Abstract
Keratinocytes are important for the acute phase of herpes simplex virus 1 (HSV-1) infection and subsequent persistence in sensory nervous tissue. In this study, we showed that keratinocytes (HEL-30) were refractory to IFNγ induction of an antiviral state to HSV-1 infection, while IFNγ did induce an antiviral state in fibroblasts (L929). This led us to examine the possible role of suppressor of cytokine signaling-1 (SOCS-1) in this refractiveness. RT-PCR analysis of SOCS-1 mRNA expression in HSV-1 infected cells showed a four-fold increase for keratinocytes while having a negligible effect on fibroblasts. A similar pattern was observed at the level of SOCS-1 protein induction. Activation of STAT1α in keratinocytes was inhibited by HSV-1 infection. A direct effect of HSV-1 on the SOCS-1 promoter was shown in a luciferase reporter gene assay. We have developed a small peptide antagonist of SOCS-1, pJAK2(1001-1013), that had both an antiviral effect in keratinocytes against HSV-1 as well as a synergistic effect on IFNγ induction of an antiviral state. HSV-1 ICP0 mutant was inhibited by IFNγ in HEL-30 cells and was less effective than wild type virus in induction of SOCS-1 promoter. We conclude that SOCS-1 plays an important role in the antiviral effect of IFNγ in keratinocytes infected with HSV-1. The use of SOCS-1 antagonist to abrogate this refractiveness could have a transformational effect on therapy against viral infections.
INTRODUCTION
Herpes Simplex Virus (HSV) is a member of a broad class of double-stranded DNA viruses that undergo replication in the cell nucleus. Examples of other members are varicella-zoster virus (VZV) and cytomegalovirus (CMV) (1). It is estimated that HSV-1 infects 60 to 80 percent of the people throughout the world, and persists for life in the infected individuals (2-4). Primary infection commonly occurs through cells of the mucous membrane and is often asymptomatic. This is followed by uptake of virus by sensory nerve fibers and retrograde transport to the cell body of the neurons in the dorsal root or trigeminal ganglion. Here, acute infection is converted to latency and from which HSV-1 periodically migrates down the nerve tissue to again infect mucosal cells for overt disease (1-4).
HSV-1 infection is characterized by a strong cytokine response in infected cells, particularly the induction of type I IFNs (4). Infection of keratinocytes, for example, results in induction of large amounts of IFNα and IFNβ as well as interleukins 1, 6, and β-chemokines (5). IFNs, macrophages, natural killer (NK) cells, and gamma/delta T cells all play an important role in host innate immune response to HSV-1 (4). Toll-like receptor (TLR) 2 is activated on the cell surface by HSV-1, while TLR-9 is activated intracellularly by viral DNA. The latter stimulus is thought to play an important role in induction of IFNα by HSV-1 (4).
The adaptive immune response plays an important role in confining HSV-1 and other herpesvirus infections to a latent state where CD8+ T cells and IFNγ play critical roles (6-8). It is functionally connected to the innate immune system where NK cells can serve as a source of IFNγ, which is also produced by CD4+ and CD 8+ T cells. IFNγ can exert direct antiviral activity as well as induce upregulation of MHC class I and class II molecules on macrophages, dendritic cells, and keratinocytes (8). Direct effects of IFNγ as per a mouse model suggest that this IFN prevents reactivation of HSV by inhibition of function of the key intermediate protein ICP0 (9). Interaction of the antigen presenting cells with CD4+ T cells induces CD8+ T cells to control HSV-1 levels in mucosal lesions (10, 11).
HSV-1 has developed several mechanisms to inhibit both the innate and adaptive immune responses to infection. HSV-1 downregulation of class I MHC expression occurs through high affinity binding of viral immediate early gene product ICP47 to the transporter associated with antigen processing (TAP) (12), which blocks IFNγ induction of cytotoxic CD8+ T cells (13). IFN signaling is also inhibited by blockage of JAK/STAT transcription factor phosphorylation by an unknown mechanism (14). ICP0 is thought to enhance proteasome-dependent degradation of IFN stimulated genes (ISGs) (15, 16). A recent study suggests that HSV-1 can exert an anti-interferon effect by activation of a protein called suppressor of cytokine signaling 3 (SOCS-3) (17).
