Abstract
Many studies demonstrate that exposure to testicular steroids such as testosterone early in life masculinizes the developing brain, leading to permanent changes in behavior. Traditionally, masculinization of the rodent brain is believed to depend on estrogen receptors (ERs) and not androgen receptors (ARs). According to the aromatization hypothesis, circulating testosterone from the testes is converted locally in the brain by aromatase to estrogens, which then activate ERs to masculinize the brain. However, an emerging body of evidence indicates that the aromatization hypothesis cannot fully account for sex differences in brain morphology and behavior, and that androgens acting on ARs also play a role. The testicular feminization mutation (Tfm) in rodents, which produces a nonfunctional AR protein, provides an excellent model to probe the role of ARs in the development of brain and behavior. Tfm rodent models indicate that ARs are normally involved in the masculinization of many sexually dimorphic brain regions and a variety of behaviors, including sexual behaviors, stress response and cognitive processing. We review the role of ARs in the development of the brain and behavior, with an emphasis on what has been learned from Tfm rodents as well as from related mutations in humans causing complete androgen insensitivity.
Keywords: complete androgen insensitivity syndrome - CAIS, vasopressin, anxiety, spatial memory, bed nucleus, medial amygdala, SDN-POA, sexual behavior, aggression, VMH
Exposure to testicular steroids such as testosterone (T) early in life masculinizes the developing brain, leading to permanent changes in behavior in a wide variety of animal models (Morris et al., 2004). According to the aromatization hypothesis, T is converted by aromatase into 17-β estradiol (E2), which then acts on estrogen receptors (ERs) to masculinize the brain (Naftolin et al., 1975). Traditionally, aromatization is believed to be the mechanism by which the rodent brain becomes masculinized and defeminized. Some sexually dimorphic regions within the hypothalamus adhere well to this hypothesis, including the sexually dimorphic nucleus of the preoptic area (SDN-POA) and anteroventral periventricular nucleus (AVPV). T spares neurons from death in the SDN-POA, while it promotes cell death in the AVPV, both through activation of ERs. The aromatization hypothesis also seems to hold for the development of some sexual and nonsexual rodent behaviors. Defeminization of rat sexual behavior, as measured by the proclivity to show lordosis (the female posture of sexual receptivity), and masculinization of aggressive behavior in rodents are largely controlled by estrogenic metabolites of T acting on ERs (Olsen, 1979; Vreeburg et al., 1977; Ogawa et al., 2000; Scordalakes and Rissman, 2004).
However, an emerging body of evidence suggests that the aromatization hypothesis cannot account for all sex differences in brain morphology and behavior. In primates, including humans, brain masculinization may be accomplished primarily via androgens acting directly on the androgen receptor (AR). For example, alpha-fetoprotein binds estrogen and prevents it from entering and masculinizing the brain in rodents (Bakker et al., 2006; Puts et al., 2006), but has very low affinity for estrogen in primates (Swartz and Soloff, 1974). If the aromatization hypothesis were generally true in primates, it would seem that ovarian estrogens would cross the blood-brain barrier and masculinize the female brain. Moreover, people with complete androgen insensitivity syndrome (CAIS) exhibit feminine behavior (see below) and morphology. CAIS individuals have a 46, XY karyotype and develop testes that remain undescended in the abdominal cavity. Despite producing normal-to-high male levels of T, individuals with CAIS have completely nonfunctional ARs, and so are phenotypically female (Imperato-McGinley et al., 1982). Because individuals with CAIS regard themselves as females, we refer to them hereafter as women Their feminine behavior suggests that functional ARs are required to masculinize the human brain. Conversely, human males with mutations rendering the aromatase enzyme dysfunctional present as normal males, despite the absence of aromatization in the brain or elsewhere (Grumbach and Auchus, 1999). Taken together, the feminine behavior of people with CAIS and the masculine behavior of men with dysfunctional aromatase suggest that ER stimulation has a very limited role, if any, in masculinizing the human brain.
In the meantime, there is growing evidence that even in rodents, androgens act on ARs to shape the brain and behavior. Traditionally, exploration into the role of ARs in brain morphology and behavior involved the administration of a nonaromatizable AR agonist (usually dihydrotestosterone: DHT) or antagonist (e.g., flutamide). Unfortunately, DHT administration does not guarantee exclusive activation of ARs. DHT can be metabolized to 3α-androstanediol (3α-diol) which has a low affinity for ARs but a high affinity for GABA receptors, and 3β-diol, an estrogenic compound which binds ERs (Kuiper et al., 1998). Even if administration of DHT did act solely upon ARs, it might not reveal a contribution of the AR in cases where T acts synergistically upon both ERs and ARs. For example, T acts upon ARs to increase aromatase activity in many brain regions (Roselli et al., 1987; Rosenfeld et al., 1977), which would provide more estrogens to stimulate ERs. Therefore DHT treatment alone would not reveal such a normal role for ARs because while it might boost aromatase activity, there would be no aromatizable androgenic precursor to be converted by the enzyme to provide estrogens for ERs. Furthermore, neonatal DHT administration may not masculinize some areas of the brain because AR expression levels in those areas may depend on ERs (McAbee and DonCarlos, 1999a). Neonatal gonadectomy in male rats decreases AR mRNA, and replacement with T or E2, but not DHT, restores AR mRNA to levels similar to gonadally intact male rats (McAbee and DonCarlos, 1999a; McAbee and DonCarlos, 1999b). These findings suggest that T normally acts through ERs to upregulate AR. Because DHT treatment fails to restore AR mRNA, DHT may fail to masculinize brain areas simply because AR levels are too low. These data also suggest reciprocal interactions between ARs and ERs in the brain, a theme emphasized in the remainder of this review.
The AR antagonist flutamide is also an imperfect test of AR contributions since its administration, while blocking ARs, also alters T production from the testes, making it difficult to attribute changes in morphology and behavior solely to AR blockade (Clos et al., 1988; Ayub and Levell, 1987).
Testicular feminization mutant (Tfm) rodents provide a unique model for examining the role of the ARs in the brain and behavior, because this mutation in the AR gene renders the protein nonfunctional. As a result of this mutation (the rodent analog of CAIS in humans), genetically male Tfm rodents appear phenotypically female: they possess nipples typical of female rodents, lack normal male genitalia, and are infertile. Since this trait is X-linked, only genetically male (XY) carriers are wholly androgen insensitive. Female Tfm carriers are heterozygous, carrying one Tfm and one wildtype (wt) allele of the AR gene, thus allowing the mutation to be passed on to future generations. Although Tfm rodents present a feminine exterior, we refer to them hereafter as Tfm males because they are genetically male, possess testes and, unlike women with CAIS, show signs of at least partial masculinization of many behaviors, as discussed below. We also refer to them as Tfm males to distinguish them from females carrying the Tfm allele.
Rat and mouse Tfm models differ in terms of the type of mutation in AR. In rats, androgen insensitivity results from a mutation involving a single base pair replacement in the AR gene (Yarbrough et al., 1990), while in the Tfm mouse the mutation results from a single base deletion, which causes a frameshift mutation (Charest et al., 1991). Thus there is only a single amino acid difference between wt and Tfm AR protein in rats resulting in expression of a normal sized but dysfunctional AR protein in Tfm rats. On the other hand, the mutation in Tfm mice introduces a premature stop codon, resulting in a shortened transcript and essentially no AR protein (He et al., 1991; Monks et al., 2007). Due to differences in the nature of these mutations, Tfm rats have some residual sensitivity to androgens through ARs, although it is greatly reduced (Yarbrough et al., 1990), while Tfm mice have virtually no sensitivity to androgen through ARs, as in CAIS women (Drews, 1998). In both models estrogen binding in the brain appears normal (Attardi et al., 1976; Olsen and Whalen, 1982).
Rat and mouse Tfm models also differ in terms of circulating T levels. Tfm male mice have significantly less endogenous T compared to their wt siblings (Jones et al., 2003), while Tfm male rats have circulating T levels in the high male range (Rosseli et al., 1987). In this regard, the Tfm rat resembles untreated humans with CAIS, who also have circulating T levels in the high-male range (Vague, 1983). In both rat and mouse Tfm models, indirect evidence suggests that T levels are near the normal male range in the perinatal period (Olsen, 1979; Goldstein and Wilson, 1972), but no one has actually measured circulating androgens in perinatal Tfm rodents.
Aromatase activity is also decreased in several areas of the adult Tfm brain compared to wt males (Roselli et al., 1987; Rosenfeld et al., 1977), so differences between wt males and Tfm males in brain morphology and behaviors associated with these regions may be caused by reduced ER activation. Therefore in the survey that follows, as we find evidence that the Tfm allele of the AR gene affects brain and behavior, we must keep in mind that such findings do not disprove a role for ER, since AR may be acting in part by affecting aromatase.
AR and Brain Morphology
Increasing evidence suggests that activation of AR also normally mediates masculinization of the nervous system and behavior. Some of the first evidence for this idea came from analysis of motoneurons of the spinal nucleus of the bulbocavernosus (SNB), which mediate penile reflexes during copulation (see Sengelaub and Forger, 2008, in this issue). There is a sex difference in the number of SNB motoneurons (males>females) that is dependent on AR activation, since the SNB system is feminized in Tfm rats (Breedlove and Arnold, 1980; Breedlove and Arnold, 1981). Recent research utilizing Tfm rodent models suggests that the absence of functional ARs also alters discrete areas of the brain. This demasculinization of specific brain regions is not due to an effect on the overall size of the brain. In both rats and mice, males have greater brain weights than do females, which probably reflect the sex difference in overall body size. We now report for the first time that the brain weight of Tfm males is fully masculine in both species (Figure 1). Interestingly, body weight of Tfm males is intermediate between that of wt males and females in both species, indicating that sexual differentiation of overall brain weight and body weight are dissociable. These results suggest that this global masculinization of brain weight may occur via ER activation or via some other mechanism not dependent on ARs. However, they also set the stage for the following results where brain regions that are typically larger in males than in females are partially or wholly feminine in Tfm males, indicating that regional brain demasculinization in Tfm animals is not a generalized effect on overall brain size, but a specific decrease in some brain areas caused by lack of AR stimulation.
Figure 1.
