that stem cells can be used to treat gliomas. One such way is for the stem cells to locally convert a nontoxic prodrug into a toxic drug that will kill tumor cells in close vicinity [29]. This strategy takes advantage of the ability of stem cells to migrate towards areas of tumor mass and distribute themselves evenly throughout the bulk of the tumor to produce high concentrations of prodrug-converting enzyme. Systemically administrated prodrug penetrates through the blood—brain barrier into the CNS and is converted into a toxic form only in the close vicinity of tumor cells, thus killing the cancer cells through a ‘bystander effect’ and sparing normal tissue from the toxic effects of the drug.
Aboody et al. demonstrated in vivo efficacy of murine NSCs transduced to express cytosine deaminase (CDA), which converts the nontoxic prodrug 5-fluorocytosine (5-FC) into 5-fluoro uracil (5-FU), which is incorporated into the newly created DNA of proliferating tumor cells, causing chain termination and subsequent cell death [29]. There was a significant reduction in tumor burden in mice with intracranial gliomas when the mice were injected intracranially with CDA-expressing NSCs and given systemic 5-FC [29]. In 2003, Baressi et al. also demonstrated regression of intracranial gliomas when they used neural progenitors genetically modified to locally convert 5-FC to 5-FU in the tumor microenvironment [134]. If the CDA-expressing NSCs were to be recruited by the tumors to contribute to the tumor bulk, CDA would function as a suicide gene for these NSCs [135]. However, tumor recruitment of these stem cells is not a concern because evidence exists that exo genous NSCs do not contribute to the tumor [136]. Another drug-activating enzyme that has been studied is thymidine kinase 135]. In 2005, Li et al. demonstrated increased survival in rats that were treated with NSCs transduced with herpes simplex virus thymidine kinase (HSV-tk) and then given systemic gancyclovir [135]. Uhl et al. demonstrated that migratory HSV-tk-transduced NSCs had the ability to eliminate glioma cells purely by means of a gap junction-mediated bystander effect and that the efficacy of the treatment correlated with connexin-43 expression in glioma cells [137]. Deoxycytosine kinase, which activates cytosine arabinoside into a toxic form, is another enzyme that has been used in viral therapies for intracerebral gliomas and has potential for stem cell therapies [138]. Furthermore, carboxylesterase, which converts convert CPT-11 (irinotecan) to SN-38, may be yet another genetic modification to consider in future NSC therapies [139-141]. Another idea that may be interesting to look into is genetically modifying stem cells to activate radiotherapy-sensitizing agents in the tumor microenvironment.
As with NSCs, MSCs can be engineered to locally convert nontoxic compounds into a toxic drug that will kill glioma cells in close vicinity [142]. In 2007, Miletic et al. demonstrated that bone marrow-derived MSCs genetically engineered to express HSV-tk were highly effective in the treatment of gliomas in rodents [142]. Tumor cells were not detected on serial histological sections in the brains of surviving mice who received the tk-MSCs with adjuvant gancyclovir. Only a cavity and scar tissue from the initial tumor mass, which had been successfully treated, was observed in long-term survivors; this tissue was found to be infiltrated by CD45+ inflammatory cells [142]. There are several more drug-activating enzymes that can be explored in MSC-based therapies for gliomas.
NSCs & MSCs as viral-delivery vehicles
The delivery of viruses via NSCs that have the ability to home in on areas of pathology and locally produce virus is a significant improvement to the older methods of viral therapies using injection of viral supernatants or immobile virus-producing fibroblasts [143]. NSCs have also been used to deliver oncolytic viruses to kill glioma cells [136]. The modified NSCs home in on tumor cells and release oncolytic viruses, thereby transfecting the tumor cells to express oncolytic genes. An advantage of this form of viral delivery to tumors is that the dispersion of NSCs throughout the tumor bulk allows more extensive delivery of the oncolytic viruses not only to the tumor bulk but also to glioma cells that have invaded into normal brain parenchyma [136]. The HSV-tk system mentioned earlier has also been used in the form of viral therapy in which replication-conditional HSV-tk viruses are delivered by murine NSCs to transfect tumor cells [136]. The systemically administered prodrug, ganciclovir, is then converted into its toxic form in tumor cells that have been transduced to express thymidine kinase [135]. Lastly, NSCs have also been used to deliver conditionally replicating adenovirus to gliomas, and studies have shown that NSC-mediated delivery of the adenovirus enhanced the intratumoral distribution of the oncolytic vector compared with injection of the virus alone [144,145].
Mesenchymal stem cells can also be engineered to deliver oncolytic viruses to glioma cells [146]. Sonabend et al. engineered human MSCs to deliver replication-competent oncolytic adenovirus (CRAd) in a model of intracranial malignant glioma in rodents and showed that, when injected away from the tumor site in vivo, MSCs migrated to the tumor and delivered 46-fold more viral copies than injection of the virus alone [146]. More studies utilizing MSCs to deliver oncolytic viruses are currently underway.
NSCs & MSCs as immunotherapy
Neural stem cells can also be used as a form of immunotherapy to elicit an immune response against tumor cells by locally secreting cytokines in proximity to the tumor cells [147]. Depending on the cytokine used, a variety of effects can be achieved, including direct cytotoxicity to the tumor cells, arrest of growth and differentiation and host immune system modulation to elicit a stronger anti-tumor response [46]. One such cytokine that has been used is IL-4, which increases T-cell-mediated response to tumor cells [147]. Benedetti et al. found that treatment of mice bearing intracranial gliomas with NSCs transduced to express and secrete the IL-4 cytokine increased survival [148]. The group of mice with C6 rat gliomas treated with IL-4-secreting NSCs had a 90-day survival of over 80%, whereas 0% of the untreated mice survived for even 30 days [148]. Likewise, similar treatment with GL261 tumors resulted in 71% of the treatment group surviving compared with 33% of tumor-bearing mice that received control NSCs with no genetic modifications [148]. IL-12 is another cytokine that has therapeutic potential [149]. It is a T-cell-stimulating factor that can stimulate the growth and function of T cells, stimulate the production of IFN-γ and TNF from T cells and natural killer (NK) cells, enhance the cytotoxic effects of NK and CD8+ cytotoxic T lymphocytes, and has anti-angiogenic activity. In 2002, Ehtesham et al. used IL-12-secreting murine NSCs to treat GL261 tumor-bearing rats [149]. The local expression and secretion of IL-12 in the tumor microenvironment elicited a significant amount of tumor infiltration by T lymphocytes [149]. Another cytokine that has been studied for stem cell therapy for tumors is IL-23, which promotes an inflammatory response by promoting angiogenesis, increasing MMP9 and reducing CD8+ T-cell infiltration [150]. There has been some concern that IL-23 may actually promote tumor incidence and growth [150]. However, Yuan et al. demonstrated that bone marrow-derived neural stem-like cells engineered to secrete IL-23-inhibited tumor growth in C57BL/6 glioma-bearing mice, and the surviving mice treated with these engineered stem cells, were resistant to tumor rechallenge [151].
TNF-related apoptosis-inducing ligand (TRAIL) is a cell membrane protein that has sparked interest in tumor therapies because it has been shown to specifically induce apoptosis in cancer cells and not in normal tissue [152,153]. Injection of TRAIL-expressing NSCs into nude mice with intracranial GBM xenografts resulted in tumor apoptosis and reduction of the tumor bulk [154]. Similar results have been achieved with human NSCs isolated from the telencephalon of human fetal cadavers (13 weeks’ gestation) [155]. Shah et al. improved on this treatment by designing a secreted version of TRAIL (S-TRAIL), which increased the delivery of TRAIL to its molecular target [156]. Intracranial injection of NSCs expressing S-TRAIL resulted in a greater than 80% decrease in tumor growth [156]. Furthermore, NSCs have been engineered to secrete S-TRAIL in combination with small hairpin RNA (shRNA) targeting BCL-2 [157] or with micro-RNA-21 inhibitors [158]. Hingtgen et al., in 2008, demonstrated that combined treatment with NSCs secreting S-TRAIL and temozolomide induced cell killing and markedly upregulated proapoptotic proteins in glioma cells that were least sensitive to TRAIL [159].
Although originally described as antiviral proteins, type I interferons (α/β) also exhibit pleiotropic anti-tumor effects, including direct tumor cytotoxicity [160], immunomodulation [161] and inhibition of angiogenesis [160,162,163]. In a study by Dickson et al., HB1.F3 NSCs engineered to deliver IFN-β were injected into tumor foci with the intent to normalize tumor vasculature before administering cytotoxic agents so that these agents would more extensively penetrate the tumor mass and thus increase the anti-tumor efficacy of the drug [164]. The delivered IFN-β resulted in the maturation of the vasculature inside the tumor, which consequently made it unable to expand to support the tumor growth [164]. The maturation of the tumor vasculature also significantly increased the delivery and anti-tumor effect of adjuvant therapy [164].
Similar to NSCs, MSCs have also been genetically engineered to deliver various immunological cytokines (FIGURE 3) [43]. One of the first studies to explore the effect of genetically altered MSCs for the treatment of gliomas was by Nakamura et al. in 2004, where they demonstrated that the MSCs were able to migrate across the corpus callosum to the contralateral hemisphere towards the glioma cells [43]. When MSCs were implanted directly into the tumor, the MSCs localized mainly at the border between the tumor cells and normal brain parenchyma, but they also infiltrated into the tumor bed [43]. Finally, the study demonstrated that genetically modifying the MSCs to secrete IL-2 limited tumor growth and prolonged the survival of tumor-bearing rats [43]. Implantation of IL-2 MSCs induced lymphocyte infiltration into the gliomas, whereas there was minimal inflammatory cell infiltration in the untreated controls [43]. Other studies have also demonstrated that genetically modified MSCs expressing IL-2 slowed tumor development when injected into intracranial gliomas [165,166]. Another cytokine that has been studied in MSC-based therapy is IL-18, which is a proinflammatory cytokine that induces the production of IFN-γ in T cells and NK cells. Xu et al. demonstrated that MSCs genetically modified to produce IL-18 inhibited glioma growth and prolonged the survival of glioma-bearing rats [167]. Transplantation of these IL-18-secreting MSCs was associated with increased T-cell infiltration of the gliomas and long-term anti-tumor immunity [167]. Yuan et al. showed that IL-23-expressing neural stem-like cells can be generated from the bone marrow of adult mice and, when implanted into the brains of mice with intracranial gliomas, these cells were able to track into the tumor mass as well as to tumor islands that had infiltrated deep into brain tissue [151]. Intratumoral implantation of bone marrow-derived NSCs transduced with IL-23 led to decreased glioma growth and significantly prolonged the survival of treated mice [151].
