Abstract
Ca2+/calmodulin-dependent protein kinase II (CaMKII) and the BK channel are enriched at the presynaptic nerve terminal, where CaMKII associates with synaptic vesicles whereas the BK channel colocalizes with voltage-sensitive Ca2+ channels (VSCCs) in the plasma membrane. Mounting evidence suggests that these two proteins play important roles in controlling neurotransmitter release. Presynaptic BK channels primarily serve as a negative regulator of neurotransmitter release. In contrast, presynaptic CaMKII either enhances or inhibits neurotransmitter release and synaptic plasticity depending on experimental/physiological conditions and properties of specific synapses. The different functions of presynaptic CaMKII appear to be mediated by distinct downstream proteins, including the BK channel.
Keywords: calcium/calmodulin-dependent protein kinase II (CaMKII), neurotransmitter release, BK channel, Slo, Slo1, SLO-1, maxi-K channel, synapsin, CaV2.1, calcium channel, ryanodine receptor, synaptotagmin, SNARE
The presynaptic nerve terminal contains a rich variety of proteins. Many of these proteins contribute to the precise control of neurotransmitter release, which is vital to proper function of the nervous system. Among presynaptic proteins that regulate neurotransmitter release, CaMKII and the BK channel are two prominent players and share the property of being activated by Ca2+. Presynaptic CaMKII and BK channel play important and complex roles in controlling synaptic strength and plasticity through interactions with a variety of other presynaptic components, including channels that provide Ca2+ for their activation and proteins that mediate their functions. This review summarizes our current understanding about the functions of presynaptic CaMKII and BK channel in regulating synaptic transmission. Two main reasons prompted me to review the functions of these two proteins in the same article: (1) presynaptic CaMKII and BK channel interact closely in regulating synaptic transmission; and (2) the functions of presynaptic CaMKII or BK channel have rarely been the focus of scientific reviews.
I. The function of presynaptic CaMKII in controlling synaptic strength and plasticity
Structural and functional properties of CaMKII
CaMKII is a serine/threonine protein kinase that is activated by Ca2+ and calmodulin. It is a holoenzyme of multiple protein subunits. Electron microscopic analyses suggest that the holoenzyme consists of 12 subunits that form two stacked hexameric rings [1, 2]. Four closely related but distinct genes (α, β, γ and δ) encode CaMKII subunits in mammals. The holoenzyme may be formed by either one or more isoforms of the CaMKII subunits [3].
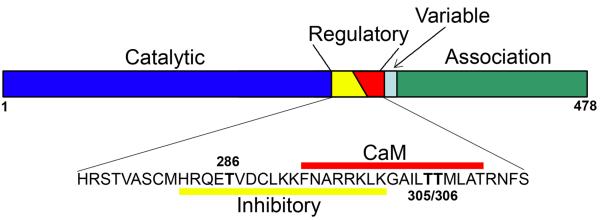
Each CaMKII subunit consists of a catalytic domain, a regulatory domain, a variable segment and an association domain (Figure 1). The regulatory domain is further divided into two partially overlapping domains: autoinhibitory domain and calmodulin-binding domain [4]. The catalytic domain has intrinsic kinase activity, and contains the substrate- and ATP-binding sites. However, under basal conditions, the kinase activity is inhibited by the autoinhibitory domain, which binds the catalytic domain as a pseudosubstrate. Upon Ca2+/calmodulin binding to the calmodulin-binding domain, the autoinhibitory domain dissociates from the catalytic domain, which unmasks the kinase activity. The variable segment differs in length among CaMKII isoforms, and may serve to localize CaMKII to specific subcellular domains and to modify the sensitivity to Ca2+/calmodulin. The association domain mediates assembly of the holoenzyme (see [5]) for a review).
Figure 1.
Schematic representation of domain structures of αCaMKII. The protein may be divided into four functional domains, including the catalytic domain, the regulatory domain, a variable segment that differs between CaMKII isoforms, and the association domain. The regulatory domain is further divided into the autoinhibitory domain and Ca2+/calmodulin (CaM)-binding domain. CaMKII may be autophosphorylated at three threonine residues. Autophosphorylation at threonine 286 (T286) allows the kinase to retain catalytic activity even after Ca2+ concentration has returned to basal level, whereas autophosphorylation at T305 and T306 blocks subsequent activation of the kinase by Ca2+/calmodulin.
The activity of CaMKII may be controlled by autophosphorylation of specific residues in the autoinhibitory domain (Figure 1). Phosphorylation of three threonine residues (T286, T305 and T306, numbered according to their positions in αCaMKII) is of particular importance. T286 is trans-autophosphorylated by the catalytic domain of a neighboring CaMKII subunit after the autoinhibitory domain has dissociated from the catalytic domain in response to Ca2+/calmodulin binding [6]. CaMKII autophosphorylated at this residue remains active even after cytoplasmic [Ca2+] falls back to the basal level [7-11]. T305 and T306 are located in the calmodulin-binding site. They may be autophosphorylated following T286 autophosphorylation and Ca2+/calmodulin dissociation from the calmodulin-binding domain. Autophosphorylation at T305 and T306 blocks subsequent activation of the kinase by Ca2+/calmodulin [12, 13]. Autophosphorylation is potentially an important mechanism in controlling the function of presynaptic CaMKII since synaptic vesicle-associated CaMKII may be similarly autophosphorylated [14] and the autophosphorylation may be enhanced by depolarization [15].
Autophosphorylation of CaMKII may be reversed by dephosphorylation catalyzed by phosphatase 1 (PP1), phosphatase 2A (PP2A) or phosphatase 2C (PP2C). Although the three phosphatases can dephosphate CaMKII at any of the three threonine residues (T286, T305 and T306) in vitro [13, 16-18], some specificity appears to exist in vivo. For example, CaMKII in the cytoplasm is primarily dephosphorylated by PP2A whereas that in the postsynaptic density is primarily dephosphorylated by PP1 [18]. This apparent specificity is probably due to the enrichment of distinct phosphatases in different subcellular compartments [18]. It remains to be determined which phosphatase(s) catalyzes the dephosphorylation of presynaptic CaMKII.
CaMKII could also become constitutively active (Ca2+-independent) following oxidation of a pair of methionine residues near the autoinhibitory domain, which was recently shown for δCaMKII [19]. However, it remains to be determined whether βCaMKII and γCaMKII, in which the two methionine residues are conserved, may be similarly modulated by the oxidation.
Presynaptic CaMKII modulates synaptic strength and plasticity
CaMKII is enriched at the presynaptic nerve terminal [15, 20], where it mainly associates with the outer surface of synaptic vesicles [21]. At least three isoforms of the CaMKII subunits (α, β and γ) associate with synaptic vesicles [22]. The function of presynaptic CaMKII in synaptic strength and plasticity has been assessed by a number of studies. Presynaptic CaMKII appears to have complex functions in synaptic transmission. While some studies suggest that presynaptic CaMKII is a positive regulator of synaptic transmission, others suggest that it is a negative or a bidirectional regulator.
Evidence for presynaptic CaMKII being a positive regulator of synaptic transmission
The earliest evidence about the function of presynaptic CaMKII came from analyses of the squid giant synapse, where injection of purified CaMKII into the presynaptic terminal increases the amplitude and rise rate of evoked postsynaptic potentials and currents (ePSPs and ePSCs), suggesting that presynaptic CaMKII enhances neurotransmitter release [23, 24]. Results of subsequent studies provide further support to the notion that presynaptic CaMKII is a positive regulator of neurotransmitter release. For example, loading of synaptosomes purified from rat cerebral cortices with autophosphorylation-activated CaMKII enhances glutamate release induced by high K+ or a Ca2+ ionophore, whereas loading with a CaMKII inhibitory peptide decreases the release [25]. Application of synthetic CaMKII inhibitory peptides to rat hippocampal brain slices strongly reduces ePSPs in hippocampal CA1 region without altering postsynaptic receptor sensitivity to glutamate [26]. At the Drosophila neuromuscular junction (NMJ), CaMKII inhibitors block the enhancement of dense core vesicle motility caused by tetanic nerve stimulation [27]. In Xenopus nerve-muscle co-culture, loading of a specific CaMKII inhibitory peptide into the presynaptic neuron but not postsynaptic myocyte blocks the stimulatory effect of neurotrophin-3 on the frequency of miniature spontaneous postsynaptic currents [28]. In cultured mouse hippocampal neurons, brief treatment with glutamate increases the frequency of miniature postsynaptic currents (mPSCs) and the number of active presynaptic boutons, which are prevented by the CaMKII inhibitor KN-93 but not its inactive analogue KN-92 [29]. These studies suggest that presynaptic CaMKII enhances vesicle motility and facilitates spontaneous as well as evoked neurotransmitter release.
Presynaptic CaMKII also plays roles in long-term potentiation (LTP). At several types of synapses examined, including synapses between Aplysia sensory and motor neurons [30], between CA3 neurons in rat hippocampal slices [31], and between cultured mouse hippocampal neurons [29], LTP may be reduced or blocked by injecting a CaMKII inhibitory peptide into the presynaptic neuron. Furthermore, injection of a recombinant αCaMKII into the presynaptic neuron induces LTP if the injection is paired with weak tetanic nerve stimulation that normally does not induce LTP [29].
Evidence for presynaptic CaMKII being a negative regulator of synaptic transmission
In contrast to the studies described above, several other studies suggest that the function of presynaptic CaMKII is to inhibit synaptic transmission. At the CA3-CA1 synapse of mouse hippocampus, targeted knockout of presynaptic CaMKII enhances neurotransmitter release evoked by repetitive presynaptic stimulation [32]. At the same synapse, universal knockout of αCaMKII enhances synaptic augmentation induced by θ-burst stimulation and decreases synaptic fatigue during repetitive stimulation. Both the augmentation and fatigue are forms of short-term synaptic plasticities. Because the origin of short-term plasticity is largely presynaptic [33-36], these observations led to the suggestion that the function of presynaptic CaMKII is to inhibit synaptic transmission [37]. Interestingly, the effects of presynaptic CaMKII on synaptic augmentation and fatigue appear to be independent of the kinase's catalytic activity [37]. In avian hippocampal slices, long-term depression (LTD) requires a rise of Ca2+ in the pre- but not postsynaptic neuron, and is blocked by the CaMKII inhibitor KN-93 [38]. In rat hippocampal slices, application of the membrane-permeant CaMKII inhibitor KN-62 to the extracellular solution but not infusion into the postsynaptic neuron blocks the LTD induced by low-frequency stimulation of the Schaffer collateral, which led to the conclusion that pre- but not postsynaptic CaMKII is needed to induce LTD [39].
