Abstract
Background & Aims
Paneth cells (PCs) secrete defensins and antimicrobial enzymes that contribute to innate immunity against pathogen infections within the mucosa of the small intestine. We examined the role of colony stimulating factor-1 (CSF-1) in PC development.
Methods
CSF-1-deficient and CSF-1 receptor (CSF-1R)-deficient mice and administration of neutralizing anti-CSF-1R antibody were used to study the requirement of CSF-1 for the development of epithelial cells of the small intestine. CSF-1 transgenic reporter mice and mice that express only the membrane-spanning, cell-surface CSF-1 isoform were used to investigate regulation by systemic versus local CSF-1.
Results
Mice deficient in CSF-1 or CSF-1R had greatly reduced numbers of mature PCs. PCs express the CSF-1R and administration of anti-CSF-1R antibody to neonatal mice significantly reduced the number of PC. Analysis of transgenic CSF-1 reporter mice showed that CSF-1-expressing cells are in close proximity to PCs. CSF-1/CSF-1R-deficient mice also had reduced numbers of the proliferating epithelial cell progenitors and lamina propria macrophages. Expression of the membrane-spanning, cell-surface CSF-1 isoform in CSF-1-deficient mice completely rescued the deficiencies of PC, proliferating progenitors and lamina propria macrophages.
Conclusions
These results indicate local regulation by CSF-1 of PC development, either directly, in a juxtacrine/paracrine manner, or indirectly, via lamina propria macrophages. Therefore, CSF-1R hyper-stimulation could be involved in hyperproliferative disorders of the small intestine, such as Crohn’s disease and ulcerative colitis.
INTRODUCTION
The small intestine (SI) progenitor cells, derived from stem cells in the crypt region, are responsible for populating the intestinal epithelium, giving rise to the three different epithelial cell types of the villi.1–5 These are the enterocytes, which secrete hydrolases, absorb nutrients and comprise more than 80% of villus epithelial cells,6 goblet cells that provide a protective mucous lining,5, 7 and rare enteroendocrine cells that secrete hormones, including serotonin, secretin and substance P.8, 9 The crypt stem cells also give rise to the Paneth cells (PC), which reside in the crypts and are the longest-lived cell type, surviving 18–22 days before degenerating and being phagocytosed by neighbouring cells. Each crypt contains 1–4 PCs, located below the stem cell zone (estimated to contain 4–6 stem cells2, 10) in the crypt base.11–14 However, recent studies with the stem cell marker, Leucine-Rich G-coupled receptor gene (Lgr-5) indicate that SI stem cells intermingle with PC at the crypt bases.15
PCs are major components of innate immunity in the gut.16–18 They produce a spectrum of anti-microbial peptides and proteins16, 17 including the α-defensins (cryptdins),19 matrilysin or matrix metalloproteinase-7 (MMP-7),20 synovial (type II) phospholipase A221 and lysozyme.22 In response to bacteria, lipopolysaccharide and cholinergic stimulation associated with feeding, PCs discharge granules containing these mediators into the crypt lumen.17, 22 PCs react to bacterial invasion by releasing α-defensins in ample quantity to kill them.23 However, the complexity of PC granule composition and the triggering of granule release by multiple stimuli, suggest that PCs may have additional functions, including regulation of intestinal inflammation, digestion, detoxification, stem cell protection and crypt development.17
CSF-1 regulation has been characterized in the CSF-1-deficient osteopetrotic (Csf1op) mutant mouse,24 which possesses an inactivating mutation in the CSF-1 gene25–28 and in mice with a targeted deletion of the sole CSF-1 cellular receptor, CSF-1R (Csf1r−/Csf1r−).29, 30 These studies showed that CSF-1 is the primary regulator of macrophage,25 osteoclast30 and Langerhans31 cell production and that all of the effects of CSF-1 are mediated via the CSF-1R.29 Immunohistochemical staining for the macrophage marker, F4/80, demonstrated that interstitial macrophage numbers are reduced in the SIs of the Csf1op/Csf1op (Csf1op/op) mice compared with their wild type (WT) littermates.25 Because of the physical proximity of interstitial macrophages to the intestinal epithelium, we investigated the role of CSF-1 and the CSF-1R in the development of the SI. We found that the absence of either CSF-1 or CSF-1R causes abnormal SI organization, reduced expression of Lgr515 and Cyclin D1, and a reduction in the number of differentiated epithelial cells. In particular, there is a dramatic reduction in PCs due to local regulation of their development and/or survival by the CSF-1.
MATERIALS AND METHODS
Mice
Csf1op/+24 and Csf1r−/+29 mice, backcrossed on the FVB/NJ background for at least 10 generations,30 were used to generate homozygous mutant and WT (+/+) control mice. Csf1op/op; TgCS5/+ mice, exclusively expressing the cell-surface isoform of CSF-1 from the TgN(CSCSF1)5Ers (TgCS5) transgene30 and TgN(Csf1-Z)9Ers homozygote (TgZ9/TgZ9) mice expressing β-gal,32 which in both cases have transgene expression driven by the same Csf1 promoter and first intron regulatory region, were also on the FVB/NJ background. The generation, genotyping and husbandry of these mice have been described previously.29, 30, 32 For the anti-CSF-1R antibody treatment, each injection comprised 100μl of 10 mg/ml of either rat anti-mouse CSF-1R (AFS-9833,34) or rat IgG (Sigma).