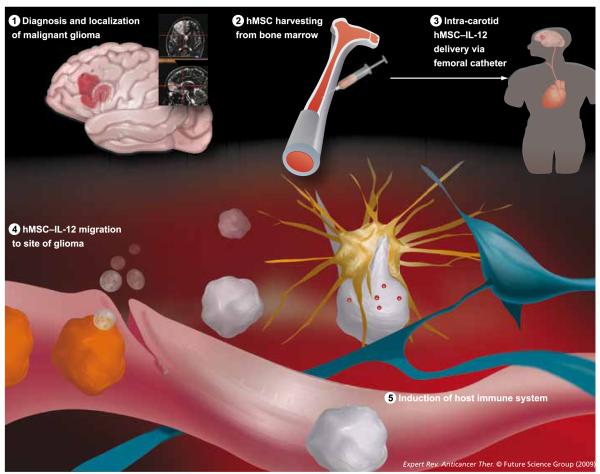
Figure 3. Potential clinical application of genetically engineered IL-12 hMSCs.
In our laboratory, we are using hMSCs harvested from bone marrow as well as from adipose tissue. The advantage to adipose-derived MSCs is the ease of harvesting patient-specific MSCs. During surgery for resection of the brain tumor, the surgeon also takes a small fat graft from the patient. These MSCs can then be cultured and transduced to express various proteins, such as IL-12. Ideally, in future therapies, the genetically modified MSCs would be injected intra-arterially through the carotid artery, and the MSCs would migrate to the site of glioma and induce an anti-tumor immune response.
hMSC: Human mesenchymal stem cell; MSC: Mesenchymal stem cell.
In 2008, Kim et al. demonstrated that human umbilical cord blood-derived MSCs that were genetically modified to secrete TRAIL significantly inhibited tumor growth and prolonged the survival of mice bearing gliomas compared with controls [168]. In 2005, Nakamizo et al. demonstrated that human MSCs transduced to express IFN-β significantly increased animal survival compared with controls in an intracranial glioma model [44]. Another study demonstrated that MSCs engineered to secrete IFN-α significantly prolonged the survival of brain tumor-bearing rats [169]. The same study found that transfection of the MSCs with EGF receptor (EGFR) enhanced the migratory responses of the MSCs in glioma-conditioned media and that EGFR MSCs were able to migrate toward GL261 gliomas or B16 melanoma in vivo, even after injection into the contralateral hemisphere of mice [169].
It is noteworthy to also mention that activating MSCs with certain cytokines, such as IL-2, IL-15 and GM-CSF, increases the cytotoxicity of genetically modified MSCs [170,171]. In Kang et al., activation of umbilical cord blood-derived MSCs with cytokines did not increase the number of differentiated immune effector cells in the tumor; however, activated umbilical cord blood-derived MSCs did secrete more immune response-related proteins such as IL-4 and IFN-γ than the MSCs that were not activated [171].
NSCs to inhibit glioma cell migration & invasion
The human metalloprotease-2 (MMP-2) fragment PEX inhibits the proliferation and migration of endothelial and glioma cells, in addition to decreasing angiogenesis [172,173]. In 2005, Kim et al. demonstrated that intratumoral injection of the hemopexin-like domain of MMP-2 (PEX)-secreting HB1.F3 NSCs reduced tumor volume by 90% and decreased angiogenesis by 45% and cell proliferation by 24% [174]. Histological ana lysis showed that these stem cells migrated to the tumor boundary [174]. This is a desirable location for the NSCs to situate themselves considering that the main goal is to inhibit tumor cell migration away from the tumor bulk. Since migration and invasion are such lethal characteristics of glioma cells, additional approaches to inhibit metalloproteases and other proteins conducive to glioma cell invasion should be explored in future therapies. This therapeutic approach should also be explored in MSCs.
Diagnostic imaging using NSCs & MSCs
Since stem cells have a tendency to track down cancer cells in the tumor mass as well as those that have migrated away from the bulk of the tumor into the brain parenchyma, studies have explored the use of stem cells as an imaging modality to determine the extent of tumor infiltration and to determine where the therapeutic stem cells are positioned (FIGURE 2) [175]. Utilizing routine bioluminescence imaging, Tang et al. were able to explore the macroscopic migratory abilities of luciferase-expressing murine neural progenitor cells toward brain tumors and demonstrated their persistence in brain tumor tissue 4 weeks after implantation in living mice [176]. Another interesting finding from the study was that intraventricular injection of the NSCs resulted in a greater number of NSCs migrating to the tumor than the intravenous, intracranial or intraperitoneal routes [176]. In a later study, the same group demonstrated that NSCs could be transduced with both S-TRAIL and luciferase to provide for the tracking of in vivo migration of the NSCs as they delivered tumoricidal S-TRAIL to glioma cells [177]. Another imaging modality relies on the internalization of tat peptide-derivatized magnetic nanoparticles in hematopoietic and neural progenitor cells and then using MRI for in vivo tracking and recovery of the cells [178]. Zhang et al. used MRI to demonstrate that neural progenitor cells and MSCs labeled with lipophilic dye-coated superparamagnetic particles migrated toward intra cranial gliomas after injection into the cisterna magna or the rat tail vein [179]. Using MRI, they were able to track the migration of as few as 1000 labeled NSCs in vivo [179]. Slotkin et al. demonstrated that fluorescent semiconductor quantum dots can be effectively used to label mammalian neural stem and progenitor cells in vivo [180]. Lastly, nanoshells are a new class of optically tunable nanoparticles that may provide imaging of NSC migration as well as therapeutic photothermal therapy to treat glioma cells [181]. Loo et al. engineered immunotargeted nanoshells that are able to scatter light in the near-infrared region (enabling imaging of the tumor) as well as absorb light (allowing selective destruction of targeted carcinoma cells through photothermal therapy) [181]. This combined imaging and therapeutic modality has great potential in gliomas.
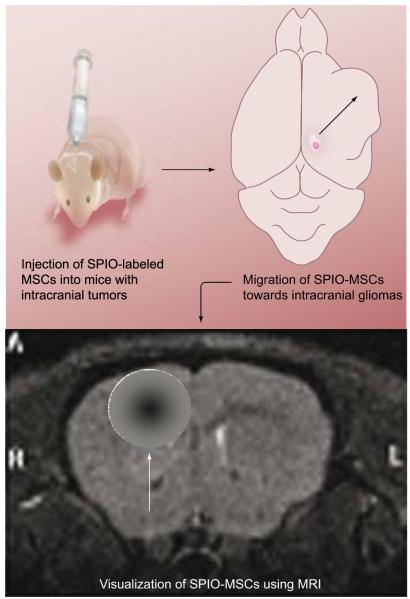
Figure 2. MSCs labeled with SPIO injected into intracranial glioma-bearing mice.
The SPIO-labeled MSCs migrate towards the area of the brain containing the tumor. This modification of MSCs and the idea that MSCs home in on tumor cells allow for another way to image brain tumors and the extent of tumor infiltration into normal brain parenchyma. More importantly, it allows for the surveillance of the location of the therapeutic stem cells. Such labeling will be important in future therapies because it will allow clinicians to know whether the therapeutic stem cells have positioned themselves in a location that will be effective for the treatment of the tumor bulk and microsatellites.
MSC: Mesenchymal stem cell; SPIO: Superparamagnetic iron oxide.
Mesenchymal stem cells can also be labeled so that they can be tracked in vivo. In 2005, Anderson et al. demonstrated that MRI can detect the incorporation of magnetically labeled bone marrow-derived precursor cells into tumor vasculature as part of ongoing angiogenesis and neovascularization [182]. This labeling of stem cells can be used to directly identify tumor neovasculature in vivo and to facilitate gene therapy by noninvasively monitoring these cells as gene-delivery vectors. In 2008, Wu et al. showed on MRI that superparamagnetic iron oxide nanoparticle-labeled MSCs, which were systemically transplanted, migrate toward gliomas with high specificity [183]. Fluorescent quantum dots are another modality for biological imaging [184] and work has already been carried out to label MSCs in vitro [185] and in vivo [186].
Concerns involving stem cell-based therapies
One of the major concerns involving stem cell therapy is that genetically modified stem cells may generate secondary malignancies [187]. Allogeneic stem cell lines used as therapy for gliomas could theoretically become tumorigenic by three mechanisms [188]:
Expression of the gene used to immortalize the cell line could transform the cells
The genetic modification of the stem cells could result in insertion of the gene into a critical locus, dysregulating the normal cell behavior and inducing cancer cell behavior
The therapeutic stem cell may fuse with endogenous cells, resulting in cells with a transformed phenotype that could potentially be cancerous
With regard to the concern that genetic modifications to immortalize cells can lead to the transformation of the stem cells, there is evidence that several cell lines immortalized with human telomerase reverse transcriptase (hTERT ) gene and simian virus 40 (SV40) large T fragment were observed to be unstable with respect to chromosome number and karyotype [189]. Nevertheless, immortalization of cell lines is important to be able to develop stem cell lines that can proliferate long enough to be well characterized, genetically modified and then amplified to therapeutic quantities [188]. Studies have shown that immortalization of stem cell lines with v-myc [188], as well as the use of cells that have only undergone a few passages [190], may decrease the risk for neoplastic transformation of the stem cells. The concern that genetic modification of the stem cells may lead to genetic insertion that will transform the cells into cancer emphasizes the need to establish cloned, extensively characterized stem cell lines in which all the insertion sites can be carefully scrutinized for their safety. Finally, with regard to therapeutic stem cells fusing with endogenous cells, resulting in cells with a transformed phenotype that could potentially be cancerous, there have been no reports of hyperdiploid cells in any of the animals treated with stem cells [188]. Embryonic stem cells do have a greater capacity for fusion than fetal or adult stem cells [191,192], which suggests that nonembryonic stem cells should preferentially be used in stem cell-based glioma therapies.
