Abstract
In multi-cellular organisms, activation of apoptosis can trigger compensatory proliferation in surrounding cells to maintain tissue homeostasis. Genetic studies in Drosophila have indicated that distinct mechanisms of compensatory proliferation are employed in apoptotic tissues of different developmental states. In proliferating eye and wing tissues, the initiator caspase Dronc coordinates cell death and compensatory proliferation through the Jun N-terminal kinase and p53. The mitogens Decapentaplegic and Wingless are induced in this process. By contrast, in differentiating eye tissues, the effector caspases DrICE and Dcp-1 activate the Hedgehog signaling pathway to induce compensatory proliferation. In this review, we summarize these findings and discuss how activation of apoptosis is linked to the process of compensatory proliferation. The developmental and pathological relevance of compensatory proliferation is also discussed.
Introduction
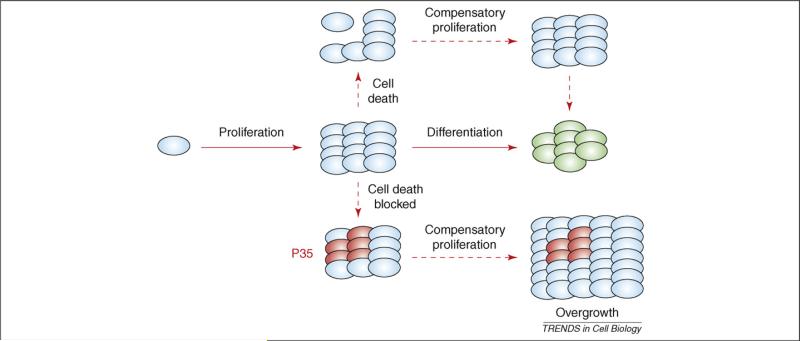
Maintenance of tissue homeostasis is crucial for the survival of multi-cellular organisms. Consequently, cell proliferation, cell growth and cell death are highly coordinated and tightly controlled during normal development. Physiologically occurring cell death, or apoptosis (see Glossary), removes cells to control cell numbers, shape morphology and eliminate injured or dangerous cells [1]. Interestingly, cell loss in response to stress and damage can induce additional divisions of the remaining cells, a process termed apoptosis-induced compensatory proliferation (Figure 1). For example, in response to irradiation-induced cell death in the Drosophila melanogaster wing imaginal disc, a larval monolayer epithelium that develops into the adult wing, cells adjacent to apoptotic cells undergo extra cell proliferation, resulting in adult wings of nearly normal size [2-4]. Even a loss of up to 60% of the total number of cells can be compensated [2]. Similar phenomena have also been observed in tissue regeneration in mammals. The conditional knockout of mdm2, a major inhibitor of p53, in the intestinal epithelium in mouse induces massive p53-dependent apoptosis. Remarkably, this apoptotic phenotype correlates with increased proliferation of non-mdm2-mutant cells, which results in a normal adult intestinal morphology [5]. Similarly, increased proliferation has been observed during intestinal regeneration after irradiation [6,7]. These observations led to the intriguing hypothesis that dying cells can communicate with their surviving neighbors to maintain tissue homeostasis. Thus, the question arose of how dying cells initiate and establish communication with neighboring cells to induce compensatory proliferation?
Figure 1.
Model of compensatory proliferation in response to apoptosis. In multi-cellular organisms, morphogenesis is composed of patterned cell proliferation followed by cell differentiation (green). Compensatory proliferation can be induced in response to cell loss to maintain tissue homeostasis. If execution of cell death is blocked, for example by expression of P35, an effector-caspase inhibitor in Drosophila, ‘undead cells’ (red) continue to secrete signals to induce proliferation, resulting in tissue overgrowth.
Although compensatory proliferation was discovered >30 years ago [2], its underlying mechanism was not revealed until recently. Using the highly accessible genetic-model organism Drosophila, several studies have started to shed some light on the mechanisms of compensatory proliferation [8-13]. Here, these findings are discussed, focusing particularly on the following four aspects: (i) the non-apoptotic function of caspases for compensatory proliferation; (ii) the growth-stimulating signals involved in compensatory proliferation; (iii) the regulators connecting caspases and growth signals; and (iv) the developmental and pathological relevance of compensatory proliferation.
Glossary.
Apoptosis: a naturally occurring form of cellular suicide.
Ark: Apaf-1 related killer; Apaf-1 homolog in Drosophila.
Autonomous: describes a genetic trait in cells, whereby only cells carrying a mutation express the phenotype.
Caspases: cell death Cys-proteases.
Compensatory proliferation: a mechanism that replaces dying cells through stimulation of proliferation.
Dronc: initiator caspase in Drosophila, caspase-9-like.
DrICE, Dcp-1: effector caspases in Drosophila; caspase-3-like.
GMR-hid: a transgene that expresses the pro-apoptotic gene hid under GMR promoter control posterior to the morphogenetic furrow.
hep: hemipterous; encodes the JNK kinase in Drosophila.
JAK–STAT: signal-transduction pathway involved in many developmental processes such as proliferation.
Non-autonomous: used in this review to describe a situation in which the behavior of cells is influenced by neighboring cells.
Notch: signaling receptor involved in many developmental processes such as proliferation.
RHG proteins: collective summary of the pro-apoptotic proteins Reaper, Hid and Grim.
Uba1: E1 ubiquitin-activating enzyme, the first enzyme in the ubiquitinconjugation pathway; required for all ubiquitin-dependent reactions.