SOCS consists of a family of inducible proteins that regulate the JAK/STAT transcription system that is critical in mediation of functions of cytokines such as the IFNs. These inducible proteins share domains of homology that characterize the SOCS family, which consists of eight identified members, SOCS-1 to SOCS-7 and cytokine induced SH2 protein (CIS) (18-20). All of the SOCS proteins contain a SH2 domain and a C terminal SOCS box domain that is involved in proteasomal degradation of SOCS-associated proteins. SOCS-1 and SOCS-3 also contain a kinase inhibitory region (KIR) of 12 amino acids that, in conjunction with SH2, inhibits JAK tyrosine kinase activity (18-20). Thus, these SOCS-1 and SOCS-3 molecules can regulate cytokine function by proteasomal degradation and inhibition of the relevant JAK activity (18, 20).
Currently, there are no effective therapeutics available against HSV infection, except the nucleoside analog acyclovir (22), which is known to have serious side effects. A search for a vaccine against HSV has remained elusive because of the successful adaptation to the host used by HSV (3). Along with direct effects, infection with HSV has been found to increase the incidence of HIV infection, probably due to HSV-associated lesions (23). Because of this interplay between HSV and HIV, it is conceivable that anti-HSV treatment may reduce the incidence of infection with HIV. Thus, the use of a SOCS-1 antagonist described here that taps into the host immune response could represent a useful strategy to treat HSV infection as well as help in the reduction of HIV-1 infections.
We have observed that fibroblast and keratinocyte cell lines derived from C3H mice (L929 fibroblasts and HEL-30 keratinocytes) respond differentially to IFNγ induction of an antiviral state against HSV-1. HEL-30 keratinocytes produced large amounts of SOCS-1 mRNA and protein, while L929 cells showed minimal increase in SOCS-1 when treated with IFNγ following infection with HSV-1. An antiviral state was induced in L929 fibroblasts but not in HEL-30 keratinocytes. We report here that the relative resistance of keratinocytes to IFN therapy is due to the hyperinduction of SOCS-1 in these cells.
MATERIALS AND METHODS
Cell Culture and Virus
HEL-30 keratinocytes (Dr. D. Germolec, NIEHS, Durham, NC), L929 fibroblasts (CCL-1, ATCC, Manassas, VA), and Vero cells (CCL-81, ATCC) were cultured in DMEM supplemented with 10% BCS. Cells were plated into 75 cm2 tissue culture flasks and incubated at 37° C, 95% air/5% CO2 in a humidified incubator. HSV-1 (syn 17+) (provided initially by Dr. Nancy Sawtell, Children's Hospital Medical Center, Cincinnati, OH) was routinely passaged and titrated in Vero cells. HSV-1 ICP0 mutant, designated as dl1403 (24), was obtained from Dr. Rick Thompson (Univ Cincinnati, Cincinnati, OH), and was grown and titrated in U2OS cells (HTB-96, ATCC). U2OS cell were grown in McCoy's 5A medium with 10% FBS. Mouse macrophage cell line RAW 264.7 was grown in RPMI with 10% FBS.
Peptides
The amino acid sequences for the peptide mimetics used in this study shown in Table I. The peptides were synthesized on an Applied Biosystems 9050 automated peptide synthesizer using conventional fluorenylmethyloxycarbonyl chemistry as previously described (25). The addition of a lipophilic group (palmitoyllysine) to the N terminus of the synthetic peptide was performed as a last step, using semiautomated protocol (26). Peptides were characterized by mass spectrometry and were purified by HPLC. All peptides were dissolved in DMSO at a concentration of 10 mg/mL. Peptides were diluted in cell culture medium prior to addition to cells.
Table I.
List of peptides used in this study.
| Peptides | Sequences |
|---|---|
| Tkip | WLVFFVIFYFFR |
| Tkip2A | WLVFFVIAYFAR |
| SOCS1-KIR | 53DTHFRTFRSHSDYRRI |
| SOCS1-KIR2A | 53DTHFATFASHSDYRRI |
| pJAK2(1001-1013) | 1001LPQDKEYYKVKEP |
| MuIFNγ(95-106) | 95AKFEVNNPQVQR |
| MuIFNγ(95-125) | 95AKFEVNNPQVQRQAFNELIRVVHQLLPESSL |
| MuIFNγ(95-132) | 95AKFEVNNPQVQRQAFNELIRVVHQLLPESSLRKRKRSR |
| MuIFNGR1(253-287) | 253TKKNSFKRKSIMLPKSLLSVVKSATLETKPESKYS |
All the peptides were synthesized with an attached lipophilic group, palmitic acid, for cell penetration. MuIFNγ(95-106), MuIFNγ(95-125), and MuIFNGR1(253-287) were used as control peptides. These peptides do not show significant biological activity in the assays for which they have been used as control peptides.