Evidence for presynaptic CaMKII being a bidirectional regulator of synaptic transmission
The studies described above suggest that presynaptic CaMKII may either enhance or inhibit synaptic transmission. There are two possibilities for the apparently opposite functions: (1) presynaptic CaMKII has different functions at different synapses; and (2) presynaptic CaMKII is a bidirectional modulator of synaptic transmission but one of these two functions predominates under specific experimental or physiological conditions. Results of at least two studies favor the second notion. In mouse hippocampal CA1 region, paired-pulse facilitation is blunted whereas posttetanic potentiation is enhanced by deletion of αCaMKII [40]. Since the origins of both paired-pulse facilitation and posttetanic potentiation are largely presynaptic [33-36], these results led to the suggestion that presynaptic CaMKII is a bidirectional modulator of neurotransmitter release depending on the pattern of presynaptic activation [40]. At the C. elegans neuromuscular junction, either gain-of-function or loss-of-function mutation of the CaMKII gene unc-43 causes a marked decrease in evoked neurotransmitter release [41], suggesting that presynaptic CaMKII is likely a bidirectional modulator of neurotransmitter release [41].
It is worth noting that several commonly used CaMKII inhibitors may have non-specific effects. For example, the prototypical CaMKII inhibitors KN-93 and KN-62 as well as the KN-93 inactive analogue KN-92 potently inhibit voltage-dependent K+ currents in vascular smooth muscle cells at concentrations that are normally used to inhibit CaMKII [42]. This effect on K+ currents is independent of CaMKII and Ca2+, and is probably due to inhibition of a delayed rectifier K+ channel [42]. Similarly, both KN-93 and KN-92 potently inhibit the activity of SLO-1 BK channels expressed in Xenopus oocytes (Liu and Wang, unpublished). In addition, KN-93 and KN-62 but not KN-92 can enhance the binding of PKC and calmodulin to A-kinase-anchoring protein 79 (Brooks and Tavalin, 2007 Annual Conference of Society for Neuroscience, presentation number 787.1). Thus, cautions should be taken when interpreting results obtained with these chemicals.
Proteins that mediate the functions of presynaptic CaMKII
Presynaptic CaMKII regulates neurotransmitter release and synaptic plasticity by acting through different downstream proteins. Generally, CaMKII modulates the functions of downstream proteins through phosphorylation. However, there is at least one example showing that the catalytic activity of CaMKII is not required. Presynaptic CaMKII may either enhance or inhibit synaptic transmission, depending on the identities of its downstream proteins.
Synapsin I
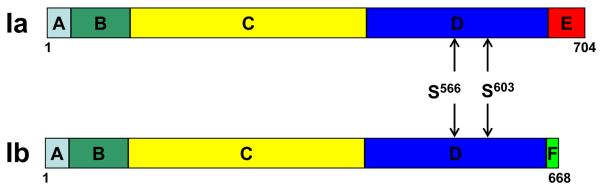
Synapsin I is perhaps the first protein implicated in the function of presynaptic CaMKII [23-25, 43]. The mammalian genome contains three distinct genes encoding synapsins (synapsins I, II and III). Among them, synapsin I is the most abundant in mature neurons [44, 45] and is the only synapsin that may be phosphorylated by CaMKII. Synapsin I has two isoforms (Ia and Ib). These two isoforms differ only at the carboxyl terminal, with Ia having a longer carboxyl terminal than Ib. Synapsins Ia and Ib may be divided into several structural domains (Figure 2). Domains A, B, and C are also found in synapsins II and III whereas domain D is unique to synapsin I. The distinct carboxyl terminals of synapsins Ia and Ib are named as domains E and F, respectively. The sites of CaMKII phosphorylation are two serine residues in domain D (Figure 2) [46].
Figure 2.
Schematic representation of domain structures of synapsin Ia and Ib. The two isoforms arise from alternative splicing of a single transcript and differ only at the carboxyl terminal. Each isoform contains five domains. Domains A, B, C and D are common to synapsin Ia and Ib whereas domain E and F are unique to Ia and Ib, respectively. CaMKII phosphorylates synapsin I at serine 566 (S566) and serine 603 (S603) in domain D [46].
Synapsin I is localized almost exclusively to nerve terminals. The great majority of presynaptic terminals, perhaps all, contain synapsin I [47]. Biochemical studies show that synapsin I primarily associates with synaptic vesicles and accounts for ∼6% of total vesicle proteins [47-50]. Synapsin I also associates with the cytoskeletal protein actin [51]. Binding of synapsin I to synaptic vesicle and actin is regulated by CaMKII-dependent phosphorylation. Dephospho-synapsin I binds to synaptic vesicles and actin, whereas phospho-synapsin I dissociates from them [51-53].
A leading hypothesis is that synapsin I tethers synaptic vesicles to the cytoskeleton by associating with actin and synaptic vesicles, and this linkage is disrupted when synapsin I is phosphorylated by CaMKII, resulting in an increase in the number of vesicles in the readily releasable pool [49]. This hypothesis is supported by results of several studies. At the squid giant synapse, injection of dephospho-synapsin I into the presynaptic terminal inhibits neurotransmitter release whereas injection of CaMKII-phosphorylated synapsin I has no effect, and injection of purified CaMKII enhances the release [23, 24]. Similar results are obtained with rat brain synaptosomes (isolated nerve terminals) [25, 43]. At rat hippocampal presynaptic terminals, GFP-tagged synapsin Ia dissociates from synaptic vesicles and disperses into axons during action potential firing but re-associates with synaptic vesicles after the synaptic activity; the rates of activity-dependent synapsin Ia dissociation/dispersion and of synaptic exocytosis are inhibited by mutations of the two CaMKII phosphorylation sites in synapsin I [54].
Despite of the abundance of synapsin I at the presynaptic terminal and its potential importance in synaptic transmission, as suggested by the studies described above, synaptic phenotypes of synapsin I knockout mice are relatively mild. Reported phenotypes include decreases in the size of synaptic vesicle reserve pool [55], in the amplitude of inhibitory postsynaptic currents evoked by isolated action potentials [56], in the number of vesicles exocytosed during brief trains of action potentials [57], and in the size of the total recycling vesicle pool [57]. However, LTP is unchanged and paired-pulse facilitation is increased in synapsin I-deficient mice [58]. Furthermore, synapsin I deficiency does not alter the amplitude of excitatory postsynaptic currents evoked by single stimuli [59, 60]. At the calyx of Held synapse, synapsins I and II double knockout mice show a greater degree of synaptic depression in response to a train of high-frequency stimuli compared with wild-type, and this difference between wild-type and mutant mice disappears following injection of either the Ca2+ chelator EGTA or a calmodulin inhibitor into the presynaptic terminal, suggesting that Ca2+/calmodulin-dependent phosphorylation of synapsins might be implicated in facilitating neurotransmitter release during high-frequency stimulation. However, there is no evidence for CaMKII involvement. Instead, Ca2+/calmodulin-dependent protein kinase I (CaMKI) was proposed as a candidate that phosphorylates synapsins because all synapsins contain a conserved CaMKI phosphorylation site in domain A [59]. Thus, analyses of synapsin knockout mice suggest that CaMKII-dependent phosphorylation of synapsin I plays only a limited role in controlling neurotransmitter release.
The BK channel
Presynaptic BK channel (large-conductance Ca2+-activated K+ channel) is a key negative regulator of neurotransmitter release at the presynaptic nerve terminal (see Section II). Several lines of evidence suggest that BK channel function might be modulated by CaMKII. First, BK channels reconstituted into artificial lipid bilayers are activated by ATP and this effect of ATP is blocked by a CaMKII inhibitor [61]. Second, Drosophila BK (dSlo1) activity channel is modulated by the protein 14-3-3, which does not interact with dSlo1 directly but via a third protein known as Slob (Slowpoke-binding protein). The binding between 14-3-3 and Slob is modulated by CaMKII [62]. Third, BK channels in glomerular mesangial cells are activated by calmodulin plus ATP in the presence but not absence of CaMKII [63]. Fourth, BK channels contribute to afterhyperpolarization of mouse vestibular nucleus neurons in a CaMKII-dependent manner [64]. Nevertheless, it is unclear from these observations whether BK channel is a direct molecular target of CaMKII phosphorylation and whether CaMKII modulates BK channels at the presynaptic terminal.
Our recent study with C. elegans shows that presynaptic CaMKII may modulate neurotransmitter release by phosphorylating the BK channel [41]. In C. elegans, neurotransmitter release is greatly reduced in an unc-43 CaMKII gain-of-function mutant [unc43(gf)]. This effect of unc43(gf) is abolished by either blocking or mutating the BK channel SLO-1. Analyses using the Xenopus oocyte expression system show that recombinant rat αCaMKII activates SLO-1 by phosphorylating SLO-1 at a threonine residue (T425) in a consensus CaMKII phosphorylation site located in the first RCK (regulator of conductance for K+) domain [65]. Expression of SLO-1 (T425A) in neurons counteracted the inhibitory effect of unc-43(gf) on neurotransmitter release in vivo as SLO-1 blockade or mutation did whereas expression of wild-type SLO-1 in neurons had no such effect. These observations suggest that presynaptic UNC-43 may downregulate neurotransmitter release by phosphorylating SLO-1 at T425. The identified CaMKII phosphorylation site is conserved in mammalian BK channels. However, it remains to be determined whether mammalian BK channels are similarly regulated by presynaptic CaMKII.
CaV2.1 (P/Q-type) voltage-sensitive Ca2+ channel
Neurotransmitter release is triggered by Ca2+. The most important source of Ca2+ triggering synaptic exocytosis is influx through VSCCs. Although several types of VSCCs have been implicated in synaptic exocytosis, CaV2.1 channel appears to be the primary one triggering neurotransmitter release at many mature synapses (for a review, see [66]). A recent study [67] shows that application of CaMKII inhibitors accelerates voltage-dependent inactivation of CaV2.1 channels, suggesting that a function of CaMKII is to decelerate voltage-dependent inactivation of the channel. Interestingly, this function of CaMKII requires its binding to CaV2.1 channels but not its catalytic activity. Conceivably, presynaptic CaMKII may be able to enhance neurotransmitter release by decelerating inactivation of CaV2.1 channels.