Another major concern is the recruitment of the stem cells to contribute to the tumor. The capacity of NSCs to self-renew and invade suggests that aberrant NSCs may transform into BTSCs, creating the bulk of the tumor [193-195]. This is an area of heavy research that many laboratories, including ours, are exploring. There is also concern that MSCs may enhance or initiate tumor growth [196,197]. Chen et al. suggested that systemic administration of human MSCs can contribute to angiogenesis in the pathological brain [198]. In 2006, Tso et al. found that a subset of primary GBM tumors and their derived tumor cell lines express cellular and molecular markers that are associated with MSCs [199]. They also found that GBM cultures can be induced to differentiate into multiple mesenchymal lineage-like cell types [199]. These findings suggest that a subset of primary glioblastomas derive from transformed stem cells containing MSC-like properties or that glioblastomas activate a series of genes resulting in the mesenchymal properties of the cancer cells and providing sustained tumor growth and malignant progression [199]. However, Hall et al. pointed out that many of these studies that have shown MSCs to possess tumorigenic properties used extensively passaged and/or genetically altered MSCs [190]. It has been demonstrated in several papers that lower-passage MSCs do not form tumors in vivo [200-202]. Whether or not NSCs and MSCs are recruited by the tumor cells to participate in the expansion of the tumor, it has been demonstrated from numerous animal studies using genetically modified NSCs or MSCs that stem cell therapy actually decreases the tumor burden.
There have also been concerns regarding the immunologic response to therapeutic NSCs or MSCs, especially that stem cells can suppress the immune response to tumors [203,204]. However, we have discussed earlier the many studies that used genetically modified NSCs or MSCs to control tumor growth and stimulate an anti-tumor immune response [44,169]. Another immunologic concern is whether these stem cells will be accepted by the recipient’s immune system. As noted by Yip et al., the NSCs used for the treatment of tumors may not necessarily need to be matched to the recipient [46]. If the tumor-infiltrating NSCs were to elicit an immune reaction against themselves, this would attract immune cells into the tumor microenvironment, increasing the immune response to the tumor as well [46]. Thus, it may be feasible to have a universal, readily available stem cell line for use as a therapy.
There are also several practical concerns about the use of these stem cell therapies for the treatment of gliomas before they can be applied to humans. For example, one major concern with NSCs is how autologous NSCs and MSCs would be obtained for patient treatment. There is concern about whether an adequate number of stem cells can be obtained from each patient and the amount of time it would take to make these stem cells ready for treatment. These harvested stem cells then need to be cultured, grown out, genetically modified and then characterized and tested for safety, all of which would take a considerable amount of time. As these stem cells are passaged and prepared for therapy, there is also concern that the original stem cells can significantly change outside their intrinsic in vivo environment. Moreover, genetic modifications may actually make these stem cells harmful to the patient rather than beneficial. The most practical way to make stem cells readily available for treatment in patients would be to create universal, immortalized stem cell lines that have been thoroughly characterized for genetic stability, biodistribution, safety and toxicity [188]. These cell lines would be kept in master cell banks and be readily available for clinical use.
Expert commentary
As noted earlier, the current standard of care for many brain tumors, which includes radiation and chemotherapy combined with surgical resection, is limited in its ability to extend survival [2,3,16-18]. Many high-grade gliomas fail to respond to therapy for a variety of reasons, including extensive seeding of microsatellites of tumor cells away from the tumor bulk into normal brain [11] and the resistance of glioma cells to radiation and chemotherapy, in particular BTSCs [36]. Stem cells represent a powerful new way to treat gliomas. Numerous studies have documented the tropism of stem cells for tumor cells and their ability to migrate to areas of pathology [29,43,44]. This behavior of stem cells is exciting in that it provides a novel way to provide therapy and/or elicit an immune response to all glioma cells, even those that have invaded deep into normal brain tissue [205].
In 1995, Brem et al. demonstrated the safety and efficacy of controlled drug delivery by biodegradable polymers that were implanted into the tumor resection cavity to release the chemotherapeutic drug carmustine [206]. In the future, we may be able to implant similar biopolymer wafers or gels into the tumor resection cavity to locally release genetically engineered stem cells to track down tumor cells and release tumoricidal substances/viruses as well as elicit a powerful immune response against the tumor cells [207]. Future stem cell therapies will probably be even more effective at tracking down tumor cells, controlling tumor growth, amplifying the anti-tumor immune response and limiting invasion of glioma cells into normal tissue.
Five-year view
Before stem cell therapy can be applied for the treatment of human brain tumors, much more research must be conducted to investigate the biology of stem cells and brain tumors. Thus, in the next 5 years, we believe that significant advances will be made in the understanding of how tumors, stem cells and the immune system interact with one another, and how these interactions can be utilized from a therapeutic standpoint. Further research needs to be carried out in finding new and more powerful genetic modifications to stem cells so that their anti-tumor effects are maximized. As these stem cell therapies advance and become more sophisticated, stem cells will probably be engineered to have multiple genetic modifications and thus be equipped to apply several different therapeutic strategies towards treating the cancer cells. More studies must also be carried out to elucidate the chemotactic factors and signaling pathways involved in the gliomatropic behavior of stem/progenitor cells. Understanding the signals involved in the tropism of stem cells for gliomas will enable better engineering of therapeutic stem cells so that they are more sensitive to signals secreted from the tumor environment and thus better able to track tumor cells. Moreover, there are still many potential applications that have not been explored. For example, there have been no studies that have looked at ways that stem cells can, for instance, increase the radiosensitivity of glioma cells.
Human trials of stem cells for the treatment of gliomas are still a long way off. Before stem cell therapy can be applied to the treatment of gliomas in humans, further in vivo human safety and efficacy data must be compiled in terms of cell distribution and duration of survival, and genomic insertion sites for exogenous genes that would minimize the risk of inducing neoplastic mutations in the cell must be identified.
Although there is still much work to be done, the progress in stem cell therapies has provided us with new hope that, in the future, we will be able to better treat aggressive brain tumors such as GBM.
Key issues.
Stem cells have a tropism for glioma cells, enabling them to home in on glioma cells in the tumor bulk as well as in distant tumor microsatellites scattered throughout normal brain tissue.
Several genetic modifications have been performed on neural and mesenchymal stem cells to increase their tumor cytotoxic effects, their stimulation of an anti-tumor immune response and their glioma-tropic tracking behavior.
Stem cells can also be used as an imaging modality to track the migration of these stem cells on tumor cells.
Further research must be performed into the exact role that neural and mesenchymal stem cells play in the tumor environment and whether there is any tumor recruitment of stem cells.
Acknowledgements
We apologize to those authors whose studies we could not cite due to space limitations.
Financial & competing interests disclosure This work was supported by grants from the Maryland Stem Cell Foundation and NIH KO8. Moreover, the Howard Hughes Medical Institute has generously supported the work of Thomas Kosztowski, Hasan Zaidi and Alfredo Quinoñes-Hinojosa. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Thomas Kosztowski, The Johns Hopkins Hospital, Department of Neurosurgery, Johns Hopkins University, CRB II, 1550 Orleans Street, Room 247, Baltimore, MD 21231, USA Tel.: +1 410 502 2906 thomas.kosztowski@jhmi.edu.
Hasan A Zaidi, The Johns Hopkins Hospital, Department of Neurosurgery, Johns Hopkins University, CRB II, 1550 Orleans Street, Room 247, Baltimore, MD 21231, USA Tel.: +1 410 502 2906 hzaidi@jhmi.edu.
Alfredo Quiñones-Hinojosa, The Johns Hopkins Hospital, Department of Neurosurgery, Johns Hopkins University, CRB II, 1550 Orleans Street, Room 247, Baltimore, MD 21231, USA Tel.: +1 410 502 2906 aquinon2@jhmi.edu.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.DeAngelis LM. Brain tumors. N. Engl. J. Med. 2001;344(2):114–123. doi: 10.1056/NEJM200101113440207. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.McGirt MJ, Than KD, Weingart JD, et al. Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme. J. Neurosurg. 2009;110(3):583–588. doi: 10.3171/2008.5.17557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kornblith PL, Walker M. Chemotherapy for malignant gliomas. J. Neurosurg. 1988;68(1):1–17. doi: 10.3171/jns.1988.68.1.0001. [DOI] [PubMed] [Google Scholar]
- 5.Wen PY, Kesari S. Malignant gliomas in adults. N. Engl. J. Med. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126.Excellent review on the pathogenesis, presentation, and treatment of malignant gliomas.