The apoptotic pathway in Drosophila
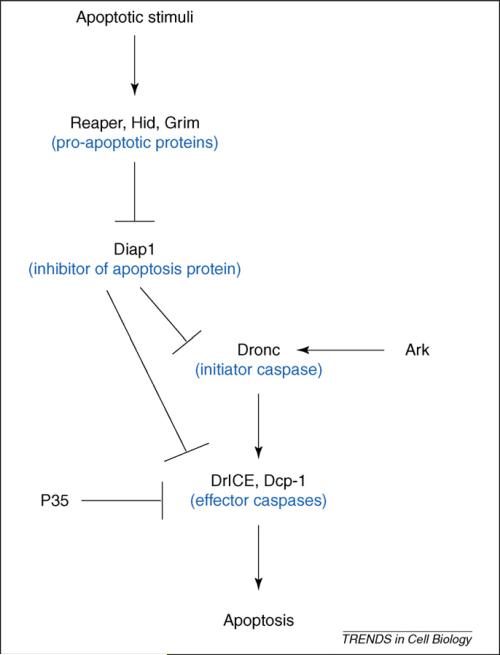
Apoptosis is a genetically controlled process and molecular studies have revealed that regulation and execution of apoptosis are conserved in metazoans [1]. One common feature in most apoptotic-cell-death programs is the activation of caspases, a highly specialized class of cell-death proteases [14]. They are produced as inactive zymogen precursors composed of a prodomain, a large and a small subunit [15]. Based on the length of the prodomain, these aspartate-specific cysteine proteases are divided into two distinct classes: initiator (long prodomains) and effector (short prodomains) caspases. Upon activation, the initiator caspases activate the effector caspases via proteolytic processing and activated effector caspases further cleave key cellular substrates to promote apoptosis [16]. The Drosophila genome contains seven caspase genes [17] but, for normal somatic apoptosis during development, only the initiator caspase Dronc (caspase-9-like) and the caspase-3-like effector caspases DrICE (Drosophila interleukin-1-converting enzyme) and Dcp-1 (death caspase-1) are required [18-21]. In surviving cells, caspases are inhibited by inhibitor-of-apoptosis proteins (IAPs), the most important one in Drosophila being Drosophila IAP1 (Diap1), which blocks apoptosis through inhibition of caspases [17,22] (Figure 2). In response to apoptotic stimuli, the pro-apoptotic proteins Reaper, Head involution defective (Hid) and Grim (RHG proteins) trigger ubiquitin-mediated degradation of Diap1, thus, releasing Dronc from Diap1 inhibition (for review, see Refs [15,23]). Together with the scaffolding protein Ark [Apaf (apoptosis-activating factor)-1 related killer], free Dronc proteolytically cleaves and activates the effector caspases DrICE and Dcp-1 to trigger cell death [15,23] (Figure 2).
Figure 2.
The apoptotic pathway in Drosophila. In response to apoptotic stimuli, expression of the pro-apoptotic genes hid, reaper and grim releases the initiator caspase Dronc from inhibition by Diap1. Free Dronc then associates with the adaptor protein Ark and proteolytically processes the effector caspases DrICE and Dcp-1 to induce apoptosis.
Non-apoptotic functions of caspases for compensatory proliferation
Because dying cells are normally quickly removed by phagocytosis, a direct analysis of how dying cells might induce compensatory proliferation is precluded. To solve this problem, using Drosophila as a model system, expression of the caspase-inhibitor P35 was used to block the execution of cell death [8-10]. P35 is a suicide substrate for effector caspases that inhibits the activity of DrICE and Dcp-1 [24-27]. Therefore, despite activation of the apoptotic pathway, the expression of P35 blocks the execution of cell death. Consequently, the dying cells are kept alive (‘undead’) and continue to secrete signals for compensatory proliferation, which results in an overgrowth phenotype (Figure 1). For example, such an overgrowth phenotype has been observed under P35-inhibited apoptotic conditions in developing wing imaginal discs or in the anterior proliferating eye imaginal discs [8-13] (Figure 3a–c).
Figure 3.
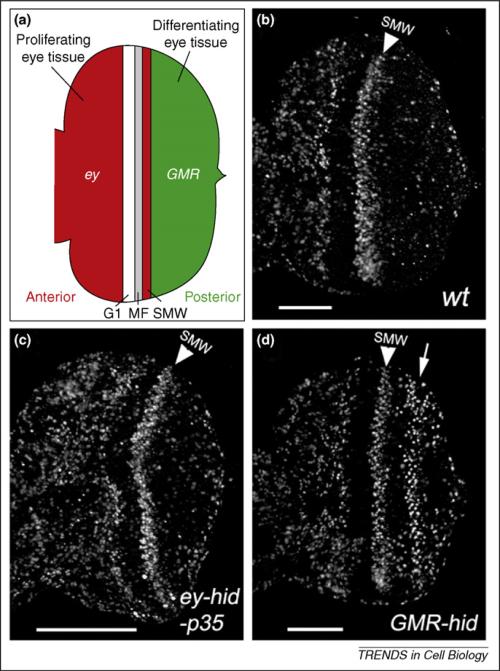
Two modes of apoptosis-induced compensatory proliferation in proliferating and differentiating eye tissues in Drosophila. (a) Schematic outline of the late-third instar eye imaginal disc. Anterior is to the left. The developing larval eye disc is composed of the anterior proliferating tissue (red) and the posterior differentiating tissue (green), which are separated by G1-arrested cells, the morphogenetic furrow (MF) and the second mitotic wave (SMW). The eyeless (ey) promoter is expressed in the anterior part (red); the GMR promoter is expressed in the posterior part (green) and in the SMW. (b) An eye imaginal disc from a 3rd instar wild-type larva labeled with BrdU as the proliferation marker. The anterior half of the disc [red in (a)] is heavily proliferating. Posterior to the SMW [green in (a)], proliferation has stopped and cells are differentiating into photoreceptor neurons and accessory cell types. The SMW is marked by a white arrowhead. (c) Co-expression of hid and P35 under ey control (ey-Gal4, UAS-hid, UAS-P35) triggers overgrowth of the anterior compartment [red in (a); compare with (b)]. The white bar indicates the extent of overgrowth [compare to (b)]. (d) Expression of hid under GMR control (GMR-hid) triggers compensatory proliferation (white arrow) posterior to the SMW [green in (a); compare with (b)]. Part (a) adapted, with permission, from Ref. [13].
