Abstract
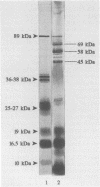
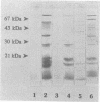
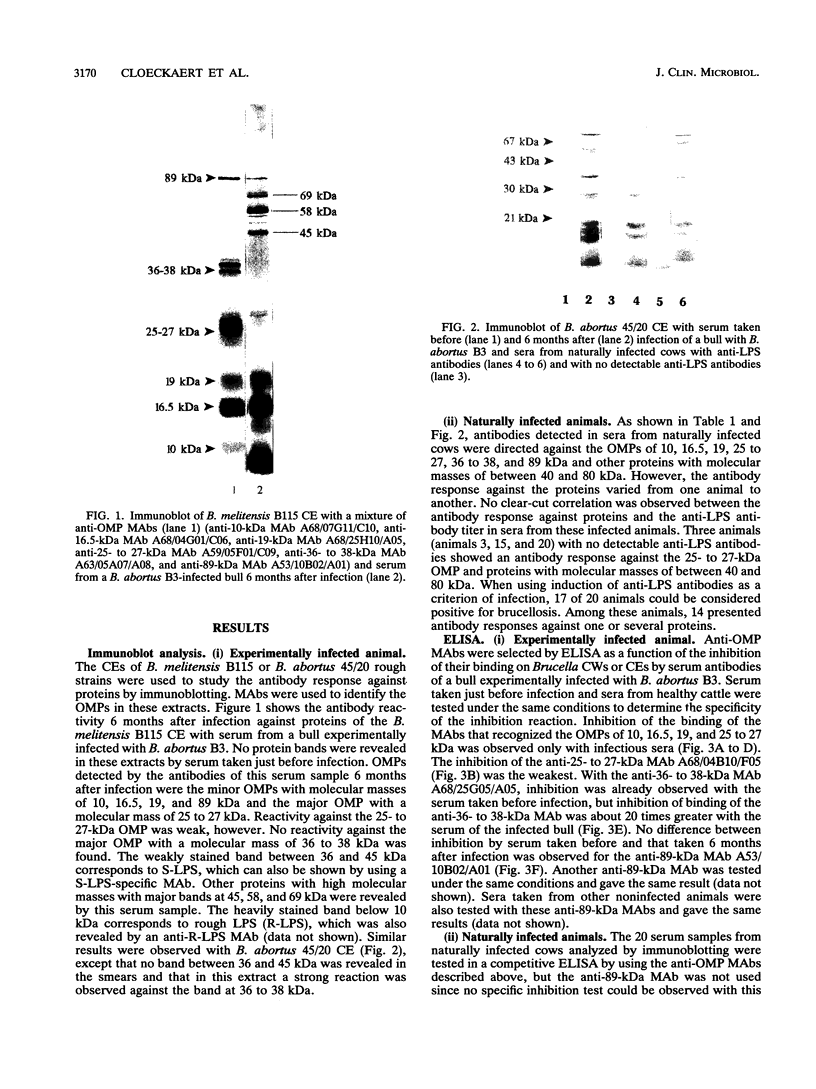
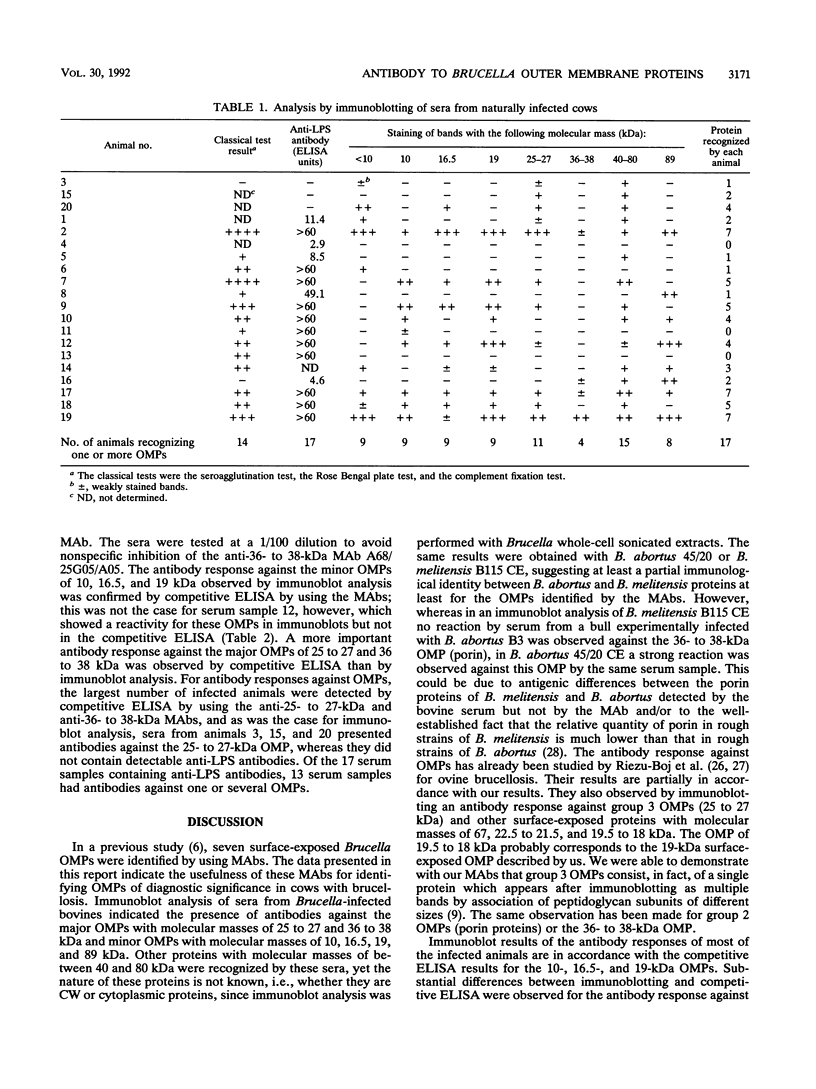
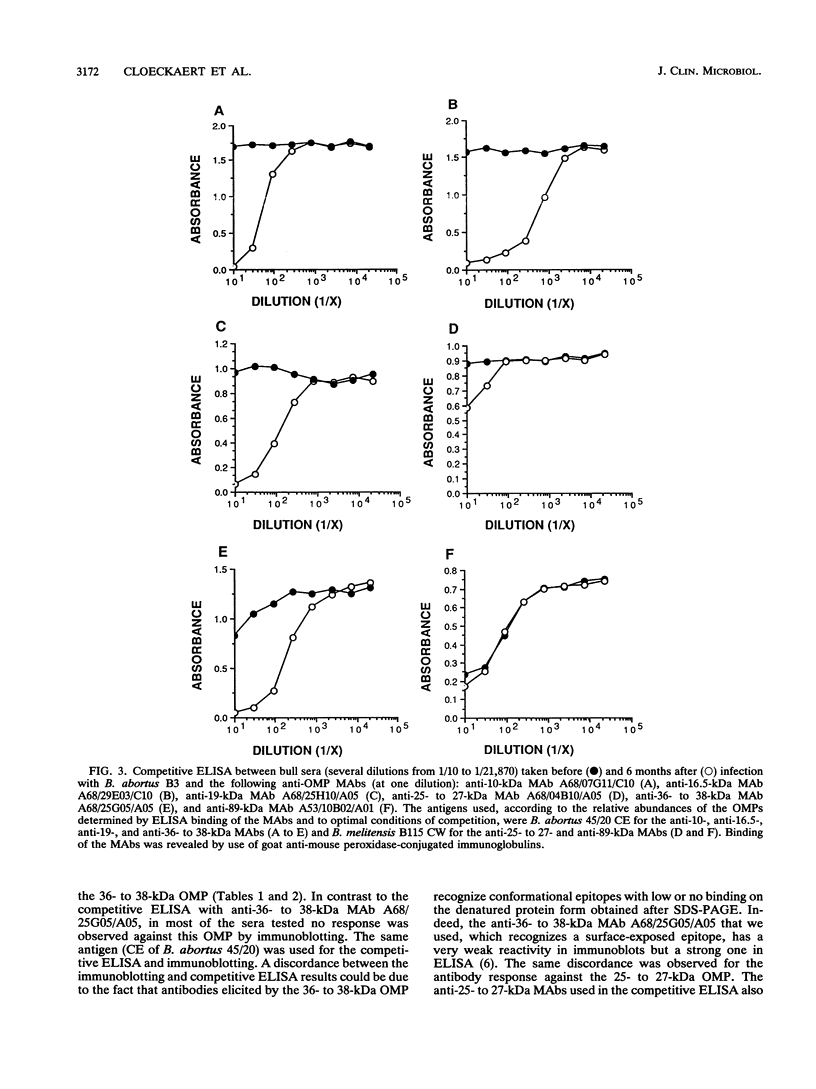
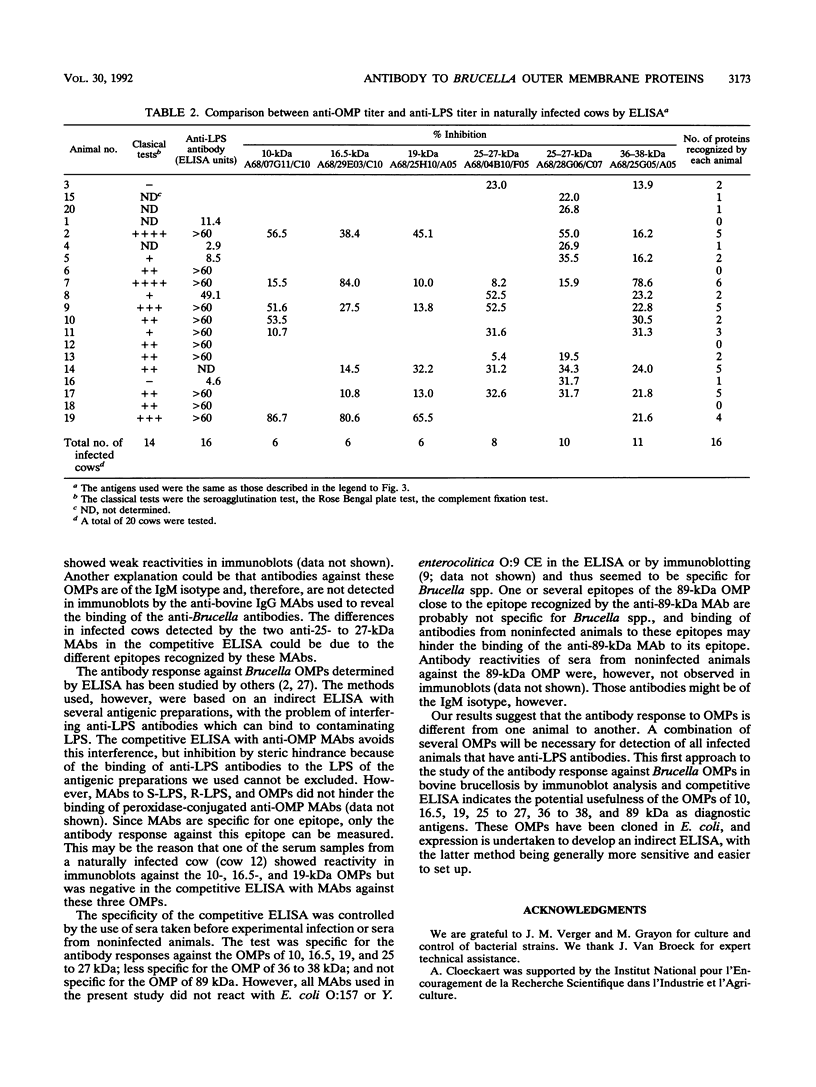
Sera from Brucella-infected bovines were analyzed by immunoblotting by using sonicated cell extracts of B. melitensis or B. abortus and a competitive enzyme-linked immunosorbent assay (ELISA) with monoclonal antibodies against outer membrane proteins (OMPs) with molecular masses of 10, 16.5, 19, 25 to 27, 36 to 38, and 89 kDa. Antibody responses against OMPs were compared with antibody responses against smooth lipopolysaccharide. Immunoblot analysis indicated that the antibody response in infected animals was largely different from one animal to another. The antigens of concern were OMPs with molecular masses of 10, 16.5, 19, 25 to 27, 36 to 38, and 89 kDa and other proteins with molecular masses of between 40 and 80 kDa. According to the specificity of the competitive ELISA, OMPs useful for the detection of infected animals are the OMPs of 10, 16.5, 19, 25 to 27, and 36 to 38 kDa. A competitive ELISA with the anti-89 kDa monoclonal antibody was not specific. Results of the competitive ELISA confirmed the individual variability of the humoral immune response against OMPs. It therefore seems that a combination of several protein antigens is necessary for the development of an immunoassay with a sensitivity comparable to that of the smooth lipopolysaccharide ELISA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araj G. F., Kaufmann A. F. Determination by enzyme-linked immunosorbent assay of immunoglobulin G (IgG), IgM, and IgA to Brucella melitensis major outer membrane proteins and whole-cell heat-killed antigens in sera of patients with brucellosis. J Clin Microbiol. 