- 6.Holland EC. Glioblastoma multiforme: the terminator. Proc. Natl Acad. Sci. USA. 2000;97(12):6242–6244. doi: 10.1073/pnas.97.12.6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaye AH, Laws ER. Historical perspective. In: Kaye AH, Laws ER, editors. Brain Tumors: An Encyclopedic Approach. Churchill Livingstone; NY, USA: 2001. pp. 3–8. [Google Scholar]
- 8.Claes A, Idema AJ, Wesseling P. Diffuse glioma growth: a guerilla war. Acta Neuropathol. 2007;114(5):443–458. doi: 10.1007/s00401-007-0293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGirt MJ, Chaichana KL, Attenello FJ, et al. Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery. 2008;63(4):700–707. doi: 10.1227/01.NEU.0000325729.41085.73. [DOI] [PubMed] [Google Scholar]
- 10.McGirt MJ, Chaichana KL, Gathinji M, et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J. Neurosurg. 2009;110(1):156–162. doi: 10.3171/2008.4.17536. [DOI] [PubMed] [Google Scholar]
- 11.Salazar OM, Rubin P. The spread of glioblastoma multiforme as a determining factor in the radiation treated volume. Int. J. Radiat. Oncol. Biol. Phys. 1976;1(7–8):627–637. doi: 10.1016/0360-3016(76)90144-9. [DOI] [PubMed] [Google Scholar]
- 12.Cedzich C, Taniguchi M, Schafer S, Schramm J. Somatosensory evoked potential phase reversal and direct motor cortex stimulation during surgery in and around the central region. Neurosurgery. 1996;38(5):962–970. doi: 10.1097/00006123-199605000-00023. [DOI] [PubMed] [Google Scholar]
- 13.Fadul C, Wood J, Thaler H, et al. Morbidity and mortality of craniotomy for excision of supratentorial gliomas. Neurology. 1988;38(9):1374–1379. doi: 10.1212/wnl.38.9.1374. [DOI] [PubMed] [Google Scholar]
- 14.Sawaya R, Hammoud M, Schoppa D, et al. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 1998;42(5):1044–1055. doi: 10.1097/00006123-199805000-00054. [DOI] [PubMed] [Google Scholar]
- 15.Dandy W. Removal of right cerebral hemispheres for certain tumors with hemiplegia: preliminary report. JAMA. 1928;90:823–825. [Google Scholar]
- 16.Karim AB, Maat B, Hatlevoll R, et al. A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) Study 22844. Int. J. Radiat. Oncol. Biol. Phys. 1996;36(3):549–556. doi: 10.1016/s0360-3016(96)00352-5. [DOI] [PubMed] [Google Scholar]
- 17.Shaw E, Arusell R, Scheithauer B, et al. Prospective randomized trial of low-versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. J. Clin. Oncol. 2002;20(9):2267–2276. doi: 10.1200/JCO.2002.09.126. [DOI] [PubMed] [Google Scholar]
- 18.DeAngelis LM, Burger PC, Green SB, Cairncross JG. Malignant glioma: who benefits from adjuvant chemotherapy? Ann. Neurol. 1998;44(4):691–695. doi: 10.1002/ana.410440418. [DOI] [PubMed] [Google Scholar]
- 19.McGirt MJ, Than KD, Weingart JD, et al. Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme. J. Neurosurg. 2009;110(3):583–588. doi: 10.3171/2008.5.17557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Recht LD, Lew R, Smith TW. Suspected low-grade glioma: is deferring treatment safe? Ann. Neurol. 1992;31(4):431–436. doi: 10.1002/ana.410310413. [DOI] [PubMed] [Google Scholar]
- 21.Laing RW, Warrington AP, Graham J, et al. Efficacy and toxicity of fractionated stereotactic radiotherapy in the treatment of recurrent gliomas (Phase I/II study) Radiother. Oncol. 1993;27(1):22–29. doi: 10.1016/0167-8140(93)90040-f. [DOI] [PubMed] [Google Scholar]
- 22.Finlay JL. The role of high-dose chemotherapy and stem cell rescue in the treatment of malignant brain tumors. Bone Marrow Transplant. 1996;18(Suppl 3):S1–S5. [PubMed] [Google Scholar]
- 23.Groothuis DR. The blood—brain and blood—tumor barriers: a review of strategies for increasing drug delivery. Neuro. Oncol. 2000;2(1):45–59. doi: 10.1093/neuonc/2.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pardridge WM. CNS drug design based on principles of blood—brain barrier transport. J. Neurochem. 1998;70(5):1781–1792. doi: 10.1046/j.1471-4159.1998.70051781.x. [DOI] [PubMed] [Google Scholar]
- 25.Lesniak MS, Brem H. Targeted therapy for brain tumours. Nat. Rev. Drug. Discov. 2004;3(6):499–508. doi: 10.1038/nrd1414. [DOI] [PubMed] [Google Scholar]
- 26.Parsa AT, Waldron JS, Panner A, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat. Med. 2007;13(1):84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 27.Fecci PE, Mitchell DA, Whitesides JF, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66(6):3294–3302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- 28.Stiles CD, Rowitch DH. Glioma stem cells: a midterm exam. Neuron. 2008;58(6):832–846. doi: 10.1016/j.neuron.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 29.Aboody KS, Brown A, Rainov NG, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc. Natl Acad. Sci. USA. 2000;97(23):12846–12851. doi: 10.1073/pnas.97.23.12846.Monumental paper proposing stem cells as treatments for brain tumors. The article demonstrated that when implanted intracranially at distant sites from the tumor, even into the contralateral hemisphere or into the cerebral ventricles, the stem cells migrated through normal tissue towards the tumor.
- 30.Dietrich J, Imitola J, Kesari S. Mechanisms of disease: the role of stem cells in the biology and treatment of gliomas. Nat. Clin. Pract. Oncol. 2008;5(7):393–404. doi: 10.1038/ncponc1132. [DOI] [PubMed] [Google Scholar]
- 31.Hemmati HD, Nakano I, Lazareff JA, et al. Cancerous stem cells can arise from pediatric brain tumors. Proc. Natl Acad. Sci. USA. 2003;100(25):15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galli R, Binda E, Orfanelli U, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64(19):7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 33.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63(18):5821–5828.Reported the identification and purification of cancer stem cells from human brain tumors of different phenotypes that possess a profound capacity for proliferation, self-renewal, and differentiation. Brain tumor stem cells (BTSCs) were isolated with the cell fraction expressing the neural stem cell surface marker CD133, and these CD133+ cells were shown to differentiate in culture into tumor cells that phenotypically resembled the tumor from the patient.
- 34.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128.Only the CD133+ brain tumor fraction, representing BTSCs, contains cells that are capable of tumor initiation in nonobese diabetic (NOD)—severe combine immunodeficiency (SCID) mouse brains. Injection of as few as 100 CD133+ cells produced a tumor that could be serially transplanted and was a phenocopy of the patient’s original tumor. On the other hand, injection of 105 CD133- cells engrafted but did not produce a tumor.
- 35.Tu SM, Lin SH, Logothetis CJ. Stem-cell origin of metastasis and heterogeneity in solid tumours. Lancet Oncol. 2002;3(8):508–513. doi: 10.1016/s1470-2045(02)00820-3. [DOI] [PubMed] [Google Scholar]
- 36.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 37.Xie Z, Chin LS. Molecular and cell biology of brain tumor stem cells: lessons from neural progenitor/stem cells. Neurosurg. Focus. 2008;24(3–4):E25. doi: 10.3171/FOC/2008/24/3-4/E24. [DOI] [PubMed] [Google Scholar]
- 38.Sakariassen PO, Immervoll H, Chekenya M. Cancer stem cells as mediators of treatment resistance in brain tumors: status and controversies. Neoplasia. 2007;9(11):882–892. doi: 10.1593/neo.07658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaichana KL, McGirt MJ, Frazier J, et al. Relationship of glioblastoma multiforme to the lateral ventricles predicts survival following tumor resection. J. Neurooncol. 2008;89(2):219–224. doi: 10.1007/s11060-008-9609-2. [DOI] [PubMed] [Google Scholar]
- 40.Bachoo RM, Maher EA, Ligon KL, et al. Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 2002;1(3):269–277. doi: 10.1016/s1535-6108(02)00046-6. [DOI] [PubMed] [Google Scholar]
- 41.Snyder EY, Taylor RM, Wolfe JH. Neural progenitor cell engraftment corrects lysosomal storage throughout the MPS VII mouse brain. Nature. 1995;374(6520):367–370. doi: 10.1038/374367a0. [DOI] [PubMed] [Google Scholar]
- 42.Yip S, Aboody KS, Burns M, et al. Neural stem cell biology may be well suited for improving brain tumor therapies. Cancer J. 2003;9(3):189–204. doi: 10.1097/00130404-200305000-00007. [DOI] [PubMed] [Google Scholar]
- 43.Nakamura K, Ito Y, Kawano Y, et al. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther. 2004;11(14):1155–1164. doi: 10.1038/sj.gt.3302276.Very important paper demonstrating that mesenchymal stem cells (MSCs) can be used as treatment for brain tumors. Confirmed the migratory capacity of MSCs in vivo and showed that when MSCs were inoculated into the contralateral hemisphere, they migrated towards the tumor through the corpus callosum. Intratumoral injection of MSCs resulted in significant inhibition of tumor growth and increased the survival of tumor-bearing rats.
- 44.Nakamizo A, Marini F, Amano T, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65(8):3307–3318. doi: 10.1158/0008-5472.CAN-04-1874.Demonstrated that human MSCs can integrate into human gliomas after intravascular or local delivery and that this tropism of human MSCs for human gliomas can be exploited to a therapeutic advantage.
