Abstract
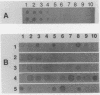
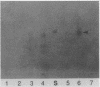
A monoclonal antibody (MAbIII604) specific to phenolic glycolipid Tb (PGL-Tb), a Mycobacterium tuberculosis-specific antigen, was produced and used in the detection of the antigen. MAbIII604 reacted with the PGL-Tb antigen but not with other phenolic glycolipids from Mycobacterium leprae, M. bovis, and M. kansasii, thus indicating the specificity of the monoclonal antibody to PGL-Tb. A dot enzyme-linked immunosorbent assay with MAbIII604 was employed to detect the PGL-Tb antigen in lipids purified from M. tuberculosis clinical isolates. Of 50 isolates, 32 (64.0%) showed clear evidence of the PGL-Tb antigen by the dot enzyme-linked immunosorbent assay, but there were marked variations in the intensities and sizes of spots. This suggests differences in PGL-Tb antigen production among M. tuberculosis strains even when they are grown in the same culture media and conditions. This was most evident from the fact that in only eight (16.0%) of the isolates examined was the PGL-Tb antigen detectable by thin-layer chromatography, which is much less sensitive for the detection of glycolipid antigens. This study shows that monoclonal antibodies specific to PGL-Tb are useful in detecting the antigen in lipid extracts and that there is a marked variation in the PGL-Tb production among M. tuberculosis clinical isolates.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen A. B., Hansen E. B. Structure and mapping of antigenic domains of protein antigen b, a 38,000-molecular-weight protein of Mycobacterium tuberculosis. Infect Immun. 1989 Aug;57(8):2481–2488. doi: 10.1128/iai.57.8.2481-2488.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird P. N., Hall L. M., Coates A. R. Cloning and sequence analysis of the 10 kDa antigen gene of Mycobacterium tuberculosis. J Gen Microbiol. 1989 Apr;135(4):931–939. doi: 10.1099/00221287-135-4-931. [DOI] [PubMed] [Google Scholar]
- Cho S. N., Cellona R. V., Fajardo T. T., Jr, Abalos R. M., dela Cruz E. C., Walsh G. P., Kim J. D., Brennan P. J. Detection of phenolic glycolipid-I antigen and antibody in sera from new and relapsed lepromatous patients treated with various drug regimens. Int J Lepr Other Mycobact Dis. 1991 Mar;59(1):25–31. [PubMed] [Google Scholar]
- Cho S. N., Shin J. S., Kim J. D., Chong Y. Production of monoclonal antibodies to lipoarabinomannan-B and use in the detection of mycobacterial antigens in sputum. Yonsei Med J. 1990 Dec;31(4):333–338. doi: 10.3349/ymj.1990.31.4.333. [DOI] [PubMed] [Google Scholar]
- Cho S. N., Yanagihara D. L., Hunter S. W., Gelber R. H., Brennan P. J. Serological specificity of phenolic glycolipid I from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect Immun. 1983 Sep;41(3):1077–1083. doi: 10.1128/iai.41.3.1077-1083.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffe M., Cho S. N., Chatterjee D., Brennan P. J. Chemical synthesis and seroreactivity of a neoantigen containing the oligosaccharide hapten of the Mycobacterium tuberculosis-specific phenolic glycolipid. J Infect Dis. 1991 Jan;163(1):161–168. doi: 10.1093/infdis/163.1.161. [DOI] [PubMed] [Google Scholar]
- Daffé M., Lacave C., Lanéelle M. A., Lanéelle G. Structure of the major triglycosyl phenol-phthiocerol of Mycobacterium tuberculosis (strain Canetti). Eur J Biochem. 1987 Aug 17;167(1):155–160. doi: 10.1111/j.1432-1033.1987.tb13317.x. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Hunter S. W., Brennan P. J. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J Bacteriol. 1981 Sep;147(3):728–735. doi: 10.1128/jb.147.3.728-735.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanyi J., Krambovitis E., Keen M. Evaluation of a monoclonal antibody (TB72) based serological test for tuberculosis. Clin Exp Immunol. 1983 Nov;54(2):337–345. [PMC free article] [PubMed] [Google Scholar]
- Jackett P. S., Bothamley G. H., Batra H. V., Mistry A., Young D. B., Ivanyi J. Specificity of antibodies to immunodominant mycobacterial antigens in pulmonary tuberculosis. J Clin Microbiol. 1988 Nov;26(11):2313–2318. doi: 10.1128/jcm.26.11.2313-2318.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarnagin J. L., Brennan P. J., Harris S. K. Rapid identification of Mycobacterium bovis by a thin-layer chromatographic technique. Am J Vet Res. 1983 Oct;44(10):1920–1922. [PubMed] [Google Scholar]
