Abstract
Cleidocranial dysplasia (CCD), an autosomal-dominant human bone disease, is thought to be caused by heterozygous mutations in runt-related gene 2 (RUNX2)/polyomavirus enhancer binding protein 2αA (PEBP2αA)/core-binding factor A1 (CBFA1). To understand the mechanism underlying the pathogenesis of CCD, we studied a novel mutant of RUNX2, CCDαA376, originally identified in a CCD patient. The nonsense mutation, which resulted in a truncated RUNX2 protein, severely impaired RUNX2 transactivation activity. We show that signal transducers of transforming growth factor β superfamily receptors, Smads, interact with RUNX2 in vivo and in vitro and enhance the transactivation ability of this factor. The truncated RUNX2 protein failed to interact with and respond to Smads and was unable to induce the osteoblast-like phenotype in C2C12 myoblasts on stimulation by bone morphogenetic protein. Therefore, the pathogenesis of CCD may be related to the impaired Smad signaling of transforming growth factor β/bone morphogenetic protein pathways that target the activity of RUNX2 during bone formation.
Polyomavirus enhancer binding protein 2 (PEBP2; ref. 1), corresponding to murine leukemia virus enhancer core-binding factor (CBF; ref. 2), is a heterodimeric transcription factor composed of α and β subunits (3). runt-related gene 2 (RUNX2), also referred to as PEBP2αA/CBFA1 (4, 5), encodes one of the three distinct mammalian α subunits of PEBP2/CBF. The other two subunits are RUNX1/PEBP2αB/CBFA2/AML1 (6–8) and RUNX3/PEBP2αC/CBFA3/AML2 (9–11). The α subunit harbors the DNA binding ability in an evolutionally conserved 128-aa region termed the Runt domain (12), which shares high homology with the products of the Drosophila genes runt (13) and lozenge (14). The Runt domain also is responsible for dimerization of the α subunit with the β subunit (12). RUNX2 is essential for osteogenesis (15), and targeted disruption of Runx2 in mice revealed the absence of mature osteoblasts and a complete lack of bone formation (16, 17).
RUNX2 was mapped to chromosome 6p21 (5, 10, 18), which also was found to be the genetic locus for cleidocranial dysplasia (CCD) syndrome (19). CCD is an autosomal-dominant human bone disease characterized by hypoplastic clavicles, patent fontanelles and sutures, and other multiple skeletal disorders (20). Previously, a γ radiation-induced mouse mutant, which showed a phenotype similar to that of CCD, was described (21). This mouse Ccd mutation was mapped to a region distal to that of H2 of chromosome 17 (22), where we previously mapped mouse homologue RUNX2 (8), and was found to result in a deletion of one of the alleles of Runx2 (17). Recently, RUNX2 was found to be heterozygously mutated in several CCD patients (18, 23). All these observations overwhelmingly suggest that the disease is caused by heteroinsufficiency of RUNX2 (15). However, the molecular mechanism underlying the pathogenesis of CCD in patients with heterozygous mutations in RUNX2 is poorly understood.
In this paper, we study a nonsense mutation, previously identified in a CCD patient, that is located downstream of the Runt domain (24). The truncated RUNX2 protein failed to interact with Smads (25–28), indicating that the pathogenesis of CCD may be related to the defect in responsiveness of RUNX2 to transforming growth factor β (TGF-β) superfamily signaling during bone formation.
Materials and Methods
Plasmids.
The human RUNX2 cDNA encoding the entire region from exon 1 to exon 7 was generated by combining the partial cDNA we isolated previously (5) with the reverse transcription–PCR fragments amplified from exon 1 and exon 6 under standard conditions (29). The mammalian expression plasmid pEF-αA was constructed by inserting the PEBP2αA cDNA into the BstXI site of pEF-Bos (30). To construct pEF-CCDαA376, the ApaI–EcoRI fragment in pEF-αA was replaced with the corresponding mutant fragment derived from the case 3 CCD patient. To make pEF-αA(88-507), the PvuII–ClaI fragment derived from the PEBP2αA cDNA was inserted into the BstXI site of the pEF-Bos vector after blunting. pEF-αA(1-424), pEF-αA(1-388), pEF-αA(1-340), pEF-αA(1-280), pEF-αA(1-221), and pEF-αA(1-214) were constructed by replacing the KpnI-BamHI fragment of pEF-αA with PCR-amplified fragments containing an artificial termination codon at the end of the indicated amino acid numbers in parentheses. pEF-β2 has been described previously (4). To construct pEF-BOSneo-αA and pEF-BOSneo-CCDαA376, the pEF-BOS backbones of pEF-αA and pEF-CCDαA376, respectively, were replaced with the pEF-BOSneo vector by using the compatible XbaI sites. To prepare the series of plasmids of RUNX2/PEBP2αA for in vitro translation, the pEF-Bos backbone of pEF-αA and its deletion constructs were replaced with the pBluescript II KS(−) vector, resulting in pKS-αA and its deletion mutants. To prepare GAL4-fusion expression plasmids, the corresponding regions of PEBP2αA (4) and Til-1 (31) were PCR-amplified and cloned into the XbaI–BamHI sites of pEF-GAL4-DBD (32). The wild-type and the mutant 1050.rOC-luc were described (33, 34). The reporter tk-GALpx3-luc was described previously (32). The expression plasmids for Flag-tagged Smad1, Smad3, Smad4 and TβR-I, and BMPR-IA have been described previously (35, 36). The luciferase reporter plasmids containing the germ line Ig Cα promoter, Cα(WT), and its mutant forms, and the expression plasmid for glutathione S-transferase (GST)-Smad3 were described elsewhere (37).