- 45.Xu F, Zhu JH. Stem cells tropism for malignant gliomas. Neurosci. Bull. 2007;23(6):363–369. doi: 10.1007/s12264-007-0054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yip S, Sabetrasekh R, Sidman RL, Snyder EY. Neural stem cells as novel cancer therapeutic vehicles. Eur. J. Cancer. 2006;42(9):1298–1308. doi: 10.1016/j.ejca.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 47.Erlandsson A, Larsson J, Forsberg-Nilsson K. Stem cell factor is a chemoattractant and a survival factor for CNS stem cells. Exp. Cell Res. 2004;301(2):201–210. doi: 10.1016/j.yexcr.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 48.Sun L, Lee J, Fine HA. Neuronally expressed stem cell factor induces neural stem cell migration to areas of brain injury. J. Clin. Invest. 2004;113(9):1364–1374. doi: 10.1172/JCI20001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Serfozo P, Schlarman MS, Pierret C, Maria BL, Kirk MD. Selective migration of neuralized embryonic stem cells to stem cell factor and media conditioned by glioma cell lines. Cancer Cell Int. 2006;6:1. doi: 10.1186/1475-2867-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heese O, Disko A, Zirkel D, Westphal M, Lamszus K. Neural stem cell migration toward gliomas in vitro. Neuro. Oncol. 2005;7(4):476–484. doi: 10.1215/S1152851704000754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Widera D, Holtkamp W, Entschladen F, et al. MCP-1 induces migration of adult neural stem cells. Eur. J. Cell. Biol. 2004;83(8):381–387. doi: 10.1078/0171-9335-00403. [DOI] [PubMed] [Google Scholar]
- 52.Palumbo R, Galvez BG, Pusterla T, et al. Cells migrating to sites of tissue damage in response to the danger signal HMGB1 require NF-κB activation. J. Cell. Biol. 2007;179(1):33–40. doi: 10.1083/jcb.200704015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kendall SE, Najbauer J, Johnston HF, et al. Neural stem cell targeting of glioma is dependent on phosphoinositide 3-kinase signaling. Stem Cells. 2008;26(6):1575–1586. doi: 10.1634/stemcells.2007-0887. [DOI] [PubMed] [Google Scholar]
- 54.Chicoine MR, Silbergeld DL. Mitogens as motogens. J. Neurooncol. 1997;35(3):249–257. doi: 10.1023/a:1005808315821. [DOI] [PubMed] [Google Scholar]
- 55.Boockvar JA, Kapitonov D, Kapoor G, et al. Constitutive EGFR signaling confers a motile phenotype to neural stem cells. Mol. Cell. Neurosci. 2003;24(4):1116–1130. doi: 10.1016/j.mcn.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 56.Schichor C, Birnbaum T, Etminan N, et al. Vascular endothelial growth factor A contributes to glioma-induced migration of human marrow stromal cells (hMSC) Exp. Neurol. 2006;199(2):301–310. doi: 10.1016/j.expneurol.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 57.Birnbaum T, Roider J, Schankin CJ, et al. Malignant gliomas actively recruit bone marrow stromal cells by secreting angiogenic cytokines. J. Neurooncol. 2007;83(3):241–247. doi: 10.1007/s11060-007-9332-4. [DOI] [PubMed] [Google Scholar]
- 58.Schmidt NO, Przylecki W, Yang W, et al. Brain tumor tropism of transplanted human neural stem cells is induced by vascular endothelial growth factor. Neoplasia. 2005;7(6):623–629. doi: 10.1593/neo.04781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ziu M, Schmidt NO, Cargioli TG, et al. Glioma-produced extracellular matrix influences brain tumor tropism of human neural stem cells. J. Neurooncol. 2006;79(2):125–133. doi: 10.1007/s11060-006-9121-5. [DOI] [PubMed] [Google Scholar]
- 60.Ries C, Egea V, Karow M, et al. MMP-2, MT1-MMP and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood. 2007;109(9):4055–4063. doi: 10.1182/blood-2006-10-051060. [DOI] [PubMed] [Google Scholar]
- 61.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat. Med. 2003;9(6):702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 62.Wysoczynski M, Reca R, Ratajczak J, et al. Incorporation of CXCR4 into membrane lipid rafts primes homing-related responses of hematopoietic stem/progenitor cells to an SDF-1 gradient. Blood. 2005;105(1):40–48. doi: 10.1182/blood-2004-04-1430. [DOI] [PubMed] [Google Scholar]
- 63.Son BR, Marquez-Curtis LA, Kucia M, et al. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 2006;24(5):1254–1264. doi: 10.1634/stemcells.2005-0271. [DOI] [PubMed] [Google Scholar]
- 64.Imitola J, Raddassi K, Park KI, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1α/CXC chemokine receptor 4 pathway. Proc. Natl Acad. Sci. USA. 2004;101(52):18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Allport JR, Shinde Patil VR, Weissleder R. Murine neuronal progenitor cells are preferentially recruited to tumor vasculature via α4-integrin and SDF-1α-dependent mechanisms. Cancer Biol. Ther. 2004;3(9):838–844. doi: 10.4161/cbt.3.9.1036. [DOI] [PubMed] [Google Scholar]
- 66.Rempel SA, Dudas S, Ge S, Gutierrez JA. Identification and localization of the cytokine SDF1 and its receptor, CXC chemokine receptor 4, to regions of necrosis and angiogenesis in human glioblastoma. Clin. Cancer Res. 2000;6(1):102–111. [PubMed] [Google Scholar]
- 67.Fears CY, Sontheimer HW, Bullard DC, Gladson CL. Could labeled neuronal progenitor cells be used to target glioma tumor endothelium? Cancer Biol. Ther. 2004;3(9):845–846. doi: 10.4161/cbt.3.9.1123. [DOI] [PubMed] [Google Scholar]
- 68.Ehtesham M, Yuan X, Kabos P, et al. Glioma tropic neural stem cells consist of astrocytic precursors and their migratory capacity is mediated by CXCR4. Neoplasia. 2004;6(3):287–293. doi: 10.1593/neo.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou Y, Larsen PH, Hao C, Yong VW. CXCR4 is a major chemokine receptor on glioma cells and mediates their survival. J. Biol. Chem. 2002;277(51):49481–49487. doi: 10.1074/jbc.M206222200. [DOI] [PubMed] [Google Scholar]
- 70.Rubin JB, Kung AL, Klein RS, et al. A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc. Natl Acad. Sci. USA. 2003;100(23):13513–13518. doi: 10.1073/pnas.2235846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee BC, Lee TH, Avraham S, Avraham HK. Involvement of the chemokine receptor CXCR4 and its ligand stromal cell-derived factor 1α in breast cancer cell migration through human brain microvascular endothelial cells. Mol. Cancer Res. 2004;2(6):327–338. [PubMed] [Google Scholar]
- 72.Liang Z, Wu T, Lou H, et al. Inhibition of breast cancer metastasis by selective synthetic polypeptide against CXCR4. Cancer Res. 2004;64(12):4302–4308. doi: 10.1158/0008-5472.CAN-03-3958. [DOI] [PubMed] [Google Scholar]
- 73.Becker AJ, Mc CE, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197:452–454. doi: 10.1038/197452a0. [DOI] [PubMed] [Google Scholar]
- 74.Siminovitch L, McCulloch EA, Till JE. The distribution of colony-forming cells among spleen colonies. J. Cell. Physiol. 1963;62:327–336. doi: 10.1002/jcp.1030620313. [DOI] [PubMed] [Google Scholar]
- 75.Weigmann A, Corbeil D, Hellwig A, Huttner WB. Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc. Natl Acad. Sci. USA. 1997;94(23):12425–12430. doi: 10.1073/pnas.94.23.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Levison SW, Goldman JE. Multipotential and lineage restricted precursors coexist in the mammalian perinatal subventricular zone. J. Neurosci. Res. 1997;48(2):83–94. [PubMed] [Google Scholar]
- 77.Doyle KL, Khan M, Cunningham AM. Expression of the intermediate filament protein nestin by sustentacular cells in mature olfactory neuroepithelium. J. Comp. Neurol. 2001;437(2):186–195. doi: 10.1002/cne.1278. [DOI] [PubMed] [Google Scholar]
- 78.Sakakibara S, Okano H. Expression of neural RNA-binding proteins in the postnatal CNS: implications of their roles in neuronal and glial cell development. J. Neurosci. 1997;17(21):8300–8312. doi: 10.1523/JNEUROSCI.17-21-08300.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Uchida K, Momiyama T, Okano H, et al. Potential functional neural repair with grafted neural stem cells of early embryonic neuroepithelial origin. Neurosci. Res. 2005;52(3):276–286. doi: 10.1016/j.neures.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 80.Palm K, Salin-Nordstrom T, Levesque MF, Neuman T. Fetal and adult human CNS stem cells have similar molecular characteristics and developmental potential. Brain Res. Mol. Brain Res. 2000;78(1–2):192–195. doi: 10.1016/s0169-328x(00)00075-9. [DOI] [PubMed] [Google Scholar]
- 81.Pevny LH, Sockanathan S, Placzek M, Lovell-Badge R. A role for SOX1 in neural determination. Development. 1998;125(10):1967–1978. doi: 10.1242/dev.125.10.1967. [DOI] [PubMed] [Google Scholar]
- 82.Zappone MV, Galli R, Catena R, et al. Sox2 regulatory sequences direct expression of a β-geo transgene to telencephalic neural stem cells and precursors of the mouse embryo, revealing regionalization of gene expression in CNS stem cells. Development. 2000;127(11):2367–2382. doi: 10.1242/dev.127.11.2367. [DOI] [PubMed] [Google Scholar]
- 83.Cai J, Wu Y, Mirua T, et al. Properties of a fetal multipotent neural stem cell (NEP cell) Dev. Biol. 2002;251(2):221–240. doi: 10.1006/dbio.2002.0828. [DOI] [PubMed] [Google Scholar]
- 84.Parker MA, anderson JK, Corliss DA, et al. Expression profile of an operationally-defined neural stem cell clone. Exp. Neurol. 2005;194(2):320–332. doi: 10.1016/j.expneurol.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 85.Gottlieb DI. Large-scale sources of neural stem cells. Annu. Rev. Neurosci. 2002;25:381–407. doi: 10.1146/annurev.neuro.25.112701.142904. [DOI] [PubMed] [Google Scholar]
- 86.Palmer TD, Schwartz PH, Taupin P, et al. Cell culture. Progenitor cells from human brain after death. Nature. 2001;411(6833):42–43. doi: 10.1038/35075141. [DOI] [PubMed] [Google Scholar]
- 87.Kukekov VG, Laywell ED, Suslov O, et al. Multipotent stem/progenitor cells with similar properties arise from two neurogenic regions of adult human brain. Exp. Neurol. 1999;156(2):333–344. doi: 10.1006/exnr.1999.7028. [DOI] [PubMed] [Google Scholar]
- 88.Eriksson PS, Perfilieva E, Bjork-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 89.Antel JP, Nalbantoglu J, Olivier A. Neuronal progenitors — learning from the hippocampus. Nat. Med. 2000;6(3):249–250. doi: 10.1038/73076. [DOI] [PubMed] [Google Scholar]
- 90.Nunes MC, Roy NS, Keyoung HM, et al. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat. Med. 2003;9(4):439–447. doi: 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- 91.Uchida N, Buck DW, He D, et al. Direct isolation of human central nervous system stem cells. Proc. Natl Acad. Sci. USA. 2000;97(26):14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Flax JD, Aurora S, Yang C, et al. Engraftable human neural stem cells respond to developmental cues, replace neurons and express foreign genes. Nat. Biotechnol. 1998;16(11):1033–1039. doi: 10.1038/3473. [DOI] [PubMed] [Google Scholar]
- 93.Tropepe V, Coles BL, Chiasson BJ, et al. Retinal stem cells in the adult mammalian eye. Science. 2000;287(5460):2032–2036. doi: 10.1126/science.287.5460.2032. [DOI] [PubMed] [Google Scholar]
- 94.Reubinoff BE, Itsykson P, Turetsky T, et al. Neural progenitors from human embryonic stem cells. Nat. Biotechnol. 2001;19(12):1134–1140. doi: 10.1038/nbt1201-1134. [DOI] [PubMed] [Google Scholar]
- 95.Quinones-Hinojosa A, Sanai N, Soriano-Navarro M, et al. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J. Comp. Neurol. 2006;494(3):415–434. doi: 10.1002/cne.20798. [DOI] [PubMed] [Google Scholar]
- 96.Sanai N, Tramontin AD, Quinones-Hinojosa A, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427(6976):740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 97.Quinones-Hinojosa A, Sanai N, Gonzalez-Perez O, Garcia-Verdugo JM. The human brain subventricular zone: stem cells in this niche and its organization. Neurosurg. Clin. N. Am. 2007;18(1):15–20. vii. doi: 10.1016/j.nec.2006.10.013.Describes the anatomic, cytoarchitectural and ultrastructural organization of the human subventricular zone neural stem cell niche.