Cytopathic Effect Inhibition Assay
L929 fibroblasts or HEL-30 keratinocytes were cultured and counted in a hemacytometer, added at densities of 2.0 ×104 to 3.0×104 to each well of a multiwell cell culture plate and incubated overnight. The following day, recombinant murine interferon gamma (IFN-γ) (Peprotech, Rocky Hill, NJ), IFN-γ peptide mimetics, SOCS-1 mimetic peptide, SOCS-1 antagonist peptide, or IFNγ mimetic peptide were added to the cultures at the indicated concentrations and incubated for 24 hours. At 100% confluence, culture medium was aspirated, cells were rinsed with PBS and HSV-1 added at an MOI of 0.1. Two days post-infection, medium was aspirated, cells were washed twice with 1x HBSS and fixed by addition of 10% formalin. Fixative was removed and cell layers were stained with crystal violet. Plates were rinsed with dH2O and dried overnight. Plates were scanned on an HP ScanJet 5300C or photographed using a Fuji LAS-300 CCD camera. Densitometry measurements of each well were computed using Multi-gauge software (FujiFilm USA, Burbank, CA) or NIH Image-J.
HSV-1 infection of Monolayer Cultures
Cells were seeded into 35 mm culture dishes at a density of 1×104 cells/cm2 and allowed to grow to ∼75% confluence. Culture medium was aspirated and monolayers washed with 1x PBS. HSV-1 diluted in minimum essential medium (MEM) containing 2% calf serum (CS) was added to the culture medium at the indicated MOI and the cell cultures incubated for 2 hours at 37°C. Medium was removed and replaced with MEM containing 10% CS.
RNA isolation and Quantitation
RNA was collected from cells at specified times after infection. Total RNA was isolated by using RNeasy mini kits (Qiagen Inc., Valencia, CA) according to the manufacturers' instructions. Samples were eluted 2x in a volume of 20 μL. RNA concentration was determined by measuring absorbance at 260 and 280 nm and purity calculated using ratios of absorbance at 260 and 280 nm (260/280). RNA integrity was checked by formaldehyde agarose gel electrophoresis. Briefly, each sample was added to one well of a 1.2% formaldehyde/agarose gel with ethidium bromide and electrophoresed at 5 V/cm. Bands were visualized with UV light and documented by capture with a CCD camera (Fujifilm USA, Burbank, CA).
RT-PCR
Briefly, 2 μg of total RNA from each experimental sample was used in a reverse transcriptase (RT) reaction. Reaction conditions were: 1x RT buffer, 0.5 mM dNTP, 1 μM oligo-dT primer, 10 U/μL RNase inhibitor, and 4 U/μL RT enzyme in a total reaction volume of 20 μL. Each sample was incubated at 37° C for 1 hour. Each completed RT reaction mix was added to a PCR master mix. The resulting PCR cocktail was aliquoted (25 μL) into PCR tubes containing appropriate primers for the gene of interest. PCR was performed with 30 cycles of the following program: 30 sec at 95° C, 30 sec at 55° C, and 30 sec at 70° C. Following the completion of PCR, 10 μL of each sample was electrophoresed through a 2% agarose gel at 5 V/cm. Images were captured using a Fuji CCD camera. Data was normalized to expression of a housekeeping gene (GAPDH) and expressed as percent of control.
Western Blotting
HEL-30 keratinocytes or L929 fibroblasts were plated into cell culture plates and allowed to grow overnight. Cells were infected with HSV-1 as described above. The virus was removed and fresh DMEM containing 10% BCS added. At the indicated time points, medium was removed and cells were rinsed 3x with PBS. Cells were then lysed with Complete Lysis Buffer M (Roche Diagnostics, Indianapolis, IN) by following manufacturer's suggestions. Equal amounts of lysate were combined with 6x Laemelli buffer and resolved by SDS-PAGE. Proteins were electro-blotted overnight onto PVDF. Membranes were blocked for one hour with 5% non-fat milk/TBS-Tween. Membranes were incubated with primary antibody to SOCS-1 (Millipore, Temecula, CA.) STAT-1, or p-STAT-1 (Santa Cruz Biotech, Santa Cruz, CA). Membranes were rinsed 3x with TBS-Tween and then incubated with secondary antibody. Protein bands were resolved by chemiluminescence. Images were captured as before using a Fuji CCD camera.