Luciferase Assay.
NIH 3T3 cells were maintained in DMEM supplemented with 10% (vol/vol) FBS. Postnatal day (P19) embryonic carcinoma cells were cultured in DMEM/F-12 medium (GIBCO) supplemented with 10% (vol/vol) FBS. For the luciferase assay, NIH 3T3 or P19 cells seeded into six-well plates were transfected by using the FuGENE 6 reagent (Boehringer Mannheim). As an internal control, 1 ng of pEF-RL luciferase reporter also was included. Cells were harvested 24 h after transfection, and luciferase activities were determined by the Dual-Luciferase Reporter Assay System (Promega). Firefly luciferase activities were normalized by renilla luciferase activities. All transfection experiments were done at least three times.
Immunoprecipitation and Western Blotting.
COS-7 cells were transiently transfected with expression plasmids by FuGENE 6. Cell extracts were prepared 48 h after transfection (36). Immunoprecipitation and Western blotting were performed as described (36). The antibodies used were anti-αA1N and anti-αA1C antisera (38), anti-αA8G5 monoclonal antibody raised against an Escherichia coli-produced recombinant PEBP2αA protein, anti-FLAG M2 antibody (Sigma), anti-Smad1 antibody (Santa Cruz Biotechnology), and anti-HA antibody (Santa Cruz Biotechnology).
Isolation of C2C12 Cells Stably Expressing αA or CCDαA376.
C2C12 cells were maintained in DMEM supplemented with 15% (vol/vol) FBS and transfected with the pEF-BOSneo alone or with pEF-BOSneo-αA or pEF-BOSneo-CCDαA376 by using FuGENE 6 reagent. Forty-eight hours after transfection, cells were subjected to G418 selection (500 μg/ml Geneticin; GIBCO) for 2 weeks and individual G418-resistant clones were picked up for further analysis.
Preparation of Recombinant Adenoviruses.
Recombinant adenoviruses carrying the cDNAs for Smad1, Smad4, and β-galactosidase, respectively, were prepared as described elsewhere (39). Infection with recombinant adenoviruses was carried out at a multiplicity of infection of 2 × 102 plaque-forming units per cell.
Bone Morphogenetic Protein (BMP).
Recombinant human BMP-7 was a gift from T. K. Sampath (Creative Biomolecules, Hopkinton, MA).
Results and Discussion
Impaired Transactivation Activity of CCDαA376.
Previously, we screened Japanese CCD patients for mutations in RUNX2 and identified five different types of heterozygous mutation in unrelated individuals (24). Mutation three, found in the case 3 patient (24), was unique and resulted in a truncated product that lacked the C-terminal 130 amino acids but left the Runt domain intact (to be referred to as CCDαA376). Here, we describe the characterization of the CCDαA376 mutant.