Histochemistry, immunohistochemistry and immunofluorescence
P14 mouse pups were perfused with periodate-lysine-2%paraformaldehyde-0.05% glutaraldehyde, pH 7.4 (PLPG),35 their intestines from the anus to stomach removed and opened longitudinally by incision along the length of the intestine, the contents removed by rinsing in PBS and the intestines fixed in PLPG overnight, prior to immersion in 70% ethanol. Paraffin embedding of the tissues was arranged such that tissue orientation could be determined. Sections were treated with periodic acid, then stained in Schiff’s reagent (0.5% pararosanaline, 1% sodium metabisulfite; PAS staining) and counterstained with hematoxylin. Sections were also stained with Alcian Blue. The mouse anti-PCNA antibody, PC-10 (1:800) (Dako, #M0879) was used to identify PCNA followed by processing with the Dako Envison+ mouse detection kit. Cryptdin 2 (1:400) and CRS4C (1:800) Immunohistochemistry was performed according to prescribed protocols,36 and developed using the ImmPress anti-rabbit detection kit (Vector Laboratories, CA, #MP-7401). Lysozyme was detected with rabbit anti-human lysozyme (1:400) (Dako, #A0099). For the immunofluorescence studies, mice were perfused with ice-cold 4% paraformaldehyde (PFA), pH 7.2 and the dissected and cleaned tissue fragments further fixed (PFA, overnight, 4°C), incubated successively in 15%, then 30% sucrose in phosphate buffered saline (PBS) (8h, 4°C each) and frozen in OCT. Sections (30 μm) were incubated with rabbit anti-human lysozyme (1/100) and affinity purified goat anti-mouse CSF-1R peptide polyclonal antibodies,37 followed by incubation with FITC-conjugated donkey anti-rabbit IgG (1/200) and TRITC-conjugated donkey anti-goat IgG (1/200) (Southern Biotech) and photography using a Olympus Bx51 upright microscope. For localization of β-galactosidase, tissues from mice perfused with PBS were fixed (1.5% PFA, 30% sucrose in PBS, pH 7.2, 8hr, 4°C), frozen in OCT and 10 μm sections stained with X-gal (overnight, 32°C) as described.38 Cell counting: longitudinal sections, blinded for genotype, were identified at 100X magnification, at which the lumen could be seen to traverse the crypt plus villus and then counted at 400X magnification. At least 40 crypts (plus villi) were examined per genotype with 3–5 mice per genotype. Data were analysed using the ANOVA program provided by the Origin 7.5R statistical package.
Quantitative RT-PCR analysis of crypt epithelium RNA
SI crypt epithelium was prepared and its purity confirmed as described.39 Real-time RT-PCR reactions were conducted on genomic DNA-depleted RNA using the Bio-Rad iQ-5 i-cycler system (Bio-Rad Laboratories, CA) and the appropriate primers (Supplementary Table 1). Gene expression was normalized to β2-microglobulin.
RESULTS
Disrupted morphology in the SIs of Csf1op/op and Csf1r−/− mice
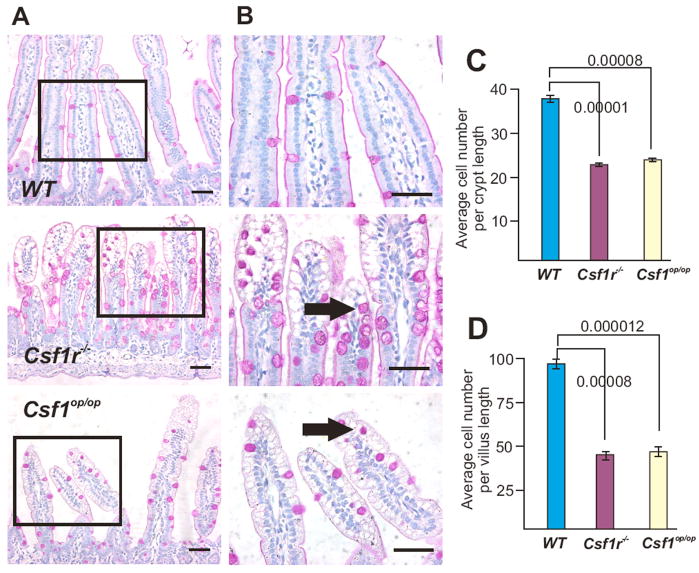
2-week-old Csf1op/op and Csf1r−/Csf1r− (Csf1r−/−) mutant FVB/NJ pups were smaller than their WT littermates, as described previously30, 40 and their SIs were shorter (Supplementary Fig. 1 online). Tissue sections stained with the neutral mucin stain, periodic acid-Schiff reagent (PAS) (Fig. 1A) or the acid mucin stain, Alcian Blue (AB) (Supplementary Fig. 2 online) revealed an aberrant goblet cell staining in the SI villi of mutant mice compared to WT littermates. The mucin-stained vacuoles in mutant villi were approximately double the diameter of those in WT villi and there was a 3.6-fold increase in the average number of PAS positive cells and a 2.3-fold increase in average AB positive cells per crypt and villus compared with WT (PAS: WT, 3.34 ± 0.29; Csf1op/op, 10.63 ± 1.33; Csf1r−/−, 11.35 ± 0.33; 50 crypts, mean ± SEM and AB: Supplementary Fig. 2 online). The villus tips were highly vacuolated with vacuoles that were mostly devoid of mucin, resembling the morphology of the villi reported in patients with malabsorption and lipid-engorgement disorders.41
Figure 1. Abnormal SI crypts and villi in two-week-old Csf1r−/− and Csf1op/op mice.
(A) Longitudinal sections stained for neutral mucins (PAS-stain) reveal intense staining of goblet cells in the SIs of the mutant mice. (B) Higher power images of the regions boxed in (A) reveal the enlarged mucin-containing deposits in goblet cells (arrows). In addition, the less ordered organization of nuclei in the mutant villi is also evident. Bars = 50 μm. (C) Numbers of cells/crypt length and (D) number of cells/villus length (mean ± SEM; n=3–5/genotype, ANOVA P-values shown).
The appearance of the mutant villi was also abnormal, with spherical enterocyte nuclei that were often more darkly basophilic than the ordered and uniformly-stained columnar nuclei in WT SI, particularly at the villus tip (Fig. 1B). The average number of cells per crypt (Fig. 1C) and per villus (Fig. 1D) was significantly reduced in both mutants. The SIs of both mutants also had reduced numbers of the rarer Chromogranin A-positive enteroendocrine cells (Supplementary Fig. 3 online), concordant with the overall reduction in enterocytes in Csf1op/op and Csf1r−/− mice. While there was no significant difference between the two mutant mice in these parameters (Fig. 1, C and D; Supplementary Fig. 3 online), the degree of architectural disorganization was more severe in Csf1r−/− than in the Csf1op/op (Fig. 1, A and B; Supplementary Fig. 2 online).