- 98.Lee A, Kessler JD, Read TA, et al. Isolation of neural stem cells from the postnatal cerebellum. Nat. Neurosci. 2005;8(6):723–729. doi: 10.1038/nn1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bedard A, Parent A. Evidence of newly generated neurons in the human olfactory bulb. Brain Res. Dev. Brain Res. 2004;151(1–2):159–168. doi: 10.1016/j.devbrainres.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 100.Colombo E, Giannelli SG, Galli R, et al. Embryonic stem-derived versus somatic neural stem cells: a comparative analysis of their developmental potential and molecular phenotype. Stem Cell. 2006;24(4):825–834. doi: 10.1634/stemcells.2005-0313. [DOI] [PubMed] [Google Scholar]
- 101.Jiang Y, Henderson D, Blackstad M, et al. Neuroectodermal differentiation from mouse multipotent adult progenitor cells. Proc. Natl Acad. Sci. USA. 2003;100(Suppl 1):11854–11860. doi: 10.1073/pnas.1834196100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kogler G, Sensken S, Airey JA, et al. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J. Exp. Med. 2004;200(2):123–135. doi: 10.1084/jem.20040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Joannides A, Gaughwin P, Schwiening C, et al. Efficient generation of neural precursors from adult human skin: astrocytes promote neurogenesis from skin-derived stem cells. Lancet. 2004;364(9429):172–178. doi: 10.1016/S0140-6736(04)16630-0. [DOI] [PubMed] [Google Scholar]
- 104.Renfranz PJ, Cunningham MG, McKay RD. Region-specific differentiation of the hippocampal stem cell line HiB5 upon implantation into the developing mammalian brain. Cell. 1991;66(4):713–729. doi: 10.1016/0092-8674(91)90116-g. [DOI] [PubMed] [Google Scholar]
- 105.Rosario CM, Yandava BD, Kosaras B, et al. Differentiation of engrafted multipotent neural progenitors towards replacement of missing granule neurons in meander tail cerebellum may help determine the locus of mutant gene action. Development. 1997;124(21):4213–4224. doi: 10.1242/dev.124.21.4213. [DOI] [PubMed] [Google Scholar]
- 106.Yandava BD, Billinghurst LL, Snyder EY. ‘Global’ cell replacement is feasible via neural stem cell transplantation: evidence from the dysmyelinated shiverer mouse brain. Proc. Natl Acad. Sci. USA. 1999;96(12):7029–7034. doi: 10.1073/pnas.96.12.7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Temple S. The development of neural stem cells. Nature. 2001;14(6859):112–117. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- 108.Ma W, Fitzgerald W, Liu QY, et al. CNS stem and progenitor cell differentiation into functional neuronal circuits in three-dimensional collagen gels. Exp. Neurol. 2004;190(2):276–288. doi: 10.1016/j.expneurol.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 109.Zlomanczuk P, Mrugala M, de la Iglesia HO, et al. Transplanted clonal neural stem-like cells respond to remote photic stimulation following incorporation within the suprachiasmatic nucleus. Exp. Neurol. 2002;174(2):162–168. doi: 10.1006/exnr.2001.7857. [DOI] [PubMed] [Google Scholar]
- 110.Ourednik J, Ourednik V, Lynch WP, Schachner M, Snyder EY. Neural stem cells display an inherent mechanism for rescuing dysfunctional neurons. Nat. Biotechnol. 2002;20(11):1103–1110. doi: 10.1038/nbt750. [DOI] [PubMed] [Google Scholar]
- 111.Kim DE, Schellingerhout D, Ishii K, Shah K, Weissleder R. Imaging of stem cell recruitment to ischemic infarcts in a murine model. Stroke. 2004;35(4):952–957. doi: 10.1161/01.STR.0000120308.21946.5D. [DOI] [PubMed] [Google Scholar]
- 112.Martinez-Serrano A, Rubio FJ, Navarro B, Bueno C, Villa A. Human neural stem and progenitor cells: in vitro and in vivo properties and potential for gene therapy and cell replacement in the CNS. Curr. Gene Ther. 2001;1(3):279–299. doi: 10.2174/1566523013348562. [DOI] [PubMed] [Google Scholar]
- 113.Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders-how to make it work. Nat. Med. 2004;10(Suppl):S42–S50. doi: 10.1038/nm1064. [DOI] [PubMed] [Google Scholar]
- 114.Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp. Hematol. 1976;4(5):267–274. [PubMed] [Google Scholar]
- 115.Gronthos S, Graves SE, Ohta S, Simmons PJ. The STRO-1+ fraction of adult human bone marrow contains the osteogenic precursors. Blood. 1994;84(12):4164–4173. [PubMed] [Google Scholar]
- 116.Krebsbach PH, Kuznetsov SA, Bianco P, Robey PG. Bone marrow stromal cells: characterization and clinical application. Crit. Rev. Oral Biol. Med. 1999;10(2):165–181. doi: 10.1177/10454411990100020401. [DOI] [PubMed] [Google Scholar]
- 117.Huss R. Perspectives on the morphology and biology of CD34-negative stem cells. J. Hematother. Stem Cell Res. 2000;9(6):783–793. doi: 10.1089/152581600750062228. [DOI] [PubMed] [Google Scholar]
- 118.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 119.Brown SE, Tong W, Krebsbach PH. The derivation of mesenchymal stem cells from human embryonic stem cells. Cells Tissues Organs. 2009;189(1–4):256–260. doi: 10.1159/000151746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tocci A, Forte L. Mesenchymal stem cell: use and perspectives. Hematol. J. 2003;4(2):92–96. doi: 10.1038/sj.thj.6200232. [DOI] [PubMed] [Google Scholar]
- 121.Conget PA, Minguell JJ. Adenoviral-mediated gene transfer into ex vivo expanded human bone marrow mesenchymal progenitor cells. Exp. Hematol. 2000;28(4):382–390. doi: 10.1016/s0301-472x(00)00134-x. [DOI] [PubMed] [Google Scholar]
- 122.Tondreau T, Meuleman N, Delforge A, et al. Mesenchymal stem cells derived from CD133-positive cells in mobilized peripheral blood and cord blood: proliferation, Oct4 expression and plasticity. Stem Cells. 2005;23(8):1105–1112. doi: 10.1634/stemcells.2004-0330. [DOI] [PubMed] [Google Scholar]
- 123.Miao Z, Jin J, Chen L, et al. Isolation of mesenchymal stem cells from human placenta: comparison with human bone marrow mesenchymal stem cells. Cell Biol. Int. 2006;30(9):681–687. doi: 10.1016/j.cellbi.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 124.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell. 2002;13(12):4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pisati F, Belicchi M, Acerbi F, et al. Effect of human skin-derived stem cells on vessel architecture, tumor growth and tumor invasion in brain tumor animal models. Cancer Res. 2007;67(7):3054–3063. doi: 10.1158/0008-5472.CAN-06-1384. [DOI] [PubMed] [Google Scholar]
- 126.Bianchi G, Muraglia A, Daga A, et al. Microenvironment and stem properties of bone marrow-derived mesenchymal cells. Wound Repair Regen. 2001;9(6):460–466. doi: 10.1046/j.1524-475x.2001.00460.x. [DOI] [PubMed] [Google Scholar]
- 127.Reyes M, Lund T, Lenvik T, et al. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood. 2001;98(9):2615–2625. doi: 10.1182/blood.v98.9.2615. [DOI] [PubMed] [Google Scholar]
- 128.Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br. J. Haematol. 2000;109(1):235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 129.Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 130.Kim DS, Kim JH, Lee JK, et al. Overexpression of CXC chemokine receptors is required for the superior glioma- tracking property of umbilical cord blood-derived mesenchymal stem cells. Stem Cells Dev. 2009;18(3):511–519. doi: 10.1089/scd.2008.0050. [DOI] [PubMed] [Google Scholar]
- 131.Glass R, Synowitz M, Kronenberg G, et al. Glioblastoma-induced attraction of endogenous neural precursor cells is associated with improved survival. J. Neurosci. 2005;25(10):2637–2646. doi: 10.1523/JNEUROSCI.5118-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Weinstein DE, Shelanski ML, Liem RK. C17, a retrovirally immortalized neuronal cell line, inhibits the proliferation of astrocytes and astrocytoma cells by a contact-mediated mechanism. Glia. 1990;3(2):130–139. doi: 10.1002/glia.440030207. [DOI] [PubMed] [Google Scholar]
- 133.Staflin K, Honeth G, Kalliomaki S, et al. Neural progenitor cell lines inhibit rat tumor growth in vivo. Cancer Res. 2004;64(15):5347–5354. doi: 10.1158/0008-5472.CAN-03-1246. [DOI] [PubMed] [Google Scholar]
- 134.Barresi V, Belluardo N, Sipione S, et al. Transplantation of prodrug-converting neural progenitor cells for brain tumor therapy. Cancer Gene Ther. 2003;10(5):396–402. doi: 10.1038/sj.cgt.7700580. [DOI] [PubMed] [Google Scholar]