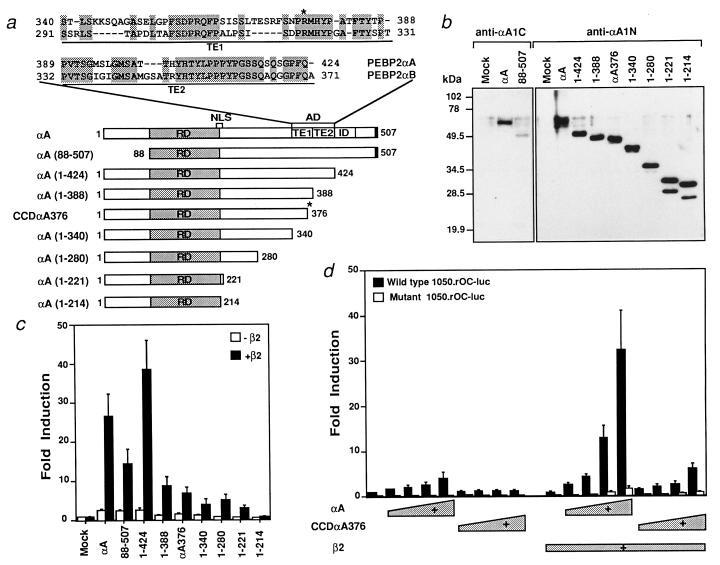
Because the DNA binding activity of this mutant was normal (data not shown), we investigated its transactivation function. Based on the high amino acid sequence similarity between RUNX1 and RUNX2, we predicted that the transactivation domain (AD) of RUNX2 lies between amino acids 340 and 424 as shown in Fig. 1a (32). A series of deletion constructs were made, and their transactivation activity was analyzed by using the rat osteocalcin promoter. In this assay, RUNX2 strongly activated reporter activity (Fig. 1 b and c), which depended on the presence of the intact PEBP2 sites (Fig. 1d). A major AD was identified between amino acids 388 and 424 of RUNX2 and corresponds to the TE2 subdomain of the AD identified in RUNX1 (32).
Figure 1.
The CCDαA376 mutant lacks transactivating ability. (a) Schematic illustration of RUNX2 (shown as αA), its truncated constructs, and the CCDαA376 mutant. The amino acid sequence comparison between ADs of PEBP2αA/RUNX2 and PEBP2αB/RUNX1 and the position of the premature stop codon in CCDαA376 (marked by an asterisk) are also shown. NLS, nuclear localization signal. (b) The protein expression pattern of RUNX2 and its deletion constructs in COS-7 cells detected by anti-αA1N and anti-αA1C antibodies. (c) Transactivation activities of RUNX2 and its deletion mutants. NIH 3T3 fibroblasts were transfected with the wild-type 1050.rOC-luc (0.5 μg) and the indicated polypeptide chain elongation factor 1α promoter (EF-BOS)-based expression plasmids (0.5 μg) with or without pEF-β2 (0.2 μg). (d) Comparison of the transactivation activities of RUNX2 and CCDαA376. Increasing amounts (0.1, 0.2, 0.4, and 0.8 μg) of pEF-αA or pEF-CCDαA376 in the absence or presence of pEF-β2 (0.2 μg) were cotransfected into NIH 3T3 cells with the wild-type 1050.rOC-luc or the mutant 1050.rOC-luc, in which all three PEBP2 binding sites were mutated.
The mutant, CCDαA376, lacking a part of the TE1 and the entire TE2 subdomain, did not show significant transactivation activity (Fig. 1 c and d), suggesting that this defect might be responsible for CCD in this patient.
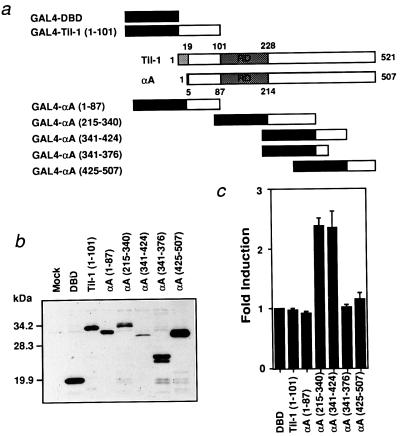
To confirm that the region between amino acids 340 and 424 of RUNX2 represents the AD, the region was fused to the GAL4 DNA binding domain (GAL4-DBD; Fig. 2a) and GAL4 binding-site-dependent transactivation was examined. As shown in Fig. 2 b and c, AD and the region between the Runt domain and AD (215-340) fused to GAL4-DBD displayed transactivation activity. The latter probably corresponds to the TE3 element described previously for RUNX1 (32). We did not examine the TE3 activity further in this study, because the activity is shared by RUNX2 and CCDαA376 and not present at a significant level in a natural form of RUNX2 (Fig. 1c). The RUNX2 gene has a distal and a proximal promoter, which leads to synthesis of two isoforms, Til-1 (31) and PEBP2αA (4), with different N-terminal regions (Fig. 2a; refs. 23 and 31). Neither of the N-terminal fragments fused to GAL4-DBD exhibited transactivation activity (Fig. 2c).
Figure 2.
The region αA(341-424) confers transactivation activity to GAL4-DBD. (a) Schematic illustration of GAL4-DBD-αA fusion constructs. The structures of Til-1 and PEBP2αA also are shown. (b) Expression of GAL4-DBD-αA fusion constructs in COS-7 cells detected by anti-GAL4-DBD antibody. (c) NIH 3T3 cells were transfected with tk-GALpx3-luc (0.4 μg) and the indicated effectors (0.2 μg).
Lack of Interaction of CCDαA376 with Smads.