Cloning of SOCS-1 reporter constructs
A DNA fragment containing the human SOCS-1 promoter was amplified using genomic DNA purified from WISH cells. The forward and reverse primers used for amplification were 5′-TTTGCTAGCTCTTCCGCAGCCGGGTAGTG-3′ and 5′-TCCAAGCTTTACAGAAGGGGCCAGCCGGA-3′, respectively. The following conditions were used for PCR. 94° C, 30 sec; 62° C, 30 sec; 68° C, 90 sec; for 30 cycles. The PCR fragment was purified and digested with Nhe I and Hind III and ligated with pGL3 basic reporter plasmid (Promega, Madison, WI) expressing firefly luciferase, digested with similar enzymes. The sequence of the reporter plasmid thus generated, which contained nucleotides −1577 to −3 of the promoter was confirmed by DNA sequencing.
Luciferase Assay
HEL-30 cells were plated into 12-well cell culture dishes and allowed to grow overnight. Cells were co-transfected with the plasmids expressing SOCS-1 promoter linked firefly luciferase and a constitutively expressed Renilla luciferase using GeneJammer (Stratagene, La Jolla, CA) transfection reagent for HEL-30 cells or Metafectene (Biontex Laboratories GmBh, Martinsreid, Germany) for L929 cells. Relative luciferase units were measured by using a dual luciferase assay kit form Promega (Madison, WI). Twenty-four hours after transfection, cells were either treated with 2000 U/mL murine IFN-γ or infected with HSV-1 at an moi of 2.0 for 4 hours prior to treatment with IFN-γ. After treatment, cells were lysed with Passive Lysis Buffer. Lysates were then assayed for luciferase activity using Dual Luciferase Assay Kit (Promega, Madison, WI). Luciferase levels were normalized to levels of the constitutive reporter.
SOCS-1 Transfection
HEL-30 cells or L929 cells were plated into 12-well cell culture dishes and allowed to grow overnight. Cells were transfected with a construct containing the full-length murine SOCS-1 gene, pFLAG-SOCS-1 (a kind gift of Dr. Douglas Hilton, Walter and Eliza Hall Institute, Victoria, Australia). Briefly, L929 cells at ∼90% confluence were transfected with indicated amounts of pFLAG-SOCS-1 using Metafectene Pro (Biontex Laboratories GmbH, Martinsreid, Germany). Twenty-four hours later, cells were lysed and extracts used for Western blotting to confirm expression of the SOCS-1 protein.
Statistics
GraphPad Prism 5 software from GraphPad software, Inc. (La Jolla, CA) was used to determine the statistical significance of different treatments.
RESULTS
IFNγ induces an antiviral state against HSV-1 in fibroblasts but not keratinocytes
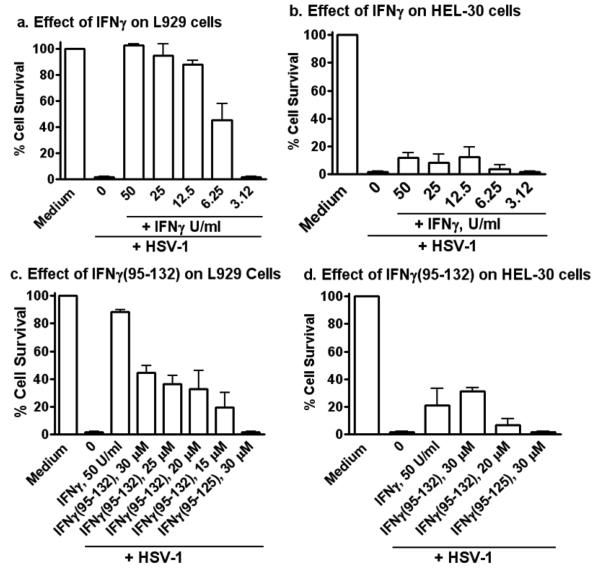
Keratinocytes are important for HSV-1 replication in the epidermis, which plays a role in infection of nervous tissue (1). We were therefore interested in determining the ability of IFNγ to inhibit HSV-1 replication in HEL-30 keratinocytes relative to L929 fibroblasts (Figure 1). IFNγ at concentrations of 12.5 to 50 U/ml protected fibroblasts infected with HSV-1 at an moi of 0.1 (Figure 1a), while HEL-30 keratinocytes were susceptible to HSV-1-mediated lysis (CPE) in the presence of IFNγ (Figure 1b). Specifically, HEL-30 keratinocytes were lysed in the presence of IFNγ, while the fibroblasts were protected. These observations suggest a possible basis for the successful pathogenesis of HSV-1 in keratinocytes even in the presence of IFNγ and also provide an approach to possible prevention of HSV-1 pathogenesis.