Severe depletion of mature PCs in the SIs of CSF-1- and CSF-1R-deficient mice
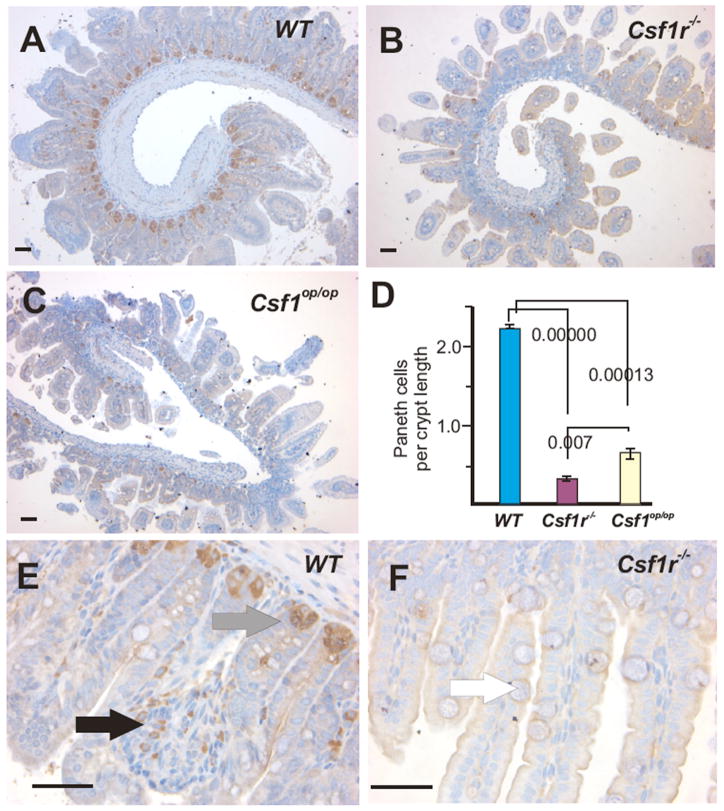
Mouse PCs appear after birth when the intestinal crypts are formed.17 PAS staining of the PC granules42 indicated that PCs were severely depleted in the mutant mouse SI crypts (data not shown). To better assess the status of PC in the mutant mice, tissue sections were stained for lysozyme,43 which showed that both mutant mice had very few PCs (Fig. 2, A–C). The number of lysozyme+ cells per crypt cross-section indicated a significant deficit in PC number that was more severe in the Csf1r−/− mice than the Csf1op/op mice (Fig. 2, D–F). Similar results were obtained with the PC markers Cryptdin 2 and CRS4C (Supplementary Figs. 4 & 5 online). The high power images also confirmed the presence of macrophage-like cells in the lamina propria of the WT25, but rarely in mutant mice (Fig. 2, E and F). The Csf1r−/− (FVB/NJ) mice do not survive beyond 4 weeks. However, a reduction in both PC and macrophages persisted in adult Csf1op/op mice (Supplementary Fig. 6 online).
Figure 2. Lysozyme staining reveals a dramatic reduction of PCs in the SI crypts of two-week-old Csf1r−/− and Csf1op/op mice.
Low power images of lysozyme+ Paneth cells in the SIs of (A) WT, (B) Csf1r−/− and (C) Csf1op/op mice. (D) Quantitation of the number of lysozyme+ cells/crypt section (mean ± SEM, n = 3, ANOVA P-values shown), revealed significant differences the number of PCs at the crypt base (grey arrow in E) between both mutant and WT, as well as between mutants. (E) Higher power images of WT crypts showing the presence of lysozyme+ cells within the lamina propria indicative of macrophages (black arrow). (F) Only background lysozyme staining is evident in the Csf1r−/− SI. White arrow indicates goblet cell with enlarged mucin body. Bar = 50μm.
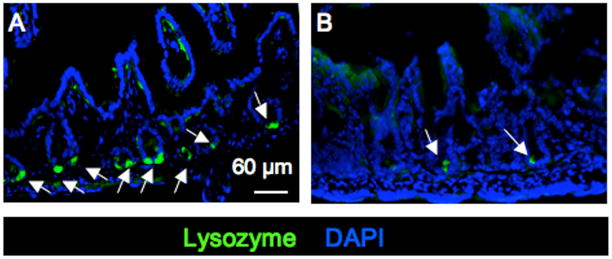
To independently evaluate the CSF-1R dependence of PC development, we subcutaneously injected neutralizing anti-CSF-1R antibody33, 34 or control immunoglobulin into WT mice from 3.5 days of age every third day until day 12.5. Mice were sacrificed at day 14 and their SIs stained for PC with anti-lysozyme antibody (Fig. 3, A and B). Consistent with the result obtained with Csf1r−/− mice (Fig. 2 D), the SIs of mice treated with anti-CSF-1R antibody versus control IgG had decreased numbers of PC (IgG-treated, 46.8 ± 7.3; CSF-1R antibody-treated, 19.0 ± 4.6; mean ± SD, counts/6 fields, p ≥ 0.005). Together, these experiments demonstrate that CSF-1 and the CSF-1R play a major role in the development and maintenance of PC.
Figure 3. Treatment of WT mice with anti-CSF-1R antibody inhibits development of PCs and villus macrophages.
Newborn mice were subcutaneously injected with (A) control IgG or (B) anti-CSF-1R antibody for 14 days prior to fixation and sectioning of the SIs and staining with anti-lysozyme antibody and DAPI. Bar = 60μm.
Reduced proliferating progenitor cells in the SIs of Csf1op/op and Csf1r−/− mice
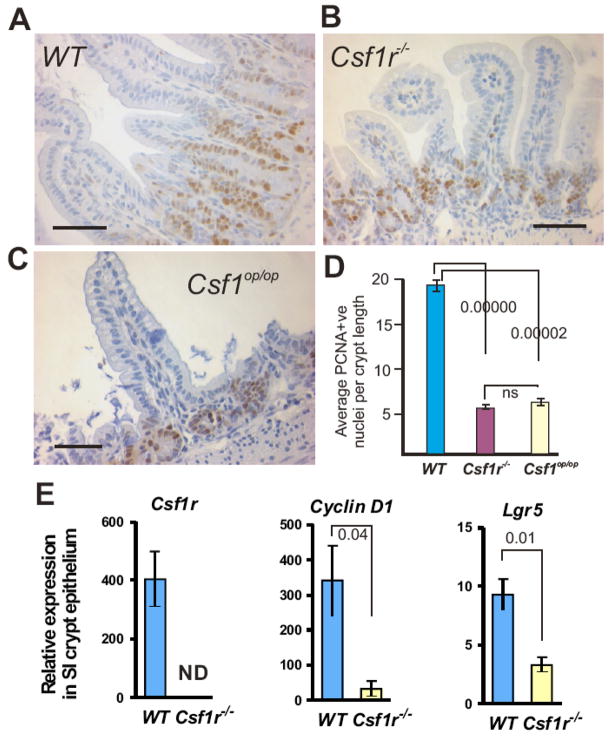
The proliferating progenitor cells that give rise to the epithelial cells of the SI (including the PC) can be identified immunohistochemically by staining for proliferating cell nuclear antigen (PCNA). Because of the smaller villus length and the reduced cell number per unit length of the crypts and villi, we examined PCNA-stained SI sections to determine the effect of the mutations on the number of proliferating progenitor cells. The distribution and extent of PCNA staining was clearly and significantly affected in the SIs of both mutant mice (Fig. 4, A–C). Apart from an overall reduction in the average number of PCNA+ cells (Fig. 4D), they were increased in frequency at the crypt base in mutant versus WT SIs at sites in which PCs are found normally. To further elucidate this proliferative defect, we isolated SI crypt epithelium and quantified the expression of a CSF-1R target gene, cyclin D144 and an intestinal stem cell marker, Lgr5, via real-time RT-PCR. Similar to the reduction of PCNA+ cell (Fig. 4D), we found a significantly decreased expression of both genes in the SI crypt of Csf1r−/− versus WT mice (Fig 4 E).