1989 Aug;27(8):1909–1912. doi: 10.1128/jcm.27.8.1909-1912.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundle D. R., Cherwonogrodzky J. W., Perry M. B. Characterization of Brucella polysaccharide B. Infect Immun. 1988 May;56(5):1101–1106. doi: 10.1128/iai.56.5.1101-1106.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundle D. R., Cherwonogrodzky J. W., Perry M. B. The structure of the lipopolysaccharide O-chain (M antigen) and polysaccharide B produced by Brucella melitensis 16M. FEBS Lett. 1987 Jun 1;216(2):261–264. doi: 10.1016/0014-5793(87)80702-0. [DOI] [PubMed] [Google Scholar]
- Cherwonogrodzky J. W., Nielsen K. H. Brucella abortus 1119-3 O-chain polysaccharide to differentiate sera from B. abortus S-19-vaccinated and field-strain-infected cattle by agar gel immunodiffusion. J Clin Microbiol. 1988 Jun;26(6):1120–1123. doi: 10.1128/jcm.26.6.1120-1123.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloeckaert A., Jacques I., Bosseray N., Limet J. N., Bowden R., Dubray G., Plommet M. Protection conferred on mice by monoclonal antibodies directed against outer-membrane-protein antigens of Brucella. J Med Microbiol. 1991 Mar;34(3):175–180. doi: 10.1099/00222615-34-3-175. [DOI] [PubMed] [Google Scholar]
- Cloeckaert A., Jacques I., de Wergifosse P., Dubray G., Limet J. N. Protection against Brucella melitensis or Brucella abortus in mice with immunoglobulin G (IgG), IgA, and IgM monoclonal antibodies specific for a common epitope shared by the Brucella A and M smooth lipopolysaccharides. Infect Immun. 1992 Jan;60(1):312–315. doi: 10.1128/iai.60.1.312-315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloeckaert A., Zygmunt M. S., de Wergifosse P., Dubray G., Limet J. N. Demonstration of peptidoglycan-associated Brucella outer-membrane proteins by use of monoclonal antibodies. J Gen Microbiol. 1992 Jul;138(7):1543–1550. doi: 10.1099/00221287-138-7-1543. [DOI] [PubMed] [Google Scholar]