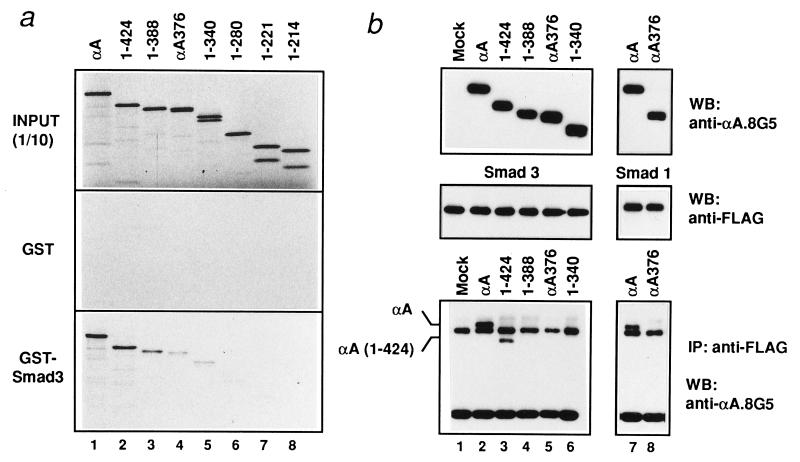
TGF-β and BMP play critical roles in osteogenesis (40, 41). Key mediators of TGF-β/BMP signaling are Smads (25–28). RUNX2, as well as RUNX1 and RUNX3, physically associate with Smad1, -2, -3, and -5 in vivo, suggesting that PEBP2/CBF is one of the major targets of TGF-β/BMP signaling (ref. 37 and also see below). Therefore, the effect of the mutation in CCDαA376 on RUNX2 association with Smads was studied. In GST–pull-down assays (Fig. 3a), the interaction of RUNX2 with Smad3 was significantly reduced when the C-terminal 119 amino acids of RUNX2 were deleted [αA(1-388)] and further reduced when CCDαA376 was used. The interaction became undetectable when the region surrounding the AD was completely removed [αA(1-221)]. The interaction of RUNX2 with Smad3 also was examined by immunoprecipitation followed by Western blotting with similar results, i.e., the interaction became undetectable when αA(1-388) or shorter constructs were used (Fig. 3b). In accordance with this result, the mutant CCDαA376 hardly interacted with Smad3 in vivo (Fig. 3b). Essentially the same result was obtained with Smad1 (Fig. 3b), Smad2, and Smad5 (data not shown). Therefore, there exists an apparent correlation between the transactivation activity of RUNX2 and the ability of RUNX2 to interact with Smads. Slight differences in the endpoint obtained by the two assays were most likely because of the difference in sensitivity between the two assays.
Figure 3.
Failure of CCDαA376 to interact with Smads. (a) The physical interaction between RUNX2 and Smad3 as examined by GST–pull-down assay (43). In vitro-translated [35S]methionine-labeled RUNX2 and its deletion mutants were incubated with GST or GST-Smad3 conjugated to glutathione Sepharose beads. The coprecipitated samples were separated by SDS/12% PAGE. INPUT (1/10), 10% of in vitro-translated products used for binding assay was loaded. (b) In vivo interaction. COS-7 cells were cotransfected with expression plasmids coding for Flag-tagged Smad3 (for lanes 1–6) or Flag-tagged Smad1 (for lanes 7 and 8), a constitutively active form of type I TGF-β (TβR-I; for lanes 1–6) or BMP receptor (BMPR-IA; for lanes 7 and 8), and RUNX2 mutants, the structures of which are shown in Fig. 2a. RUNX2 proteins were immunoprecipitated from cell lysates with anti-Flag antibody followed by immunoblotting (WB) using anti-αA8G5 antibody. (Bottom) The coimmunoprecipitation (IP) of RUNX2 and αA(1-424) with Smads. (Top and Middle) Expression levels of individual proteins are shown.
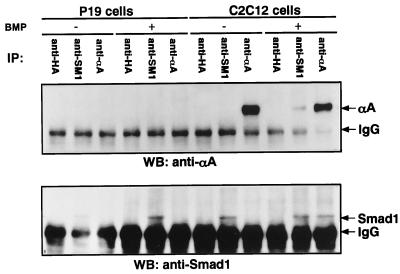
We then examined whether endogenously expressed RUNX2 and Smads would interact. P19 embryonal carcinoma cells did not express RUNX2 at an appreciable level (Fig. 4 Upper, lanes 3 and 6), whereas they expressed Smad1 (Fig. 4 Lower, lanes 2 and 5). On the contrary, C2C12 myoblasts expressed both proteins. When C2C12 cell extracts were subjected to immunoprecipitation with RUNX2- or Smad1-specific antibodies, no coprecipitation of either protein was observed (Fig. 4, lanes 8 and 9). Interestingly, endogenous RUNX2 and Smad1 coprecipitated Smad1 (Fig. 4 Lower, lane 12) and RUNX2 (Fig. 4 Upper, lane11), respectively, after the cells were treated for one hour with BMP. The ligand-dependent interaction between RUNX2 and Smad1 suggests that the RUNX2/Smad1 interaction is likely to represent one of the physiological pathways through which signals from BMP receptors are transmitted to target genes.