Figure 4. SI crypts of two-week-old Csf1r−/− and Csf1op/op mice display hypoproliferative defects.
PCNA staining of (A) WT (B) Csf1r−/− and (C) Csf1op/op SI reveals proliferation deficits in the mutant crypts. (D) Reduced numbers of PCNA+ nuclei/Csf1r−/− or Csf1op/op crypt compared with WT littermate crypts (mean ± SEM; n=3/genotype, 25 crypt/villi per mouse counted, ANOVA P-values shown). Bar = 50μm. (E) Quantitative RT-PCR analysis of Csf1r, cyclin D1 and Lgr5 in crypts of Csf1r−/− and WT littermates (mean ± SEM; ND = no data, n=3, ANOVA P-values shown).
PCs and SI macrophages express the CSF-1R
To determine whether the decreased numbers of PCs, enterocytes and enteroendocrine cells in Csf1op/op and Csf1r−/− mice were possibly due to the direct regulation by CSF-1, we stained WT sections using a specific (Supplementary Fig. 7 online), anti-CSF-1R peptide antibody. There was strong CSF-1R staining of macrophages in the lamina propria and below the crypt base, as previously reported25 and of PC, that co-localized with anti-lysozyme antibody staining (Fig. 5, A–C), but no staining of other SI cell types. As reported previously for lysozyme,45 the PC staining was within and outside granules (Supplementary Fig. 8 online).
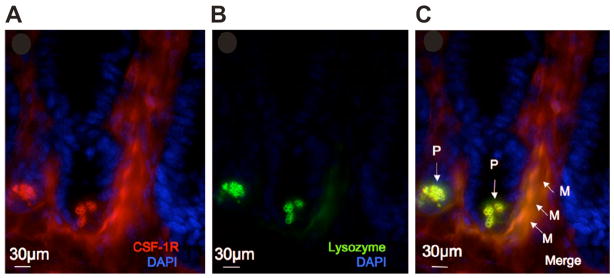
Figure 5. PCs and SI macrophages express the CSF-1R.
Immunofluorescence micrography showing: (A) CSF-1R staining (red) of cells within the crypt (PC), below the crypt and within interstitial regions of the villi (macrophages), (B) staining for lysozyme (green) and (C) merged images, showing co-localization of the CSF-1R and lysozyme staining. P, PCs; M, macrophages. Nuclei counterstained with DAPI (blue). Bar = 30μm.
CSF-1-synthesizing cells are in close proximity to PCs
Since PC express the CSF-1R, it was likely that there were CSF-1-synthesizing cells nearby. To detect them, a transgenic mouse line (TgZ9) that expresses the beta-galactosidase gene (β-gal) under the control of the Csf1 promoter and first intron was used in which nuclear expression of the β-gal reporter is driven in a cellular pattern recapitulating expression of the endogenous Csf1 gene.32 Histochemical staining of β-gal in the SIs of TgZ9/TgZ9 and non-transgenic control mice showed that CSF-1 promoter activity was absent in control tissue (Fig. 6A), but present in cells that are in close proximity to the crypt base in the region of PCs in the SIs of transgenic mice (Fig. 6, B–D).
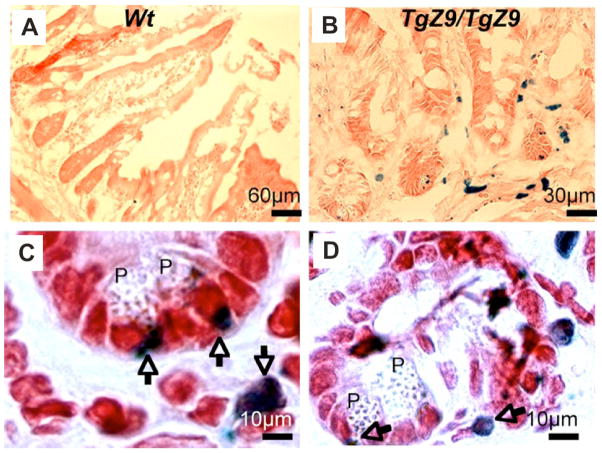
Figure 6. CSF-1 is expressed in close proximity to PCs.
Histochemical staining for beta-galactosidase in the SIs of two-week-old (A) control WT and (B–D) TgZ9/TgZ9 mice. (B), Low power and (C) and (D), high power fields, showing the close proximity of β-gal staining (blue, nuclear, arrowed) to the PCs (P). Note: There is spill-over of nuclear beta-galactosidase expression into cytoplasm in some cells.
PCs are locally regulated by cell-surface CSF-1 isoform-expressing cells
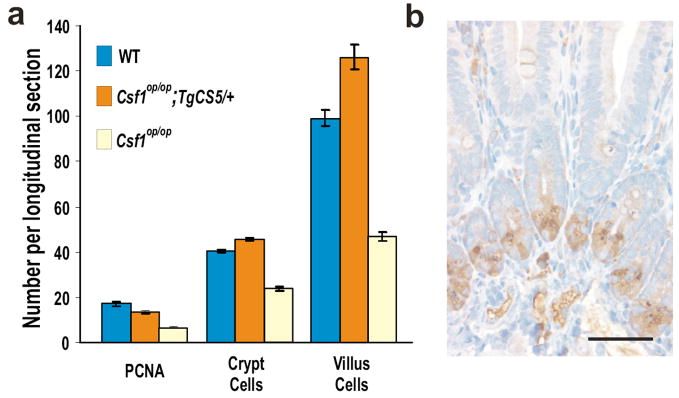
The close proximity of the CSF-1-synthesizing cells to PC suggested that the PC might be locally regulated by them. We therefore examined Csf1op/op; TgCS5/+ mice that exclusively express the cell surface isoform of CSF-1 (csCSF-1) driven by the same CSF-1 promoter and first intron sequence as used in TgZ9, and that fail to express detectable circulating CSF-1.46 Rescue of the proliferation, crypt and villus defects of Csf1op/op mice by expression of csCSF-1 was essentially complete (Fig. 7A). Importantly, normal PC numbers were also restored in mice exclusively expressing csCSF-1 (Fig. 7B) as were lamina propria macrophages (Supplementary Fig. 9 online). These results demonstrate that local expression of csCSF-1 is sufficient for PC development and/or maintenance.