- Cloeckaert A., de Wergifosse P., Dubray G., Limet J. N. Identification of seven surface-exposed Brucella outer membrane proteins by use of monoclonal antibodies: immunogold labeling for electron microscopy and enzyme-linked immunosorbent assay. Infect Immun. 1990 Dec;58(12):3980–3987. doi: 10.1128/iai.58.12.3980-3987.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz R., Garatea P., Jones L. M., Moriyon I. Radial immunodiffusion test with a Brucella polysaccharide antigen for differentiating infected from vaccinated cattle. J Clin Microbiol. 1979 Jul;10(1):37–41. doi: 10.1128/jcm.10.1.37-41.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas J. T., Rosenberg E. Y., Nikaido H., Verstreate D. R., Winter A. J. Porins of Brucella species. Infect Immun. 1984 Apr;44(1):16–21. doi: 10.1128/iai.44.1.16-21.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubray G., Charriaut C. Evidence of three major polypeptide species and two major polysaccharide species in the Brucella outer membrane. Ann Rech Vet. 1983;14(3):311–318. [PubMed] [Google Scholar]
- Dubray G., Limet J. Evidence of heterogeneity of lipopolysaccharides among Brucella biovars in relation to A and M specificities. Ann Inst Pasteur Microbiol. 1987 Jan-Feb;138(1):27–37. doi: 10.1016/0769-2609(87)90051-2. [DOI] [PubMed] [Google Scholar]
- Jacques I., Cloeckaert A., Limet J. N., Dubray G. Protection conferred on mice by combinations of monoclonal antibodies directed against outer-membrane proteins or smooth lipopolysaccharide of Brucella. J Med Microbiol. 1992 Aug;37(2):100–103. doi: 10.1099/00222615-37-2-100. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leong D., Diaz R., Milner K., Rudbach J., Wilson J. B. Some structural and biological properties of Brucella endotoxin. Infect Immun. 1970 Feb;1(2):174–182. doi: 10.1128/iai.1.2.174-182.