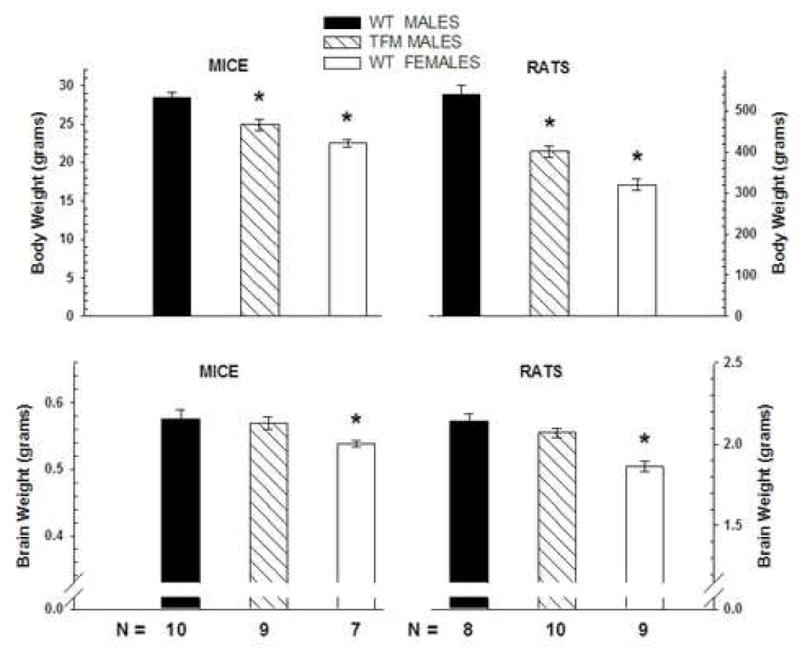
(Left) Brain and body weights of adult wildtype (wt) male, Tfm male, and wt female mice and (Right) rats. Note different scales on the Y axes for the two species. All animals were between 120–150 days old at the time of sacrifice. Brain weight measurements did not include the olfactory bulb and were taken after prolonged postfixation in 10% formalin. Wt males show an increased brain weight compared to females, and Tfm males resemble wt males, in both mice and rats. Tfm males show a body weight intermediate between wt males and females. * indicates p< .05 compared to wt males.
Posterodorsal medial amygdala (MePD)
Morphology of the posterodorsal medial amygdala (MePD), which receives olfactory and pheromonal information and is important for some aspects of male sexual behavior, is highly dependent on adult hormones. MePD volume is 1.5 times greater in male rats than in females, but this sex difference can be abolished by castration of adult males and/or administration of T to adult females (Cooke et al., 1999). Structural plasticity in the adult MePD appears to be mediated through activation of both ARs and ERs. Cooke et al. (2003) found that treating castrated adult male rats with E2 and the nonaromatizable androgen DHT each masculinize aspects of MePD morphology. Treatment with E2 increases both volume and soma size of MePD cells compared to untreated castrates, while DHT treatment increased MePD soma size but did not affect volume. MePD volume and soma size have also been examined in Tfm male rats (Morris et al., 2005) and both were found to be partially demasculinized, significantly different from both wt males and females (Figure 2a–f). In contrast, the rostrocaudal extent of the MePD, which is greater in wt males than females, is fully masculinized in Tfm males. Because T levels are in the high male range and aromatase activity is normal in the medial amygdala of Tfm male rats (Rosseli et al., 1987), adequate amounts of estrogens should be available for ER activation. Given that both MePD soma size and volume are smaller in Tfm males than in wt males, T activity through ERs appears insufficient to fully masculinize these characteristics (Morris et al., 2005). On the other hand, masculinization of the rostrocaudal extent of the MePD appears to be independent of AR activation, with Tfm and wt males equivalent (Morris et al., 2005). Similarly, Tfm and wt male rats also show an asymmetry, with MePD volumes greater in the right hemisphere than the left. This laterality in MePD volume is not found in females, indicating that its presence may depend on some masculinizing factor unrelated to AR activation, the leading candidate being ER activation (Morris et al., 2005).
Figure 2.
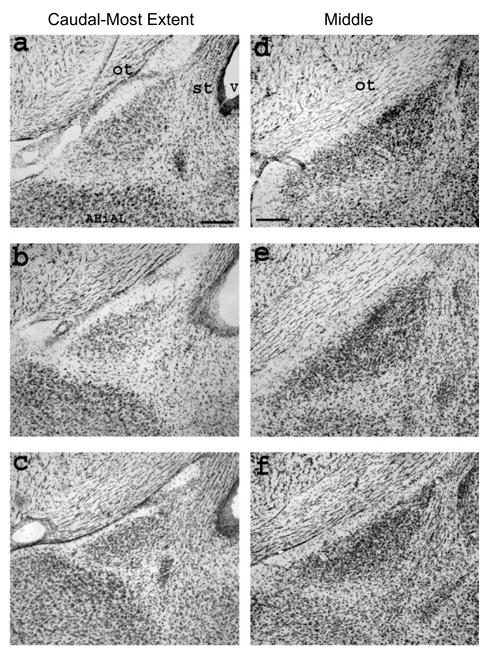
The posterodorsal medial amygdala (MePD) in Nissl-stained coronal sections from a wildtype (wt) male (top), a Tfm male with a dysfunctional androgen receptor (middle), and a female rat (bottom). The panels on the left are from the caudalmost appearance of the MePD, which served as an anchor point to assess changes in the nucleus across the rostrocaudal dimension. The appearance of the MePD, as well as the optic tract (ot), the stria terminalis (st), the anterolateral part of the amygdalohippocampal transition area (AHiAL), and the lateral ventricle (v) are equivalent in wt males (a), Tfm males (b), and females (c), indicating that the caudal termination of the MePD occurs in the homologous region of the brain across groups. The panels on the right are from the approximate middle of the rostrocaudal extent of the MePD where the nucleus is larger in wt males (d) than in females (f) and is intermediate in size in Tfm males (e). The MePD also extends farther rostrally in wt males and Tfm males than in females (Morris et al., 2005). Scale bar = 250 m in a (applies to a–c), d (applies to d–f).
Suprachiasmatic Nucleus
Sexual dimorphisms have been found in the brain’s biological clock, the suprachiasmatic nucleus (SCN), in both humans and rodents. The vasoactive intestinal polypeptide (VIP) containing subnucleus of the SCN is twice as large in men as in women, and the vasopressin containing subnucleus of the SCN has a different shape in men than in women (Swaab et al., 1994; Swaab et al., 1985). Sexual orientation has also been correlated with the size of the human SCN. Swaab and Hofman (1990) found the vasopressin subnucleus of the SCN of homosexual men to be 1.7 times as large and contain twice as many cells as that of heterosexual men. Studies of sex differences in the SCN of rats have yielded somewhat conflicting findings in which males have a greater SCN volume than females (Robinson et al., 1986; Gorski et al., 1978) or there were no differences (Madeira et al., 1995; Bloch and Gorski, 1988). These discrepancies suggest that sex differences in the rat SCN may either be small and/or strain dependent. In gerbils, volume of the male SCN was twice that of the female SCN (Holman and Hutchison, 1991). Furthermore, castration of gerbils on the day of birth reduced SCN volume to female levels in adulthood, suggesting that the SCN is influenced by early androgen exposure. Morris et al. (2005) analyzed the SCN in Tfm male rats and found that volume and neuronal soma size was decreased compared to wt male rats, indicating that functional ARs are essential for the full masculinization of this brain area. However, given that aromatase activity is decreased in the SCN of Tfm male rats (Roselli et al., 1987), it is possible that the main role the AR plays in masculinization of the SCN is to regulate the level of aromatase and that ERs mediate downstream changes in SCN structure. This scenario would still indicate an important role for ARs in normal masculinization of the SCN, but it would also suggest that sufficient stimulation of ERs, such as with exogenous estrogen, can fully masculinize the nucleus.
Ventromedial Hypothalamus
The ventromedial hypothalamus (VMH) is another sexually differentiated region that is involved in sexual and parental behaviors. VMH volume and soma size are greater in males than in females, and males also have a higher concentration of ARs in the VMH than do females (Matsumoto and Arai, 1983; Madeira et al., 2001). The VMH contains four morphologically distinct regions, including the anterior (VMHa), dorsomedial (VMHdm), ventrolateral (VMHvl), and central (VMHc) subdivisions, which have distinct connectivity patterns to other brain regions and may affect a variety of functions (McClellan et al., 2006). In a recent study, volume and neuronal soma size of the entire VMH and its four subdivisions were compared between wt males, females, and Tfm male rats (Dugger et al., 2007). Confirming earlier findings, overall VMH volume was greater in males than in females. This difference was accounted for by a larger male VMHvl and a marginally greater male volume in the VMHdm. In Tfm males, overall VMH volume was intermediate between wt males and females, but did not significantly differ from either group. However, the VMHvl, which accounts for most of the sexual dimorphism in VMH volume as a whole, was significantly smaller in Tfm males than wt males and similar to that of females. Analysis of neuronal soma size revealed a sex difference in which males had a greater soma size than females in three of the four VMH subdivisions (VMHvl, VMHc, and VMHdm). Tfm males had significantly smaller somata than wt males in the VMHdm and marginally smaller somata in the VMHvl and VMHc. Together these data suggest that functional ARs normally play a role in the full masculinization of the VMH. In fact, ARs may be the predominant steroid receptor mediating masculinization of the VMH, since Tfm males did not significantly differ from females on any measure. However, differences in AR-induced aromatase activity, as discussed for the SCN, may also influence VMH morphology. Finally, these results from the VMH, along with those discussed for the MePD and SCN, emphasize the fact that different morphological traits of a given brain region may or may not be affected by ARs, and that the effects of the AR on individual cells within a region may not affect overall volume.
Bed Nucleus of the Stria Terminalis
The bed nucleus of the stria terminalis (BST) is a sexually dimorphic region within the hypothalamus that plays a role in social and reproductive behaviors (De Vries and Panzica, 2006). A portion of this region, the posteromedial nucleus of the bed nucleus of the stria terminalis (BSTMPM) shows several sexual dimorphisms, including greater AR density and regional volume in males compared to females (Hines et al., 1992; Lisciotto and Morrell, 1994; Roselli, 1991). The sex difference in volume appears to result from hormone exposure both perinatally and in adulthood. Gonadectomy of newborn males decreases BSTMPM volume, and androgen treatment of newborn females increases it (Guillamon et al., 1988b), while gonadectomy of adult males also decreases regional volume (Malsbury and McKay, 1994). Based on neuroanatomical similarities of the BSTMPM and the MePD (proximity and high AR content), and similar hormone responsiveness between the two regions, it was hypothesized that ARs may also play a role in the sexual differentiation of this region. Durazzo et al. (2007) compared characteristics of the BSTMPM in wt male, wt female, and Tfm male rats and found group differences in regional volume, but not neuronal soma size. There was a sex difference in BSTMPM volume in both the left and right hemisphere (males > females), while volume was increased in wt males compared to Tfm males only in the left hemisphere. These results suggest that, as in the MePD, ARs normally contribute to the full masculinization of the BSTMPM in males.
Arginine vasopressin (AVP) innervation of the septal area (including the BST), which plays a role in pair bonding, parental, and aggressive behavior (De Vries and Panzica, 2006), is also sexually differentiated. AVP innervation of this area is greater in males than in females and is dependent upon androgens in both early development and adulthood. Castration of male rats in adulthood decreases AVP innervation, although not as extensively as does castration neonatally (Wang et al., 1993). Androgens contribute to the sex difference in AVP via aromatized metabolites of T acting on ERs, although ARs also appear to contribute to the perinatal organization of this system to some degree. For example, gonadectomized rat pups treated in the early postnatal period with DHT show a partial masculinization of BST vasopressin, although this masculinization is less than that found in rat pups treated with E2 either alone or combined with DHT (Han and De Vries, 2003). On the other hand, Tfm male mice are not different from wt males after adult castration and treatment with E2 (Scordalakes and Rissman, 2004). These results suggest that the role of ARs in the masculinization of AVP innervation of the septal area in mice is minimal.