Figure 4.
Ligand-dependent interaction between endogenous RUNX2 and Smad1. Two 15-cm plates each of P19 or C2C12 cells (1 × 108) were prepared. One plate each was treated with BMP-7 for 1 h and the other was mock-treated. Immunoprecipitation from 10% of the cell extracts each was performed by using either polyclonal anti-αA or polyclonal anti-Smad1. Western blotting of the immunopresipitates was performed by using monoclonal antibodies specific to RUNX2 (Upper) or Smad1 (Lower). The positions of RUNX2 (shown as αA) and Smad 1 are indicated. Nonspecific bands representing IgG also are indicated.
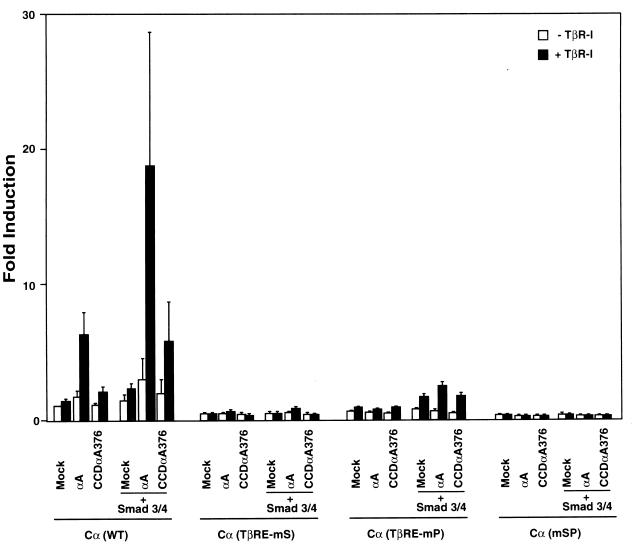
The functional relationship between PEBP2 and Smads has been successfully studied by using the germ line Ig Cα promoter, because the TGF-β response element in this promoter consists only of PEBP2 and Smad binding sites (37). The Cα promoter was cooperatively stimulated by RUNX2 and Smads in a TGF-β-dependent manner (Fig. 5). However, CCDαA376 displayed greatly reduced responsiveness to TGF-β, which is in agreement with the impaired interaction between CCDαA376 and Smads (Fig. 3). The result established that RUNX2 has the capacity to functionally cooperate with Smads and that CCDαA376 is severely impaired in this ability.
Figure 5.
Impaired responsiveness of CCDαA376 to TGF-β signaling. P19 cells were cotransfected with the reporter plasmid of Cα (WT), Cα (TβRE-mS), Cα (TβRE-mP), or Cα (mSP) (0.2 μg) and the expression plasmids for RUNX2 (shown as αA) or CCDαA376 (0.1 μg) and Smad3/4 (0.1 μg each) with or without TβR-I (0.1 μg). Relative luciferase activities are shown as -fold induction.
Inability of CCDαA376 to Induce Osteogenesis in Myoblasts Stimulated with BMP.
The myoblastic cell line, C2C12, undergoes differentiation to form multinucleated myotubes in response to low mitogen concentrations in culture medium. BMP inhibits myotube formation of C2C12 cells and induces osteoblastic phenotypes (42). Therefore, C2C12 cells provide a useful tool to study the ligand-specific signaling mechanisms of BMP. We examined the properties of CCDαA376 in this system.