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limet J. N., Bosseray N., Garin-Bastuji B., Dubray G., Plommet M. Humoral immunity in mice mediated by monoclonal antibodies against the A and M antigens of Brucella. J Med Microbiol. 1989 Sep;30(1):37–43. doi: 10.1099/00222615-30-1-37. [DOI] [PubMed] [Google Scholar]
- Limet J., Plommet A. M., Dubray G., Plommet M. Immunity conferred upon mice by anti-LPS monoclonal antibodies in murine brucellosis. Ann Inst Pasteur Immunol. 1987 May-Jun;138(3):417–424. doi: 10.1016/s0769-2625(87)80052-1. [DOI] [PubMed] [Google Scholar]
- Montaraz J. A., Winter A. J., Hunter D. M., Sowa B. A., Wu A. M., Adams L. G. Protection against Brucella abortus in mice with O-polysaccharide-specific monoclonal antibodies. Infect Immun. 1986 Mar;51(3):961–963. doi: 10.1128/iai.51.3.961-963.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyon I., Berman D. T. Isolation, purification, and partial characterization of Brucella abortus matrix protein. Infect Immun. 1983 Jan;39(1):394–402. doi: 10.1128/iai.39.1.394-402.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- Nielsen K., Cherwonogrodzky J. W., Duncan J. R., Bundle D. R. Enzyme-linked immunosorbent assay for differentiation of the antibody response of cattle naturally infected with Brucella abortus or vaccinated with strain 19. Am J Vet Res. 1989 Jan;50(1):5–9. [PubMed] [Google Scholar]
- Phillips M., Deyoe B. L., Canning P. C. Protection of mice against Brucella abortus infection by inoculation with monoclonal antibodies recognizing Brucella O-antigen. Am J Vet Res. 1989 Dec;50(12):2158–2161. [PubMed] [Google Scholar]