Figure 7. Expression of the membrane-spanning, cell-surface isoform of CSF-1 is sufficient to rescue the SI defects of two-week-old Csf1op/op mice.
(A) Rescue of proliferating (PCNA), crypt and villus cells. (B) Rescue of PCs. Bar = 50μm.
DISCUSSION
PCs play a critical role in the innate immune response to bacteria.16–18 CSF-1 regulation of macrophages,25 trophoblastic cells,47 and Langerhans cells31 is important for innate immunity in many tissues.48 We have shown that both PCs and macrophages in the SI express high levels of the CSF-1R and that, as for macrophages,25 the development of PCs is largely dependent on the CSF-1R. Furthermore, CSF-1-synthesizing cells are found in close proximity to PCs and normal regulation of PC development was observed when, in the absence of circulating CSF-1, these cells exclusively expressed membrane-spanning csCSF-1. These results indicate that CSF-1 locally regulates PC development.
Apart from the PC and macrophage deficiency, other changes contributed to the disruption of SI architecture observed in the Csf1op/op and/or Csf1r−/− mice. First, there was a reduction in the major epithelial cell population, the villus columnar cells. Second, there was an increase in both the number of the goblet cells and their cytoplasmic volume. Third, there was a reduction in Chromogranin A-positive enteroendocrine cells. Fourth, consistent with the overall reduction in mature SI epithelial cells, the number of proliferating progenitor cells in the crypt was significantly reduced. Finally, concordant with the decrease in proliferating progenitor cells, we observed a reduction in the expression of cyclin D1 and Lgr5 in the SI crypts of Csf1r−/− mice.
The increase in the number of goblet cells may be a consequence of CSF-1R-regulated PC development. Previous studies showed that PCs and goblet cells share an intermediate cell precursor with shared features of both cell types.14, 49 When PC differentiation of these or other precursors was inhibited by transgenic, PC-specific expression of SV40 T antigen, the survival, proliferation and differentiation of the intermediate cell to mature goblet cells in the upper crypt and lower half of the villus was increased,49 Thus, the increase in the number of goblet cells we observed when PC development was inhibited by removal of the CSF-1R, is consistent with a default fate of the intermediate cell precursor to goblet cell differentiation.
The reduction in non-PC epithelial cells of the columnar/enterocyte and enteroendocrine lineages, together with the overall decrease in progenitor cell proliferation and reduction in expression of the intestinal stem cell marker, Lgr515, indicates that CSF-1R signaling controls progenitor cell proliferation and may regulate lineage determination at the stem/progenitor cell level. However, CSF-1R expression was detected only in PC and interstitial macrophages (Fig. 5 and data not shown) suggesting that this regulation of the CSF-1R-negative stem/progenitor cells is indirect. It is unlikely that the deficits we have observed in the Csf1r−/− mice are secondary to the loss of PC, since transgenic ablation of PCs had no qualitative or quantitative effects on the other three SI cell lineages or on the stem cell compartment.49 On the other hand, given growing evidence for the role of CSF-1 and macrophages in the development50 and regeneration51 of epithelia and in the regulation of malignant epithelial cells,52 it is possible that the interstitial macrophages of the lamina propria contribute to the regulation of progenitor cell expansion and commitment and that their loss in CSF-1/CSF-1R deficiency leads to the deficits in these SI lineages. Indeed, while PC expression of the CSF-1R, their close proximity to CSF-1-expressing cells and their normal development in mice exclusively expressing csCSF-1 are consistent with direct juxtacrine/paracrine regulation of PC by CSF-1, our experiments do not formally exclude indirect regulation of PC development by the lamina propria macrophages, which are also co-ordinately rescued by csCSF-1 expression.
The demonstration that locally produced CSF-1 regulates PC development is consistent with studies suggesting that terminal differentiation of the SI epithelial lineages is largely cell non-autonomous and apparently dependent on signals from specific positions along the crypt-villus axis.53 Previous studies have implicated the WNT signaling pathway transduced through β-catenin/TCF4, in maintaining the undifferentiated state of intestinal epithelial progenitor cells,54, 55 as well as PC specification56. Furthermore, loss of the adenomatous polyposis coli (Apc) gene, which normally negatively regulates WNT signalling,5 perturbed differentiation along the enterocyte, goblet and enteroendocrine lineages, while promoting partial differentiation to the PC lineage through β-catenin/TCF4 regulated transcription of specific markers of PC.57 Also, conditional removal of the common Notch pathway transcription factor, CSL/RBP-J decreases proliferative cells and increases goblet cells.58 It will be of interest to determine how regulation by the CSF-1R is related to WNT- and Notch- regulated differentiation.
The CSF-1R could directly or indirectly mediate commitment to the PC lineage, support the survival of committed cells, or regulate aspects of PC differentiation. While CSF-1 plays a major role in PC development, the effects of CSF-1R deficiency were more severe than those of CSF-1 deficiency (Fig. 2 D). It has been suggested that this difference, observed for other phenotypes, is due to the existence of another CSF-1R ligand.29 Indeed, it is possible that regulation by the novel CSF-1R ligand, interleukin-34,59 is responsible. In addition, the small residual PC development in Csf1r−/− mice suggests a secondary role of a non-CSF-1R ligand.