This elegant experimental design with P35 enables the identification of the components in the apoptotic pathway that are linked to compensatory proliferation. Initially, two studies came to different conclusions regarding a requirement of Dronc [8] versus Diap1 [9] as the coordinator mediating apoptosis and compensatory proliferation; however, two recent studies have provided more evidence to support a requirement of Dronc in this capacity [11,12] (Figure 4a). Interestingly, loss of one gene copy of dronc substantially suppressed compensatory proliferation, although, effector caspases were still activated [11]. This indicates that compensatory proliferation requires higher activity of Dronc than apoptosis, and further implies that compensatory proliferation occurs after induction of apoptosis.
Figure 4.
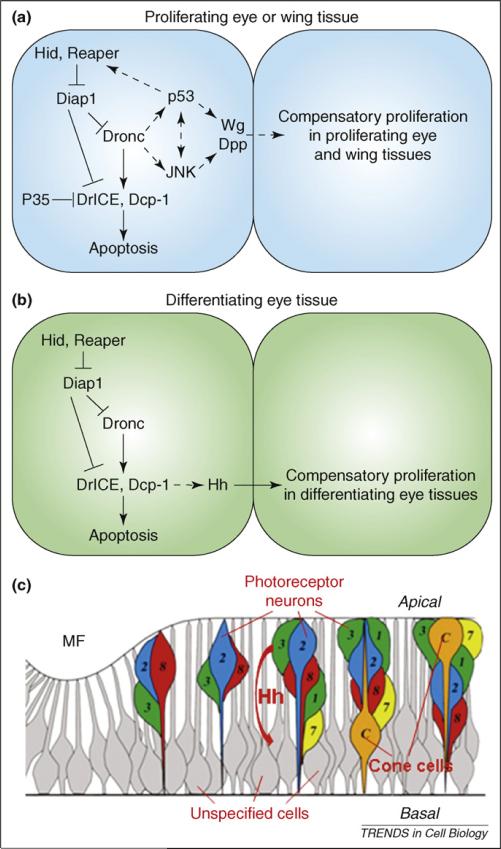
Models of distinct mechanisms of compensatory proliferation in proliferating versus differentiating tissues in Drosophila. (a,b) Molecular mechanisms of apoptosis-induced compensatory proliferation in proliferating tissues (a) and differentiating tissues (b). An apoptotic cell (left) and a cell that is induced to undergo compensatory proliferation (right) are shown. Dashed arrows indicate unknown interactions. The scaffolding protein Ark is omitted for clarity (Figure 2). (c) Schematic outline of various cell types in the differentiating eye tissue. Hh produced in apical dying photoreceptor neurons (numbered colored cells) non-autonomously induces cell-cycle re-entry of basally-located unspecified cells (gray cells). ‘Apical’ and ‘basal’ refer to the apical and basal sides of the disc. The SMW is omitted for clarity. Part (c) adapted, with permission, from Ref. [13].
In addition to Dronc functioning as a coordinator to connect apoptosis and compensatory proliferation, it has recently been demonstrated that the effector caspases DrICE and Dcp-1 can also have such a role [13]. This study was carried out in the developing larval eye imaginal disc. In eye imaginal discs, an indentation, known as the morphogenetic furrow (MF), sweeps from posterior to anterior across the eye discs. In the MF, cells are cell-cycle-arrested in G1 and the first five photoreceptor neurons are specified. After that, the remaining cells synchronously re-enter the cell cycle for one additional cell division and form the second mitotic wave (SMW) (Figure 3a,b). After completion of the SMW, all cells permanently exit the cell cycle and are recruited into additional photoreceptors, cone cells and pigment cells [28]. In the aforementioned study [13], the pro-apoptotic gene hid (Figure 2) was expressed under control of the glass-multimer-reporter (GMR)-promoter (GMR-hid), which is expressed in all cells posterior to the MF [29] (Figure 3a). Expression of GMR-hid causes massive cell death and compensatory proliferation posterior to the MF [13] (Figure 3d). In this study, compensatory proliferation was characterized without the use of P35. In fact, expression of P35 blocked compensatory proliferation in GMR-hid eye discs, indicating that the P35 targets DrICE and Dcp-1 are required for compensatory proliferation in GMR-hid eye discs. This was indeed confirmed in drICE dcp-1 double mutants [13].