Figure 1.
Differential response to IFNγ and IFNγ mimetic peptide in fibroblasts (L929) and keratinocytes (HEL-30). L929 fibroblasts (a and c) and HEL-30 keratinocytes (b and d) were grown overnight and treated with IFN-γ (a and b), or IFNγ mimetic (c and d) at the indicated concentrations for 24 hours, after which HSV-1 (syn 17+) was added at an moi of 0.1. Cells were incubated for 48 hours, rinsed with HBSS, fixed and stained with crystal violet. Densitometry measurements of each well were computed using Multi-gauge software for quantitation of cell survival of the various treatment groups. Data are expressed as percentage of background corrected for medium control. Error bars indicate standard error of the means. The experiments were carried out in triplicate and data are representative of two independent experiments. There were statistically significant differences between IFNγ and or IFNγ peptide treated cells when compared to the untreated cells (P < 0.001) as determined by Mann-Whitney signed rank test.
We have developed a small peptide mimetic of mouse IFNγ that consists of the C-terminus of IFNγ with an attached palmitate for plasma membrane penetration (Table I) (25). The mimetic contains an essential alpha helix and polycationic nuclear localization sequence. It binds to the cytoplasmic domain of the IFNγ receptor subunit, IFNGR1, and participates in activation of STAT1α and transport of a complex of STAT1α and IFNGR1 to the GAS promoter element in genes specifically activated by IFNγ (28). The mimetic peptide, IFNγ (95-132), inhibited HSV-1-induced CPE at 15 to 30 μM in L929 fibroblasts in a manner similar to IFNγ (Figure 1c), while having little or no antiviral effect in HEL-30 keratinocytes (Figure 1d). Thus, the IFNγ mimetic showed similar but slightly less HSV-1 inhibition patterns to IFNγ in keratinocytes versus fibroblasts.
HSV-1 infection induces SOCS-1 expression in HEL-30 keratinocytes but not in L929 fibroblasts
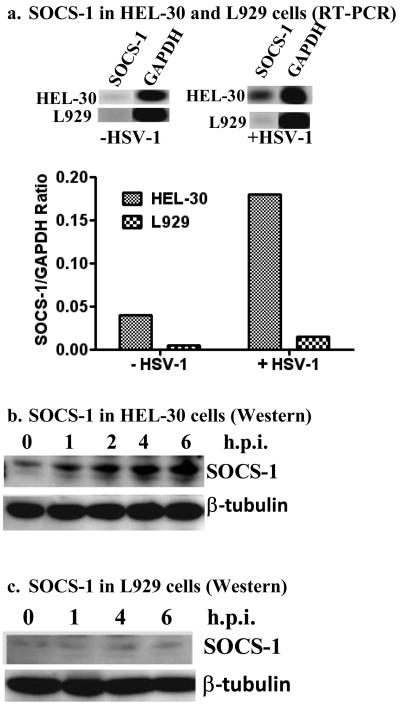
IFN activity is negatively regulated by SOCS-1, so we infected HEL-30 keratinocytes and L929 fibroblasts with HSV-1 to investigate possible differential induction of SOCS-1. RT-PCR analysis of SOCS-1 mRNA expression in HSV-1 (moi of 1) infected fibroblasts and keratinocytes showed an increase of 4-fold for keratinocytes while having a negligible effect on fibroblasts (Figure 2a). Expression of SOCS-1 mRNA was normalized to the levels of GAPDH mRNA expression and presented as fold induction over uninfected cells. Levels of GAPDH or another housekeeping gene β-tubulin did not change during these treatments. At the protein level, HSV-1 infected HEL-30 fibroblasts showed increased levels by Western blots up to 6 hours post-infection as shown in Figure 2b. By comparison, there was minimal to no effect on induction of SOCS-1 protein in L929 fibroblasts (Figure 2c). RT-PCR analysis of SOCS-1 mRNA over time showed that similar levels were observed from 1 to 6 hours post-infection with HSV-1 (Figure 2d). Further, the level of SOCS-1 gene activation was similar at 1 and 2 moi and enhanced at 5 moi (data not shown). Thus, HSV-1 differentially induced the activation of SOCS-1 as per RT-PCR and Western blot analysis of HEL-30 and L929 cells, which is consistent with the ability of IFNγ to inhibit HSV-1 induced CPE in L929 fibroblasts but not in HEL-30 keratinocytes.