Sexually Dimorphic Nucleus of the Preoptic Area (SDN-POA)
Even the SDN-POA, in which abundant literature suggests that sex differences depend on the metabolism of T into E2 and activation of ERs, may normally depend on ARs for full masculinization. Regional volume and soma size of SDN-POA neurons are greater in male than in female rats (Gorski et al., 1980; Madeira et al., 1995), and perinatal hormone manipulations suggest that ERs, not ARs, are important in the masculinization of SDN-POA volume (Dohler et al., 1986). However, Tfm male rats show only a partial masculinization of the SDN-POA: volume is fully masculine but neuronal soma size is not, with Tfm males having smaller neurons in the SDN-POA than wt males (Morris et al., 2005). Again, as in several other brain regions, aromatase activity is decreased in the medial preoptic area of Tfm male rats (Roselli et al., 1987), so ARs may not be directly necessary for the masculinization of neuronal soma size in the SDN-POA, if sufficient estrogen is provided. Nevertheless, ARs appear to contribute normally to the full masculinization of the rat SDN-POA.
Locus Coeruleus
The locus coeruleus (LC), a brainstem nucleus implicated in the physiological response to stress and panic, is an example of a sexual dimorphism in which females show a larger volume and a greater number of neurons than do males (Guillamon et al., 1988a). This dimorphism appears to be under the control of both testicular and ovarian hormones, with testicular steroids decreasing and ovarian steroids increasing growth and survival of LC cells. Both neonatal and postpubertal gonadectomy of females decrease the number of neurons in the LC (De Blas et al., 1990; De Blas et al., 1995), while pre- and postnatal androgen administration also decreases LC neuron number (Guillamon et al., 1988a). In males, ARs rather than ERs appear to play a critical role in the masculinization of the LC. Garcia-Falgueras et al. (2005) found that, compared to wt male littermates, Tfm males had a larger volume and greater number of neurons in the LC. Based on these results, Guillamon’s group suggests that when a sexual dimorphism exists in which females show more neurons and/or greater volume it may be at least partially mediated via AR activation in males.
Hippocampus
The hippocampus is another sexually dimorphic area of the brain in which males show a greater overall volume than females (Madeira et al., 1995; Nunez et al., 2000). One area of the hippocampus, the dentate gyrus, is sexually dimorphic in some strains of mice in which males show a greater number, size, and density of cells within this region (Wimer and Wimer, 1984; Wimer et al., 1988; Wimer and Wimer, 1989). Study of Tfm male mice suggests that ARs may play some role in the development of the mouse dentate gyrus. Although no sex difference was reported in volume of the granule cell layer of the dentate gyrus (GCL) in C57BL6J mice, both wt males and females had a larger right than left GCL, a laterality that was absent in Tfm males and partially androgen insensitive Tfm carrier female mice (Tabibnia et al., 1999). These results suggest that ARs play a role in establishing laterality in the mouse GCL regardless of whether a sexual dimorphism exists. In the rat dentate gyrus, the presence of functional ARs also appears to affect morphology, despite a lack of sexual dimorphism. Jones and Watson (2005) found that Tfm male rats show a greater GCL volume than do females, although there was no sex difference in GCL volume. Why GCL volume is greatest in Tfm rats is unclear, but the authors suggest it may result from increased ER activation within the GCL due to the higher levels of T in Tfm male rats.
Androgens also increase the pyramidal cell dendritic spine density (PSSD) in CA1 of the hippocampus in male and female rats (MacLusky et al., 2004; Leranth et al., 2004; Hajszan et al., in this issue). In females, estrogens can also increase CA1 PSSD, though they have no effect in males (Leranth et al., 2003; MacLusky et al., 2005). This result indicates that the androgen-induced increase in PSSD in males may be mediated through ARs. However, Tfm males with dysfunctional ARs showed a PSSD comparable to wt males (MacLuscky et al., 2006). Interestingly, DHT administration increased CA1 PSSD in both wt and Tfm males, suggesting that androgens may exert their influence through nonclassical pathways.
Our review of the literature indicates that, for every known sexual dimorphism in the rat and mouse nervous system that has been examined in Tfm models so far, results indicate that ARs normally contribute to masculinization of at least some aspects of morphology. In several cases, both regional volume and neuronal soma size are significantly demasculinized in Tfm males (SNB, MePD, VMH, SCN). Sometimes volume is demasculinized while neuronal soma size is not, sometimes the reverse is true. Our conclusion is that ARs are integral to masculine structural development throughout the rodent brain. These studies are summarized in Table 1.
Table 1.
Key morphological findings in the Tfm rodent brain
| Morphological findings in the TFM Rodent Central Nervous System | ||||
|---|---|---|---|---|
| Region | Species | Morphological Characteristics | Tfm ♂s are: | References |
| MePD | Rat | Volume: wt ♂ > Tfm ♂ > wt ♀. Soma Size: wt ♂ > Tfm ♂ > wt ♀. |
Intermediate Intermediate |
Morris et al, 2005 |
| SCN | Volume: wt ♂ > Tfm ♂ ≈ wt ♀. Soma Size: wt ♂ > Tfm ♂ ≈ wt ♀, but wt ♂ ≈ wt ♀. |
Feminine Feminine? |
Morris et al, 2005 | |
| VMHdm | Volume: No significant group differences. Soma Size: wt ♂ > Tfm ♂ ≈ wt ♀. |
--- Feminine |
Dugger et al, 2007 | |
| VMHvl | Volume: wt ♂ > Tfm ♂ ≈ wt ♀. Soma Size: wt ♂ > wt ♀, but Tfm ♂ not significantly different from either sex. |
Feminine Intermediate? |
Dugger et al, 2007 | |
| BSTMPM(Left Hemisphere) | Volume: wt ♂ > Tfm ♂ ≈ wt ♀. Soma Size: No significant group differences. |
Feminine --- |
Durazzo et al, 2007 | |
| SDN-POA | Volume: wt ♂ ≈ Tfm ♂ > wt ♀. Soma Size: wt ♂ > Tfm ♂ ≈ wt ♀, but wt ♂ ≈ wt ♀. |
Masculine Feminine? |
Morris et al, 2005 | |
| LC | Volume: wt ♂ < Tfm ♂ ≈ wt ♀. Neuron Number: wt ♂ < Tfm ♂ ≈ wt ♀. |
Feminine Feminine |
Garcia-Falgueras et al, 2005 | |
| DG | Volume: wt ♂ ≈ Tfm ♂ > wt ♀, but wt ♂ not significantly different from either group. | Masculine? | Jones and Watson, 2005 | |
| SNB | Neuron Number: wt ♂ > Tfm ♂ ≈ wt ♀. Soma Size: wt ♂ > Tfm ♂ ≈ wt ♀. |
Feminine Feminine |
Breedlove and Arnold, 1981 | |
| LS | Mouse | AVP Density: wt ♂ ≈ Tfm ♂ > wt ♀. | Masculine | Scordalakes and Rissman, 2004 |
MePD: posterodorsal medial amygdala; SCN: suprachiasmatic nucleus; VMHdm: dorsomedial aspect of the ventromedial hypothalamus; VMHvl: ventrolateral aspect of the ventromedial hypothalamus; BSTMPM: posteromedial nucleus of the bed nucleus of the stria terminalis; SDN-POA: sexually dimorphic nucleus of the preoptic area; LC: locus coeruleus; DG: dentate gyrus; SNB: spinal nucleus of the bulbocavernosus; LS: lateral septum; AVP: arginine vasopressin.
AR and Behavior
In line with the emerging role of the AR in shaping brain morphology, evidence also suggests that the AR is important in shaping behavior, both during its early organization and its later activation in adulthood. Although aromatization may account for many sex differences in rodent behavior, research using Tfm models suggests that the AR normally plays a critical role in a variety of behaviors ranging from sexual to cognitive.
Spatial Memory
Males outperform females in spatial memory performance in both rodents and humans (Rizk-Jackson et al., 2006; Roof, 1993; Astur et al., 1998; Jonasson, 2005). The sex differences in rodents have generally been attributed to the organizational effects of androgens, since neonatal castration of males or administration of T to newborn females eliminates such sex differences (Isgor and Sengelaub, 2003). The aromatization of androgens to estrogens may be particularly important in the development of the sex difference, since neonatal administration of E2 masculinizes spatial ability in female rats (Williams et al., 1990; Williams and Meck, 1991). However, other evidence suggests that perinatal AR activation may also play a role in enhancing male spatial memory performance. Isgor & Sengelaub (1998) found that prenatal E2 treatment did not masculinize performance in the Morris Water Maze (MWM) in female Sprague-Dawley rats, whereas treatment with either T or DHT did. In addition, males administered AR antagonists prenatally show poorer MWM performance than do control males, eliminating the sex difference in this behavior (Isgor and Sengelaub, 1998; Joseph et al., 1978).
Hormonal influence on such behaviors, however, may not be solely organizational. Adult circulating androgens and estrogens can also affect certain aspects of spatial memory performance and these effects may, at least partially, be mediated through activation of ARs in adulthood (Gibbs, 2005; Sandstrom et al., 2006; Naghdi et al., 2001). To further explore the role of ARs in spatial memory, performance in the MWM has been examined in both the rat and mouse Tfm models. Jones and Watson (2005) compared MWM performance in wt male, female, and Tfm male rats and found that Tfm males showed an intermediate pattern of performance in which they took longer to reach male-typical performance levels. Tfm male mice also show impaired MWM performance compared to partially androgen-insensitive Tfm carrier females, although sex differences in wt mice were not found (Rizk et al., 2005). Together, these findings suggest that the AR is necessary for the full masculinization of spatial memory.
Other facets of memory also appear to be influenced by androgens, such as object memory in rodents, which is strongly influenced by circulating androgens and estrogens in adulthood (Frye and Lacey, 2001; Walf et al., 2006). Use of the Tfm models would further elucidate the role of T and its mechanism of action in this and other memory tasks.
Sex differences in human spatial ability also appear to be mediated by androgens (Puts et al., 2007; Puts et al., in press), and evidence supports a role of ARs in this sex difference. In the only published study of CAIS and spatial ability to date, CAIS females performed significantly worse on spatial tasks than did their male relatives (Imperato-McGinley et al., 1991). Although this finding suggests that androgens masculinize spatial ability via ARs, it is also possible that CAIS women exhibit less masculine spatial abilities because they were socialized in a manner concordant with their phenotypic gender. A more powerful comparison is that between CAIS women and their unaffected (46, XX) female relatives. If spatial ability is AR-mediated, then CAIS women should perform significantly worse on tests of spatial ability than their unaffected female relatives, who are also socialized as females yet produce and receive some androgen stimulation. In fact, this is what was found (Imperato-McGinley et al., 1991). Even this comparison must be interpreted cautiously however, as it is possible that ovarian hormone production in unaffected females caused them to differ from their CAIS relatives.
Anxiety-Related Behavior
In humans, anxiety disorders are estimated to affect about 1 in 5 people (Kessler et al., 1994), and there are striking sex differences in the prevalence of these disorders. Women suffer more from social anxiety disorder than do men (Weinstock, 1999) and tend to receive the majority of diagnoses for specific phobias (e.g., arachnophobia; DSM-IV, 1994). Differences in circulating sex hormones, E2 and T, are likely involved in the occurrence of gender differences in anxiety. Estrogen replacement has repeatedly been shown to increase mood and decrease anxiety in postmenopausal women (Yazici et al., 2003). Other studies indicate that androgens also play a role in the regulation of anxiety in humans. Chemical castration through androgen blockade therapy in men with prostate cancer was correlated with an increase in anxiety. When treatment ceased, anxiety levels decreased (Almeida et al., 2004).