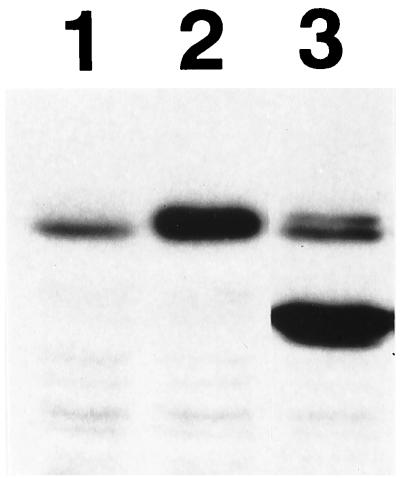
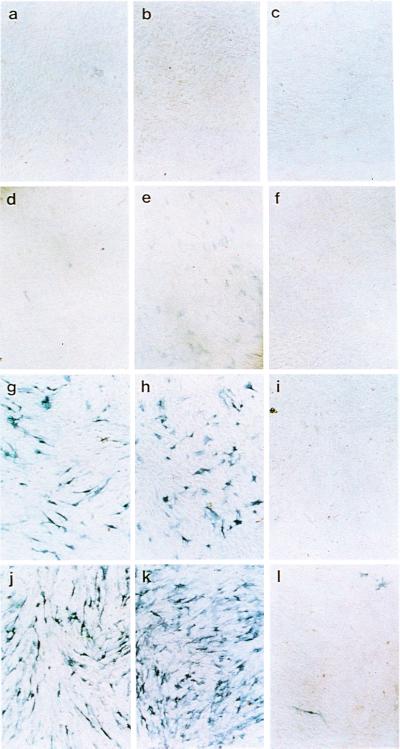
C2C12 cells stably expressing Runx2 or CCDαA376 at high levels were obtained (Fig. 6). Induction of alkaline phosphatase (ALP) activity after BMP treatment is considered to be an indicator of an early stage of osteoblastic differentiation (15). Exogenous expression of Smads 1 and 4, with recombinant adenovirus vectors induced ALP activity in C2C12 cells (Fig. 7g). Treatment of these adenovirus-infected cells with BMP-7 significantly stimulated this ALP activity (Fig. 7j), even at a BMP-7 concentration that failed to induce ALP activity in control cells (Fig. 7d), suggesting that BMP activated the exogenously expressed Smad1. C2C12 cells stably expressing Runx2 did not express ALP activity at a detectable level (Fig. 7b). Exogenous expression of Smads 1 and 4 in cells stably expressing RUNX2 showed slightly higher levels of ALP activity than that in parental C2C12 cells, suggesting a possible cooperation between Smads and RUNX2 (Fig. 7h). On the contrary, exogenous expression of Smads 1 and 4 in cells stably expressing CCDαA376 did not induce a detectable level of ALP activity (Fig. 7i). Even after subsequent BMP treatment, the induction of ALP activity was severely restricted (Fig. 7l). It seems that the high ALP activity induced by Smads 1 and 4 and BMP-7 in control C2C12 cells (Fig. 7j) was strongly inhibited by the stable high-level expression of CCDαA376 (Fig. 7l). The most plausible explanation for the observed inhibition would be that CCDαA376 interfered with the DNA binding of endogenous Runx2. Unlike Runx2, CCDαA376, which is unable to interact with Smads, can probably heterodimerize freely with PEBP2β and thereby bind to DNA more easily than Runx2. This property makes CCDαA376 an efficient transdominant-interfering molecule (1). The results suggest that Runx2 and Smads cooperate to induce osteoblastic cells and that they are critical elements in an elaborate molecular switch that triggers differentiation into the osteoblast lineage after BMP treatment.
Figure 6.
Western blot showing expression of RUNX2 and CCDαA376 in C2C12 cells stably expressing the genes. Lane 1, mock; lane 2, RUNX2; lane 3, CCDαA376.
Figure 7.
Alkaline phosphatase activities (42) induced by cooperation between Runx2 and Smads. (a, d, g, and j) Control C2C12 cells. (b, e, h, and k) C2C12 cells stably expressing Runx2. (c, f, i, and l) C2C12 cells stably expressing CCDAα376. (a–f) Infected with recombinant adenovirus expressing β-galactosidase as a control of unrelated protein. (d–f) C2C12 cells treated with BMP-7 (150 ng/ml) for 3 days. (g–i) C2C12 cells infected with recombinant adenoviruses expressing Smads 1 and 4 for 24 h and incubated for 3 more days. (j–l) C2C12 cells infected with recombinant adenoviruses expressing Smads 1 and 4 for 24 h and then treated with BMP-7 (150 ng/ml) for 3 days.
The results of the present study suggest that interaction of RUNX2 with signal transducers of TGF-β/BMP pathways, Smads, is critically important for the function of this transcription transactivator. The pathological basis of the CCD syndrome may be attributable, at least in part, to haploinsufficiency of responsiveness of RUNX2 to TGF-β superfamily signaling.
Acknowledgments
We thank G. Stein, J. Stein, A. van Wijnen, J. Lian, and S. Gutierrez for the rat osteocalcin promoter; and N. Nozaki, K. Sasaguri, and Y. Yamaji for producing the monoclonal antibody anti-αA8G5. This work was supported by Grant-in-Aid 09253220 for Priority Area in Cancer Research from Ministry of Education, Science, Sports and Culture, Japan. Y.-W.Z. is supported by a fellowship from the Japan Society for Promotion of Science.