Figure 2.
HSV-1 infection causes induction of SOCS-1 in keratinocytes (HEL-30 cells), but not in fibroblasts (L929 cells). (a). HEL-30 and L929 cells were infected with HSV-1 at an moi of 1 for 12 hours. Total RNA was extracted and used as a template for RT-PCR using primers specific for SOCS-1 or GAPDH, the control. Data are presented as the ratio of SOCS-1 to GAPDH. HEL-30 (b). and L929 (c). cells exhibit differences in SOCS-1 protein expression as analyzed by Western blot analysis. Cells were infected with HSV-1 at an moi of 2. At the indicated time points, cells were harvested, washed, lysed, and whole-cell extracts were isolated. Lysates were subjected to 10% SDS-PAGE. Proteins were blotted onto a PVDF membrane and probed with an antibody specific for SOCS-1. Membranes were stripped and re-probed with β-tubulin to control for equal loading of samples. (d) Time course of SOCS-1 induction in HEL-30 cells. Cells were infected at an moi of 2 for the times indicated. RNA was extracted and used for RT-PCR as in (a) above. Results are indicative of three independent experiments.
IFNγ activation of STAT1α is inhibited in HEL-30 keratinocytes infected with HSV-1
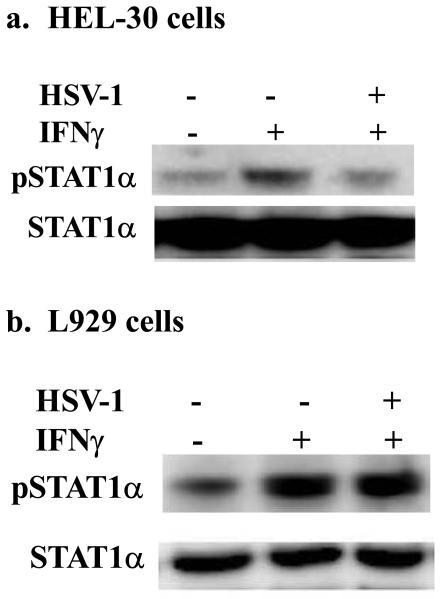
Activation of STAT1α transcription factor by the IFNγ/IFNγ receptor complex is critical for induction of the antiviral state (28). Accordingly, we determined the effect of HSV-1 infection of HEL-30 keratinocytes and L929 fibroblasts on IFNγ activation of STAT1α in these cells. As shown in Figure 3a, treatment of HEL-30 keratinocytes with IFNγ activated STAT1α as indicated by tyrosine phosphorylation. Treatment of cells that were infected with HSV-1 reduced phosphorylation to the basal level. By contrast, the activation of STAT1α in the presence of HSV-1 infection of L929 cells was not inhibited (Figure 3b). Activation of STAT1α is dependent on the tyrosine kinase JAK2 and we have shown that SOCS-1 via the kinase inhibitory region (KIR) inhibits JAK2 function by binding to its activation loop (29). Thus, induction of SOCS-1 in HEL-30 keratinocytes is consistent with inhibition of STAT1α activation by IFNγ and the resultant failure of IFNγ to induce an antiviral state in HEL-30 cells.
Figure 3.
Inhibition of STAT1α phosphorylation upon HSV-1 infection in HEL-30, but not in L929 cells. HEL-30 (a) or L929 (b) cells were mock treated or infected with HSV-1 at an moi of 2 was used in 4 hour incubation. Some of the infected cells were also treated with 1000 U/ml IFN-γ for 10 minutes. Cells were washed in PBS, harvested, lysed, and whole-cell extracts were isolated. Extracts were subjected to 10% SDS-PAGE. Proteins were blotted onto a PVDF membrane and probed with an antibody specific for pSTAT-1α (Tyr 701). Filters were stripped and re-probed with antibody to total STAT1α to ascertain equal loading of proteins.