Ryanodine receptors
The ryanodine receptor (RyR) is a Ca2+-releasing channel in the endoplasmic reticulum (ER) membrane. It is a homomeric complex of four subunits. There are three isoforms of RyRs in mammals: RyR1, RyR2 and RyR3, which are encoded by three different genes. All of them are expressed in the nervous system [68, 69]. The isoforms of RyRs at the presynaptic terminal may vary between synapses. One study shows that presynaptic RyRs are of the RyR2 isoform [70] whereas another study shows that all the three isoforms of RyRs exist at the presynaptic terminal with RyR1 being the most abundant [71]. Pharmacological and genetic analyses suggest that presynaptic RyRs may have several functions in synaptic transmission: (1) promoting spontaneous (i. e. action potential-independent) synaptic exocytosis [72-74]; (2) enhancing evoked neurotransmitter release [74, 75]; and (3) contributing to short-term and long-term synaptic plasticities [76, 77] (see [66] for a review).
The RyR is mainly activated by Ca2+-induced Ca2+ release, a process that links Ca2+ influx through VSCCs or receptor-operated Ca2+ channels in the plasma membrane to Ca2+ release from the ER [78]. CaMKII may modulate Ca2+ release from the ER by phosphorylating RyRs, as shown for RyR2. The CaMKII phosphorylation site of RyR2 was initially thought to be serine 2809 (S2809) [79, 80]. However, a subsequent study showed that serine 2815 (S2815) rather than S2809 is phosphorylated by CaMKII [81]. Phosphorylation of S2815 increases RyR2's Ca2+ sensitivity and open probability [81]. Because RyR2 exists at the presynaptic terminal [70, 71], CaMKII could potentially enhance neurotransmitter release by phosphorylating RyR2. However, this possibility has not been experimentally tested.
Synaptotagmin
Synaptotagmin 1 (Syt1) is an integral membrane protein of synaptic vesicles. A large body of evidence suggests that Syt1 is the key Ca2+ sensor for the fast and synchronous phase of synaptic exocytosis [82-86] (see [87] and [88] for reviews,). Binding of Syt1 to phospholipids and SNAREs (soluble N-ethylmaleimide-sensitive factor attachment receptor) are important to its function [87]. CaMKII may phosphorylate Syt1 at threonine 112 (T112) [89], which increases the interaction of Syt1 with the SNAREs syntaxin and SNAP-25 in vitro, particularly when Ca2+ is present [90]. However, substituting T112 of Syt1 with either alanine, which cannot be phosphorylated, or aspartate, which is a phosphomimetic, does not alter Syt1's ability of rescuing a secretion defect observed in Syt1 null chromaffin cells, suggesting that CaMKII-dependent phosphorylation of Syt1 does not play an obvious role in exocytosis [91]. Further analyses are needed to determine whether CaMKII-dependent phosphorylation of Syt1 plays a role in regulating neurotransmitter release.
SNAREs
The SNARE proteins include VAMP (synaptobrevin), syntaxin and SNAP-25. VAMP is also known as v-SNARE because it is a vesicle-associated protein whereas syntaxin and SNAP-25 are also known as t-SNAREs because they associate with the target (plasma) membrane. These proteins play a central role in synaptic vesicle fusion by forming an α-helical bundle called the SNARE complex or core complex [92]. All three SNARE proteins may be phosphorylated by CaMKII in vitro [93, 94]. It has been shown that autophosphorylated CaMKII binds syntaxin in a Ca2+-dependent manner, and microinjection of a short peptide that is identical to the CaMKII binding domain of syntaxin inhibits exocytosis in neurons and chromaffin cells, suggesting that binding of CaMKII to syntaxin is important to synaptic exocytosis [95].
Others
Several other presynaptic proteins are also phosphorylated by CaMKII in vitro, including NSF (N-ethylmaleimide sensitive factor), αSNAP (α soluble NSF attachment protein)[94], synaptophysin [96] and rabphilin [97]. These proteins are also implicated in synaptic vesicle cycle. However, it is unknown whether phosphorylation of these proteins by CaMKII regulates synaptic transmission.
Possible sources of Ca2+ for activating presynaptic CaMKII
The potential sources of Ca2+ for activating presynaptic CaMKII include entry of extracellular Ca2+ and release from intracellular stores. However, existing evidence has only implicated Ca2+ release from the ER in activating presynaptic CaMKII. At the Drosophila NMJ, brief tetanus nerve stimulation causes an increase of dense-core vesicle motility, which is dependent on RyR-mediated Ca2+ release and CaMKII activation but independent of external Ca2+ influx. In this reaction, CaMKII appears to function downstream of RyRs [27]. At the frog NMJ, neurotrophin-3 increases the frequency of spontaneous postsynaptic currents, and this effect of neurotrophin-3 is independent of extracellular Ca2+ but may be prevented by inhibiting either inositol 1, 4, 5-trisphosphate receptors (IP3Rs) or RyRs, or by loading a CaMKII inhibitory peptide into presynaptic motoneurons but not postsynaptic myocytes. These observations led to the suggestion that presynaptic CaMKII is activated by IP3R- and/or RYR-mediated Ca2+ release from the ER [28]. At Aplysia sensory-motor neuron synapses, a form of homosynaptic potentiation involves several synaptic proteins, including presynaptic RyRs and CaMKII [30]. While these observations favor the notion that presynaptic CaMKII is activated by Ca2+ release from the ER, it may be premature to exclude potential contributions from Ca2+ entry via VSCCs. Further analyses are needed to better understand the source(s) of Ca2+ that activates presynaptic CaMKII.
II. The function of presynaptic BK channels in regulating neurotransmitter release
Structural and functional properties of the BK channel
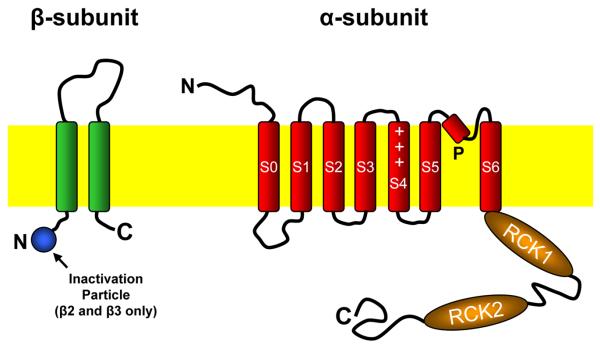
BK channel (Slo1) is a member of the Slo family of K+ channels, which has four members in mammals, including Slo1, Slo2.1 (also known as Slick), Slo2.2 (also known as Slack), and Slo3 [98]. BK channel was first cloned from Drosophila, in which the channel is encoded by the slowpoke (slo) locus [99-101]. BK channel has large single-channel conductance (often >200 pS), and is activated by membrane depolarization and elevation of cytoplasmic free [Ca2+]. The central components of a BK channel are four α-subunits. Each α-subunit contains 7 putative membrane-spanning domains (S0–S6). A pore domain exists between S5 and S6, and three positively charged residues occur at regular intervals in the putative S4 voltage sensor [99, 102, 103]. The carboxyl terminal following S6 is located on the cytoplasmic side and contains two RCK domains and a “Ca2+ bowl” located within the second RCK domain (Figure 3) [65, 104, 105]. Both RCK domains, including the Ca2+ bowl, are important for Ca2+-dependent channel gating [65, 104-107]. Mammalian BK channel may also contain a second protein called the β-subunit, which has two membrane-spanning domains (Figure 3). Four different BK channel β-subunits have been identified [108-111]. The β-subunits may modulate several properties of the channel, including the apparent Ca2+ sensitivity [111, 112], inactivation [108, 110, 113], and sensitivities to charybdotoxin and iberiotoxin [113, 114](for a review on the Slo family of K+ channels, see [98]).
Figure 3.
Diagram showing the structure of BK channel α- and β-subunits. The α-subunit contains an extracellular amino terminal, seven membrane spanning domains (S0 to S6) linked by intracellular and extracellular loops, a pore domain (P) between S5 and S6, and a long cytosolic carboxyl terminal following S6. The putative voltage sensor S4 contains three arginine residues (indicated by the “+” sign). The carboxyl terminal contains two RCK domains. The β-subunit has two putative membrane-spanning domains, an extracellular loop between the membrane-spanning domains, and intracellular amino and carboxyl terminals. The amino terminals of β2 and β3-subunits contain sequences that cause channel inactivation.
BK channels are enriched at the presynaptic terminal
Many electrophysiological analyses show that the presynaptic terminal contains a K+ channel with hallmarks of the BK channel, including large single-channel conductance (> 200 pS), voltage- and Ca2+- dependence, and blockade by charybdotoxin or iberiotoxin [115-125]. Besides the BK channel, the presynaptic terminal often contains several other voltage-gated K+ (KV) channels (for a review, see [126]). It is sometimes necessary to block other voltage-gated K+ channels to unmask BK channel function [116, 118, 121].
The existence of BK channels at the presynaptic terminal has been confirmed by immunocytochemistry and labeling with biotin-conjugated charybdotoxin. In rat brain, immunostaining with BK channel-specific antibodies shows that specific immunoreactivity is concentrated in axons and nerve terminals [127, 128]. At the frog NMJ, labeling with biotin-conjugated charybdotoxin reveals a banding pattern of BK channel distribution at the presynaptic terminal, which mirrors the distribution pattern of α-bungarotoxin-labeled postsynaptic acetylcholine receptors [129]. At the Drosophila NMJ, immunostaining with a BK channel-specific antibody shows that BK channels are enriched in synaptic boutons [62]. At excitatory synapses of rat hippocampus, immunoelectron microscopy analyses show that BK channels are localized to the presynaptic plasma membrane facing postsynaptic glutamate receptors [130]. In cultured rat hippocampal neurons, immunostaining shows that heterologously-expressed human BK channels are initially targeted to the axonal surface membrane but become localized in presynaptic terminals with further development [128].
BK channels colocalize with VSCCs at the presynaptic nerve terminal. For example, at the frog NMJ, presynaptic BK channels and N-type Ca2+ channels show an identical and overlapping banding pattern of distribution [129]; blocking N-type Ca2+ channels with ω-conotoxin GVIA inhibits neurotransmitter release as well as BK channel currents [123]. At the presynaptic site of frog saccular hair cells, BK channels and VSCCs cluster together in the active zone [124], with each cluster containing on average 40 BK channels and 90 VSCCs [131]. The colocalization allows activation of presynaptic BK channels by the Ca2+ micro- or nanodomains resulting from Ca2+ entry via neighboring VSCCs (see [66, 132] for reviews on Ca2+ micro- and nanodomains). This property has been explored by using BK channel activity to track Ca2+ dynamics at the presynaptic terminals of Xenopus NMJs [133] and frog saccular hair cells [131].