- 135.Li S, Tokuyama T, Yamamoto J, et al. Bystander effect-mediated gene therapy of gliomas using genetically engineered neural stem cells. Cancer Gene Ther. 2005;12(7):600–607. doi: 10.1038/sj.cgt.7700826. [DOI] [PubMed] [Google Scholar]
- 136.Herrlinger U, Woiciechowski C, Sena-Esteves M, et al. Neural precursor cells for delivery of replication-conditional HSV-1 vectors to intracerebral gliomas. Mol. Ther. 2000;1(4):347–357. doi: 10.1006/mthe.2000.0046. [DOI] [PubMed] [Google Scholar]
- 137.Uhl M, Weiler M, Wick W, et al. Migratory neural stem cells for improved thymidine kinase-based gene therapy of malignant gliomas. Biochem. Biophys. Res. Commun. 2005;328(1):125–129. doi: 10.1016/j.bbrc.2004.12.164. [DOI] [PubMed] [Google Scholar]
- 138.Manome Y, Wen PY, Dong Y, et al. Viral vector transduction of the human deoxycytidine kinase cDNA sensitizes glioma cells to the cytotoxic effects of cytosine arabinoside in vitro and in vivo. Nat. Med. 1996;2(5):567–573. doi: 10.1038/nm0596-567. [DOI] [PubMed] [Google Scholar]
- 139.Aghi M, Hochberg F, Breakefield XO. Prodrug activation enzymes in cancer gene therapy. J. Gene Med. 2000;2(3):148–164. doi: 10.1002/(SICI)1521-2254(200005/06)2:3<148::AID-JGM105>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 140.Davidson BL, Breakefield XO. Viral vectors for gene delivery to the nervous system. Nat. Rev. Neurosci. 2003;4(5):353–364. doi: 10.1038/nrn1104. [DOI] [PubMed] [Google Scholar]
- 141.Rooseboom M, Commandeur JN, Vermeulen NP. Enzyme-catalyzed activation of anticancer prodrugs. Pharmacol. Rev. 2004;56(1):53–102. doi: 10.1124/pr.56.1.3. [DOI] [PubMed] [Google Scholar]
- 142.Miletic H, Fischer Y, Litwak S, et al. Bystander killing of malignant glioma by bone marrow-derived tumor-infiltrating progenitor cells expressing a suicide gene. Mol. Ther. 2007;15(7):1373–1381. doi: 10.1038/sj.mt.6300155. [DOI] [PubMed] [Google Scholar]
- 143.Lynch WP, Sharpe AH, Snyder EY. Neural stem cells as engraftable packaging lines can mediate gene delivery to microglia: evidence from studying retroviral env-related neurodegeneration. J. Virol. 1999;73(8):6841–6851. doi: 10.1128/jvi.73.8.6841-6851.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tyler MA, Ulasov IV, Sonabend AM, et al. Neural stem cells target intracranial glioma to deliver an oncolytic adenovirus in vivo. Gene Ther. 2009;16(2):262–278. doi: 10.1038/gt.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Arnhold S, Hilgers M, Lenartz D, et al. Neural precursor cells as carriers for a gene therapeutical approach in tumor therapy. Cell Transplant. 2003;12(8):827–837. doi: 10.3727/000000003771000174. [DOI] [PubMed] [Google Scholar]
- 146.Sonabend AM, Ulasov IV, Tyler MA, et al. Mesenchymal stem cells effectively deliver an oncolytic adenovirus to intracranial glioma. Stem Cells. 2008;26(3):831–841. doi: 10.1634/stemcells.2007-0758. [DOI] [PubMed] [Google Scholar]
- 147.Faber C, Terao E, Morga E, Heuschling P. Interleukin-4 enhances the in vitro precursor cell recruitment for tumor-specific T lymphocytes in patients with glioblastoma. J. Immunother. 2000;23(1):11–16. doi: 10.1097/00002371-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 148.Benedetti S, Pirola B, Pollo B, et al. Gene therapy of experimental brain tumors using neural progenitor cells. Nat. Med. 2000;6(4):447–450. doi: 10.1038/74710. [DOI] [PubMed] [Google Scholar]
- 149.Ehtesham M, Kabos P, Kabosova A, et al. The use of interleukin 12-secreting neural stem cells for the treatment of intracranial glioma. Cancer Res. 2002;62(20):5657–5663. [PubMed] [Google Scholar]
- 150.Langowski JL, Zhang X, Wu L, et al. IL-23 promotes tumour incidence and growth. Nature. 2006;442(7101):461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 151.Yuan X, Hu J, Belladonna ML, Black KL, Yu JS. Interleukin-23-expressing bone marrow-derived neural stem-like cells exhibit antitumor activity against intracranial glioma. Cancer Res. 2006;66(5):2630–2638. doi: 10.1158/0008-5472.CAN-05-1682. [DOI] [PubMed] [Google Scholar]
- 152.Ashkenazi A, Pai RC, Fong S, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J. Clin. Invest. 1999;104(2):155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Walczak H, Miller RE, Ariail K, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat. Med. 1999;5(2):157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 154.Ehtesham M, Kabos P, Gutierrez MA, et al. Induction of glioblastoma apoptosis using neural stem cell-mediated delivery of tumor necrosis factor-related apoptosis-inducing ligand. Cancer Res. 2002;62(24):7170–7174. [PubMed] [Google Scholar]
- 155.Kim I, Kim H, S. I, Snyder EY, Park KI. Induction of intracranial glioblastoma apoptosis by transplantation of TRAIL (tumor necrosis factor-related apoptosis-inducing ligand) expressing human neural stem cells (NSCs). Presented at: Annual Meeting of Society for Neuroscience; 23–27 October 2004; San Diego, CA, USA. [Google Scholar]
- 156.Shah K, Tung CH, Breakefield XO, Weissleder R. In vivo imaging of S-TRAIL-mediated tumor regression and apoptosis. Mol. Ther. 2005;11(6):926–931. doi: 10.1016/j.ymthe.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 157.Kock N, Kasmieh R, Weissleder R, Shah K. Tumor therapy mediated by lentiviral expression of shBcl-2 and S-TRAIL. Neoplasia. 2007;9(5):435–442. doi: 10.1593/neo.07223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Corsten MF, Miranda R, Kasmieh R, et al. MicroRNA-21 knockdown disrupts glioma growth in vivo and displays synergistic cytotoxicity with neural precursor cell delivered S-TRAIL in human gliomas. Cancer Res. 2007;67(19):8994–9000. doi: 10.1158/0008-5472.CAN-07-1045. [DOI] [PubMed] [Google Scholar]
- 159.Hingtgen S, Ren X, Terwilliger E, et al. Targeting multiple pathways in gliomas with stem cell and viral delivered S-TRAIL and Temozolomide. Mol. Cancer Ther. 2008;7(11):3575–3585. doi: 10.1158/1535-7163.MCT-08-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu. Rev. Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 161.Belardelli F, Ferrantini M, Proietti E, Kirkwood JM. Interferon-α in tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13(2):119–134. doi: 10.1016/s1359-6101(01)00022-3. [DOI] [PubMed] [Google Scholar]
- 162.Dvorak HF, Gresser I. Microvascular injury in pathogenesis of interferon-induced necrosis of subcutaneous tumors in mice. J. Natl Cancer Inst. 1989;81(7):497–502. doi: 10.1093/jnci/81.7.497. [DOI] [PubMed] [Google Scholar]
- 163.Streck CJ, Dickson PV, Ng CY, et al. Antitumor efficacy of AAV-mediated systemic delivery of interferon-β. Cancer Gene Ther. 2006;13(1):99–106. doi: 10.1038/sj.cgt.7700878. [DOI] [PubMed] [Google Scholar]
- 164.Dickson PV, Hamner JB, Burger RA, et al. Intravascular administration of tumor tropic neural progenitor cells permits targeted delivery of interferon-β and restricts tumor growth in a murine model of disseminated neuroblastoma. J. Pediatr. Surg. 2007;42(1):48–53. doi: 10.1016/j.jpedsurg.2006.09.050. [DOI] [PubMed] [Google Scholar]
- 165.Kurozumi K, Nakamura K, Tamiya T, et al. Mesenchymal stem cells that produce neurotrophic factors reduce ischemic damage in the rat middle cerebral artery occlusion model. Mol. Ther. 2005;11(1):96–104. doi: 10.1016/j.ymthe.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 166.Honma T, Honmou O, Iihoshi S, et al. Intravenous infusion of immortalized human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Exp. Neurol. 2006;199(1):56–66. doi: 10.1016/j.expneurol.2005.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Xu G, Jiang XD, Xu Y, et al. Adenoviral-mediated interleukin-18 expression in mesenchymal stem cells effectively suppresses the growth of glioma in rats. Cell Biol. Int. 2008;33(4):466–474. doi: 10.1016/j.cellbi.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 168.Kim SM, Lim JY, Park SI, et al. Gene therapy using TRAIL-secreting human umbilical cord blood-derived mesenchymal stem cells against intracranial glioma. Cancer Res. 2008;68(23):9614–9623. doi: 10.1158/0008-5472.CAN-08-0451. [DOI] [PubMed] [Google Scholar]
- 169.Sato H, Kuwashima N, Sakaida T, et al. Epidermal growth factor receptor-transfected bone marrow stromal cells exhibit enhanced migratory response and therapeutic potential against murine brain tumors. Cancer Gene Ther. 2005;12(9):757–768. doi: 10.1038/sj.cgt.7700827. [DOI] [PubMed] [Google Scholar]