- Riezu-Boj J. I., Moriyón I., Blasco J. M., Gamazo C., Díaz R. Antibody response to Brucella ovis outer membrane proteins in ovine brucellosis. Infect Immun. 1990 Feb;58(2):489–494. doi: 10.1128/iai.58.2.489-494.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riezu-Boj J. I., Moriyón I., Blasco J. M., Marín C. M., Diaz R. Comparison of lipopolysaccharide and outer membrane protein-lipopolysaccharide extracts in an enzyme-linked immunosorbent assay for the diagnosis of Brucella ovis infection. J Clin Microbiol. 1986 May;23(5):938–942. doi: 10.1128/jcm.23.5.938-942.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos J. M., Verstreate D. R., Perera V. Y., Winter A. J. Outer membrane proteins from rough strains of four Brucella species. Infect Immun. 1984 Oct;46(1):188–194. doi: 10.1128/iai.46.1.188-194.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowa B. A., Kelly K. A., Ficht T. A., Frey M., Adams L. G. SDS-soluble and peptidoglycan-bound proteins in the outer membrane-peptidoglycan complex of Brucella abortus. Vet Microbiol. 1991 May;27(3-4):351–369. doi: 10.1016/0378-1135(91)90160-h. [DOI] [PubMed] [Google Scholar]
- Verstreate D. R., Creasy M. T., Caveney N. T., Baldwin C. L., Blab M. W., Winter A. J. Outer membrane proteins of Brucella abortus: isolation and characterization. Infect Immun. 1982 Mar;35(3):979–989. doi: 10.1128/iai.35.3.979-989.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreate D. R., Winter A. J. Comparison of sodium dodecyl sulfate-polyacrylamide gel electrophoresis profiles and antigenic relatedness among outer membrane proteins of 49 Brucella abortus strains. Infect Immun. 1984 Oct;46(1):182–187. doi: 10.1128/iai.46.1.182-187.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter A. J., Rowe G. E., Duncan J. R., Eis M. J., Widom J., Ganem B., Morein B. Effectiveness of natural and synthetic complexes of porin and O polysaccharide as vaccines against Brucella abortus in mice. Infect Immun. 1988 Nov;56(11):2808–2817. doi: 10.1128/iai.56.11.2808-2817.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]