BK channels are activated by Ca2+ entry through VSCCs. However, identities of VSCCs performing this function appear to vary from cell to cell. In pyramidal neurons of rat hippocampal CA1 region, BK channel activity coincides with the opening of N-type Ca2+ channels, suggesting that BK channels are activated by colocalized N-type Ca2+ channels [134]. In sympathetic neurons of rat superior cervical ganglia, blocking either BK channels with charybdotoxin or L-type Ca2+ channels with nifedipine increases the half-width of action potentials whereas blocking other VSCCs has no such effect, suggesting that Ca2+ entry via L-type channels selectively activates the BK channel [135]. In rat cerebellar Purkinje neurons, blocking P/Q-type Ca2+ channels suppresses afterhyperpolarization [136], which is mainly contributed by the BK channel [137], suggesting that Ca2+ entry via P/Q-type channels may activate the BK channel. In rat medial vestibular nucleus neurons, the ratio of evoked firing rate to input current is reduced by increasing extracellular Ca2+ and increased by lowering extracellular Ca2+, blocking BK channels, or blocking T-type Ca2+ channels; blocking BK channels occludes the firing response gain via the T-type but not other VSCCs, suggesting that BK channels are activated by Ca2+ entry via T-type Ca2+ channels [138]. In plasma membrane-enriched fractions prepared from rat whole brain, subunits of several Ca2+ channels, including CaV1.2 (L-type), CaV2.1 (P/Q-type), and CaV2.2 (N-type), are copurified with BK channels [139]. When BK channels are coexpressed with CaV2.1 or CaV2.2 channels in Xenopus oocytes and analyzed in inside-out patches, a large outward BK current is preceded by a small inward Ca2+ current at each voltage step above the activation threshold of Ca2+ channels; normalized current-voltage relationship of the BK channel is bell-shaped and mirrors that of the Ca2+ channel but at an inverted direction [139]. The diversity of Ca2+ channels that may mediate BK channel activation suggests that activation of BK channels by colocalized Ca2+ channels is not a unique property of a specific type of VSCC; rather, the apparent specificity of coupling reflects the identity of VSCCs that are coexpressed and colocalized with the BK channel.
Relatively little is known about the identity of VSCCs that activates BK channels at the presynaptic terminal. At frog NMJs, N-type Ca2+ channels play an important role in triggering neurotransmitter release [121, 123, 140-142]. At this synapse, blocking N-type Ca2+ channels with ω-conotoxin GVIA significantly inhibits BK currents [121, 123], suggesting that Ca2+ entry through N-type channels has the dual functions of triggering synaptic exocytosis and activating colocalized BK channels.
Presynaptic BK channels regulate neurotransmitter release
Neurotransmitter release is triggered by action potentials and the resultant Ca2+ entry through presynaptic VSCCs. BK channels are especially suited to serving as a negative regulator of neurotransmitter release because they colocalize with VSCCs at the active zone and are activated by membrane depolarization as well as elevation of cytosolic free [Ca2+]. Indeed, such a function of presynaptic BK channels has been shown by a number of studies. For example, blocking BK channels with charybdotoxin and/or iberiotoxin increases Ca2+ entry into the presynaptic terminal as well as the amplitude of end-plate potentials at frog NMJs [129, 143]. The stimulatory effect of charybdotoxin on neurotransmitter release is prevented by pretreatment with BAPTA-AM [1,2-bis(2-amino-5-fluorophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl) ester] but not EGTA-AM [ethyleneglycol-bis(β-aminoethyl)-N,N,N′,N′-tetraacetoxymethyl ester] [129]. These two agents are membrane-permeant Ca2+ chelators. Each molecule of BAPTA-AM or EGTA-AM has four ester groups attached to the Ca2+-binding site. The ester groups confer membrane permeability and keep the Ca2+ chelator inactive. BAPTA-AM and EGTA-AM become active and are trapped inside the cells once the ester groups are removed by intracellular esterases [144]. Although BAPTA and EGTA bind Ca2+ with similar affinities (Kd ≈ 160 nM), BAPTA has a 100-fold faster on-rate and a greater effect at the mouth of Ca2+ channels than EGTA [145, 146]. Thus, the blockage of charybdotoxin's effect by BAPTA but not EGTA suggests that presynaptic BK channels are located very close to VSCCs at frog NMJs. In C. elegans, the amplitude of ePSCs at the NMJ is increased in slo-1 loss-of-function mutant but decreased in slo-1 gain-of-function mutant [41]. At hippocampal excitatory synapses between CA3 and CA1 neurons, and between CA3 and CA3 neurons, blocking BK channels increases the amplitude of ePSPs or ePSCs [130, 147]. In addition, blocking BK channels decreases paired-pulse ratio of ePSPs or ePSCs, especially at synapses with high release probabilities [130, 147], which is also a sign of elevated neurotransmitter release probability [126, 148, 149]. Because blocking BK channels causes spike broadening in neurons [130, 147, 150-152], including the presynaptic terminal [118], presynaptic BK channels likely downregulate neurotransmitter release by shortening the duration of depolarization that allows Ca2+ entry through VSCCs.
The importance of presynaptic BK channels as a negative regulator of neurotransmitter release is well illustrated by a genetic screen in C. elegans. In a genetic a screen designed to isolate mutants that suppress the lethargic phenotype of a hypomorphic syntaxin mutant, which restricts neurotransmitter release, six slo-1 loss-of-function alleles but no mutants of other K+ channel genes were identified [153]. Given that there are approximately 70 K+ channels in C. elegans [154], the isolation of only slo-1 mutants from this whole-genome-based genetic screen suggests that presynaptic SLO-1 plays an important, if not unique, role in regulating neurotransmitter release.
The function of presynaptic BK channels in regulating neurotransmitter release may vary from synapse to synapse. While blocking or mutating BK channels alone is sufficient to enhance neurotransmitter release at frog and C. elegans NMJs, and at glutamatergic synapses between hippocampal CA1 and CA3 neurons [41, 129, 147, 153], addition of 4-aminopyridine to inhibit other K+ channels is a prerequisite for BK channel blockers to increase neurotransmitter release at hippocampal CA3-CA3 synapses [130]. One potential cause for the differential dependence on 4-aminopyridine between CA1-CA3 and CA3-CA3 synapses is a difference in experimental conditions, as suggested by others [126].
Surprisingly, blocking BK channels decreases the amplitude of excitatory postsynaptic currents evoked by light at salamander rod synapses [155] and by direct nerve stimulation in frog nerve-muscle co-culture [156], suggesting that the function of presynaptic BK channels is to facilitate neurotransmitter release. The apparent positive role of presynaptic BK channels in neurotransmitter release might reflect unusual synaptic properties or nonphysiological experimental conditions. For example, salamander rod photoreceptors are non-spiking neurons; special structural properties of the rod synapse might allow BK channels to enhance Ca2+ entry and neurotransmitter release by increasing extracellular K+ concentration in the synaptic cleft, as speculated by the authors [155]. Frogs NMJs in-culture may be different from those in vivo or in situ in some functional properties, as suggested by the opposite effects of BK channel blockers on frog neuromuscular transmission in situ [129] and in-culture [156].
Concluding remarks
The studies discussed above indicate that presynaptic CaMKII and BK channel play important roles in regulating synaptic transmission. The molecular mechanisms of presynaptic CaMKII and BK channel functions are summarized in a diagram (Figure 4). Some major questions remain to be answered about presynaptic CaMKII and BK channel. For example, what are the molecular bases of localizing CaMKII to synaptic vesicles and BK channels to the presynaptic nerve terminal? Is the presynaptic localization of CaMKII or BK channels regulated by synaptic activity? What factors determine whether the predominant function of presynaptic CaMKII is to enhance or inhibit neurotransmitter release under physiological conditions? What is the physiological significance of CaMKII-dependent phosphorylation of some presynaptic proteins that may be phosphorylated by CaMKII in vitro? How are the functions of presynaptic CaMKII and BK channel regulated under physiological conditions? An elucidation of the functions of presynaptic CaMKII and BK channel will help us to understand how neurotransmitter release and synaptic function are precisely controlled.
Figure 4.
Diagram showing molecular mechanisms of presynaptic CaMKII and BK channel functions. Presynaptic CaMKII may modulate neurotransmitter release through several possible mechanisms. First, CaMKII may promote synaptic vesicle translocation from the reserve pool to the readily releasable pool by phosphorylating synapsin I (SYN). Dephospho-synapsin I binds to synaptic vesicles (SVs) and keeps SVs in the reserve pool by associating with actin in the cytoskeleton whereas phosphor-synapsin I dissociates from actin and SVs, allowing SVs to translocate to the readily releasable pool. Second, CaMKII may increase Ca2+ entry by decelerating CaV2.1 (P/Q-type) channel inactivation. This effect of CaMKII is independent of its catalytic activity. Third, CaMKII may phosphorylate ryanodine receptors (RYR) to enhance Ca2+ release from the endoplasmic reticulum (ER). Fourth, CaMKII may downregulate neurotransmitter release by phosphorylating and activating the BK channel. Several other presynaptic proteins, including synaptotagmin (syt) and SNAREs, are also phosphorylated by CaMKII in vitro; however, the physiological significance is unclear. Presynaptic BK channel is activated by membrane depolarization and Ca2+ entry through colocalized voltage-sensitive Ca2+ channels (e. g. the P/Q-type channel). The BK channel downregulates neurotransmitter release by shortening the duration of action potentials that allow Ca2+ entry through voltage-sensitive Ca2+ channels. For clarity, CaMKII, syt, and SNAREs are depicted as stand-alone proteins although they normally associate with SVs or the plasma membrane.
Acknowledgements
This work was supported by the National Science Foundation (0619427) and National Institute of Health (GM083049).