Therefore, there are two distinct forms of apoptosis-induced compensatory proliferation; one dependent on the initiator caspase Dronc, and the other dependent on the effector caspases DrICE and Dcp-1 (Figure 4b). Under what conditions are these two forms of compensatory proliferation used? One likely explanation lies in the developmental state of the apoptotic tissue. The studies in the developing wing (leading to Dronc-dependent compensatory proliferation) were performed at a time when this tissue is largely proliferating [30,31]. By contrast, cell-death induction in GMR-hid eye discs (leading to DrICE- and Dcp-1-dependent compensatory proliferation) occurs posterior to the MF, in which most cells are post-mitotic and differentiating, except in the SMW (Figure 3a,b). However, the developing eye is well suited to a study of both forms of compensatory proliferation because the tissue anterior to the MF is largely proliferating, similarly to the wing (Figure 3a,b). Consistently, induction of apoptosis anterior to the MF triggers Dronc-dependent compensatory proliferation [13]. To summarize, apoptosis induction in proliferating wing and eye tissue triggers Dronc-dependent compensatory proliferation whereas, in differentiating eye tissue, DrICE- and Dcp-1-dependent compensatory proliferation is induced. In addition, these observations provide another example of non-apoptotic functions of caspases, which have been recently demonstrated in many other processes [32,33].
Distinct signaling pathways are employed to activate compensatory proliferation
Given that caspases coordinate cell death and compensatory proliferation, the next intriguing question is: what signaling pathways are activated by caspases to trigger compensatory proliferation? Two major mitogens, Decapentaplegic (Dpp; Drosophila homolog of TGF-β) and Wingless (Wg; Drosophila Wnt homolog), were found to be up-regulated or ectopically induced in dying cells [9,10] (Figure 4a). Interestingly, the Wnt and TGF-β signaling pathways, homologs of Drosophila Wg and Dpp, have been shown to be involved in tissue regeneration in both worms and vertebrates [34,35]. However, the role of Dpp and Wg for compensatory proliferation is not entirely clear. Initial support for a requirement of Wg for compensatory proliferation came from expression of a dominant-negative mutant of the T-cell factor (TCF), the transcription factor mediating Wg signaling. In this situation, Wg signaling is inhibited and, accordingly, hid-induced compensatory proliferation is diminished [9]. However, a recent study observed that, although Dpp and Wg are ectopically induced in dying cells, expression of their downstream targets such as phospho-Mad and Vestigial is actually reduced, which indicates that both Dpp and Wg activity is suppressed in cells undergoing compensatory proliferation [11]. Hence, despite being induced, the exact role of Dpp and Wg signals in compensatory proliferation still needs to be further investigated.
In differentiating eye tissues in GMR-hid- expressing animals, both transcription and protein levels of Hedgehog (Hh), but not Dpp and Wg, are up-regulated in an effector-caspase-dependent manner [13]. hh loss-of-function, either by reducing hh activity or by blocking transduction of the Hh signal, leads to loss of compensatory proliferation, which indicates that hh is required in this paradigm. Interestingly, induction of hh expression in GMR-hid is restricted to differentiating photoreceptor neurons [13]. However, photoreceptor neurons are not undergoing compensatory proliferation. Instead, through secretion of Hh, dying photoreceptor neurons stimulate neighboring cells located at the basal side of the disc to proliferate (Figure 4c). These neighboring cells are normally cell-cycle arrested, but have not been specified yet. Thus, expression of Hh in dying photoreceptor neurons triggers non-autonomous cell-cycle re-entry of these cells (Figure 4c). This also indicates that photoreceptor neurons and unspecified cells have different capacities to proliferate in response to apoptosis (see later). However, up-regulation of Hh alone is not sufficient to induce proliferation that is similar to GMR-hid eye discs. Therefore, in addition to the Hh activity, other factors in the apoptotic background are also required for compensatory proliferation.
Recently, several other signaling pathways such as Notch and JAK (Janus kinase)–STAT (signal transducers and activators of transcription) have also been implicated in non-autonomous proliferation, which might be induced by apoptosis. For example, mutations of the vacuolar-protein-sorting genes vps23 and vps25 in Drosophila induce apoptosis autonomously and tissue overgrowth non-autonomously [36-39]. Vps 23 and Vps25 are required during endosomal protein sorting for inactivation of cell-surface receptors [40,41]. Loss of vps23 and vps25 function causes endosomal defects, which results in accumulation of cell-surface receptors at the endosome. Particularly sensitive to endosomal disturbances is the Notch receptor. Endosomal accumulation causes inappropriate Notch activity, triggering the secretion of interleukin-6-like signaling molecules, which then activate the JAK–STAT pathway in neighboring cells, causing non-autonomous proliferation and overgrowth.
Similarly, loss of the entire ubiquitin-conjugation pathway triggers apoptosis, but also, surprisingly, strong non-autonomous overgrowth in Drosophila. Mutations in the E1 ubiquitin-activating enzyme Uba1, which catalyzes the activation step in the ubiquitin-conjugation pathway, induce apoptosis autonomously and tissue overgrowth non-autonomously [42,43]. Similar to vps23 and vps25, Notch and JAK–STAT signals are activated in Uba1 mutant cells, and genetic analyses indicate that they are required for non-autonomous tissue overgrowth. However, it is unknown how Notch and JAK–STAT signaling become activated if ubiquitylation is blocked. Because Uba1-mutant cells undergo apoptosis, it is possible that the non-autonomous phenotypes could be caused by apoptosis-induced compensatory proliferation.