Gonadal hormones also play a role in the display of anxiety-related behavior in rodents as assessed by open field testing, exposure to a novel object, the elevated plus maze task, and light dark box (Edinger and Frye, 2004; Walf and Frye, 2005; Lund et al., 2005; Bridges and Starkey, 2004; Frye and Lacey, 2001). In general, increases in either T or E2 in both male and female rodents are correlated with a decrease in anxiety-related behaviors (Frye and Walf, 2004; Bing et al., 1998; Frye et al. 2007). Recent reports have shed some light on the hormone receptors that are activated to provide anxiolysis. Imwalle et al. (2005) reported that estrogen receptor beta (ERβ) knockout male mice exhibit greater anxiety on an elevated plus maze anxiety test compared to their wt littermates. Furthermore, pharmacological activation of ERβ in rats decreased, while estrogen receptor alpha (ERα) activation increased, anxiety-related behaviors in an open field and when exposed to a novel object (Lund et al., 2005). Together these studies suggest that estrogen action via ERα and ERβ subtypes differentially regulates anxiety-related behaviors.
ARs also appear to be involved in anxiety in rodents. In rats, DHT treatment reduces anxiety behavior in the elevated plus maze (Edinger and Frye, 2004; Frye and Edinger, 2004). However, as previously mentioned, DHT can also interact with other hormone and non-hormone receptors via further metabolism. DHT can be metabolized to 3α-androstanediol (3α-diol) which has a low affinity for ARs but a high affinity for GABA receptors, and treatment with 3α-diol increases anxiolysis in gonadectomized rats (Frye and Edinger, 2004). 3β-diol, which can also be derived from DHT, binds ERs, with greater affinity for ERβ than ERα (Kuiper et al., 1998; Lund et al., 2004). Given what is known about the role of ERβ in the display of anxiety, it is likely that increases in 3β-diol levels following DHT administration can also alter this behavior (see Handa et al., in this issue). Consequently, Tfm male rodents with nonfunctional ARs provide a useful and novel method for exploring the role of the AR in anxiety-related behaviors, while avoiding some limitations of DHT treatment.
Studies using Tfm mice indeed suggest that ARs are involved in the anxiolytic actions of androgens. Rizk et al. (2005) found that, as a group, mice carrying the Tfm allele (Tfm males and Tfm carrier females) showed increased indices of anxiety on the elevated plus maze compared to wt male and female mice. Furthermore, in a novel object exposure test conducted in our laboratory, Tfm male mice tend to spend less time exploring the novel object in an open field compared to wt males, indicating that mice without functional ARs show greater anxiety-related behavior (Fig 3a). This difference does not appear to depend on inherent differences in circulating T levels between Tfm and wt males, as androgen treatment, which increases the time spent with a novel object in castrated wt males, does not alter the behavior of Tfm males in this test (Fig 3b).
Figure 3.
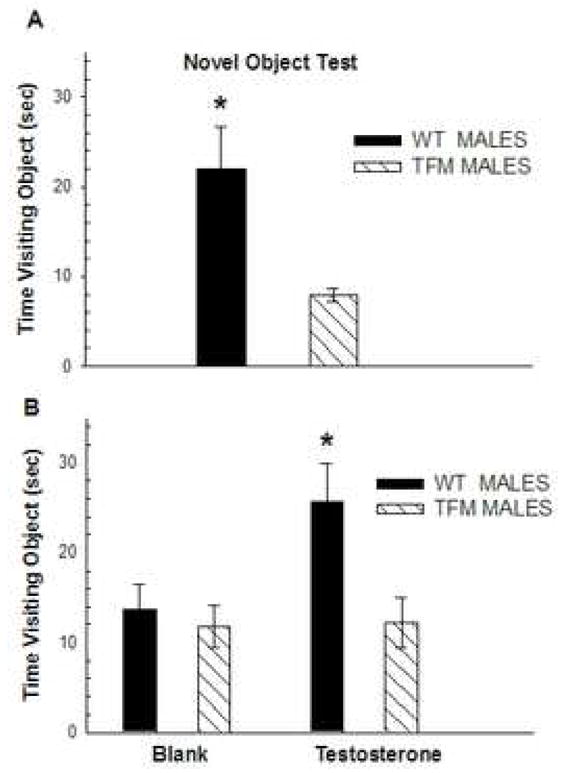
Time spent visiting a novel object in adult male wt and Tfm mice that were placed first in an empty open field and after five minutes were briefly removed and placed back into the open field that contained a small object (a 2″× 2″Petri dish with red and blue tape) in the center. (a) Upon exposure to a novel object, wt males spent more time exploring the novel object than did Tfm males. (b) Wt male mice castrated as adults spent less time visiting the object, an effect that was averted if they were treated with testosterone (T) rather than blank capsules. T had no effect in Tfm males, indicating that androgen receptors mediate this effect. * indicates p< .05 compared to (a) Tfm males or (b) T-treated Tfm males.
Androgens play a critical role in the regulation of the hypothalamic-pituitary-adrenal (HPA) axis, with administration of T or DHT reducing the rise of stress hormones (adrenocorticotropic hormone (ACTH) and corticosterone) following exposure to a stressful situation (Handa et al., 1994; Lund et al., 2004; Lund et al., 2005; Lund et al., 2006). Increased HPA axis activation is often correlated with increased anxiety (Lund et al., 2005; Salome et al., 2004), which suggests that androgens may regulate the display of anxiety-related behavior by depressing HPA axis activation. In a recent study comparing activation of the HPA axis in Tfm and wt male mice, we found that Tfm males show an increased release of corticosterone following exposure to an open field with a novel object (Fig 4), indicating that the increased anxiety-related behavior in Tfm male mice described above may be related to hyperactivation of the HPA axis.
Figure 4.
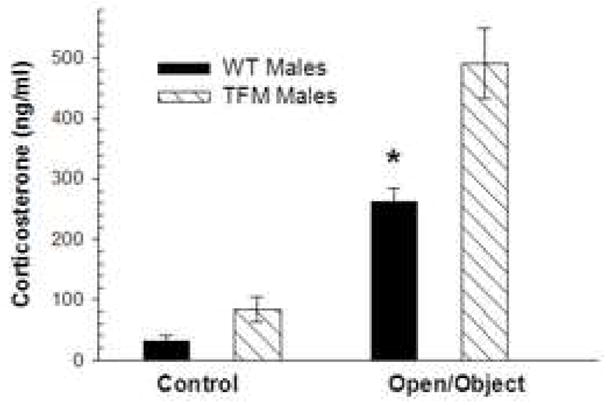
Plasma corticosterone levels 20 minutes after initial exposure to the 10 minute open field/novel object test were significantly greater in Tfm male mice compared to wt male mice. * indicates p< .01 compared to Tfm males.
Play Fighting and Aggression
Play fighting (which includes two major components, playful attack and playful defense) is a common juvenile behavior in many mammalian species and appears to be regulated by gonadal hormones. In rats, play fighting, also called rough-and-tumble play, peaks between postnatal days 30–40 (Thor and Holloway, 1984), and occurs more frequently in males than females (Olioff and Stewart, 1978; Pellis and Pellis, 1990). Play fighting is decreased in male rats castrated at birth and its frequency begins to decline at the onset of puberty (Beatty et al., 1981; Meaney, 1988; Pellis and Pellis, 1990). Analysis of Tfm male rats suggests that ARs are involved in the development of play fighting behavior since, as juveniles, Tfm males show decreased play fighting behavior compared to wt males (Meaney et al., 1983; Meaney, 1988), although recent data suggest that this difference may depend on the testing paradigm (Field et al., 2006). Tfm males also fail to show the male-typical decline in playful attack with age, but like wt males they do show a decline in some aspects of playful defense, indicating that ARs are involved in some but not all aspects of play fighting (Field et al., 2006).
Unlike juvenile play fighting behavior, aggression in adulthood may rely more heavily on the activation of ERs. In male mice, castration decreases aggressive behavior while administration of T, E2, DHT, or a combination of E2 and DHT have been shown to increase male aggressive behavior (Gandelman, 1980; Matochik et al., 1994; Burge and Edwards, 1971; Finney and Erpino, 1976; Luttge and Hall, 1973). Gonadally intact Tfm mice also show decreased aggressive behavior compared to intact males, suggesting that ARs play a role in mediating this behavior (Ohno et al., 1974). However, when adult wt and Tfm male mice were castrated and supplemented with exogenous E2, they showed similar levels of aggression, indicating that low levels of circulating testicular hormones, and not dysfunctional ARs, underlies decreased aggression in gonadally intact Tfm male mice (Scordalakes and Rissman, 2004). Studies using hormone receptor knockout mice also indicate that aggression may be mostly, if not entirely, mediated through activation of estrogen receptors, with enhancement of aggression via ERα activation and inhibition via ERβ activation (Scordalakes and Rissman, 2004; Nomura et al., 2002).
Sexual/Social Behavior
The masculinization and defeminization of male sexual behavior in rodents are traditionally believed to rely on the conversion of androgens to estrogens and the subsequent activation of ERs during the perinatal period, a hypothesis most recently supported by data from mice deficient in either aromatase or ERs (Ogawa et al., 2000; Matsumoto et al., 2003; Ogawa et al., 1997). However, newborn male rats administered an aromatase inhibitor retain masculine sexual behaviors (Dominguez-Salazar et al., 2002), while genetically produced AR knockout mice (ARKO) show less masculine sexual behavior (Sato et al., 2004), suggesting that ARs are also involved in male sexual behavior. Tfm male mice, similar to ARKO mice, also display impaired male sexual behavior (Ohno et al., 1974), but this impairment in coital behavior (mounts and thrusts), is not observed after E2 replacement in adulthood (Bodo and Rissman, 2007). This finding indicates that AR activation during development is of little importance for the expression of coital male behavior in mice, or that sufficient ER stimulation can overcome a lack of AR activation.
Masculinization of other sex-related behaviors, such as partner preference in mice, does appear to rely on the presence of a functional AR during development. For example, while wt males prefer females, Tfm males act like wt females in showing no partner preference, even when E2 is equated across groups (Bodo and Rissman, 2007). Tfm males and females also prefer to explore male-soiled bedding, while males prefer exploring female-soiled bedding (Bodo and Rissman, 2007). Expression of the immediate early gene c-Fos in the MPOA and BST was also elevated in Tfm males and females but not in wt males after exposure to male-soiled bedding (Bodo and Rissman, 2007). Together these data suggest that ARs are necessary for the masculinization of at least some aspects of sexual and social behavior in mice. Similarly, people with CAIS do not differ from genetic (XX) females in sexual orientation, are just as likely to be married, and as children were involved in female typical play (Money et al., 1968; Hines et al., 2003).