References
- 1.Kolodziej SJ, Hudmon A, Waxham MN, Stoops JK. Three-dimensional reconstructions of calcium/calmodulin-dependent (CaM) kinase IIα and truncated CaM kinase IIα reveal a unique organization for its structural core and functional domains. J Biol Chem. 2000;275:14354–14359. doi: 10.1074/jbc.275.19.14354. [DOI] [PubMed] [Google Scholar]
- 2.Morris EP, Torok K. Oligomeric structure of α-calmodulin-dependent protein kinase II. J Mol Biol. 2001;308:1–8. doi: 10.1006/jmbi.2001.4584. [DOI] [PubMed] [Google Scholar]
- 3.Brocke L, Chiang LW, Wagner PD, Schulman H. Functional implications of the subunit composition of neuronal CaM kinase II. J Biol Chem. 1999;274:22713–22722. doi: 10.1074/jbc.274.32.22713. [DOI] [PubMed] [Google Scholar]
- 4.Griffith LC. CaMKII: new tricks for an old dog. Cell. 2008;133:397–399. doi: 10.1016/j.cell.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- 6.Rich RC, Schulman H. Substrate-directed function of calmodulin in autophosphorylation of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 1998;273:28424–28429. doi: 10.1074/jbc.273.43.28424. [DOI] [PubMed] [Google Scholar]
- 7.Miller SG, Patton BL, Kennedy MB. Sequences of autophosphorylation sites in neuronal type II CaM kinase that control Ca2+-independent activity. Neuron. 1988;1:593–604. doi: 10.1016/0896-6273(88)90109-2. [DOI] [PubMed] [Google Scholar]
- 8.Fong YL, Taylor WL, Means AR, Soderling TR. Studies of the regulatory mechanism of Ca2+/calmodulin-dependent protein kinase II. Mutation of threonine 286 to alanine and aspartate. J Biol Chem. 1989;264:16759–16763. [PubMed] [Google Scholar]
- 9.Hanson PI, Kapiloff MS, Lou LL, Rosenfeld MG, Schulman H. Expression of a multifunctional Ca2+/calmodulin-dependent protein kinase and mutational analysis of its autoregulation. Neuron. 1989;3:59–70. doi: 10.1016/0896-6273(89)90115-3. [DOI] [PubMed] [Google Scholar]
- 10.Waxham MN, Aronowski J, Westgate SA, Kelly PT. Mutagenesis of Thr-286 in monomeric Ca2+/calmodulin-dependent protein kinase II eliminates Ca2+/calmodulin-independent activity. Proc Natl Acad Sci U S A. 1990;87:1273–1277. doi: 10.1073/pnas.87.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohsako S, Nakazawa H, Sekihara S, Ikai A, Yamauchi T. Role of threonine-286 as autophosphorylation site for appearance of Ca2+-independent activity of calmodulin-dependent protein kinase II α subunit. J Biochem. 1991;109:137–143. doi: 10.1093/oxfordjournals.jbchem.a123334. [DOI] [PubMed] [Google Scholar]
- 12.Colbran RJ, Soderling TR. Calcium/calmodulin-independent autophosphorylation sites of calcium/calmodulin-dependent protein kinase II. Studies on the effect of phosphorylation of threonine 305/306 and serine 314 on calmodulin binding using synthetic peptides. J Biol Chem. 1990;265:11213–11219. [PubMed] [Google Scholar]
- 13.Patton BL, Miller SG, Kennedy MB. Activation of type II calcium/calmodulin-dependent protein kinase by Ca2+/calmodulin is inhibited by autophosphorylation of threonine within the calmodulin-binding domain. J Biol Chem. 1990;265:11204–11212. [PubMed] [Google Scholar]
- 14.Benfenati F, Onofri F, Czernik AJ, Valtorta F. Biochemical and functional characterization of the synaptic vesicle-associated form of Ca2+/calmodulin-dependent protein kinase II. Brain Res Mol Brain Res. 1996;40:297–309. doi: 10.1016/0169-328x(96)00053-8. [DOI] [PubMed] [Google Scholar]
- 15.Gorelick FS, Wang JK, Lai Y, Nairn AC, Greengard P. Autophosphorylation and activation of Ca2+/calmodulin-dependent protein kinase II in intact nerve terminals. J Biol Chem. 1988;263:17209–17212. [PubMed] [Google Scholar]
- 16.Shields SM, Ingebritsen TS, Kelly PT. Identification of protein phosphatase 1 in synaptic junctions: dephosphorylation of endogenous calmodulin-dependent kinase II and synapse-enriched phosphoproteins. J Neurosci. 1985;5:3414–3422. doi: 10.1523/JNEUROSCI.05-12-03414.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukunaga K, Kobayashi T, Tamura S, Miyamoto E. Dephosphorylation of autophosphorylated Ca2+/calmodulin-dependent protein kinase II by protein phosphatase 2C. J Biol Chem. 1993;268:133–137. [PubMed] [Google Scholar]
- 18.Strack S, Barban MA, Wadzinski BE, Colbran RJ. Differential inactivation of postsynaptic density-associated and soluble Ca2+/calmodulin-dependent protein kinase II by protein phosphatases 1 and 2A. J Neurochem. 1997;68:2119–2128. doi: 10.1046/j.1471-4159.1997.68052119.x. [DOI] [PubMed] [Google Scholar]
- 19.Erickson JR, Joiner M.-l.A., Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O'Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, L. H-J, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walaas SI, Gorelick FS, Greengard P. Presence of calcium/calmodulin-dependent protein kinase II in nerve terminals of rat brain. Synapse. 1989;3:356–362. doi: 10.1002/syn.890030409. [DOI] [PubMed] [Google Scholar]
- 21.Ouimet CC, McGuinness TL, Greengard P. Immunocytochemical localization of calcium/calmodulin-dependent protein kinase II in rat brain. Proc Natl Acad Sci U S A. 1984;81:5604–5608. doi: 10.1073/pnas.81.17.5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takamori S, Holt M, Stenius K, Lemke EA, Gronborg M, Riedel D, Urlaub H, Schenck S, Brugger B, Ringler P, Muller SA, Rammner B, Grater F, Hub JS, De Groot BL, Mieskes G, Moriyama Y, Klingauf J, Grubmuller H, Heuser J, Wieland F, Jahn R. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 23.Llinas R, McGuinness TL, Leonard CS, Sugimori M, Greengard P. Intraterminal injection of synapsin I or calcium/calmodulin-dependent protein kinase II alters neurotransmitter release at the squid giant synapse. Proc Natl Acad Sci U S A. 1985;82:3035–3039. doi: 10.1073/pnas.82.9.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Llinas R, Gruner JA, Sugimori M, McGuinness TL, Greengard P. Regulation by synapsin I and Ca2+-calmodulin-dependent protein kinase II of the transmitter release in squid giant synapse. J Physiol. 1991;436:257–282. doi: 10.1113/jphysiol.1991.sp018549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nichols RA, Sihra TS, Czernik AJ, Nairn AC, Greengard P. Calcium/calmodulin-dependent protein kinase II increases glutamate and noradrenaline release from synaptosomes. Nature. 1990;343:647–651. doi: 10.1038/343647a0. [DOI] [PubMed] [Google Scholar]
- 26.Waxham MN, Malenka RC, Kelly PT, Mauk MD. Calcium/calmodulin-dependent protein kinase II regulates hippocampal synaptic transmission. Brain Res. 1993;609:1–8. doi: 10.1016/0006-8993(93)90847-g. [DOI] [PubMed] [Google Scholar]
- 27.Shakiryanova D, Klose MK, Zhou Y, Gu T, Deitcher DL, Atwood HL, Hewes RS, Levitan ES. Presynaptic ryanodine receptor-activated calmodulin kinase II increases vesicle mobility and potentiates neuropeptide release. J Neurosci. 2007;27:7799–7806. doi: 10.1523/JNEUROSCI.1879-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He X, Yang F, Xie Z, Lu B. Intracellular Ca2+ and Ca2+/calmodulin-dependent kinase II mediate acute potentiation of neurotransmitter release by neurotrophin-3. J Cell Biol. 2000;149:783–792. doi: 10.1083/jcb.149.4.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ninan I, Arancio O. Presynaptic CaMKII is necessary for synaptic plasticity in cultured hippocampal neurons. Neuron. 2004;42:129–141. doi: 10.1016/s0896-6273(04)00143-6. [DOI] [PubMed] [Google Scholar]
- 30.Jin I, Hawkins RD. Presynaptic and postsynaptic mechanisms of a novel form of homosynaptic potentiation at Aplysia sensory-motor neuron synapses. J Neurosci. 2003;23:7288–7297. doi: 10.1523/JNEUROSCI.23-19-07288.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu FM, Hawkins RD. Presynaptic and postsynaptic Ca2+ and CaMKII contribute to long-term potentiation at synapses between individual CA3 neurons. Proc Natl Acad Sci U S A. 2006;103:4264–4269. doi: 10.1073/pnas.0508162103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hinds HL, Goussakov I, Nakazawa K, Tonegawa S, Bolshakov VY. Essential function of α-calcium/calmodulin-dependent protein kinase II in neurotransmitter release at a glutamatergic central synapse. Proc Natl Acad Sci U S A. 2003;100:4275–4280. doi: 10.1073/pnas.0530202100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 34.von Gersdorff H, Borst JG. Short-term plasticity at the calyx of held. Nat Rev Neurosci. 2002;3:53–64. doi: 10.1038/nrn705. [DOI] [PubMed] [Google Scholar]
- 35.Schneggenburger R, Sakaba T, Neher E. Vesicle pools and short-term synaptic depression: lessons from a large synapse. Trends Neurosci. 2002;25:206–212. doi: 10.1016/s0166-2236(02)02139-2. [DOI] [PubMed] [Google Scholar]
- 36.Xu J, He L, Wu LG. Role of Ca2+ channels in short-term synaptic plasticity. Curr Opin Neurobiol. 2007;17:352–359. doi: 10.1016/j.conb.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Hojjati MR, van Woerden GM, Tyler WJ, Giese KP, Silva AJ, Pozzo-Miller L, Elgersma Y. Kinase activity is not required for αCaMKII-dependent presynaptic plasticity at CA3-CA1 synapses. Nat Neurosci. 2007;10:1125–1127. doi: 10.1038/nn1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Margrie TW, Rostas JA, Sah P. Presynaptic long-term depression at a central glutamatergic synapse: a role for CaMKII. Nat Neurosci. 1998;1:378–383. doi: 10.1038/1589. [DOI] [PubMed] [Google Scholar]
- 39.Stanton PK, Gage AT. Distinct synaptic loci of Ca2+/calmodulin-dependent protein kinase II necessary for long-term potentiation and depression. J Neurophysiol. 1996;76:2097–2101. doi: 10.1152/jn.1996.76.3.2097. [DOI] [PubMed] [Google Scholar]
- 40.Chapman PF, Frenguelli BG, Smith A, Chen CM, Silva AJ. The α-Ca2+/calmodulin kinase II: a bidirectional modulator of presynaptic plasticity. Neuron. 1995;14:591–597. doi: 10.1016/0896-6273(95)90315-1. [DOI] [PubMed] [Google Scholar]