Abbreviations
- CCD
cleidocranial dysplasia
- AML
acute myeloid leukemia
- TGF-β
transforming growth factor β
- BMP
bone morphogenetic protein
- GST
glutathione S-transferase
- ALP
alkaline phosphatase
- AD
transactivation domain
- Pn
postnatal day n
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.180309597.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.180309597
References
- 1.Ito Y. Gene Cells. 1999;4:685–696. doi: 10.1046/j.1365-2443.1999.00298.x. [DOI] [PubMed] [Google Scholar]
- 2.Speck N A, Stacy T. Crit Rev Eukaryotic Gene Expression. 1995;5:337–364. doi: 10.1615/critreveukargeneexpr.v5.i3-4.60. [DOI] [PubMed] [Google Scholar]
- 3.Kamachi Y, Ogawa E, Asano M, Ishida S, Murakami Y, Satake M, Ito Y, Shigesada K. J Virol. 1990;64:4808–4819. doi: 10.1128/jvi.64.10.4808-4819.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogawa E, Maruyama M, Kagoshima H, Inuzuka M, Lu J, Satake M, Shigesada K, Ito Y. Proc Natl Acad Sci USA. 1993;90:6859–6863. doi: 10.1073/pnas.90.14.6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y-W, Bae S-C, Takahashi E, Ito Y. Oncogene. 1997;15:367–371. doi: 10.1038/sj.onc.1201352. [DOI] [PubMed] [Google Scholar]
- 6.Miyoshi H, Shimizu K, Maseki N, Kaneko Y, Ohki M. Proc Natl Acad Sci USA. 1991;88:10431–10434. doi: 10.1073/pnas.88.23.10431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bae S C, Yamaguchi-Iwai Y, Ogawa E, Maruyama M, Inuzuka M, Kagoshima H, Shigesada K, Satake M, Ito Y. Oncogene. 1993;8:809–814. [PubMed] [Google Scholar]
- 8.Bae S-C, Ogawa E, Maruyama M, Oka H, Satake M, Shigesada K, Jenkins N A, Gilbert D J, Copeland N G, Ito Y. Mol Cell Biol. 1994;14:3242–3252. doi: 10.1128/mcb.14.5.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bae S C, Takahashi E, Zhang Y W, Ogawa E, Shigesada K, Namba Y, Satake M, Ito Y. Gene. 1995;159:245–248. doi: 10.1016/0378-1119(95)00060-j. [DOI] [PubMed] [Google Scholar]
- 10.Levanon D, Negreanu V, Bernstein Y, Bar-Am I, Avivi L, Groner Y. Genomics. 1994;23:425–432. doi: 10.1006/geno.1994.1519. [DOI] [PubMed] [Google Scholar]
- 11.Wijimenga C, Speck N A, Dracopoli N C, Hofker M H, Liu P, Collins F S. Genomics. 1995;26:611–614. doi: 10.1016/0888-7543(95)80185-o. [DOI] [PubMed] [Google Scholar]
- 12.Kagoshima H, Shigesada K, Satake M, Ito Y, Miyoshi H, Ohki M, Pepling M, Gergen P. Trends Genet. 1993;9:338–341. doi: 10.1016/0168-9525(93)90026-e. [DOI] [PubMed] [Google Scholar]
- 13.Kania M A, Bonner A S, Duffy J B, Gergen J P. Genes Dev. 1990;4:1701–1713. doi: 10.1101/gad.4.10.1701. [DOI] [PubMed] [Google Scholar]
- 14.Daga A, Kariovich C A, Dumstrei K, Banerjee U. Genes Dev. 1996;10:1194–1205. doi: 10.1101/gad.10.10.1194. [DOI] [PubMed] [Google Scholar]
- 15.Rodan G A, Harada S. Cell. 1997;89:677–680. doi: 10.1016/s0092-8674(00)80249-4. [DOI] [PubMed] [Google Scholar]
- 16.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson R T, Gao Y-H, Inada M, et al. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 17.Otto F, Thornell A P, Crompton T, Denzel A, Gilmour K C, Rosewell I R, Stamp G W H, Beddington R S P, Mundlos S, Olsen B R, et al. Cell. 1997;89:765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 18.Lee B, Thirunavukkarasu K, Zhou L, Pastore L, Baldini A, Hecht J, Geoffroy V, Ducy P, Karsenty G. Nat Genet. 1997;16:307–310. doi: 10.1038/ng0797-307. [DOI] [PubMed] [Google Scholar]
- 19.Mundlos S, Mulliken J B, Abramson D L, Warman M L, Knoll J H M, Olsen B R. Hum Mol Genet. 1995;4:71–75. doi: 10.1093/hmg/4.1.71. [DOI] [PubMed] [Google Scholar]
- 20.Jones K L. Smith's Recognizable Patterns of Human Malformation. Philadelphia: Saunders; 1997. [Google Scholar]