Independent of its regulation of PC development, CSF-1 could also regulate PC function. CSF-1 is known to prime the macrophage response to bacterial lipopolysaccharide60 and lipopolysaccharide enhances CSF-1 synthesis and release from CSF-1-synthesizing cells.61 If CSF-1 has a similar role in regulating the PC response to bacteria, increased bacteria-induced local expression of CSF-1 in the SI can be expected to contribute to regulation of the anti-bacterial response of PC. The CSF-1-synthesizing cell type in the crypt region is not known. A possible candidate is the subepithelial myofibroblast,62 a morphological hybrid of a smooth muscle cell and a fibroblast, that continuously migrate upward from the crypt base.63
The demonstration that CSF-1 is required for PC genesis and normal SI development has important implications for our understanding of intestinal development and disease. Accumulating evidence suggests that defective PC differentiation and function underpins intestinal disease.16, 18, 64–67 For example, diminished defensin production has recently been associated with Crohn’s disease and ulcerative colitis.66 PCs also secrete the anti-bacterial, pro-inflammatory phospholipase A2, expression of which also suppresses the development of intestinal adenomas in APCmin mutant mice16 as well as matrilysin, a protease essential for the correct processing of the multiple defensin precursor proteins and for maximum tumor invasion.68 In addition, relevant to our findings, administration of anti-CSF-1 antibody inhibits the development of dextran sulfate sodium-induced colitis in mice.69 Taken together, these data indicate a regulatory role of CSF-1 in PC development and the need for further investigation of the biological roles of CSF-1 in the intestine.
Supplementary Material
Acknowledgments
Grant support: This work was supported by the National Health and Medical Research Council of Australia, the Cancer Council of Victoria (R.G.R.), National Institutes of Health grant CA32551 (E.R.S), the Albert Einstein College of Medicine Cancer Center grant 5P30-CA13330, an American Society of Hematology Fellow Scholar Award (X-M. D.), and a Leukaemia and Lymphoma Society Special Fellow Award (X-M.D). We thank Drs. Paul Jubinsky and Y. G. Yeung for critically reviewing the manuscript and Dr. Xiao-Hua Zong and Ranu Basu for technical assistance and Dr. Jenny Karlsson Sjöberg (Karolinska Institutet, Sweden) for the Cryptdin 2 and CRS4C antibodies.
Abbreviations used in this paper
- AB
alcian blue
- CSF-1
colony stimulating factor
- CSF-1R
colony stimulating factor receptor
- PAS
periodic acid Schiff
- PC
Paneth cell
- PCNA
proliferating cell nuclear antigen
- SI
small intestine
Footnotes
Financial disclosure: All authors report no conflicting financial interest concerning the submitted material.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat. 1974;141:537–61. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- 2.Potten CS. Stem cells in gastrointestinal epithelium: numbers, characteristics and death. Philos Trans R Soc Lond B Biol Sci. 1998;353:821–30. doi: 10.1098/rstb.1998.0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Potten CS. Radiation, the ideal cytotoxic agent for studying the cell biology of tissues such as the small intestine. Radiat Res. 2004;161:123–36. doi: 10.1667/rr3104. [DOI] [PubMed] [Google Scholar]
- 4.Sancho E, Batlle E, Clevers H. Live and let die in the intestinal epithelium. Curr Opin Cell Biol. 2003;15:763–70. doi: 10.1016/j.ceb.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Sancho E, Batlle E, Clevers H. Signaling pathways in intestinal development and cancer. Annu Rev Cell Dev Biol. 2004;20:695–723. doi: 10.1146/annurev.cellbio.20.010403.092805. [DOI] [PubMed] [Google Scholar]
- 6.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. I Columnar cell. Am J Anat. 1974;141:461–79. doi: 10.1002/aja.1001410403. [DOI] [PubMed] [Google Scholar]
- 7.Cheng H. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. II Mucous cells. Am J Anat. 1974;141:481–501. doi: 10.1002/aja.1001410404. [DOI] [PubMed] [Google Scholar]
- 8.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. III Entero-endocrine cells. Am J Anat. 1974;141:503–19. doi: 10.1002/aja.1001410405. [DOI] [PubMed] [Google Scholar]
- 9.Hocker M, Raychowdhury R, Plath T, Wu H, O’Connor DT, Wiedenmann B, Rosewicz S, Wang TC. Sp1 and CREB mediate gastrin-dependent regulation of chromogranin A promoter activity in gastric carcinoma cells. J Biol Chem. 1998;273:34000–7. doi: 10.1074/jbc.273.51.34000. [DOI] [PubMed] [Google Scholar]
- 10.Marshman E, Booth C, Potten CS. The intestinal epithelial stem cell. Bioessays. 2002;24:91–8. doi: 10.1002/bies.10028. [DOI] [PubMed] [Google Scholar]
- 11.Cheng H. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. IV Paneth cells. Am J Anat. 1974;141:521–35. doi: 10.1002/aja.1001410406. [DOI] [PubMed] [Google Scholar]
- 12.Cheng H, Merzel J, Leblond CP. Renewal of Paneth cells in the small intestine of the mouse. Am J Anat. 1969;126:507–25. doi: 10.1002/aja.1001260409. [DOI] [PubMed] [Google Scholar]
- 13.Elmes ME. The Paneth cell population of the small intestine of the rat-effects of fasting and zinc deficiency on total count and on dithizone-reactive count. J Pathol. 1976;118:183–91. doi: 10.1002/path.1711180308. [DOI] [PubMed] [Google Scholar]
- 14.Troughton WD, Trier JS. Paneth and goblet cell renewal in mouse duodenal crypts. J Cell Biol. 1969;41:251–68. doi: 10.1083/jcb.41.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–7. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 16.Keshav S. Paneth cells: leukocyte-like mediators of innate immunity in the intestine. J Leukoc Biol. 2006;80:500–8. doi: 10.1189/jlb.1005556. [DOI] [PubMed] [Google Scholar]
- 17.Porter EM, Bevins CL, Ghosh D, Ganz T. The multifaceted Paneth cell. Cell Mol Life Sci. 2002;59:156–70. doi: 10.1007/s00018-002-8412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wehkamp J, Stange EF. Paneth cells and the innate immune response. Curr Opin Gastroenterol. 2006;22:644–50. doi: 10.1097/01.mog.0000245541.95408.86. [DOI] [PubMed] [Google Scholar]