Other regulatory factors connecting caspases and their downstream signaling pathways
The studies described raise another intriguing question: how do initiator and effector caspases connect to the downstream growth-signaling pathways to activate compensatory proliferation? One candidate for this process is the Jun N-terminal kinase (JNK) pathway. The JNK pathway is an evolutionarily conserved mitogen-activated-protein-kinase (MAPK) pathway that has roles in multiple cellular processes including apoptosis, cell proliferation and cell migration [44,45]. JNK signaling is activated during compensatory proliferation in developing wing discs [9]. Expression of puckered (puc), encoding a phosphatase that negatively regulates JNK [46], blocks Wg induction and growth stimulation in compensatory proliferation. By contrast, increasing JNK activity by loss of one copy of puc enhances compensatory proliferation. Moreover, expression of hepCA, a constitutively active form of Drosophila JNK kinase [47], is sufficient to induce compensatory proliferation [9]. These data indicate that the JNK pathway is required for activation of compensatory proliferation. Moreover, co-expression of Dronc and P35 in the developing wing disc activates JNK and induces tissue overgrowth, mimicking compensatory proliferation [12]. These data indicate that activation of JNK signaling during compensatory proliferation is a downstream event of Dronc activation (Figure 4a). Notably, Mst proteins, upstream kinases of the JNK pathway, can be activated by both initiator caspases and effector caspases through evolutionarily conserved caspase-cleavage sites [48-50].It is, therefore, conceivable that Dronc can activate upstream kinases in the JNK pathway for compensatory proliferation. However, it is currently unknown how JNK regulates Wg or Dpp. Interestingly, although the molecular mechanisms are not clear yet, genetic analyses have shown that canonical Wg, Dpp and JNK signaling pathways interact with each other to promote dorsal closure and ventral patterning during Drosophila embryogenesis [51]. Moreover, in addition to Wnt and TGF-β [35], the activity of JNK signaling has also been shown to be involved in mammalian liver regeneration [52-54].
Interestingly, the tumor suppressor p53 has also been implicated in compensatory proliferation [11]. ‘Undead’ cells that express the pro-apoptotic genes hid or reaper together with P35 are temporarily arrested in G2 before they undergo compensatory proliferation. This cell-cycle arrest prompted an analysis of the possible role of the DNA-damage-sensing pathway in the process of compensatory proliferation [11]. Although mutations of crucial genes in the DNA-damage-sensing pathway, such as atm and chk2, do not block compensatory proliferation, transcription of p53 was dramatically induced in ‘undead’ cells. Importantly, loss of p53 completely suppresses cell-cycle arrest and compensatory proliferation. Further investigation revealed a regulatory loop, including the pro-apoptotic genes hid and reaper, the initiator caspase dronc, and p53 (Figure 4a). Expression of hid and P35 induces expression of reaper whereas, conversely, expression of reaper and P35 induces expression of hid. Dronc and p53 are required in this feedback process. Moreover, expression of Dronc and P35 induces expression of p53 and compensatory proliferation, although, loss of p53 blocks these [11]. Therefore, p53 is required downstream of Dronc to sustain activation of apoptosis and compensatory proliferation.
Because the JNK pathway is induced downstream of Dronc, are there any interactions between p53 and the JNK pathway? In mammals, on the one hand, upon exposure to stressful stimuli, JNK phosphorylates p53, thereby leading to p53-mediated cellular responses. On the other hand, p53 can activate phosphatases to regulate JNK signaling [55]. By contrast, it has recently been shown that caspase-3 can cleave a JNK upstream kinase, MEKK1 (MAP ERK kinase kinase 1), to promote p53 transcriptional activity via JNK-independent mechanisms [56]. Moreover, JNK can regulate the stability and activity of p73, a p53 homolog [57]. It has also been shown that p53 is both necessary and sufficient for radiation-induced JNK activation in Drosophila [58]. Therefore, the interaction between p53 and the JNK pathway can be bi-directional and context-dependent. It will be of particular interest to analyze whether p53 interacts with JNK directly and regulates other downstream growth-stimulating pathways for compensatory proliferation. It is also conceivable that, in addition to p53 and JNK, other unknown factors are required to establish the connection between caspases and the downstream growth signals. A possible systematic screen to identify positive and negative regulators of compensatory proliferation in Drosophila might provide fascinating insights.
Concluding remarks
During development of multi-cellular organisms, compensatory proliferation is crucial to restore tissue homeostasis in response to cellular stress and tissue damage. However, compensatory proliferation can not be induced by developmentally programmed apoptosis, which is also important to maintain tissue homeostasis by removing extra cells or shape morphology. Therefore, an intrinsic mechanism might exist that controls activation of compensatory proliferation in response to stress-induced apoptosis, but not developmental apoptosis. Nevertheless, because animals are frequently exposed to environmental stress, such as UV radiation, or suffer accidental tissue damage in nature, compensatory proliferation might have important roles for tissue recovery and organismal survival.
Another intriguing link of compensatory proliferation is tissue regeneration. Notably, although the underlying mechanism might be somehow conserved, the capacity of successful regeneration varies among species and among tissues within the body [34,59]. Interestingly, the degree of apoptosis sensitivity and of proliferation potential in developing tissue seems to correlate. For example, the highly proliferative cells in developing wing and anterior eye discs are very sensitive to apoptotic stimuli, but are quickly replaced by compensatory proliferation in response to apoptosis. By contrast, differentiating photoreceptor neurons are more resistant to cell death and do not undergo compensatory proliferation. However, through secretion of Hh, dying photoreceptors stimulate neighboring unspecified cells to re-enter the cell cycle for compensatory proliferation [13] (Figure 4c). Therefore, distinct mechanisms of compensatory proliferation in tissues with different developmental states might partially explain the different regenerative capacity of various tissues or organs.