- 41.Liu Q, Chen B, Ge Q, Wang ZW. Presynaptic Ca2+/calmodulin-dependent protein kinase II modulates neurotransmitter release by activating BK channels at Caenorhabditis elegans neuromuscular junction. J Neurosci. 2007;27:10404–10413. doi: 10.1523/JNEUROSCI.5634-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ledoux J, Chartier D, Leblanc N. Inhibitors of calmodulin-dependent protein kinase are nonspecific blockers of voltage-dependent K+ channels in vascular myocytes. J Pharmacol Exp Ther. 1999;290:1165–1174. [PubMed] [Google Scholar]
- 43.Nichols RA, Chilcote TJ, Czernik AJ, Greengard P. Synapsin I regulates glutamate release from rat brain synaptosomes. J Neurochem. 1992;58:783–785. doi: 10.1111/j.1471-4159.1992.tb09788.x. [DOI] [PubMed] [Google Scholar]
- 44.Chin LS, Li L, Ferreira A, Kosik KS, Greengard P. Impairment of axonal development and of synaptogenesis in hippocampal neurons of synapsin I-deficient mice. Proc Natl Acad Sci U S A. 1995;92:9230–9234. doi: 10.1073/pnas.92.20.9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferreira A, Kao HT, Feng J, Rapoport M, Greengard P. Synapsin III: developmental expression, subcellular localization, and role in axon formation. J Neurosci. 2000;20:3736–3744. doi: 10.1523/JNEUROSCI.20-10-03736.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Czernik AJ, Pang DT, Greengard P. Amino acid sequences surrounding the cAMP-dependent and calcium/calmodulin-dependent phosphorylation sites in rat and bovine synapsin I. Proc Natl Acad Sci U S A. 1987;84:7518–7522. doi: 10.1073/pnas.84.21.7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huttner WB, Schiebler W, Greengard P, De Camilli P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol. 1983;96:1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valtorta F, Villa A, Jahn R, De Camilli P, Greengard P, Ceccarelli B. Localization of synapsin I at the frog neuromuscular junction. Neuroscience. 1988;24:593–603. doi: 10.1016/0306-4522(88)90353-3. [DOI] [PubMed] [Google Scholar]
- 49.Greengard P, Valtorta F, Czernik AJ, Benfenati F. Synaptic vesicle phosphoproteins and regulation of synaptic function. Science. 1993;259:780–785. doi: 10.1126/science.8430330. [DOI] [PubMed] [Google Scholar]
- 50.De Camilli P, Harris SM, Jr., Huttner WB, Greengard P. Synapsin I (Protein I), a nerve terminal-specific phosphoprotein. II. Its specific association with synaptic vesicles demonstrated by immunocytochemistry in agarose-embedded synaptosomes. J Cell Biol. 1983;96:1355–1373. doi: 10.1083/jcb.96.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bahler M, Greengard P. Synapsin I bundles F-actin in a phosphorylation-dependent manner. Nature. 1987;326:704–707. doi: 10.1038/326704a0. [DOI] [PubMed] [Google Scholar]
- 52.Schiebler W, Jahn R, Doucet JP, Rothlein J, Greengard P. Characterization of synapsin I binding to small synaptic vesicles. J Biol Chem. 1986;261:8383–8390. [PubMed] [Google Scholar]
- 53.Benfenati F, Valtorta F, Rubenstein JL, Gorelick FS, Greengard P, Czernik AJ. Synaptic vesicle-associated Ca2+/calmodulin-dependent protein kinase II is a binding protein for synapsin I. Nature. 1992;359:417–420. doi: 10.1038/359417a0. [DOI] [PubMed] [Google Scholar]
- 54.Chi P, Greengard P, Ryan TA. Synapsin dispersion and reclustering during synaptic activity. Nat Neurosci. 2001;4:1187–1193. doi: 10.1038/nn756. [DOI] [PubMed] [Google Scholar]
- 55.Li L, Chin LS, Shupliakov O, Brodin L, Sihra TS, Hvalby O, Jensen V, Zheng D, McNamara JO, Greengard P, et al. Impairment of synaptic vesicle clustering and of synaptic transmission, and increased seizure propensity, in synapsin I-deficient mice. Proc Natl Acad Sci U S A. 1995;92:9235–9239. doi: 10.1073/pnas.92.20.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baldelli P, Fassio A, Valtorta F, Benfenati F. Lack of synapsin I reduces the readily releasable pool of synaptic vesicles at central inhibitory synapses. J Neurosci. 2007;27:13520–13531. doi: 10.1523/JNEUROSCI.3151-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ryan TA, Li L, Chin LS, Greengard P, Smith SJ. Synaptic vesicle recycling in synapsin I knock-out mice. J Cell Biol. 1996;134:1219–1227. doi: 10.1083/jcb.134.5.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosahl TW, Geppert M, Spillane D, Herz J, Hammer RE, Malenka RC, Sudhof TC. Short-term synaptic plasticity is altered in mice lacking synapsin I. Cell. 1993;75:661–670. doi: 10.1016/0092-8674(93)90487-b. [DOI] [PubMed] [Google Scholar]
- 59.Sun J, Bronk P, Liu X, Han W, Sudhof TC. Synapsins regulate use-dependent synaptic plasticity in the calyx of Held by a Ca2+/calmodulin-dependent pathway. Proc Natl Acad Sci U S A. 2006 doi: 10.1073/pnas.0511300103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gitler D, Takagishi Y, Feng J, Ren Y, Rodriguiz RM, Wetsel WC, Greengard P, Augustine GJ. Different presynaptic roles of synapsins at excitatory and inhibitory synapses. J Neurosci. 2004;24:11368–11380. doi: 10.1523/JNEUROSCI.3795-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muller M, Madan D, Levitan IB. State-dependent modulation of mSlo, a cloned calcium-dependent potassium channel. Neuropharmacology. 1996;35:877–886. doi: 10.1016/0028-3908(96)00091-3. [DOI] [PubMed] [Google Scholar]
- 62.Zhou Y, Schopperle WM, Murrey H, Jaramillo A, Dagan D, Griffith LC, Levitan IB. A dynamically regulated 14-3-3, Slob, and Slowpoke potassium channel complex in Drosophila presynaptic nerve terminals. Neuron. 1999;22:809–818. doi: 10.1016/s0896-6273(00)80739-4. [DOI] [PubMed] [Google Scholar]
- 63.Sansom SC, Ma R, Carmines PK, Hall DA. Regulation of Ca2+-activated K+ channels by multifunctional Ca2+/calmodulin-dependent protein kinase. Am J Physiol Renal Physiol. 2000;279:F283–288. doi: 10.1152/ajprenal.2000.279.2.F283. [DOI] [PubMed] [Google Scholar]
- 64.Nelson AB, Gittis AH, du Lac S. Decreases in CaMKII activity trigger persistent potentiation of intrinsic excitability in spontaneously firing vestibular nucleus neurons. Neuron. 2005;46:623–631. doi: 10.1016/j.neuron.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 65.Jiang Y, Pico A, Cadene M, Chait BT, MacKinnon R. Structure of the RCK domain from the E. coli K+ channel and demonstration of its presence in the human BK channel. Neuron. 2001;29:593–601. doi: 10.1016/s0896-6273(01)00236-7. [DOI] [PubMed] [Google Scholar]
- 66.Wang ZW, Chen B, Ge Q. Roles and sources of calcium in synaptic exocytosis. In: Wang ZW, editor. Molecular Mechanisms of Neurotransmitter Release. Humana Press; Totowa: 2008. pp. 61–84. [Google Scholar]
- 67.Jiang X, Lautermilch NJ, Watari H, Westenbroek RE, Scheuer T, Catterall WA. Modulation of CaV2.1 channels by Ca2+/calmodulin-dependent protein kinase II bound to the C-terminal domain. Proc Natl Acad Sci U S A. 2008;105:341–346. doi: 10.1073/pnas.0710213105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Furuichi T, Furutama D, Hakamata Y, Nakai J, Takeshima H, Mikoshiba K. Multiple types of ryanodine receptor/Ca2+ release channels are differentially expressed in rabbit brain. J Neurosci. 1994;14:4794–4805. doi: 10.1523/JNEUROSCI.14-08-04794.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Giannini G, Conti A, Mammarella S, Scrobogna M, Sorrentino V. The ryanodine receptor/calcium channel genes are widely and differentially expressed in murine brain and peripheral tissues. J Cell Biol. 1995;128:893–904. doi: 10.1083/jcb.128.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ouyang Y, Martone ME, Deerinck TJ, Airey JA, Sutko JL, Ellisman MH. Differential distribution and subcellular localization of ryanodine receptor isoforms in the chicken cerebellum during development. Brain Res. 1997;775:52–62. doi: 10.1016/s0006-8993(97)00840-8. [DOI] [PubMed] [Google Scholar]
- 71.De Crescenzo V, Fogarty KE, Zhuge R, Tuft RA, Lifshitz LM, Carmichael J, Bellve KD, Baker SP, Zissimopoulos S, Lai FA, Lemos JR, Walsh JV., Jr. Dihydropyridine receptors and type 1 ryanodine receptors constitute the molecular machinery for voltage-induced Ca2+ release in nerve terminals. J Neurosci. 2006;26:7565–7574. doi: 10.1523/JNEUROSCI.1512-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sharma G, Vijayaraghavan S. Modulation of presynaptic store calcium induces release of glutamate and postsynaptic firing. Neuron. 2003;38:929–939. doi: 10.1016/s0896-6273(03)00322-2. [DOI] [PubMed] [Google Scholar]
- 73.Llano I, Gonzalez J, Caputo C, Lai FA, Blayney LM, Tan YP, Marty A. Presynaptic calcium stores underlie large-amplitude miniature IPSCs and spontaneous calcium transients. Nat Neurosci. 2000;3:1256–1265. doi: 10.1038/81781. [DOI] [PubMed] [Google Scholar]
- 74.Liu Q, Chen B, Yankova M, Morest DK, Maryon E, Hand AR, Nonet ML, Wang ZW. Presynaptic ryanodine receptors are required for normal quantal size at the Caenorhabditis elegans neuromuscular junction. J Neurosci. 2005;25:6745–6754. doi: 10.1523/JNEUROSCI.1730-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Galante M, Marty A. Presynaptic ryanodine-sensitive calcium stores contribute to evoked neurotransmitter release at the basket cell-Purkinje cell synapse. J Neurosci. 2003;23:11229–11234. doi: 10.1523/JNEUROSCI.23-35-11229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Emptage NJ, Reid CA, Fine A. Calcium stores in hippocampal synaptic boutons mediate short-term plasticity, store-operated Ca2+ entry, and spontaneous transmitter release. Neuron. 2001;29:197–208. doi: 10.1016/s0896-6273(01)00190-8. [DOI] [PubMed] [Google Scholar]