In rats, defeminization of sexual behavior appears to be AR-independent. Olsen (1979) found that neonatally castrated Tfm male rats, compared to intact Tfm males, showed an increased display of lordosis, indicating that functional ARs are not necessary for the defeminization of this behavior, but rather androgens normally act through another mechanism (presumably ERs) to defeminize lordosis. The role of ARs in the development of masculine sexual behavior (i.e., mounting behavior), however, is not as clear. Tfm male rats have been reported to show variable, but consistently reduced sexual behavior compared to wt males (Beach and Buehler, 1977; Hamson et al., 2005; Olsen and Whalen, 1981; Olsen, 1992; Shapiro et al., 1976). Interestingly, c-Fos expression following a sexual encounter was similar in relevant brain areas (such as the medial preoptic area (MPOA) and medial amygdala (MeA)) of wt and Tfm males that showed comparable levels of sexual behavior (Hamson et al., 2005), suggesting that activation of relevant neural circuitry may be normal in Tfm rats despite the lack of functional ARs.
Diminished sexual behavior in Tfm male rats may actually have little to do with their motivation, since partner preference is masculinized in Tfm male rats (Hamson et al., 2005; unlike in Tfm male mice), and more to do with a female’s disinterest in them. Tfm male rats make fewer ultrasonic vocalizations during sexual encounters compared to wt males, a deficit that may reduce the receptivity of estrous females (Hamson et al., 2006). For Tfm animals in both species, we also cannot discount the influence of having a female genital phenotype on male sexual behavior because, as Frank Beach was fond of saying, “it’s hard to be a good carpenter without a hammer”.
In conclusion, just as Tfm models indicate that AR normally contributes to masculine development of every neural region examined so far, Tfm models also indicate that AR normally contributes to masculine development of a wide range of behaviors. We summarize these findings in Table 2.
Table 2.
Key behavioral findings in Tfm rodents
| Behavioral Findings in Tfm Rodents | |||
|---|---|---|---|
| Behavior (Test) | Species | Key Findings in Adult Rats or Mice | References |
| Spatial Memory | |||
| Morris Water Maze | Rat | Tfm ♂’s show an intermediate spatial memory performance between wt ♂’s and ♀’s, with wt ♂’s displaying superior performance. | Jones and Watson, 2005 |
| Mouse | Tfm ♂’s show decreased spatial memory performance compared to Tfm carrier ♀’s, though there was no sex difference in wt mice. | Rizk et al., 2005 | |
|
| |||
| Anxiety-related behavior | |||
| Novel Object Exposure | Mouse | Tfm ♂’s show greater anxiety-related behavior than wt ♂’s even when treated with T. | Present Study |
| Elevated Plus Maze | Mouse | Tfm mice (Tfm ♂’s and carrier ♀’s) show increased indices of anxiety compared to wt mice (♂’s and ♀’s). | Rizk et al., 2005 |
|
| |||
| Play Fighting | |||
| Juvenile play fighting | Rat | Tfm ♂’s show decreased play fighting compared to wt ♂’s. | Meaney et al, 1983 |
| Rat | Tfm ♂’s fail to reduce the amount of playful attacks or playful defense (complete rotations) with aging, as do wt ♂’s. | Field et al, 2006 | |
|
| |||
| Aggression | |||
| Resident-intruder test | Mouse | Similar indices of aggression in Tfm and wt ♂’s; both more aggressive than ♀’s. | Scordalakes and Rissman, 2004 |
|
| |||
| Social/Sexual Behavior | |||
| Feminine mating behavior | Rat | Tfm ♂’s are similar to wt ♂’s, displaying little or no lordosis response. | Olsen, 1979 |
| Masculine mating behavior | Rat | Tfm ♂’s show reduced masculine sexual behavior compared to wt ♂’s. | Beach and Buehler, 1977; Olsen and Whalen, 1981; Olsen, 1992; Shapiro et al. 1976 |
| Mouse | Tfm ♂’s show reduced masculine sexual behavior compared to wt ♂’s, a difference that is eliminated in E2 treated mice. | Ono et al, 1974; Bodo and Rissman, 2007 | |
| Partner Preference | Rat | Tfm ♂’s show a masculinized partner preference. | Hamson et al, 2005 |
| Mouse | Tfm ♂’s show a demasculinized partner preference even when treated with E2. | Bodo and Rissman, 2007 | |
| Olfactory preference test | Mouse | Tfm ♂’s show a demasculinized preference for female soiled bedding even when treated with E2. | Bodo and Rissman, 2007 |
Structure/function relationships in Tfm rodents
Thus far, little evidence has been generated to suggest a strong correlation between behavior and brain morphology in Tfm males. However, morphological differences have been found in brain areas that have been associated with particular behaviors. One such example includes differences in hippocampal morphology and spatial memory. Studies in Tfm mice and rats suggest that functional ARs are beneficial for optimal spatial memory performance (Jones and Watson, 2005; Rizk et al., 2005), and differences in GCL morphology have been reported in both species (Jones and Watson, 2005; Tabibnia et al., 1999). However, morphological differences are not the same in the two Tfm models and it is difficult to interpret how an increased GCL volume in Tfm male rats, or a lack of GCL laterality in Tfm male mice, could result in spatial memory impairment. It is possible that morphological differences in unexamined hippocampal regions, such as CA1 of Tfm males, contribute to this deficit.
As reviewed above, several brain areas believed to regulate sexual behavior in rodents (MePD, VMH, SDN-POA, BST) are demasculinized in Tfm male rats, and thus may contribute to their reduced sexual behavior (Beach and Buehler, 1977; Shapiro et al., 1976; Hamson et al., 2005). In this instance, it is easier to infer how demasculinization of sex behavior-related circuitry could lead to a demasculinization of behavior. Ultrasonic vocalizations, which are disrupted in Tfm males, appear to rely heavily on androgens acting in the VMH (Harding and McGinnis, 2003; Harding and McGinnis, 2004), so the demasculinization of this nucleus in Tfm males (Dugger et al., 2007) may contribute to this deficit in masculine behavior. Furthermore, along with its role in sexual behavior, the BST is also an important component of the circuitry involved in the regulation of the HPA axis and anxiety-related behavior. Therefore, altered BST morphology in Tfm males could potentially underlie differences in HPA axis physiology and anxiety. Of course, it is also possible that structural changes in the brains of Tfm male rats and mice are unrelated to the differences observed in their behavior.
Conclusion
The Tfm models have provided overwhelming evidence that functional ARs are indeed necessary for the full masculinization of rodent brain and behavior, and studies of CAIS strongly implicate a role of ARs in human behavior as well. Certain rodent brain sexual dimorphisms rely heavily on the presence of functional ARs (MePD, VMH, LC), whereas others do to a lesser extent (SDN-POA), and yet others appear largely unaffected by ARs (septal AVP innervation).
However, there is more to be learned, and there are many holes in the Tfm story. In terms of differences in brain morphology, very little has been examined in the Tfm mouse for at least two reasons: (1) sex differences in the rat brain are better documented, and (2) Tfm male mice also have much lower T levels than wt males in adulthood, making it more difficult to attribute morphological differences to the AR per se. Few studies of Tfm mouse behavior have provided exogenous T to equilibrate this factor as was done for Figure 3. It is also not known when during development T titers begin to decline in Tfm male mice, although, as mentioned earlier, indirect evidence suggests that T levels are normal during the pre- and early postnatal period (Goldstein and Wilson, 1972). One important benefit of the Tfm mouse model is that, unlike in the Tfm rat, the AR is completely nonfunctional, making it easier to detect contributions of ARs. Furthermore, genetic tools and approaches are already available in mice, offering more opportunities to dissect the differing contributions of the AR in various tissues.
For the behaviors discussed in this review, analysis has often been performed in only one of the two models or in some cases experiments were performed using Tfm mice that were not supplemented with hormones in adulthood to equate levels in wt and Tfm males. The strength and generalizabilty of previous findings would benefit from addressing these issues. There are undoubtedly other differences in the brain and behavior of Tfm and wt males that have yet to be discovered, some of which could involve non-neuronal mechanisms. It is possible that AR deficiency could affect the morphology and number of glia such as astrocytes, and in turn influence behavior.
In many ways, the Tfm rodent model can serve as a starting point upon which the role of ARs in brain morphology and behavior can be explored, but it has its limitations. Standard use of these models does not resolve the issue of whether any differences are the result of organizational or activational influences of hormones, since the Tfm defect is present throughout ontogeny. We know that in the MePD, volume and soma size are dependent on adult androgens, so in other cases in which brain morphology differs in Tfm males, the notion that adult AR activation can alter morphology should not be discounted. Another question concerns cellular targets that mediate a particular androgen effect, and this is not readily answered with traditional Tfm models. For example, the cell type(s) (neurons, astroctyes, oligodendrocytes) that are the site of action for ARs to alter morphology or behavior are difficult to determine using this model. The development of conditional knockout models (in which the AR is deleted only in neurons, or only in astrocytes, or in both at particular times in the lifespan) could help answer such questions (see Juntti et al., 2008, in this issue). First, however, it would be beneficial to learn more about the role of global AR deficiency using the Tfm rodent models. Future research will undoubtedly add to what we have already learned from the Tfm models which, simply stated, is that AR does play an important role in the masculinization of rodent brains and behaviors.