However, the reasons why and how these distinct forms of compensatory proliferation are employed in tissues with different developmental states are still unknown. It is possible that an endogenous monitoring system ensures the activation of compensatory proliferation in a proper way in various tissues depending on their sensitivity to damage and/or their potential to proliferate and regenerate. For example, the differentiating photoreceptor neurons in the eye disc are more resistant to cell death compared with proliferating cells. This might contribute to the employment of effector caspases instead of initiator caspases for compensatory proliferation in differentiating eye tissues. Identification of other regulatory elements will be crucial for understanding how the communication between caspases and their downstream growth-stimulating signaling pathways is established. In the long term, this knowledge will be crucial for developing therapeutic treatments for diseases associated with either lack of regeneration, such as neurodegeneration, or inappropriate regeneration, such as stroke in the aging brain [60,61].
Compensatory proliferation might also be of pathological relevance for the growth of tumors. Many tumor cells are resistant to cell death even if they are induced to undergo apoptosis, because essential components of the apoptotic pathway, such as Apaf-1 and caspases, are defective in these cells [62-64]. This is very similar to P35-inhibited apoptosis in proliferating Drosophila wing and eye tissues. In this situation, ‘undead’ cells continue to emit growth-stimulating signals to induce uncontrolled proliferation. Thus, ‘undead’ tumor cells might also secrete mitogens to induce compensatory proliferation for tumor growth. In this context, apoptosis-induced compensatory proliferation might resemble inflammation-induced cancer in humans [65]. Moreover, deregulation of developmentally important signaling pathways such as Wnt, TGF-β and Hh have been shown to be involved in various types of human cancers [66]. Thus, dissecting the molecular mechanisms of apoptosis-induced compensatory proliferation could contribute to the understanding of several common diseases and might provide potential therapeutic targets for treatment.
Acknowledgements
We apologize to our colleagues for omitting many relevant publications owing to space limitations. We thank Yasmine A. Valentin-Vega and Guillermina Lozano for sharing unpublished data. This work was supported by grant R01 GM068016 from the National Institute of General Medical Sciences (NIGMS) and the Robert A. Welch Foundation (G-1496).
Footnotes
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier's archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
References
- 1.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 2.Haynie JL, Bryant PJ. The effects of X-rays on the proliferation dynamics of cells in the imaginal disc of Drosophila melanagaster. Rouxs Arch. Dev. Biol. 1977;183:85–100. doi: 10.1007/BF00848779. [DOI] [PubMed] [Google Scholar]
- 3.James AA, Bryant PJ. A quantitative study of cell death and mitotic inhibition in γ-irradiated imaginal wing discs of Drosophila melanogaster. Radiat. Res. 1981;87:552–564. [PubMed] [Google Scholar]
- 4.Milan M, et al. Developmental parameters of cell death in the wing disc of Drosophila. Proc. Natl. Acad. Sci. U. S. A. 1997;94:5691–5696. doi: 10.1073/pnas.94.11.5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valentin-Vega YA, et al. The intestinal epithelium compensates for p53-mediated cell death and guarantees organismal survival. Cell Death Differ. 2008 doi: 10.1038/cdd.2008.109. DOI: 10.1038/cdd.2008.109 (http://www.nature.com/cdd) [DOI] [PMC free article] [PubMed]
- 6.Ijiri K, Potten CS. Radiation-hypersensitive cells in small intestinal crypts; their relationships to clonogenic cells. Br. J. Cancer. 1986;7:20–22. [PMC free article] [PubMed] [Google Scholar]
- 7.Ruifrok AC, et al. Spatial and temporal patterns of expression of epidermal growth factor, transforming growth factor a and transforming growth factor b 1−3 and their receptors in mouse jejunum after radiation treatment. Radiat. Res. 1997;147:1–12. [PubMed] [Google Scholar]
- 8.Huh JR, et al. Compensatory proliferation induced by cell death in the Drosophila wing disc requires activity of the apical cell death caspase Dronc in a nonapoptotic role. Curr. Biol. 2004;14:1262–1266. doi: 10.1016/j.cub.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 9.Ryoo HD, et al. Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Dev. Cell. 2004;7:491–501. doi: 10.1016/j.devcel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 10.Perez-Garijo A, et al. Caspase inhibition during apoptosis causes abnormal signalling and developmental aberrations in Drosophila. Development. 2004;131:5591–5598. doi: 10.1242/dev.01432. [DOI] [PubMed] [Google Scholar]
- 11.Wells BS, et al. Compensatory proliferation in Drosophila imaginal discs requires Dronc-dependent p53 activity. Curr. Biol. 2006;16:1606–1615. doi: 10.1016/j.cub2006.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kondo S, et al. DRONC coordinates cell death and compensatory proliferation. Mol. Cell. Biol. 2006;26:7258–7268. doi: 10.1128/MCB.00183-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan Y, Bergmann A. Distinct mechanisms of apoptosis-induced compensatory proliferation in proliferating and differentiating tissues in the Drosophila eye. Dev. Cell. 2008;14:399–410. doi: 10.1016/j.devcel.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Degterev A, et al. A decade of caspases. Oncogene. 2003;22:8543–8567. doi: 10.1038/sj.onc.1207107. [DOI] [PubMed] [Google Scholar]