HSV-1 infection of HEL-30 keratinocytes increases transcription from the SOCS-1 promoter
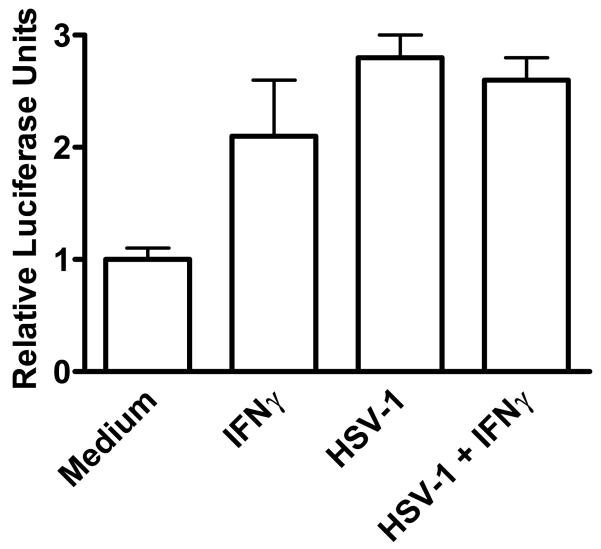
The dramatic increase in SOCS-1 mRNA and protein in HSV-1 infected HEL-30 keratinocytes, would suggest an effect on the SOCS-1 promoter. Accordingly, we fused the SOCS-1 promoter (nucleotides -1577 to -3) to the luciferase reporter gene and transfected this reporter plasmid into HEL-30 cells. As shown in Figure 4, treatment of the cells with 2000 U/ml of IFNγ caused a two-fold increase in relative luciferase activity. Remarkably, infection of the cells with HSV-1 increased luciferase activity approximately three-fold that was not reduced in the presence of IFNγ. These results are consistent with the induction of endogenous SOCS-1 message and protein in HEL-30 keratinocytes infected with HSV-1 in the absence and presence of IFNγ and is explanatory of the refractiveness to the induction of an antiviral state to HSV-1 in these cells.
Figure 4.
HSV-1 infection increases transcription from the SOCS-1 promoter. HEL-30 cells were cultured overnight in 12-well plates and transfected with a luciferase reporter construct containing the full-length SOCS-1 promoter. Cells were incubated for 24 hours and then mock-infected or infected with HSV-1 at an moi of 2 for 4 hours, after which they were treated with IFN-γ at 2000 U/ml for 2 hours. Cell lysates were collected and luciferase activity was measured in a single-tube luminometer. Values given are expressed as luciferase units measured from the SOCS-1 reporter divided by luciferase units measured from a co-transfected constitutive reporter and subsequently normalized to medium controls. Values are representative of triplicate wells of the two independent experiments. The experiments were carried out in triplicate and data are representative of two independent experiments. There were statistically significant differences between IFNγ, HSV-1, and HSV-1 + IFNγ treated cells when compared to the untreated cells (P < 0.001) as determined by Mann-Whitney signed rank test.
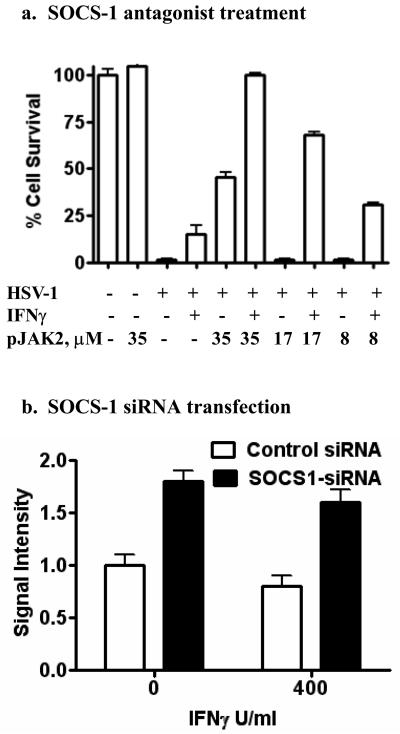
A peptide antagonist of SOCS-1 synergizes with IFNγ to induce an antiviral state against HSV-1 in HEL-30 keratinocytes
We have developed an antagonist of SOCS-1 that consists of a peptide corresponding to the activation loop of JAK2 (27). We synthesized the peptide, pJAK2(1001-1013), with a phosphotyrosine at position 1007, corresponding to the activation state of JAK2 (30). pJAK2(1001-1013) binds to the KIR region of SOCS-1, enhances IFNγ activity, reverses SOCS-1 inhibition of STAT activation, and enhances GAS promoter activity of IFNγ (27). We attached a palmitate group to pJAK2(1001-1013) for cell penetration and determined if it could synergize with IFNγ in induction of an antiviral state in HEL-30 cells infected with HSV-1.