- 77.Unni VK, Zakharenko SS, Zablow L, DeCostanzo AJ, Siegelbaum SA. Calcium release from presynaptic ryanodine-sensitive stores is required for long-term depression at hippocampal CA3-CA3 pyramidal neuron synapses. J Neurosci. 2004;24:9612–9622. doi: 10.1523/JNEUROSCI.5583-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 79.Witcher DR, Kovacs RJ, Schulman H, Cefali DC, Jones LR. Unique phosphorylation site on the cardiac ryanodine receptor regulates calcium channel activity. J Biol Chem. 1991;266:11144–11152. [PubMed] [Google Scholar]
- 80.Rodriguez P, Bhogal MS, Colyer J. Stoichiometric phosphorylation of cardiac ryanodine receptor on serine 2809 by calmodulin-dependent kinase II and protein kinase A. J Biol Chem. 2003;278:38593–38600. doi: 10.1074/jbc.C301180200. [DOI] [PubMed] [Google Scholar]
- 81.Wehrens XH, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res. 2004;94:e61–70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- 82.Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Sudhof TC. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- 83.Nishiki T, Augustine GJ. Synaptotagmin I synchronizes transmitter release in mouse hippocampal neurons. J Neurosci. 2004;24:6127–6132. doi: 10.1523/JNEUROSCI.1563-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mackler JM, Drummond JA, Loewen CA, Robinson IM, Reist NE. The C2B Ca2+-binding motif of synaptotagmin is required for synaptic transmission in vivo. Nature. 2002;418:340–344. doi: 10.1038/nature00846. [DOI] [PubMed] [Google Scholar]
- 85.Yoshihara M, Littleton JT. Synaptotagmin I functions as a calcium sensor to synchronize neurotransmitter release. Neuron. 2002;36:897–908. doi: 10.1016/s0896-6273(02)01065-6. [DOI] [PubMed] [Google Scholar]
- 86.Maximov A, Sudhof TC. Autonomous function of synaptotagmin 1 in triggering synchronous release independent of asynchronous release. Neuron. 2005;48:547–554. doi: 10.1016/j.neuron.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 87.Loewen C, Reist N. Synaptotagmin: transducing Ca2+-binding to vesicle fusion. In: Wang ZW, editor. Molecular Mechanisms of Neurotransmitter Release. Humana Press; Totowa: 2008. pp. 107–134. [Google Scholar]
- 88.Sudhof TC, Rizo J. Synaptotagmins: C2-domain proteins that regulate membrane traffic. Neuron. 1996;17:379–388. doi: 10.1016/s0896-6273(00)80171-3. [DOI] [PubMed] [Google Scholar]
- 89.Hilfiker S, Pieribone VA, Nordstedt C, Greengard P, Czernik AJ. Regulation of synaptotagmin I phosphorylation by multiple protein kinases. J Neurochem. 1999;73:921–932. doi: 10.1046/j.1471-4159.1999.0730921.x. [DOI] [PubMed] [Google Scholar]
- 90.Verona M, Zanotti S, Schafer T, Racagni G, Popoli M. Changes of synaptotagmin interaction with t-SNARE proteins in vitro after calcium/calmodulin-dependent phosphorylation. J Neurochem. 2000;74:209–221. doi: 10.1046/j.1471-4159.2000.0740209.x. [DOI] [PubMed] [Google Scholar]
- 91.Nagy G, Kim JH, Pang ZP, Matti U, Rettig J, Sudhof TC, Sorensen JB. Different effects on fast exocytosis induced by synaptotagmin 1 and 2 isoforms and abundance but not by phosphorylation. J Neurosci. 2006;26:632–643. doi: 10.1523/JNEUROSCI.2589-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Palfreyman MT, Jorgensen EM. Roles of SNARE proteins in synaptic vesicle fusion. In: Wang ZW, editor. Molecular Mechanisms of Neurotransmitter Release. Humana Press; Totowa: 2008. pp. 35–59. [Google Scholar]
- 93.Nielander HB, Onofri F, Valtorta F, Schiavo G, Montecucco C, Greengard P, Benfenati F. Phosphorylation of VAMP/synaptobrevin in synaptic vesicles by endogenous protein kinases. J Neurochem. 1995;65:1712–1720. doi: 10.1046/j.1471-4159.1995.65041712.x. [DOI] [PubMed] [Google Scholar]
- 94.Hirling H, Scheller RH. Phosphorylation of synaptic vesicle proteins: modulation of the α SNAP interaction with the core complex. Proc Natl Acad Sci U S A. 1996;93:11945–11949. doi: 10.1073/pnas.93.21.11945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ohyama A, Hosaka K, Komiya Y, Akagawa K, Yamauchi E, Taniguchi H, Sasagawa N, Kumakura K, Mochida S, Yamauchi T, Igarashi M. Regulation of exocytosis through Ca2+/ATP-dependent binding of autophosphorylated Ca2+/calmodulin-activated protein kinase II to syntaxin 1A. J Neurosci. 2002;22:3342–3351. doi: 10.1523/JNEUROSCI.22-09-03342.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rubenstein JL, Greengard P, Czernik AJ. Calcium-dependent serine phosphorylation of synaptophysin. Synapse. 1993;13:161–172. doi: 10.1002/syn.890130207. [DOI] [PubMed] [Google Scholar]
- 97.Fykse EM, Li C, Sudhof TC. Phosphorylation of rabphilin-3A by Ca2+/calmodulin- and cAMP-dependent protein kinases in vitro. J Neurosci. 1995;15:2385–2395. doi: 10.1523/JNEUROSCI.15-03-02385.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nat Rev Neurosci. 2006;7:921–931. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- 99.Adelman JP, Shen KZ, Kavanaugh MP, Warren RA, Wu YN, Lagrutta A, Bond CT, North RA. Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron. 1992;9:209–216. doi: 10.1016/0896-6273(92)90160-f. [DOI] [PubMed] [Google Scholar]
- 100.Atkinson NS, Robertson GA, and Ganetzky B. A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science. 1991;253:551–555. doi: 10.1126/science.1857984. [DOI] [PubMed] [Google Scholar]
- 101.Elkins T, Ganetzky B, Wu CF. A Drosophila mutation that eliminates a calcium-dependent potassium current. Proc Natl Acad Sci U S A. 1986;83:8415–8419. doi: 10.1073/pnas.83.21.8415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meera P, Wallner M, Song M, Toro L. Large conductance voltage- and calcium-dependent K+ channel, a distinct member of voltage-dependent ion channels with seven N-terminal transmembrane segments (S0-S6), an extracellular N terminus, and an intracellular (S9-S10) C terminus. Proc Natl Acad Sci U S A. 1997;94:14066–14071. doi: 10.1073/pnas.94.25.14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Butler A, Tsunoda S, McCobb DP, Wei A, Salkoff L. mSlo, a complex mouse gene encoding “maxi” calcium-activated potassium channels. Science. 1993;261:221–224. doi: 10.1126/science.7687074. [DOI] [PubMed] [Google Scholar]
- 104.Schreiber M, Salkoff L. A novel calcium-sensing domain in the BK channel. Biophys J. 1997;73:1355–1363. doi: 10.1016/S0006-3495(97)78168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yusifov T, Savalli N, Gandhi CS, Ottolia M, Olcese R. The RCK2 domain of the human BKCa channel is a calcium sensor. Proc Natl Acad Sci U S A. 2008;105:376–381. doi: 10.1073/pnas.0705261105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Krishnamoorthy G, Shi J, Sept D, Cui J. The NH2 terminus of RCK1 domain regulates Ca2+-dependent BKCa channel gating. J Gen Physiol. 2005;126:227–241. doi: 10.1085/jgp.200509321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xia XM, Zeng X, Lingle CJ. Multiple regulatory sites in large-conductance calcium-activated potassium channels. Nature. 2002;418:880–884. doi: 10.1038/nature00956. [DOI] [PubMed] [Google Scholar]
- 108.Uebele VN, Lagrutta A, Wade T, Figueroa DJ, Liu Y, McKenna E, Austin CP, Bennett PB, Swanson R. Cloning and functional expression of two families of beta-subunits of the large conductance calcium-activated K+ channel. J Biol Chem. 2000;275:23211–23218. doi: 10.1074/jbc.M910187199. [DOI] [PubMed] [Google Scholar]
- 109.Knaus HG, Garcia-Calvo M, Kaczorowski GJ, Garcia ML. Subunit composition of the high conductance calcium-activated potassium channel from smooth muscle, a representative of the mSlo and slowpoke family of potassium channels. J Biol Chem. 1994;269:3921–3924. [PubMed] [Google Scholar]
- 110.Xia XM, Ding JP, Lingle CJ. Molecular basis for the inactivation of Ca2+- and voltage-dependent BK channels in adrenal chromaffin cells and rat insulinoma tumor cells. J Neurosci. 1999;19:5255–5264. doi: 10.1523/JNEUROSCI.19-13-05255.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brenner R, Jegla TJ, Wickenden A, Liu Y, Aldrich RW. Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. J Biol Chem. 2000;275:6453–6461. doi: 10.1074/jbc.275.9.6453. [DOI] [PubMed] [Google Scholar]
- 112.McManus OB, Helms LM, Pallanck L, Ganetzky B, Swanson R, Leonard RJ. Functional role of the β subunit of high conductance calcium-activated potassium channels. Neuron. 1995;14:645–650. doi: 10.1016/0896-6273(95)90321-6. [DOI] [PubMed] [Google Scholar]
- 113.Xia XM, Ding JP, Zeng XH, Duan KL, Lingle CJ. Rectification and rapid activation at low Ca2+ of Ca2+-activated, voltage-dependent BK currents: consequences of rapid inactivation by a novel beta subunit. J Neurosci. 2000;20:4890–4903. doi: 10.1523/JNEUROSCI.20-13-04890.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Meera P, Wallner M, Toro L. A neuronal beta subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc Natl Acad Sci U S A. 2000;97:5562–5567. doi: 10.1073/pnas.100118597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Farley J, Rudy B. Multiple types of voltage-dependent Ca2+-activated K+ channels of large conductance in rat brain synaptosomal membranes. Biophys J. 1988;53:919–934. doi: 10.1016/S0006-3495(88)83173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lindgren CA, Moore JW. Identification of ionic currents at presynaptic nerve endings of the lizard. J Physiol. 1989;414:201–222. doi: 10.1113/jphysiol.1989.sp017684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tabti N, Bourret C, Mallart A. Three potassium currents in mouse motor nerve terminals. Pflugers Arch. 1989;413:395–400. doi: 10.1007/BF00584489. [DOI] [PubMed] [Google Scholar]