- 15.Cashio P, et al. Genetic control of programmed cell death in Drosophila melanogaster. Semin. Cell Dev. Biol. 2005;16:225–235. doi: 10.1016/j.semcdb.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Kumar S. Caspase function in programmed cell death. Cell Death Differ. 2007;14:32–43. doi: 10.1038/sj.cdd.4402060. [DOI] [PubMed] [Google Scholar]
- 17.Salvesen GS, Abrams JM. Caspase activation - stepping on the gas or releasing the brakes? Lessons from humans and flies. Oncogene. 2004;23:2774–2784. doi: 10.1038/sj.onc.1207522. [DOI] [PubMed] [Google Scholar]
- 18.Xu D, et al. The CARD-carrying caspase Dronc is essential for most, but not all, developmental cell death in Drosophila. Development. 2005;132:2125–2134. doi: 10.1242/dev.01790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muro I, et al. The Drosophila caspase Ice is important for many apoptotic cell deaths and for spermatid individualization, a nonapoptotic process. Development. 2006;133:3305–3315. doi: 10.1242/dev.02495. [DOI] [PubMed] [Google Scholar]
- 20.Xu D, et al. The effector caspases drICE and dcp-1 have partially overlapping functions in the apoptotic pathway in Drosophila. Cell Death Differ. 2006;13:1697–1706. doi: 10.1038/sj.cdd.4401920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laundrie B, et al. Germline cell death is inhibited by P element insertions disrupting the dcp-1/pita nested gene pair in Drosophila. Genetics. 2003;165:1881–1888. doi: 10.1093/genetics/165.4.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hay BA, et al. Drosophila homologs of baculovirus inhibitor of apoptosis proteins function to block cell death. Cell. 1995;83:1253–1262. doi: 10.1016/0092-8674(95)90150-7. [DOI] [PubMed] [Google Scholar]
- 23.Hay BA, Guo M. Caspase-dependent cell death in Drosophila. Annu. Rev. Cell Dev. Biol. 2006;22:623–650. doi: 10.1146/annurev.cellbio.21.012804.093845. [DOI] [PubMed] [Google Scholar]
- 24.Bump NJ, et al. Inhibition of ICE family proteases by baculovirus antiapoptotic protein p35. Science (New York N.Y.) 1995;269:1885–1888. doi: 10.1126/science.7569933. [DOI] [PubMed] [Google Scholar]
- 25.Xue D, Horvitz HR. Inhibition of the Caenorhabditis elegans cell-death protease CED-3 by a CED-3 cleavage site in baculovirus p35 protein. Nature. 1995;377:248–251. doi: 10.1038/377248a0. [DOI] [PubMed] [Google Scholar]
- 26.Yoo SJ, et al. Hid, Rpr and Grim negatively regulate DIAP1 levels through distinct mechanisms. Nat. Cell Biol. 2002;4:416–424. doi: 10.1038/ncb793. [DOI] [PubMed] [Google Scholar]
- 27.Yu SY, et al. A pathway of signals regulating effector and initiator caspases in the developing Drosophila eye. Development. 2002;129:3269–3278. doi: 10.1242/dev.129.13.3269. [DOI] [PubMed] [Google Scholar]
- 28.Baker NE. Patterning signals and proliferation in Drosophila imaginal discs. Curr. Opin. Genet. Dev. 2007;17:287–293. doi: 10.1016/j.gde.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Ellis MC, et al. Expression of Drosophila glass protein and evidence for negative regulation of its activity in non-neuronal cells by another DNA-binding protein. Development. 1993;119:855–865. doi: 10.1242/dev.119.3.855. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Bellido A, Merriam JR. Parameters of the wing imaginal disc development of Drosophila melanogaster. Dev. Biol. 1971;24:61–87. doi: 10.1016/0012-1606(71)90047-9. [DOI] [PubMed] [Google Scholar]
- 31.Milan M, et al. Cell cycling and patterned cell proliferation in the Drosophila wing during metamorphosis. Proc. Natl. Acad. Sci. U. S. A. 1996;93:11687–11692. doi: 10.1073/pnas.93.21.11687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamkanfi M, et al. Caspases in cell survival, proliferation and differentiation. Cell Death Differ. 2007;14:44–55. doi: 10.1038/sj.cdd.4402047. [DOI] [PubMed] [Google Scholar]
- 33.Kuranaga E, Miura M. Nonapoptotic functions of caspases: caspases as regulatory molecules for immunity and cell-fate determination. Trends Cell Biol. 2007;17:135–144. doi: 10.1016/j.tcb.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Birnbaum KD, Sanchez Alvarado A. Slicing across kingdoms: regeneration in plants and animals. Cell. 2008;132:697–710. doi: 10.1016/j.cell.2008.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stoick-Cooper CL, et al. Advances in signaling in vertebrate regeneration as a prelude to regenerative medicine. Genes Dev. 2007;21:1292–1315. doi: 10.1101/gad.1540507. [DOI] [PubMed] [Google Scholar]
- 36.Moberg KH, et al. Mutations in erupted, the Drosophila ortholog of mammalian tumor susceptibility gene 101, elicit non-cell-autonomous overgrowth. Dev. Cell. 2005;9:699–710. doi: 10.1016/j.devcel.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 37.Thompson BJ, et al. Tumor suppressor properties of the ESCRT-II complex component Vps25 in Drosophila. Dev. Cell. 2005;9:711–720. doi: 10.1016/j.devcel.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 38.Vaccari T, Bilder D. The Drosophila tumor suppressor vps25 prevents nonautonomous overproliferation by regulating notch trafficking. Dev. Cell. 2005;9:687–698. doi: 10.1016/j.devcel.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 39.Herz HM, et al. vps25 mosaics display non-autonomous cell survival and overgrowth, and autonomous apoptosis. Development. 2006;133:1871–1880. doi: 10.1242/dev.02356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Babst M. A protein's final ESCRT. Traffic. 2005;6:2–9. doi: 10.1111/j.1600-0854.2004.00246.x. [DOI] [PubMed] [Google Scholar]