- 21.Selby P B, Selby P R. Mutat Res. 1978;51:199–236. doi: 10.1016/s0027-5107(78)80019-0. [DOI] [PubMed] [Google Scholar]
- 22.Mundlos S, Huang L F, Selby P, Olsen B R. Ann NY Acad Sci. 1996;785:301–302. doi: 10.1111/j.1749-6632.1996.tb56290.x. [DOI] [PubMed] [Google Scholar]
- 23.Mundlos S, Otto F, Mundlos C, Mulliken J B, Aylsworth A S, Albright S, Lindhout D, Cole W G, Henn W, Knoll J H M, et al. Cell. 1997;89:773–779. doi: 10.1016/s0092-8674(00)80260-3. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y-W, Yasui N, Kakazu N, Abe T, Takada K, Imai S, Sato M, Nomura S, Ochi T, Okuzumi S, et al. Gene. 2000;244:21–28. doi: 10.1016/s0378-1119(99)00558-2. [DOI] [PubMed] [Google Scholar]
- 25.Heldin C-H, Miyazono K, ten Dijke P. Nature (London) 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 26.Attisano L, Wrana J L. Curr Opin Cell Biol. 1998;10:188–194. doi: 10.1016/s0955-0674(98)80141-5. [DOI] [PubMed] [Google Scholar]
- 27.Massague J. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 28.Derynck R, Zhang Y, Feng X-H. Cell. 1998;95:737–740. doi: 10.1016/s0092-8674(00)81696-7. [DOI] [PubMed] [Google Scholar]
- 29.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current Protocols in Molecular Biology. New York: Wiley; 1994. [Google Scholar]
- 30.Mizushima S, Nagata S. Nucleic Acids Res. 1990;18:5322. doi: 10.1093/nar/18.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stewart M, Terry A, Hu M, O'Hara M, Blyth K, Baxter E, Cameron E, Onions D E, Neil J C. Proc Natl Acad Sci USA. 1997;94:8646–8651. doi: 10.1073/pnas.94.16.8646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanno T, Kanno Y, Chen L F, Ogawa E, Kim W Y, Ito Y. Mol Cell Biol. 1998;18:2444–2454. doi: 10.1128/mcb.18.5.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Towler D A, Rutledge S J, Rodan G A. Mol Endocrinol. 1994;8:1484–1493. doi: 10.1210/mend.8.11.7877617. [DOI] [PubMed] [Google Scholar]
- 34.Javed A, Gutierrez S, Montecino M, van Wijnen A J, Stein J L, Stein G S, Lian J. Mol Cell Biol. 1999;19:7491–7500. doi: 10.1128/mcb.19.11.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imamura T, Takase M, Nishihara A, Oeda E, Hanai J, Kawabata M, Miyazono K. Nature (London) 1997;389:622–626. doi: 10.1038/39355. [DOI] [PubMed] [Google Scholar]
- 36.Kawabata M, Inoue H, Hanyu A, Imamura T, Miyazono K. EMBO J. 1998;17:4056–4065. doi: 10.1093/emboj/17.14.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanai J-I, Chen L F, Kanno T, Ohtani-Fujita N, Kim W Y, Guo W-H, Imamura T, Ishidou Y, Fukuchi M, Shi M-J, et al. J Biol Chem. 1999;274:31577–31582. doi: 10.1074/jbc.274.44.31577. [DOI] [PubMed] [Google Scholar]
- 38.Lu J, Maruyama M, Satake M, Bae S-C, Ogawa E, Kagoshima H, Shigesada K, Ito Y. Mol Cell Biol. 1995;15:1651–1661. doi: 10.1128/mcb.15.3.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujii M, Takeda K, Imamura T, Aoki H, Sampath T K, Enomoto S, Kawabata M, Kato M, Ichijo H, Miyazono K. Mol Biol Cell. 1999;10:3801–3813. doi: 10.1091/mbc.10.11.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonewald L F. Principles of Bone Biology. Vol. 46. San Diego: Academic; 1996. pp. 647–659. [Google Scholar]
- 41.Hogan B L M. Genes Dev. 1996;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- 42.Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, Rosen V, Wozney J M, Fujisawa-Sehara A, Suda T. J Cell Biol. 1994;127:1755–1766. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y-W, Bae S-C, Huang G, Fu Y-X, Lu J, Ahn M-Y, Kanno Y, Kanno T, Ito Y. Mol Cell Biol. 1997;7:4133–4145. doi: 10.1128/mcb.17.7.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]