- Kadival G. V., Chaparas S. D. Production, characterization, and species specificity of five monoclonal antibodies to Mycobacterium tuberculosis. J Clin Microbiol. 1987 Jan;25(1):76–80. doi: 10.1128/jcm.25.1.76-80.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston A. E., Salgame P. R., Mitchison N. A., Colston M. J. Immunological activity of a 14-kilodalton recombinant protein of Mycobacterium tuberculosis H37Rv. Infect Immun. 1987 Dec;55(12):3149–3154. doi: 10.1128/iai.55.12.3149-3154.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolk A. H., Ho M. L., Klatser P. R., Eggelte T. A., Kuijper S., de Jonge S., van Leeuwen J. Production and characterization of monoclonal antibodies to Mycobacterium tuberculosis, M. bovis (BCG) and M. leprae. Clin Exp Immunol. 1984 Dec;58(3):511–521. [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Lee T. Y., Cho S. N., Yoon K. H., Shin J. S., Kim J. D. Comparison of DNA fragment patterns between the phenolic glycolipid-Tb producers and non-producers of Mycobacterium tuberculosis. Yonsei Med J. 1991 Sep;32(3):243–249. doi: 10.3349/ymj.1991.32.3.243. [DOI] [PubMed] [Google Scholar]
- Martín Casabona N., Gonzalez Fuente T., Arcalis Arce L., Otal Entraigas J., Vidal Pla R. Evaluation of a phenolglycolipid antigen (PGL-Tb 1) from M. tuberculosis in the serodiagnosis of tuberculosis: comparison with PPD antigen. Acta Leprol. 1989;7 (Suppl 1):89–93. [PubMed] [Google Scholar]
- Neill M. A., Klebanoff S. J. The effect of phenolic glycolipid-1 from Mycobacterium leprae on the antimicrobial activity of human macrophages. J Exp Med. 1988 Jan 1;167(1):30–42. doi: 10.1084/jem.167.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olds G. R., Sanson A. J., Daniel T. M. Characterization of Mycobacterium tuberculosis antigen 5 epitopes by using a panel of 19 monoclonal antibodies. J Clin Microbiol. 1987 Mar;25(3):471–475. doi: 10.1128/jcm.25.3.471-475.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa F., Laszlo A., David H. L., Daffé M. Serological specificity of Mycobacterium tuberculosis glycolipids. Acta Leprol. 1989;7 (Suppl 1):98–101. [PubMed] [Google Scholar]
- Papa F., Luquin M., David H. L. DOT-ELISA for detection of phenolic glycolipid PGL-Tb1 and diacyl-trehalose antigens of Mycobacterium tuberculosis. Res Microbiol. 1992 Mar-Apr;143(3):327–331. doi: 10.1016/0923-2508(92)90024-i. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M. The 65-kilodalton antigen of Mycobacterium tuberculosis. J Bacteriol. 1987 Mar;169(3):1080–1088. doi: 10.1128/jb.169.3.1080-1088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgal-Garcia J., David H. L., Papa F. Preliminary evaluation of a Mycobacterium tuberculosis phenolglycolipid antigen in the serologic diagnosis of tuberculosis. Ann Inst Pasteur Microbiol. 1988 May-Jun;139(3):289–294. doi: 10.1016/0769-2609(88)90020-8. [DOI] [PubMed] [Google Scholar]
- Torgal-Garcia J., Papa F., David H. L. The use of PGL-Tb 1 from M. tuberculosis in the serological diagnosis of tuberculous meningitis: a preliminary report. Acta Leprol. 1989;7 (Suppl 1):136–137. [PubMed] [Google Scholar]
- Vismara D., Mezzopreti M. F., Gilardini Montani M. S., Gilardini M. S., Del Porto P., Lombardi G., Piccolella E., Damiani G., Rappuoli R., Colizzi V. Identification of a 35-kilodalton Mycobacterium tuberculosis protein containing B- and T-cell epitopes. Infect Immun. 1990 Jan;58(1):245–251. doi: 10.1128/iai.58.1.245-251.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. B., Harnisch J. P., Knight J., Buchanan T. M. Detection of phenolic glycolipid I in sera from patients with lepromatous leprosy. J Infect Dis. 1985 Nov;152(5):1078–1081. doi: 10.1093/infdis/152.5.1078. [DOI] [PubMed] [Google Scholar]
- Young D. B., Khanolkar S. R., Barg L. L., Buchanan T. M. Generation and characterization of monoclonal antibodies to the phenolic glycolipid of Mycobacterium leprae. Infect Immun. 1984 Jan;43(1):183–188. doi: 10.1128/iai.43.1.183-188.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]