Acknowledgments
This work was supported by NIH grant RO1-NS28421, RO1-NS045195 and Predoctoral NRSA Grant F31 MH78273.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almeida OP, Waterreus A, Spry N, Flicker L, Martins RN. One year follow-up study of the association between chemical castration, sex hormones, beta-amyloid, memory and depression in men. Psychoneuroendocrinology. 2004;29:1071–81. doi: 10.1016/j.psyneuen.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Astur RS, Ortiz ML, Sutherland RJ. A characterization of performance by men and women in a virtual Morris water task: A large and reliable sex difference. Behav Brain Res. 1998;93(1–2):185–190. doi: 10.1016/s0166-4328(98)00019-9. [DOI] [PubMed] [Google Scholar]
- Attardi B, Geller LN, Ohno S. Androgen and estrogen receptors in brain cytosol from male, female, and testicular feminized (Tfm/y hermaphrodite) mice. Endocrinology. 1976;98(4):864–74. doi: 10.1210/endo-98-4-864. [DOI] [PubMed] [Google Scholar]
- Ayub M, Levell MJ. Inhibition of rat testicular 17 alpha-hydroxylase and 17,20-lyase activities by anti-androgens (flutamide, hydroxyflutamide, RU23908, cyproterone acetate) in vitro. J Steroid Biochem. 1987;28(1):43–7. doi: 10.1016/0022-4731(87)90122-1. [DOI] [PubMed] [Google Scholar]
- Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, Szpirer C. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci. 2006;9(2):220–6. doi: 10.1038/nn1624. [DOI] [PubMed] [Google Scholar]
- Beach FA, Buehler MG. Male rats with inherited insensitivity to androgen show reduced sexual behavior. Endocrinology. 1977;100(1):197–200. doi: 10.1210/endo-100-1-197. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Dodge AM, Traylor KL, Meaney MJ. Temporal boundary of the sensitive period for hormonal organization of social play in juvenile rats. Physiol Behav. 1981;26(2):241–3. doi: 10.1016/0031-9384(81)90017-2. [DOI] [PubMed] [Google Scholar]
- Bing O, Heilig M, Kakoulidis P, Sundblad C, Wiklund L, Eriksson E. High doses of testosterone increase anticonflict behaviour in rat. European Neuropsychopharmacology. 1998;8:321–3. doi: 10.1016/s0924-977x(97)00095-3. [DOI] [PubMed] [Google Scholar]
- Bloch GJ, Gorski RA. Estrogen/progesterone treatment in adulthood affects the size of several components of the medial preoptic area in the male rat. J Comp Neurol. 1988;275(4):613–22. doi: 10.1002/cne.902750409. [DOI] [PubMed] [Google Scholar]
- Bodo C, Rissman EF. Androgen receptor is essential for sexual differentiation of responses to olfactory cues in mice. Eur J Neurosci. 2007;25(7):2182–90. doi: 10.1111/j.1460-9568.2007.05484.x. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Hormone accumulation in a sexually dimorphic motor nucleus of the rat spinal cord. Science. 1980;210(4469):564–6. doi: 10.1126/science.7423210. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Sexually dimorphic motor nucleus in the rat lumbar spinal cord: response to adult hormone manipulation, absence in androgen-insensitive rats. Brain Res. 1981;225(2):297–307. doi: 10.1016/0006-8993(81)90837-4. [DOI] [PubMed] [Google Scholar]
- Bridges NJ, Starkey NJ. Sex differences in Mongolian gerbils in four tests of anxiety. Physiol Behav. 2004;83(1):119–27. doi: 10.1016/j.physbeh.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Burge KG, Edwards DA. The adrenal gland and the pre and post castrational aggressive behavior of male mice. Physiol Behav. 1971;7(6):885–8. doi: 10.1016/0031-9384(71)90058-8. [DOI] [PubMed] [Google Scholar]
- Charest NJ, Zhou ZX, Lubahn DB, Olsen KL, Wilson EM, French FS. A frameshift mutation destabilizes androgen receptor messenger RNA in the Tfm mouse. Mol Endocrinol. 1991;5(4):573–81. doi: 10.1210/mend-5-4-573. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Breedlove SM, Jordan CL. Both estrogen receptors and androgen receptors contribute to testosterone-induced changes in the morphology of the medial amygdala and sexual arousal in male rats. Horm Behav. 2003;43(2):336–46. doi: 10.1016/s0018-506x(02)00047-8. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Tabibnia G, Breedlove SM. A brain sexual dimorphism controlled by adult circulating androgens. Proc Natl Acad Sci U S A. 1999;96(13):7538–40. doi: 10.1073/pnas.96.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clos V, Esteve A, Jane F, Salva P. Microsomal effects of cyproterone acetate and flutamide in rat testis. Gen Pharmacol. 1988;19(3):393–7. doi: 10.1016/0306-3623(88)90035-3. [DOI] [PubMed] [Google Scholar]
- De Blas MR, Rodriguez-Zafra M, Perez-Laso C, Collado P, Del Abril A, Del Cerro MCR, Segovia S, Guillamon A. Feminization of the rat locus coeruleus: the role of estradiol. Conf Reprod Behav. 1995;27:112. (Abstract) [Google Scholar]
- De Blas MR, Segovia S, Guillamon A. Effect of postpuberal gonadectomy on cell population of the locus coeruleus in the rat. Med Sci Res. 1990;18:355–356. [Google Scholar]
- De Vries GJ, Panzica GC. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: different mechanisms, similar endpoints. Neuroscience. 2006;138(3):947–55. doi: 10.1016/j.neuroscience.2005.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diagnostic and statistical manual of mental disorders, Fourth edition (DSM-IV) Washington, DC: APA Press; 1994. [Google Scholar]
- Dohler KD, Coquelin A, Davis F, Hines M, Shryne JE, Sickmoller PM, Jarzab B, Gorski RA. Pre- and postnatal influence of an estrogen antagonist and an androgen antagonist on differentiation of the sexually dimorphic nucleus of the preoptic area in male and female rats. Neuroendocrinology. 1986;42(5):443–8. doi: 10.1159/000124484. [DOI] [PubMed] [Google Scholar]
- Domínguez-Salazar E, Portillo W, Baum MJ, Bakker J, Paredes RG. Effect of prenatal androgen receptor antagonist or aromatase inhibitor on sexual behavior, partner preference and neuronal Fos responses to estrous female odors in the rat accessory olfactory system. Physiol Behav. 2002;75(3):337–46. doi: 10.1016/s0031-9384(01)00674-6. [DOI] [PubMed] [Google Scholar]
- Drews U. Direct and mediated effects of testosterone: analysis of sex reversed mosaic mice heterozygous for testicular feminization. Cytogenet Cell Genet. 1998;80:68–74. doi: 10.1159/000014959. [DOI] [PubMed] [Google Scholar]
- Dugger BN, Morris JA, Jordan CL, Breedlove SM. Androgen receptors are required for full masculinization of the ventromedial hypothalamus (VMH) in rats. Horm Behav. 2007;51(2):195–201. doi: 10.1016/j.yhbeh.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo A, Morris JA, Breedlove SM, Jordan CL. Effects of the testicular feminization mutation (Tfm) of the androgen receptor gene on BSTMPM volume and morphology in rats. Neurosci Lett. 2007;419(2):168–71. doi: 10.1016/j.neulet.2007.04.033. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Testosterone’s analgesic, anxiolytic, and cognitive-enhancing effects may be due in part to actions of its 5alpha-reduced metabolites in the hippocampus. Behav Neurosci. 2004;118:1352–64. doi: 10.1037/0735-7044.118.6.1352. [DOI] [PubMed] [Google Scholar]
- Field EF, Whishaw IQ, Pellis SM, Watson NV. Play fighting in androgen-insensitive Tfm rats: evidence that androgen receptors are necessary for the development of adult playful attack and defense. Dev Psychobiol. 2006;48(2):111–20. doi: 10.1002/dev.20121. [DOI] [PubMed] [Google Scholar]
- Finney HC, Erpino MJ. Synergistic effect of estradiol benzoate and dihydrotestosterone on aggression in mice. Horm Behav. 1976;7(4):391–400. doi: 10.1016/0018-506x(76)90010-6. [DOI] [PubMed] [Google Scholar]
- Frye CA, Edinger KL. Testosterone’s metabolism in the hippocampus may mediate its anti-anxiety effects in male rats. Pharmacol Biochem Behav. 2004;78:473–81. doi: 10.1016/j.pbb.2004.04.019. [DOI] [PubMed] [Google Scholar]
- Frye CA, Edinger K, Sumida K. Androgen Administration to Aged Male Mice Increases Anti-Anxiety Behavior and Enhances Cognitive Performance. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301498. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Lacey EH. Posttraining androgens’ enhancement of cognitive performance is temporally distinct from androgens’ increases in affective behavior. Cogn Affect Behav Neurosci. 2001;1(2):172–82. doi: 10.3758/cabn.1.2.172. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf CA. Estrogen and/or progesterone administered systemically or to the amygdala can have anxiety-, fear-, and pain-reducing effects in ovariectomized rats. Behav Neurosci. 2004;118(2):306–13. doi: 10.1037/0735-7044.118.2.306. [DOI] [PubMed] [Google Scholar]
- Gandelman R. Gonadal hormones and the induction of intraspecific fighting in mice. Neurosci Biobehav Rev. 1980;4(2):133–40. doi: 10.1016/0149-7634(80)90011-1. [DOI] [PubMed] [Google Scholar]
- Garcia-Falgueras A, Pinos H, Collado P, Pasaro E, Fernandez R, Jordan CL, Segovia S, Guillamon A. The role of the androgen receptor in CNS masculinization. Brain Res. 2005;1035(1):13–23. doi: 10.1016/j.brainres.2004.11.060. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Testosterone and estradiol produce different effects on cognitive performance in male rats. Horm Behav. 2005;48(3):268–77. doi: 10.1016/j.yhbeh.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JL, Wilson JD. Studies on the pathogenesis of the pseudohermaphroditism in the mouse with testicular feminization. J Clin Invest. 1972;51(7):1647–58. doi: 10.1172/JCI106966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski RA, Gordon JH, Shryne JE, Southam AM. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 1978;148(2):333–46. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- Gorski RA, Harlan RE, Jacobson CD, Shryne JE, Southam AM. Evidence for the existence of a sexually dimorphic nucleus in the preoptic area of the rat. J Comp Neurol. 1980;193(2):529–39. doi: 10.1002/cne.901930214. [DOI] [PubMed] [Google Scholar]
- Grumbach MM, Auchus RJ. Estrogen: consequences and implications of human mutations in synthesis and action. J Clin Endocrinol Metab. 1999;84(12):4677–94. doi: 10.1210/jcem.84.12.6290. [DOI] [PubMed] [Google Scholar]
- Guillamon A, de Blas MR, Segovia S. Effects of sex steroids on the development of the locus coeruleus in the rat. Brain Res. 1988a;468(2):306–10. doi: 10.1016/0165-3806(88)90143-5. [DOI] [PubMed] [Google Scholar]
- Guillamon A, Segovia S, del Abril A. Early effects of gonadal steroids on the neuron number in the medial posterior region and the lateral division of the bed nucleus of the stria terminalis in the rat. Brain Res Dev Brain Res. 1988b;44(2):281–90. doi: 10.1016/0165-3806(88)90226-x. [DOI] [PubMed] [Google Scholar]
- Hajszan T, MacLusky NJ, Leranth C. Role of Androgens and the Androgen Receptor in Remodeling of Spine Synapses in Limbic Brain Areas. Horm Behav. 2008 doi: 10.1016/j.yhbeh.2007.12.007. (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamson DK, Csupity AS, Ali FT, Watson NV. Mating behavior and CNS morphology in rats carrying the testicular feminization mutation. Society for Neuroscience. 2005;320.19 (abstract) [Google Scholar]