- 41.Fischer JA, et al. Endocytosis, endosome trafficking, and the regulation of Drosophila development. Annu. Rev. Cell Dev. Biol. 2006;22:181–206. doi: 10.1146/annurev.cellbio.22.010605.093205. [DOI] [PubMed] [Google Scholar]
- 42.Lee TV, et al. The E1 ubiquitin-activating enzyme Uba1 in Drosophila controls apoptosis autonomously and tissue growth nonautonomously. Development. 2008;135:43–52. doi: 10.1242/dev.011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfleger CM, et al. Mutation of the gene encoding the ubiquitin activating enzyme Uba1 causes tissue overgrowth in Drosophila. Fly. 2007;1:95–105. doi: 10.4161/fly.4285. [DOI] [PubMed] [Google Scholar]
- 44.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 45.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr. Opin. Cell Biol. 2007;19:142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 46.Martin-Blanco E, et al. puckered encodes a phosphatase that mediates a feedback loop regulating JNK activity during dorsal closure in Drosophila. Genes Dev. 1998;12:557–570. doi: 10.1101/gad.12.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adachi-Yamada T, et al. Distortion of proximodistal information causes JNK-dependent apoptosis in Drosophila wing. Nature. 1999;400:166–169. doi: 10.1038/22112. [DOI] [PubMed] [Google Scholar]
- 48.Graves JD, et al. Both phosphorylation and caspase-mediated cleavage contribute to regulation of the Ste20-like protein kinase Mst1 during CD95/Fas-induced apoptosis. J. Biol. Chem. 2001;276:14909–14915. doi: 10.1074/jbc.M010905200. [DOI] [PubMed] [Google Scholar]
- 49.Lee KK, et al. MST, a physiological caspase substrate, highly sensitizes apoptosis both upstream and downstream of caspase activation. J. Biol. Chem. 2001;276:19276–19285. doi: 10.1074/jbc.M005109200. [DOI] [PubMed] [Google Scholar]
- 50.Song JJ, Lee YJ. Differential cleavage of Mst1 by caspase-7/-3 is responsible for TRAIL-induced activation of the MAPK superfamily. Cell. Signal. 2008;20:892–906. doi: 10.1016/j.cellsig.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McEwen DG, et al. The canonical Wg and JNK signaling cascades collaborate to promote both dorsal closure and ventral patterning. Development. 2000;127:3607–3617. doi: 10.1242/dev.127.16.3607. [DOI] [PubMed] [Google Scholar]
- 52.Maeda S, et al. IKKb couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 53.Sakurai T, et al. Loss of hepatic NF-kB activity enhances chemical hepatocarcinogenesis through sustained c-Jun N-terminal kinase 1 activation. Proc.Natl. Acad.Sci.U.S.A. 2006;103:10544–10551. doi: 10.1073/pnas.0603499103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhong Z, et al. Liver regeneration is suppressed in small-for-size liver grafts after transplantation: involvement of c-Jun N-terminal kinase, cyclin D1, and defective energy supply. Transplantation. 2006;82:241–250. doi: 10.1097/01.tp.0000228867.98158.d2. [DOI] [PubMed] [Google Scholar]
- 55.Wu GS. The functional interactions between the p53 and MAPK signaling pathways. Cancer Biol. Ther. 2004;3:156–161. doi: 10.4161/cbt.3.2.614. [DOI] [PubMed] [Google Scholar]
- 56.Zebrowski DC, et al. Caspase-3 mediated cleavage of MEKK1 promotes p53 transcriptional activity. J. Mol. Cell. Cardiol. 2006;40:605–618. doi: 10.1016/j.yjmcc.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 57.Toh WH, et al. c-Jun regulates the stability and activity of the p53 homologue, p73. J. Biol. Chem. 2004;279:44713–44722. doi: 10.1074/jbc.M407672200. [DOI] [PubMed] [Google Scholar]
- 58.McEwen DG, Peifer M. Puckered, a Drosophila MAPK phosphatase, ensures cell viability by antagonizing JNK-induced apoptosis. Development. 2005;132:3935–3946. doi: 10.1242/dev.01949. [DOI] [PubMed] [Google Scholar]
- 59.Tsonis PA. Regeneration in vertebrates. Dev. Biol. 2000;221:273–284. doi: 10.1006/dbio.2000.9667. [DOI] [PubMed] [Google Scholar]
- 60.Johansson BB. Regeneration and plasticity in the brain and spinal cord. J. Cereb. Blood Flow Metab. 2007;27:1417–1430. doi: 10.1038/sj.jcbfm.9600486. [DOI] [PubMed] [Google Scholar]
- 61.Popa-Wagner A, et al. The response of the aged brain to stroke: too much, too soon? Curr. Neurovasc. Res. 2007;4:216–227. doi: 10.2174/156720207781387213. [DOI] [PubMed] [Google Scholar]
- 62.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 63.Hajra KM, Liu JR. Apoptosome dysfunction in human cancer. Apoptosis. 2004;9:691–704. doi: 10.1023/B:APPT.0000045786.98031.1d. [DOI] [PubMed] [Google Scholar]
- 64.Anichini A, et al. APAF-1 signaling in human melanoma. Cancer Lett. 2006;238:168–179. doi: 10.1016/j.canlet.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 65.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kelleher FC, et al. Common critical pathways in embryogenesis and cancer. Acta Oncol. 2006;45:375–388. doi: 10.1080/02841860600602946. [DOI] [PubMed] [Google Scholar]