- 19.Huttner KM, Selsted ME, Ouellette AJ. Structure and diversity of the murine cryptdin gene family. Genomics. 1994;19:448–53. doi: 10.1006/geno.1994.1093. [DOI] [PubMed] [Google Scholar]
- 20.Wilson CL, Heppner KJ, Rudolph LA, Matrisian LM. The metalloproteinase matrilysin is preferentially expressed by epithelial cells in a tissue-restricted pattern in the mouse. Mol Biol Cell. 1995;6:851–69. doi: 10.1091/mbc.6.7.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiyohara H, Egami H, Shibata Y, Murata K, Ohshima S, Ogawa M. Light microscopic immunohistochemical analysis of the distribution of group II phospholipase A2 in human digestive organs. J Histochem Cytochem. 1992;40:1659–64. doi: 10.1177/40.11.1431054. [DOI] [PubMed] [Google Scholar]
- 22.Satoh Y, Ishikawa K, Tanaka H, Oomori Y, Ono K. Immunohistochemical observations of lysozyme in the Paneth cells of specific-pathogen-free and germ-free mice. Acta Histochem. 1988;83:185–8. doi: 10.1016/S0065-1281(88)80055-2. [DOI] [PubMed] [Google Scholar]
- 23.Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol. 2000;1:113–8. doi: 10.1038/77783. [DOI] [PubMed] [Google Scholar]
- 24.Marks SC, Jr, Lane PW. Osteopetrosis, a new recessive skeletal mutation on chromosome 12 of the mouse. J Hered. 1976;67:11–18. doi: 10.1093/oxfordjournals.jhered.a108657. [DOI] [PubMed] [Google Scholar]
- 25.Cecchini MG, Dominguez MG, Mocci S, Wetterwald A, Felix R, Fleisch H, Chisholm O, Hofstetter W, Pollard JW, Stanley ER. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development. 1994;120:1357–72. doi: 10.1242/dev.120.6.1357. [DOI] [PubMed] [Google Scholar]
- 26.Pollard JW, Stanley ER. Pleiotropic roles for CSF-1 in development defined by the mouse mutation osteopetrotic. Advances in Developmental Biochemistry. 1996;4:153–193. [Google Scholar]
- 27.Wiktor-Jedrzejczak W, Bartocci A, Ferrante AW, Jr, Ahmed-Ansari A, Sell KW, Pollard JW, Stanley ER. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci U S A. 1990;87:4828–32. doi: 10.1073/pnas.87.12.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo T, Shultz LD, Nishikawa S. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345:442–4. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- 29.Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, Sylvestre V, Stanley ER. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002;99:111–20. doi: 10.1182/blood.v99.1.111. [DOI] [PubMed] [Google Scholar]
- 30.Dai XM, Zong XH, Akhter MP, Stanley ER. Osteoclast deficiency results in disorganized matrix, reduced mineralization, and abnormal osteoblast behavior in developing bone. J Bone Miner Res. 2004;19:1441–51. doi: 10.1359/JBMR.040514. [DOI] [PubMed] [Google Scholar]
- 31.Ginhoux F, Tacke F, Angeli V, Bogunovic M, Loubeau M, Dai XM, Stanley ER, Randolph GJ, Merad M. Langerhans cells arise from monocytes in vivo. Nat Immunol. 2006;7:265–73. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryan GR, Dai XM, Dominguez MG, Tong W, Chuan F, Chisholm O, Russell RG, Pollard JW, Stanley ER. Rescue of the colony-stimulating factor 1 (CSF-1)-nullizygous mouse (Csf1(op)/Csf1(op)) phenotype with a CSF-1 transgene and identification of sites of local CSF-1 synthesis. Blood. 2001;98:74–84. doi: 10.1182/blood.v98.1.74. [DOI] [PubMed] [Google Scholar]
- 33.Sudo T, Nishikawa S, Ogawa M, Kataoka H, Ohno N, Izawa A, Hayashi S, Nishikawa S. Functional hierarchy of c-kit and c-fms in intramarrow production of CFU-M. Oncogene. 1995;11:2469–76. [PubMed] [Google Scholar]
- 34.Murayama T, Yokode M, Kataoka H, Imabayashi T, Yoshida H, Sano H, Nishikawa S, Nishikawa S, Kita T. Intraperitoneal administration of anti-c-fms monoclonal antibody prevents initial events of atherogenesis but does not reduce the size of advanced lesions in apolipoprotein E-deficient mice. Circulation. 1999;99:1740–6. doi: 10.1161/01.cir.99.13.1740. [DOI] [PubMed] [Google Scholar]
- 35.McLean IW, Nakane PK. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974;22:1077–83. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- 36.Karlsson J, Putsep K, Chu H, Kays RJ, Bevins CL, Andersson M. Regional variations in Paneth cell antimicrobial peptide expression along the mouse intestinal tract. BMC Immunol. 2008;9:37. doi: 10.1186/1471-2172-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buscher D, Dello Sbarba P, Hipskind RA, Rapp UR, Stanley ER, Baccarini M. v-raf confers CSF-1 independent growth to a macrophage cell line and leads to immediate early gene expression without MAP-kinase activation. Oncogene. 1993;8:3323–32. [PubMed] [Google Scholar]
- 38.Hennighausen L, Wall RJ, Tillmann U, Li M, Furth PA. Conditional gene expression in secretory tissues and skin of transgenic mice using the MMTV-LTR and the tetracycline responsive system. J Cell Biochem. 1995;59:463–72. doi: 10.1002/jcb.240590407. [DOI] [PubMed] [Google Scholar]
- 39.Whitehead RH, Demmler K, Rockman SP, Watson NK. Clonogenic growth of epithelial cells from normal colonic mucosa from both mice and humans. Gastroenterology. 1999;117:858–65. doi: 10.1016/s0016-5085(99)70344-6. [DOI] [PubMed] [Google Scholar]
- 40.Nandi S, Akhter MP, Seifert MF, Dai XM, Stanley ER. Developmental and functional significance of the CSF-1 proteoglycan chondroitin sulfate chain. Blood. 2006;107:786–95. doi: 10.1182/blood-2005-05-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Owens SR, Greenson JK. The pathology of malabsorption: current concepts. Histopathology. 2007;50:64–82. doi: 10.1111/j.1365-2559.2006.02547.x. [DOI] [PubMed] [Google Scholar]
- 42.Sorokin SP, Hoyt RF., Jr PAS-lead hematoxylin as a stain for small-granule endocrine cell populations in the lungs, other pharyngeal derivatives and the gut. Anat Rec. 1978;192:245–59. doi: 10.1002/ar.1091920205. [DOI] [PubMed] [Google Scholar]