- Hamson DK, Csupity AS, Watson NV. Patterns of ultrasonic vocalization in androgen-insensitive XY rats compared with normal males and females. Society for Neuroscience. 2006:578.5. (abstract) [Google Scholar]
- Han TM, De Vries GJ. Organizational effects of testosterone, estradiol, and dihydrotestosterone on vasopressin mRNA expression in the bed nucleus of the stria terminalis. J Neurobiol. 2003;54(3):502–10. doi: 10.1002/neu.10157. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O’Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28(4):464–76. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Pak TR, Kudwa AE, Lund TE, Hinds L. An alternate pathway for androgen regulation of brain function: Activation of estrogen receptor beta by the metabolite of dihydrotestosterone, 5α-androstane-3β,17β-diol. Horm Behav. 2008 doi: 10.1016/j.yhbeh.2007.09.012. (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SM, McGinnis MY. Effects of testosterone in the VMN on copulation, partner preference, and vocalizations in male rats. Horm Behav. 2003;43(2):327–35. doi: 10.1016/s0018-506x(02)00049-1. [DOI] [PubMed] [Google Scholar]
- Harding SM, McGinnis MY. Androgen receptor blockade in the MPOA or VMN: effects on male sociosexual behaviors. Physiol Behav. 2004;81(4):671–80. doi: 10.1016/j.physbeh.2004.03.008. [DOI] [PubMed] [Google Scholar]
- He WW, Kumar MV, Tindall DJ. A frame-shift mutation in the androgen receptor gene causes complete androgen insensitivity in the testicular-feminized mouse. Nucleic Acids Res. 1991;19(9):2373–8. doi: 10.1093/nar/19.9.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines M, Ahmed SF, Hughes IA. Psychological outcomes and gender-related development in complete androgen insensitivity syndrome. Arch Sex Behav. 2003;32(2):93–101. doi: 10.1023/a:1022492106974. [DOI] [PubMed] [Google Scholar]
- Hines M, Allen LS, Gorski RA. Sex differences in subregions of the medial nucleus of the amygdala and the bed nucleus of the stria terminalis of the rat. Brain Res. 1992;579:321–326. doi: 10.1016/0006-8993(92)90068-k. [DOI] [PubMed] [Google Scholar]
- Holman SD, Hutchison JB. Differential effects of neonatal castration on the development of sexually dimorphic brain areas in the gerbil. Brain Res Dev Brain Res. 1991;61(1):147–50. doi: 10.1016/0165-3806(91)90125-3. [DOI] [PubMed] [Google Scholar]
- Imperato-McGinley J, Peterson RE, Gautier T, Cooper G, Danner R, Arthur A, Morris PL, Sweeney WJ, Shackleton C. Hormonal evaluation of a large kindred with complete androgen insensitivity: evidence for secondary 5 alpha-reductase deficiency. J Clin Endocrinol Metab. 1982;54(5):931–41. doi: 10.1210/jcem-54-5-931. [DOI] [PubMed] [Google Scholar]
- Imperato-McGinley J, Pichardo M, Gautier T, Voyer D, Bryden MP. Cognitive abilities in androgen-insensitive subjects: Comparison with control males and females from the same kindred. Clin Endocrinol. 1991;34(5):341–347. Imwalle, D.B., Gustafsson, J.A., Rissman, E.F., 2005. Lack of functional estrogen receptor beta influences anxiety behavior and serotonin content in female mice. Physiol. Behav. 84(1):157–63. doi: 10.1111/j.1365-2265.1991.tb00303.x. [DOI] [PubMed] [Google Scholar]
- Imwalle DB, Gustafsson JA, Rissman EF. Lack of functional estrogen receptor beta influences anxiety behavior and serotonin content in female mice. Physiol Behav. 2005;84(1):157–63. doi: 10.1016/j.physbeh.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Isgor C, Sengelaub DR. Prenatal gonadal steroids affect adult spatial behavior, CA1 and CA3 pyramidal cell morphology in rats. Horm Behav. 1998;34(2):183–98. doi: 10.1006/hbeh.1998.1477. [DOI] [PubMed] [Google Scholar]
- Isgor C, Sengelaub DR. Effects of neonatal gonadal steroids on adult CA3 pyramidal neuron dendritic morphology and spatial memory in rats. J Neurobiol. 2003;55(2):179–90. doi: 10.1002/neu.10200. [DOI] [PubMed] [Google Scholar]
- Jonasson Z. Meta-analysis of sex differences in rodent models of learning and memory: A review of behavioral and biological data. Neurosci Biobehav Rev. 2005;28(8):811–825. doi: 10.1016/j.neubiorev.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Jones BA, Watson NV. Spatial memory performance in androgen insensitive male rats. Physiol Behav. 2005;85(2):135–41. doi: 10.1016/j.physbeh.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Jones RD, Pugh PJ, Hall J, Channer KS, Jones TH. Altered circulating hormone levels, endothelial function and vascular reactivity in the testicular feminised mouse. Eur J Endocrinol. 2003;148:111–20. doi: 10.1530/eje.0.1480111. [DOI] [PubMed] [Google Scholar]
- Joseph R, Hess S, Birecree E. Effects of hormone manipulations and exploration on sex differences in maze learning. Behav Biol. 1978;24(3):364–77. doi: 10.1016/s0091-6773(79)90223-2. [DOI] [PubMed] [Google Scholar]
- Juntti SA, Coats JK, Shah NM. A genetic approach to dissect sexually dimorphic behaviours. Horm Behav. 2008 doi: 10.1016/j.yhbeh.2007.12.012. (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51(1):8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Leranth C, Hajszan T, MacLusky NJ. Androgens increase spine synapse density in the CA1 hippocampal subfield of ovariectomized female rats. J Neurosci. 2004;24(2):495–9. doi: 10.1523/JNEUROSCI.4516-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J Neurosci. 2003;23(5):1588–92. doi: 10.1523/JNEUROSCI.23-05-01588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisciotto CA, Morrell JI. Sex differences in the distribution and projections of testosterone target neurons in the medial preoptic area and the bed nucleus of the stria terminalis of rats. Horm Behav. 1994;28:492–502. doi: 10.1006/hbeh.1994.1047. [DOI] [PubMed] [Google Scholar]
- Lund TD, Hinds LR, Handa RJ. The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. J Neurosci. 2006;26(5):1448–56. doi: 10.1523/JNEUROSCI.3777-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund TD, Munson DJ, Haldy ME, Handa RJ. Dihydrotestosterone may inhibit hypothalamo-pituitary-adrenal activity by acting through estrogen receptor in the male mouse. Neurosci Lett. 2004;365(1):43–7. doi: 10.1016/j.neulet.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor-beta on anxiety-related behaviors. Endocrinology. 2005;146:797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- Luttge WG, Hall NR. Androgen-induced agonistic behavior in castrate male Swiss-Webster mice: comparison of four naturally occurring androgens. Behav Biol. 1973;8(6):725–32. doi: 10.1016/s0091-6773(73)80114-2. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Hajszan T, Johansen JA, Jordan CL, Leranth C. Androgen effects on hippocampal CA1 spine synapse numbers are retained in Tfm male rats with defective androgen receptors. Endocrinology. 2006;147(5):2392–8. doi: 10.1210/en.2005-0673. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Hajszan T, Leranth C. Effects of dehydroepiandrosterone and flutamide on hippocampal CA1 spine synapse density in male and female rats: implications for the role of androgens in maintenance of hippocampal structure. Endocrinology. 2004;145(9):4154–61. doi: 10.1210/en.2004-0477. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Luine VN, Hajszan T, Leranth C. The 17alpha and 17beta isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005;146(1):287–93. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- Madeira MD, Ferreira-Silva L, Paula-Barbosa MM. Influence of sex and estrus cycle on the sexual dimorphisms of the hypothalamic ventromedial nucleus: stereological evaluation and Golgi study. J Comp Neurol. 2001;432(3):329–45. doi: 10.1002/cne.1106. [DOI] [PubMed] [Google Scholar]
- Madeira MD, Sousa N, Santer RM, Paula-Barbosa MM, Gundersen HJ. Age and sex do not affect the volume, cell numbers, or cell size of the suprachiasmatic nucleus of the rat: an unbiased stereological study. J Comp Neurol. 1995;361(4):585–601. doi: 10.1002/cne.903610404. [DOI] [PubMed] [Google Scholar]
- Malsbury CW, McKay K. Neurotrophic effects of testosterone on the medial nucleus of the amygdala in adult male rats. J Neuroendocrinol. 1994;6(1):57–69. doi: 10.1111/j.1365-2826.1994.tb00555.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y. Sex difference in volume of the ventromedial nucleus of the hypothalamus in the rat. Endocrinol Jpn. 1983;30(3):277–80. doi: 10.1507/endocrj1954.30.277. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Honda S, Harada N. Alteration in sex-specific behaviors in male mice lacking the aromatase gene. Neuroendocrinology. 2003;77(6):416–24. doi: 10.1159/000071313. [DOI] [PubMed] [Google Scholar]
- Matochik JA, Sipos ML, Nyby JG, Barfield RJ. Intracranial androgenic activation of male-typical behaviors in house mice: motivation versus performance. Behav Brain Res. 1994;60(2):141–9. doi: 10.1016/0166-4328(94)90141-4. [DOI] [PubMed] [Google Scholar]
- McAbee MD, Doncarlos LL. Estrogen, but not androgens, regulates androgen receptor messenger ribonucleic acid expression in the developing male rat forebrain. Endocrinology. 1999a;140(8):3674–81. doi: 10.1210/endo.140.8.6901. [DOI] [PubMed] [Google Scholar]
- McAbee MD, Doncarlos LL. Regulation of androgen receptor messenger ribonucleic acid expression in the developing rat forebrain. Endocrinology. 1999b;140(4):1807–14. doi: 10.1210/endo.140.4.6632. [DOI] [PubMed] [Google Scholar]
- McClellan KM, Parker KL, Tobet S. Development of the ventromedial nucleus of the hypothalamus. Front Neuroendocrinol. 2006;27(2):193–209. doi: 10.1016/j.yfrne.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. The sexual differentiation of social play. Trends Neurosci. 1988;11(2):54–8. doi: 10.1016/0166-2236(88)90164-6. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Stewart J, Poulin P, McEwen BS. Sexual differentiation of social play in rat pups is mediated by the neonatal androgen-receptor system. Neuroendocrinology. 1983;37(2):85–90. doi: 10.1159/000123524. [DOI] [PubMed] [Google Scholar]
- Money J, Ehrhardt AA, Masica DN. Fetal feminization induced by androgen insensitivity in the testicular feminizing syndrome: effect on marriage and maternalism. Johns Hopkins Med J. 1968;123(3):105–14. [PubMed] [Google Scholar]
- Monks DA, Johansen JA, Mo K, Rao P, Eagleson B, Yu Z, Lieberman AP, Breedlove SM, Jordan CL. Overexpression of wild-type androgen receptor in muscle recapitulates polyglutamine disease. Proc Natl Acad Sci U S A. 2007;104(46):18259–64. doi: 10.1073/pnas.0705501104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat Neurosci. 2004;7(10):1034–9. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Partial demasculinization of several brain regions in adult male (XY) rats with a dysfunctional androgen receptor gene. J Comp Neurol. 2005;487(2):217–26. doi: 10.1002/cne.20558. [DOI] [PubMed] [Google Scholar]
- Naftolin F, Ryan KJ, Davies IJ, Petro Z, Kuhn M. The formation and metabolism of estrogens in brain tissues. Adv Biosci. 1975;15:105–21. [PubMed] [Google Scholar]