- 118.Morita K, Barrett EF. Evidence for two calcium-dependent potassium conductances in lizard motor nerve terminals. J Neurosci. 1990;10:2614–2625. doi: 10.1523/JNEUROSCI.10-08-02614.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sivaramakrishnan S, Bittner GD, Brodwick MS. Calcium-activated potassium conductance in presynaptic terminals at the crayfish neuromuscular junction. J Gen Physiol. 1991;98:1161–1179. doi: 10.1085/jgp.98.6.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wangemann P, Takeuchi S. Maxi-K+ channel in single isolated cochlear efferent nerve terminals. Hear Res. 1993;66:123–129. doi: 10.1016/0378-5955(93)90133-l. [DOI] [PubMed] [Google Scholar]
- 121.Katz E, Ferro PA, Cherksey BD, Sugimori M, Llinas R, Uchitel OD. Effects of Ca2+ channel blockers on transmitter release and presynaptic currents at the frog neuromuscular junction. J Physiol. 1995;486(Pt 3):695–706. doi: 10.1113/jphysiol.1995.sp020845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sun XP, Schlichter LC, Stanley EF. Single-channel properties of BK-type calcium-activated potassium channels at a cholinergic presynaptic nerve terminal. J Physiol. 1999;518(Pt 3):639–651. doi: 10.1111/j.1469-7793.1999.0639p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yazejian B, DiGregorio DA, Vergara JL, Poage RE, Meriney SD, Grinnell AD. Direct measurements of presynaptic calcium and calcium-activated potassium currents regulating neurotransmitter release at cultured Xenopus nerve-muscle synapses. J Neurosci. 1997;17:2990–3001. doi: 10.1523/JNEUROSCI.17-09-02990.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Issa NP, Hudspeth AJ. Clustering of Ca2+ channels and Ca2+-activated K+ channels at fluorescently labeled presynaptic active zones of hair cells. Proc Natl Acad Sci U S A. 1994;91:7578–7582. doi: 10.1073/pnas.91.16.7578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sun XP, Yazejian B, Grinnell AD. Electrophysiological properties of BK channels in Xenopus motor nerve terminals. J Physiol. 2004;557:207–228. doi: 10.1113/jphysiol.2003.060509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Trussell LO, Roberts MT. The role of potassium channels in the regulation of neurotransmitter release. In: Wang ZW, editor. Molecular Mechanisms of Neurotransmitter Release. Humana Press; Totowa: 2008. pp. 171–185. [Google Scholar]
- 127.Knaus HG, Schwarzer C, Koch RO, Eberhart A, Kaczorowski GJ, Glossmann H, Wunder F, Pongs O, Garcia ML, Sperk G. Distribution of high-conductance Ca2+-activated K+ channels in rat brain: targeting to axons and nerve terminals. J Neurosci. 1996;16:955–963. doi: 10.1523/JNEUROSCI.16-03-00955.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Misonou H, Menegola M, Buchwalder L, Park EW, Meredith A, Rhodes KJ, Aldrich RW, Trimmer JS. Immunolocalization of the Ca2+-activated K+ channel Slo1 in axons and nerve terminals of mammalian brain and cultured neurons. J Comp Neurol. 2006;496:289–302. doi: 10.1002/cne.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Robitaille R, Garcia ML, Kaczorowski GJ, Charlton MP. Functional colocalization of calcium and calcium-gated potassium channels in control of transmitter release. Neuron. 1993;11:645–655. doi: 10.1016/0896-6273(93)90076-4. [DOI] [PubMed] [Google Scholar]
- 130.Hu H, Shao LR, Chavoshy S, Gu N, Trieb M, Behrens R, Laake P, Pongs O, Knaus HG, Ottersen OP, Storm JF. Presynaptic Ca2+-activated K+ channels in glutamatergic hippocampal terminals and their role in spike repolarization and regulation of transmitter release. J Neurosci. 2001;21:9585–9597. doi: 10.1523/JNEUROSCI.21-24-09585.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Roberts WM, Jacobs RA, Hudspeth AJ. Colocalization of ion channels involved in frequency selectivity and synaptic transmission at presynaptic active zones of hair cells. J Neurosci. 1990;10:3664–3684. doi: 10.1523/JNEUROSCI.10-11-03664.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Augustine GJ, Santamaria F, Tanaka K. Local calcium signaling in neurons. Neuron. 2003;40:331–346. doi: 10.1016/s0896-6273(03)00639-1. [DOI] [PubMed] [Google Scholar]
- 133.Yazejian B, Sun XP, Grinnell AD. Tracking presynaptic Ca2+ dynamics during neurotransmitter release with Ca2+-activated K+ channels. Nat Neurosci. 2000;3:566–571. doi: 10.1038/75737. [DOI] [PubMed] [Google Scholar]
- 134.Marrion NV, Tavalin SJ. Selective activation of Ca2+-activated K+ channels by co-localized Ca2+ channels in hippocampal neurons. Nature. 1998;395:900–905. doi: 10.1038/27674. [DOI] [PubMed] [Google Scholar]
- 135.Davies PJ, Ireland DR, McLachlan EM. Sources of Ca2+ for different Ca2+-activated K+ conductances in neurones of the rat superior cervical ganglion. J Physiol. 1996;495(Pt 2):353–366. doi: 10.1113/jphysiol.1996.sp021599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Womack MD, Chevez C, Khodakhah K. Calcium-activated potassium channels are selectively coupled to P/Q-type calcium channels in cerebellar Purkinje neurons. J Neurosci. 2004;24:8818–8822. doi: 10.1523/JNEUROSCI.2915-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Edgerton JR, Reinhart PH. Distinct contributions of small and large conductance Ca2+-activated K+ channels to rat Purkinje neuron function. J Physiol. 2003;548:53–69. doi: 10.1113/jphysiol.2002.027854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Smith MR, Nelson AB, Du Lac S. Regulation of firing response gain by calcium-dependent mechanisms in vestibular nucleus neurons. J Neurophysiol. 2002;87:2031–2042. doi: 10.1152/jn.00821.2001. [DOI] [PubMed] [Google Scholar]
- 139.Berkefeld H, Sailer CA, Bildl W, Rohde V, Thumfart JO, Eble S, Klugbauer N, Reisinger E, Bischofberger J, Oliver D, Knaus HG, Schulte U, Fakler B. BKCa-Cav channel complexes mediate rapid and localized Ca2+-activated K+ signaling. Science. 2006;314:615–620. doi: 10.1126/science.1132915. [DOI] [PubMed] [Google Scholar]
- 140.Koyano K, Abe T, Nishiuchi Y, Sakakibara S. Effects of synthetic ω-conotoxin on synaptic transmission. Eur J Pharmacol. 1987;135:337–343. doi: 10.1016/0014-2999(87)90683-2. [DOI] [PubMed] [Google Scholar]
- 141.Kerr LM, Yoshikami D. A venom peptide with a novel presynaptic blocking action. Nature. 1984;308:282–284. doi: 10.1038/308282a0. [DOI] [PubMed] [Google Scholar]
- 142.Robitaille R, Adler EM, Charlton MP. Strategic location of calcium channels at transmitter release sites of frog neuromuscular synapses. Neuron. 1990;5:773–779. doi: 10.1016/0896-6273(90)90336-e. [DOI] [PubMed] [Google Scholar]
- 143.Robitaille R, Charlton MP. Presynaptic calcium signals and transmitter release are modulated by calcium-activated potassium channels. J Neurosci. 1992;12:297–305. doi: 10.1523/JNEUROSCI.12-01-00297.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tsien RY. A non-disruptive technique for loading calcium buffers and indicators into cells. Nature. 1981;290:527–528. doi: 10.1038/290527a0. [DOI] [PubMed] [Google Scholar]
- 145.Deisseroth K, Bito H, Tsien RW. Signaling from synapse to nucleus: postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron. 1996;16:89–101. doi: 10.1016/s0896-6273(00)80026-4. [DOI] [PubMed] [Google Scholar]
- 146.Neher E. In: In Calcium Electrogenesis and Neuronal Functioning, Experimental Brain Research Series. Heinemann U, editor. Vol. 14. Springer; Berlin: 1986. pp. 1659–1663. [Google Scholar]
- 147.Raffaelli G, Saviane C, Mohajerani MH, Pedarzani P, Cherubini E. BK potassium channels control transmitter release at CA3-CA3 synapses in the rat hippocampus. J Physiol. 2004;557:147–157. doi: 10.1113/jphysiol.2004.062661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Regehr WG, Delaney KR, Tank DW. The role of presynaptic calcium in short-term enhancement at the hippocampal mossy fiber synapse. J Neurosci. 1994;14:523–537. doi: 10.1523/JNEUROSCI.14-02-00523.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schulz PE, Cook EP, Johnston D. Changes in paired-pulse facilitation suggest presynaptic involvement in long-term potentiation. J Neurosci. 1994;14:5325–5337. doi: 10.1523/JNEUROSCI.14-09-05325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shao LR, Halvorsrud R, Borg-Graham L, Storm JF. The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J Physiol. 1999;521(Pt 1):135–146. doi: 10.1111/j.1469-7793.1999.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Faber ES, Sah P. Ca2+-activated K+ (BK) channel inactivation contributes to spike broadening during repetitive firing in the rat lateral amygdala. J Physiol. 2003;552:483–497. doi: 10.1113/jphysiol.2003.050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Loane DJ, Lima PA, Marrion NV. Co-assembly of N-type Ca2+ and BK channels underlies functional coupling in rat brain. J Cell Sci. 2007;120:985–995. doi: 10.1242/jcs.03399. [DOI] [PubMed] [Google Scholar]
- 153.Wang ZW, Saifee O, Nonet ML, Salkoff L. SLO-1 potassium channels control quantal content of neurotransmitter release at the C. elegans neuromuscular junction. Neuron. 2001;32:867–881. doi: 10.1016/s0896-6273(01)00522-0. [DOI] [PubMed] [Google Scholar]
- 154.Salkoff L, Wei AD, Baban B, Butler A, Fawcett G, Ferreira G, Santi CM. TheCelegansResearchCommunity, editor. Potassium channels in C. elegans. Wormbook. 2005 doi: 10.1895/wormbook.1.42.1. doi/10.1895/wormbook.1.42.1, http://www.wormbook.org.) [DOI] [PMC free article] [PubMed]
- 155.Xu JW, Slaughter MM. Large-conductance calcium-activated potassium channels facilitate transmitter release in salamander rod synapse. J Neurosci. 2005;25:7660–7668. doi: 10.1523/JNEUROSCI.1572-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pattillo JM, Yazejian B, DiGregorio DA, Vergara JL, Grinnell AD, Meriney SD. Contribution of presynaptic calcium-activated potassium currents to transmitter release regulation in cultured Xenopus nerve-muscle synapses. Neuroscience. 2001;102:229–240. doi: 10.1016/s0306-4522(00)00453-x. [DOI] [PubMed] [Google Scholar]