- 43.Chung LP, Keshav S, Gordon S. Cloning the human lysozyme cDNA: inverted Alu repeat in the mRNA and in situ hybridization for macrophages and Paneth cells. Proc Natl Acad Sci U S A. 1988;85:6227–31. doi: 10.1073/pnas.85.17.6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsushime H, Roussel MF, Ashmun RA, Sherr CJ. Colony-stimulating factor 1 regulates novel cyclins during the G1 phase of the cell cycle. Cell. 1991;65:701–13. doi: 10.1016/0092-8674(91)90101-4. [DOI] [PubMed] [Google Scholar]
- 45.Mathan M, Hughes J, Whitehead R. The morphogenesis of the human Paneth cell. An immunocytochemical ultrastructural study. Histochemistry. 1987;87:91–6. doi: 10.1007/BF00518730. [DOI] [PubMed] [Google Scholar]
- 46.Dai XM, Zong XH, Sylvestre V, Stanley ER. Incomplete restoration of colony-stimulating factor 1 (CSF-1) function in CSF-1-deficient Csf1op/Csf1op mice by transgenic expression of cell surface CSF-1. Blood. 2004;103:1114–23. doi: 10.1182/blood-2003-08-2739. [DOI] [PubMed] [Google Scholar]
- 47.Guleria I, Pollard JW. The trophoblast is a component of the innate immune system during pregnancy. Nat Med. 2000;6:589–93. doi: 10.1038/75074. [DOI] [PubMed] [Google Scholar]
- 48.Chitu V, Stanley ER. Colony-stimulating factor-1 in immunity and inflammation. Curr Opin Immunol. 2006;18:39–48. doi: 10.1016/j.coi.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 49.Garabedian EM, Roberts LJ, McNevin MS, Gordon JI. Examining the role of Paneth cells in the small intestine by lineage ablation in transgenic mice. J Biol Chem. 1997;272:23729–40. doi: 10.1074/jbc.272.38.23729. [DOI] [PubMed] [Google Scholar]
- 50.Lin EY, Gouon-Evans V, Nguyen AV, Pollard JW. The macrophage growth factor CSF-1 in mammary gland development and tumor progression. J Mammary Gland Biol Neoplasia. 2002;7:147–62. doi: 10.1023/a:1020399802795. [DOI] [PubMed] [Google Scholar]
- 51.Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci U S A. 2005;102:99–104. doi: 10.1073/pnas.0405979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goswami S, Sahai E, Wyckoff JB, Cammer M, Cox D, Pixley FJ, Stanley ER, Segall JE, Condeelis JS. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 2005;65:5278–83. doi: 10.1158/0008-5472.CAN-04-1853. [DOI] [PubMed] [Google Scholar]
- 53.Hermiston ML, Wong MH, Gordon JI. Forced expression of E-cadherin in the mouse intestinal epithelium slows cell migration and provides evidence for nonautonomous regulation of cell fate in a self-renewing system. Genes Dev. 1996;10:985–96. doi: 10.1101/gad.10.8.985. [DOI] [PubMed] [Google Scholar]
- 54.Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–20. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 55.Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–83. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 56.Andreu P, Peignon G, Slomianny C, Taketo MM, Colnot S, Robine S, Lamarque D, Laurent-Puig P, Perret C, Romagnolo B. A genetic study of the role of the Wnt/beta-catenin signalling in Paneth cell differentiation. Dev Biol. 2008;324:288–96. doi: 10.1016/j.ydbio.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 57.Andreu P, Colnot S, Godard C, Gad S, Chafey P, Niwa-Kawakita M, Laurent-Puig P, Kahn A, Robine S, Perret C, Romagnolo B. Crypt-restricted proliferation and commitment to the Paneth cell lineage following Apc loss in the mouse intestine. Development. 2005;132:1443–51. doi: 10.1242/dev.01700. [DOI] [PubMed] [Google Scholar]
- 58.van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, Clevers H. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–63. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 59.Lin H, Lee E, Hestir K, Leo C, Huang M, Bosch E, Halenbeck R, Wu G, Zhou A, Behrens D, Hollenbaugh D, Linnemann T, Qin M, Wong J, Chu K, Doberstein SK, Williams LT. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science. 2008;320:807–11. doi: 10.1126/science.1154370. [DOI] [PubMed] [Google Scholar]
- 60.Sweet MJ, Hume DA. CSF-1 as a regulator of macrophage activation and immune responses. Arch Immunol Ther Exp (Warsz) 2003;51:169–77. [PubMed] [Google Scholar]
- 61.Roth P, Bartocci A, Stanley ER. Lipopolysaccharide induces synthesis of mouse colony-stimulating factor-1 in vivo. J Immunol. 1997;158:3874–80. [PubMed] [Google Scholar]
- 62.Adegboyega PA, Mifflin RC, DiMari JF, Saada JI, Powell DW. Immunohistochemical study of myofibroblasts in normal colonic mucosa, hyperplastic polyps, and adenomatous colorectal polyps. Arch Pathol Lab Med. 2002;126:829–36. doi: 10.5858/2002-126-0829-ISOMIN. [DOI] [PubMed] [Google Scholar]
- 63.Marsh MN, Trier JS. Morphology and cell proliferation of subepithelial fibroblasts in adult mouse jejunum. II Radioautographic studies. Gastroenterology. 1974;67:636–45. [PubMed] [Google Scholar]
- 64.Grimm MC, Pavli P. NOD2 mutations and Crohn’s disease: are Paneth cells and their antimicrobial peptides the link? Gut. 2004;53:1558–60. doi: 10.1136/gut.2004.043307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wehkamp J, Harder J, Weichenthal M, Schwab M, Schaffeler E, Schlee M, Herrlinger KR, Stallmach A, Noack F, Fritz P, Schroder JM, Bevins CL, Fellermann K, Stange EF. NOD2 (CARD15) mutations in Crohn’s disease are associated with diminished mucosal alpha-defensin expression. Gut. 2004;53:1658–64. doi: 10.1136/gut.2003.032805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wehkamp J, Schmid M, Fellermann K, Stange EF. Defensin deficiency, intestinal microbes, and the clinical phenotypes of Crohn’s disease. J Leukoc Biol. 2005;77:460–5. doi: 10.1189/jlb.0904543. [DOI] [PubMed] [Google Scholar]
- 67.Wehkamp J, Schmid M, Stange EF. Defensins and other antimicrobial peptides in inflammatory bowel disease. Curr Opin Gastroenterol. 2007;23:370–8. doi: 10.1097/MOG.0b013e328136c580. [DOI] [PubMed] [Google Scholar]
- 68.Ii M, Yamamoto H, Adachi Y, Maruyama Y, Shinomura Y. Role of matrix metalloproteinase-7 (matrilysin) in human cancer invasion, apoptosis, growth, and angiogenesis. Exp Biol Med (Maywood) 2006;231:20–7. doi: 10.1177/153537020623100103. [DOI] [PubMed] [Google Scholar]
- 69.Marshall D, Cameron J, Lightwood D, Lawson AD. Blockade of colony stimulating factor-1 (CSF-I) leads to inhibition of DSS-induced colitis. Inflamm Bowel Dis. 2007;13:219–24. doi: 10.1002/ibd.20055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.