- Naghdi N, Nafisy N, Majlessi N. The effects of intrahippocampal testosterone and flutamide on spatial localization in the Morris water maze. Brain Res. 2001;897(1–2):44–51. doi: 10.1016/s0006-8993(00)03261-3. [DOI] [PubMed] [Google Scholar]
- Nomura M, Durbak L, Chan J, Smithies O, Gustafsson JA, Korach KS, Pfaff DW, Ogawa S. Genotype/age interactions on aggressive behavior in gonadally intact estrogen receptor beta knockout (betaERKO) male mice. Horm Behav. 2002;41(3):288–96. doi: 10.1006/hbeh.2002.1773. [DOI] [PubMed] [Google Scholar]
- Nunez JL, Koss WA, Juraska JM. Hippocampal anatomy and water maze performance are affected by neonatal cryoanesthesia in rats of both sexes. Horm Behav. 2000;37(3):169–78. doi: 10.1006/hbeh.2000.1572. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Chester AE, Hewitt SC, Walker VR, Gustafsson JA, Smithies O, Korach KS, Pfaff DW. Abolition of male sexual behaviors in mice lacking estrogen receptors alpha and beta (alpha beta ERKO) Proc Natl Acad Sci U S A. 2000;97(26):14737–41. doi: 10.1073/pnas.250473597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Lubahn DB, Korach KS, Pfaff DW. Behavioral effects of estrogen receptor gene disruption in male mice. Proc Natl Acad Sci U S A. 1997;94(4):1476–81. doi: 10.1073/pnas.94.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S, Geller LN, Lai EV. Tfm mutation and masculinization versus feminization of the mouse central nervous system. Cell. 1974;3(3):235–42. doi: 10.1016/0092-8674(74)90137-8. [DOI] [PubMed] [Google Scholar]
- Olioff M, Stewart J. Sex differences in the play behavior of prepubescent rats. Physiol Behav. 1978;20(2):113–5. doi: 10.1016/0031-9384(78)90060-4. [DOI] [PubMed] [Google Scholar]
- Olsen KL. Androgen-insensitive rats are defeminised by their testes. Nature. 1979;279(5710):238–9. doi: 10.1038/279238a0. [DOI] [PubMed] [Google Scholar]
- Olsen KL. Genetic influences on sexual behavior differentiation. In: Gerall A, Moltz H, Ward IL, editors. Sexual Differentiation: a Life-Span Approach, Handbook of Behavioral Neurobiology. Plenum Press; New York: 1992. pp. 1–40. [Google Scholar]
- Olsen KL, Whalen RE. Hormonal control of the development of sexual behavior in androgen-insensitive (Tfm) rats. Physiol Behav. 1981;27(5):883–6. doi: 10.1016/0031-9384(81)90058-5. [DOI] [PubMed] [Google Scholar]
- Olsen KL, Whalen RE. Estrogen binds to hypothalamic nuclei of androgen-insensitive (Tfm) rats. Experientia. 1982;38(1):139–40. doi: 10.1007/BF01944575. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pellis VC. Differential rates of attack, defense, and counterattack during the developmental decrease in play fighting by male and female rats. Dev Psychobiol. 1990;23(3):215–31. doi: 10.1002/dev.420230303. [DOI] [PubMed] [Google Scholar]
- Puts DA, Gaulin SJ, Breedlove SM. Sex differences in spatial ability: Evolution, hormones and the brain. In: Platek SM, Keenan JP, Shackelford TK, editors. Evolutionary Cognitive Neuroscience. MIT Press; Cambridge, Massachusetts: 2007. pp. 329–379. [Google Scholar]
- Puts DA, Jordan CL, Breedlove SM. Defending the brain from estrogen. Nat Neurosci. 2006;9(2):155–6. doi: 10.1038/nn0206-155. [DOI] [PubMed] [Google Scholar]
- Puts DA, McDaniel MA, Jordan CL, Breedlove SM. Spatial ability and prenatal androgens: Meta-analyses of congenital adrenal hyperplasia and digit ratio (2D:4D) studies. Archives of Sexual Behavior (Special Issue) doi: 10.1007/s10508-007-9271-3. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizk A, Robertson J, Raber J. Behavioral performance of Tfm mice supports the beneficial role of androgen receptors in spatial learning and memory. Brain Res. 2005;1034(1–2):132–8. doi: 10.1016/j.brainres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Rizk-Jackson AM, Acevedo SF, Inman D, Howieson D, Benice TS, Raber J. Effects of sex on object recognition and spatial navigation in humans. Behav Brain Res. 2006;173(2):181–90. doi: 10.1016/j.bbr.2006.06.029. [DOI] [PubMed] [Google Scholar]
- Robinson SM, Fox TO, Dikkes P, Pearlstein RA. Sex differences in the shape of the sexually dimorphic nucleus of the preoptic area and suprachiasmatic nucleus of the rat: 3-D computer reconstructions and morphometrics. Brain Res. 1986;371(2):380–4. doi: 10.1016/0006-8993(86)90380-x. [DOI] [PubMed] [Google Scholar]
- Roof RL. Neonatal exogenous testosterone modifies sex difference in radial arm and Morris water maze performance in prepubescent and adult rats. Behav Brain Res. 1993;53(1–2):1–10. doi: 10.1016/s0166-4328(05)80261-x. [DOI] [PubMed] [Google Scholar]
- Roselli CE. Sex-differences in androgen receptors and aromatase-activity in microdissected regions of the rat-brain. Endocrinology. 1991;128:1310–1316. doi: 10.1210/endo-128-3-1310. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Salisbury RL, Resko JA. Genetic evidence for androgen-dependent and independent control of aromatase activity in the rat brain. Endocrinology. 1987;121:2205–10. doi: 10.1210/endo-121-6-2205. [DOI] [PubMed] [Google Scholar]
- Rosenfeld JM, Daley JD, Ohno S, YoungLai EV. Central aromatization of testosterone in testicular feminized mice. Experientia. 1977;33(10):1392–3. doi: 10.1007/BF01920200. [DOI] [PubMed] [Google Scholar]
- Salomé N, Salchner P, Viltart O, Sequeira H, Wigger A, Landgraf R, Singewald N. Neurobiological correlates of high (HAB) versus low anxiety-related behavior (LAB): differential Fos expression in HAB and LAB rats. Biol Psychiatry. 2004;55(7):715–23. doi: 10.1016/j.biopsych.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Kim JH, Wasserman MA. Testosterone modulates performance on a spatial working memory task in male rats. Horm Behav. 2006;50(1):18–26. doi: 10.1016/j.yhbeh.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Sato T, Matsumoto T, Kawano H, Watanabe T, Uematsu Y, Sekine K, Fukuda T, Aihara K, Krust A, Yamada T, Nakamichi Y, Yamamoto Y, Nakamura T, Yoshimura K, Yoshizawa T, Metzger D, Chambon P, Kato S. Brain masculinization requires androgen receptor function. Proc Natl Acad Sci U S A. 2004;101(6):1673–8. doi: 10.1073/pnas.0305303101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scordalakes EM, Rissman EF. Aggression and arginine vasopressin immunoreactivity regulation by androgen receptor and estrogen receptor alpha. Genes Brain Behav. 2004;3(1):20–6. doi: 10.1111/j.1601-183x.2004.00036.x. [DOI] [PubMed] [Google Scholar]
- Sengelaub DR, Forger NG. The spinal nucleus of the bulbocavernosus: Firsts in androgen-dependent neural sex differences. Horm Behav. 2008 doi: 10.1016/j.yhbeh.2007.11.008. (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro BH, Goldman AS, Steinbeck HF, Neumann F. Is feminine differentiation of the brain hormonally determined? Experientia. 1976;32(5):650–1. doi: 10.1007/BF01990214. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Fliers E, Fisser B. The vasopressin containing neurons in the human brain; changes during ageing and senile dementia. Br J Clin Pract Suppl. 1985;39:7–10. [PubMed] [Google Scholar]
- Swaab DF, Hofman MA. An enlarged suprachiasmatic nucleus in homosexual men. Brain Res. 1990;537(1–2):141–8. doi: 10.1016/0006-8993(90)90350-k. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Zhou JN, Ehlhart T, Hofman MA. Development of vasoactive intestinal polypeptide neurons in the human suprachiasmatic nucleus in relation to birth and sex. Brain Res Dev Brain Res. 1994;79(2):249–59. doi: 10.1016/0165-3806(94)90129-5. [DOI] [PubMed] [Google Scholar]
- Swartz SK, Soloff MS. The lack of estrogen binding by human alpha fetoprotein. J Clin Endocrinol Metab. 1974;39:589–591. doi: 10.1210/jcem-39-3-589. [DOI] [PubMed] [Google Scholar]
- Tabibnia G, Cooke BM, Breedlove SM. Sex difference and laterality in the volume of mouse dentate gyrus granule cell layer. Brain Res. 1999;827(1–2):41–5. doi: 10.1016/s0006-8993(99)01262-7. [DOI] [PubMed] [Google Scholar]
- Thor DH, Holloway WR., Jr Social play in juvenile rats: a decade of methodological and experimental research. Neurosci Biobehav Rev. 1984;8(4):455–64. doi: 10.1016/0149-7634(84)90004-6. [DOI] [PubMed] [Google Scholar]
- Vague J. Testicular feminization syndrome. An experimental model for the study of hormone action on sexual behavior. Horm Res. 1983;18:62–8. doi: 10.1159/000179779. [DOI] [PubMed] [Google Scholar]
- Vreeburg JT, van der Vaart PD, van der Schoot P. Prevention of central defeminization but not masculinization in male rats by inhibition neonatally of oestrogen biosynthesis. J Endocrinol. 1977;74(3):375–82. doi: 10.1677/joe.0.0740375. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Antianxiety and antidepressive behavior produced by physiological estradiol regimen may be modulated by hypothalamic-pituitary-adrenal axis activity. Neuropsychopharmacology. 2005;30(7):1288–301. doi: 10.1038/sj.npp.1300708. [DOI] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem. 2006;86(1):35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Bullock NA, De Vries GJ. Sexual differentiation of vasopressin projections of the bed nucleus of the stria terminals and medial amygdaloid nucleus in rats. Endocrinology. 1993;132(6):2299–306. doi: 10.1210/endo.132.6.8504734. [DOI] [PubMed] [Google Scholar]
- Weinstock LS. Gender differences in the presentation and management of social anxiety disorder. J Clin Psychiatry. 1999;60:9–13. [PubMed] [Google Scholar]
- Williams CL, Barnett AM, Meck WH. Organizational effects of early gonadal secretions on sexual differentiation in spatial memory. Behav Neurosci. 1990;104(1):84–97. doi: 10.1037//0735-7044.104.1.84. [DOI] [PubMed] [Google Scholar]
- Williams CL, Meck WH. The organizational effects of gonadal steroids on sexually dimorphic spatial ability. Psychoneuroendocrinology. 1991;16(1–3):155–176. doi: 10.1016/0306-4530(91)90076-6. [DOI] [PubMed] [Google Scholar]
- Wimer CC, Wimer RE. On the sources of strain and sex differences in granule cell number in the dentate area of house mice. Dev Brain Res. 1989;48:167–176. doi: 10.1016/0165-3806(89)90073-4. [DOI] [PubMed] [Google Scholar]
- Wimer RE, Wimer CC. The sex dimorphisms in the granule cell layer of the hippocampus in house mice. Brain Research. 1984;328:105–109. doi: 10.1016/0006-8993(85)91328-9. [DOI] [PubMed] [Google Scholar]
- Wimer RE, Wimer CC, Alameddine LA. On the development of strain and sex differences in granule cell number in the area dentata in house mice. Dev Brain Res. 1988;42:191–197. doi: 10.1016/0165-3806(88)90237-4. [DOI] [PubMed] [Google Scholar]
- Yarbrough WG, Quarmby VE, Simental JA, Joseph DR, Sar M, Lubahn DB, Olsen KL, French FS, Wilson EM. A single base mutation in the androgen receptor gene causes androgen insensitivity in the testicular feminized rat. J Biol Chem. 1990;265(15):8893–900. [PubMed] [Google Scholar]
- Yazici K, Pata O, Yazici A, Aktas A, Tot S, Kanik A. The effects of hormone replacement therapy in menopause on symptoms of anxiety and depression. Turkish Journal of Psychiatry. 2003;14:101–5. [PubMed] [Google Scholar]