- 170.Kang SG, Jeun SS, Lim JY, et al. Cytotoxicity of rat marrow stromal cells against malignant glioma cells. Childs Nerv. Syst. 2005;21(7):528–538. doi: 10.1007/s00381-005-1216-3. [DOI] [PubMed] [Google Scholar]
- 171.Kang SG, Jeun SS, Lim JY, et al. Cytotoxicity of human umbilical cord blood-derived mesenchymal stem cells against human malignant glioma cells. Childs Nerv. Syst. 2008;24(3):293–302. doi: 10.1007/s00381-007-0515-2. [DOI] [PubMed] [Google Scholar]
- 172.Brooks PC, Silletti S, von Schalscha TL, Friedlander M, Cheresh DA. Disruption of angiogenesis by PEX, a noncatalytic metalloproteinase fragment with integrin binding activity. Cell. 1998;92(3):391–400. doi: 10.1016/s0092-8674(00)80931-9. [DOI] [PubMed] [Google Scholar]
- 173.Bello L, Lucini V, Carrabba G, et al. Simultaneous inhibition of glioma angiogenesis, cell proliferation and invasion by a naturally occurring fragment of human metalloproteinase-2. Cancer Res. 2001;61(24):8730–8736. [PubMed] [Google Scholar]
- 174.Kim SK, Cargioli TG, Machluf M, et al. PEX-producing human neural stem cells inhibit tumor growth in a mouse glioma model. Clin. Cancer Res. 2005;11(16):5965–5970. doi: 10.1158/1078-0432.CCR-05-0371. [DOI] [PubMed] [Google Scholar]
- 175.Weissleder R, Ntziachristos V. Shedding light onto live molecular targets. Nat. Med. 2003;9(1):123–128. doi: 10.1038/nm0103-123. [DOI] [PubMed] [Google Scholar]
- 176.Tang Y, Shah K, Messerli SM, et al. In vivo tracking of neural progenitor cell migration to glioblastomas. Hum. Gene Ther. 2003;14(13):1247–1254. doi: 10.1089/104303403767740786. [DOI] [PubMed] [Google Scholar]
- 177.Shah K, Bureau E, Kim DE, et al. Glioma therapy and real-time imaging of neural precursor cell migration and tumor regression. Ann. Neurol. 2005;57(1):34–41. doi: 10.1002/ana.20306. [DOI] [PubMed] [Google Scholar]
- 178.Lewin M, Carlesso N, Tung CH, et al. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat. Biotechnol. 2000;18(4):410–414. doi: 10.1038/74464. [DOI] [PubMed] [Google Scholar]
- 179.Zhang Z, Jiang Q, Jiang F, et al. In vivo magnetic resonance imaging tracks adult neural progenitor cell targeting of brain tumor. Neuroimage. 2004;23(1):281–287. doi: 10.1016/j.neuroimage.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 180.Slotkin JR, Chakrabarti L, Dai HN, et al. In vivo quantum dot labeling of mammalian stem and progenitor cells. Dev. Dyn. 2007;236(12):3393–3401. doi: 10.1002/dvdy.21235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Loo C, Lowery A, Halas N, West J, Drezek R. Immunotargeted nanoshells for integrated cancer imaging and therapy. Nano Lett. 2005;5(4):709–711. doi: 10.1021/nl050127s. [DOI] [PubMed] [Google Scholar]
- 182.Anderson SA, Glod J, Arbab AS, et al. Noninvasive MR imaging of magnetically labeled stem cells to directly identify neovasculature in a glioma model. Blood. 2005;105(1):420–425. doi: 10.1182/blood-2004-06-2222. [DOI] [PubMed] [Google Scholar]
- 183.Wu X, Hu J, Zhou L, et al. In vivo tracking of superparamagnetic iron oxide nanoparticle-labeled mesenchymal stem cell tropism to malignant gliomas using magnetic resonance imaging. Laboratory investigation. J. Neurosurg. 2008;108(2):320–329. doi: 10.3171/JNS/2008/108/2/0320. [DOI] [PubMed] [Google Scholar]
- 184.Jaiswal JK, Simon SM. Potentials and pitfalls of fluorescent quantum dots for biological imaging. Trends Cell. Biol. 2004;14(9):497–504. doi: 10.1016/j.tcb.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 185.Muller-Borer BJ, Collins MC, Gunst PR, Cascio WE, Kypson AP. Quantum dot labeling of mesenchymal stem cells. J. Nanobiotechnology. 2007;5:9. doi: 10.1186/1477-3155-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ohyabu Y, Kaul Z, Yoshioka T, et al. Stable and non-disruptive in vitro/in vivo labeling of mesenchymal stem cells by internalizing quantum dots. Hum. Gene Ther. 2009;20(3):217–224. doi: 10.1089/hum.2008.100. [DOI] [PubMed] [Google Scholar]
- 187.Pike-Overzet K, van der Burg M, Wagemaker G, van Dongen JJ, Staal FJ. New insights and unresolved issues regarding insertional mutagenesis in X-linked SCID gene therapy. Mol. Ther. 2007;15(11):1910–1916. doi: 10.1038/sj.mt.6300297. [DOI] [PubMed] [Google Scholar]
- 188.Aboody KS, Najbauer J, Danks MK. Stem and progenitor cell-mediated tumor selective gene therapy. Gene Ther. 2008;15(10):739–752. doi: 10.1038/gt.2008.41. [DOI] [PubMed] [Google Scholar]
- 189.Yeager TR, Reddel RR. Constructing immortalized human cell lines. Curr. Opin Biotechnol. 1999;10(5):465–469. doi: 10.1016/s0958-1669(99)00011-7. [DOI] [PubMed] [Google Scholar]
- 190.Hall B, Dembinski J, Sasser AK, et al. Mesenchymal stem cells in cancer: tumor-associated fibroblasts and cell-based delivery vehicles. Int. J. Hematol. 2007;86(1):8–16. doi: 10.1532/IJH97.06230. [DOI] [PubMed] [Google Scholar]
- 191.Terada N, Hamazaki T, Oka M, et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416(6880):542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- 192.Ying QL, Nichols J, Evans EP, Smith AG. Changing potency by spontaneous fusion. Nature. 2002;416(6880):545–548. doi: 10.1038/nature729. [DOI] [PubMed] [Google Scholar]
- 193.Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N. Engl. J. Med. 2005;353(8):811–822. doi: 10.1056/NEJMra043666. [DOI] [PubMed] [Google Scholar]
- 194.Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nat. Rev. Cancer. 2006;6(6):425–436. doi: 10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]
- 195.Fomchenko EI, Holland EC. Stem cells and brain cancer. Exp. Cell Res. 2005;306(2):323–329. doi: 10.1016/j.yexcr.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 196.Parham DM. Pathologic classification of rhabdomyosarcomas and correlations with molecular studies. Mod. Pathol. 2001;14(5):506–514. doi: 10.1038/modpathol.3880339. [DOI] [PubMed] [Google Scholar]
- 197.Iacobuzio-Donahue CA, Argani P, Hempen PM, Jones J, Kern SE. The desmoplastic response to infiltrating breast carcinoma: gene expression at the site of primary invasion and implications for comparisons between tumor types. Cancer Res. 2002;62(18):5351–5357. [PubMed] [Google Scholar]
- 198.Chen J, Zhang ZG, Li Y, et al. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ. Res. 2003;92(6):692–699. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- 199.Tso CL, Shintaku P, Chen J, et al. Primary glioblastomas express mesenchymal stem-like properties. Mol. Cancer Res. 2006;4(9):607–619. doi: 10.1158/1541-7786.MCR-06-0005. [DOI] [PubMed] [Google Scholar]
- 200.Ohlsson LB, Varas L, Kjellman C, Edvardsen K, Lindvall M. Mesenchymal progenitor cell-mediated inhibition of tumor growth in vivo and in vitro in gelatin matrix. Exp. Mol. Pathol. 2003;75(3):248–255. doi: 10.1016/j.yexmp.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 201.De Kok IJ, Drapeau SJ, Young R, Cooper LF. Evaluation of mesenchymal stem cells following implantation in alveolar sockets: a canine safety study. Int. J. Oral Maxillofac. Implants. 2005;20(4):511–518. [PubMed] [Google Scholar]
- 202.Chen J, Wang C, Lu S, et al. In vivo chondrogenesis of adult bone-marrow-derived autologous mesenchymal stem cells. Cell Tissue Res. 2005;319(3):429–438. doi: 10.1007/s00441-004-1025-0. [DOI] [PubMed] [Google Scholar]
- 203.Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 204.Kim DW, Chung YJ, Kim TG, Kim YL, Oh IH. Cotransplantation of third-party mesenchymal stromal cells can alleviate single-donor predominance and increase engraftment from double cord transplantation. Blood. 2004;103(5):1941–1948. doi: 10.1182/blood-2003-05-1601. [DOI] [PubMed] [Google Scholar]
- 205.Bexell D, Gunnarsson S, Tormin A, et al. Bone marrow multipotent mesenchymal stroma cells act as pericyte-like migratory vehicles in experimental gliomas. Mol. Ther. 2009;17(1):183–190. doi: 10.1038/mt.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Brem H, Piantadosi S, Burger PC, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet. 1995;345(8956):1008–1012. doi: 10.1016/s0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- 207.Park KI, Teng YD, Snyder EY. The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nat. Biotechnol. 2002;20(11):1111–1117. doi: 10.1038/nbt751. [DOI] [PubMed] [Google Scholar]