As shown in Figure 5a, HEL-30 cells infected with HSV-1 were minimally protected by 100 U/ml of IFNγ alone. pJAK2(1001-1013) at 35 μM showed approximately 40 percent protection, while 17 and 8 μM were not protective. Combined treatment of infected cells with 100 U/ml of IFNγ and 35 μM of pJAK2(1001-1013) resulted in 100 percent protection against HSV-1. This protection was concentration-dependent as 17 and 8 μM of pJAK2(1001-1013) and 100 U/ml IFNγ resulted in approximately 70 and 25 percent protection, respectively. The IFNγ mimetic also synergized with the SOCS-1 antagonist (data not shown). These results provide further evidence that the refractiveness to induction of an antiviral state in HEL-30 cells to HSV-1 is due to induction of SOCS-1. Additionally, the results suggest an approach to counter the induction of SOCS-1 by viruses as a mechanism to avoid IFN induction of an antiviral state in cells.
Figure 5.
A peptide antagonist of SOCS-1 (a) or siRNA for SOCS-1 (b) reduced HSV-1-induced CPE in HEL-30 cells. (a). HEL-30 cells were cultured overnight in 96-well plates and treated with 100 U/ml IFN γ alone or different concentrations of pJAK2(1001-1013) with or without 100 U/ml of IFNγ. Following treatment for 24 hours, cells were mock-infected or infected with HSV-1 at an moi of 0.1. Plates were incubated 48 hours, washed with PBS, fixed and stained with crystal violet. Absorbance units of each well were calculated using Multi-Gauge. Values are expressed as percent cell survival relative to mock-infected controls. Values are representative of duplicate wells of two independent experiments. (b). HSV-1-induced cytopathic effect is reduced by treatment with SOCS-1 siRNA. HEL-30 cells were transfected with control or SOCS-1 siRNA, incubated for 48 hours, then treated with IFN-γ for 6 hours, and subsequently infected with 100 pfu HSV-1 (syn17+). At 72 hours post-infection, the cells were fixed and stained with crystal violet. Plates were scanned using a flatbed scanner. Densitometry measurements of each well were made using NIH Image J.
As a correlate to the peptide antagonist experiment, we transfected HEL-30 cells with SOCS-1 siRNA and determined the relative protection against HSV-1 in the presence and absence of 400 U/ml of IFNγ. As shown in Figure 5b, siRNA alone provided similar protection to that observed in combination with IFNγ. A control siRNA, by comparison was relatively non-protective. Thus, the pJAK2(1001-1013) protection is supported by similar protection with SOCS-1 siRNA transfected HEL-30 cells infected with HSV-1.
Consistent with inhibition of HSV-1 replication in L929 fibroblasts by IFNγ mimetic peptide IFNγ(95-132) as determined by CPE, yield reductions in L929 cells demonstrate that the IFNγ mimetic at 50 μM and 25 μM inhibited plaque formation by 77- fold and by 14-fold, respectively (Table II). The SOCS-1 antagonist pJAK2(1001-1013) had a negligible effect on HSV-1 replication in L929 cells at 25 μM (also at 50 μM, data not shown). This is consistent with the lack of induction of SOCS-1 in these cells by HSV-1. The yield reduction assay indicates that the protections observed were due to inhibition of virus replication rather than to some nonreplicative toxic effect of HSV-1 on the cells.
Table II.
Yield reduction of HSV-1 in L929 fibroblasts treated with IFNγ mimetic and SOCS-1 antagonist.
| Treatment | Virus Yield (PFU/ml) |
Fold Reduction |
|---|---|---|
| Untreated | 7 × 107 | - |
| pJAK2 (25 μM) | 4 × 107 | 2 |
| IFNγ(95-132) (25 μM) | 5 × 106 | 14 |
| IFNγ(95-132) (50 μM) | 9 × 105 | 77 |
L929 cells (1.6 × 107 per well in 6-well plates) were treated with peptides for 24 h, followed by infection with HSV-1 at moi 0.001 for 24 h. Cell lysates and supernatants were collected and viral yield was determined by standard plaque assay.
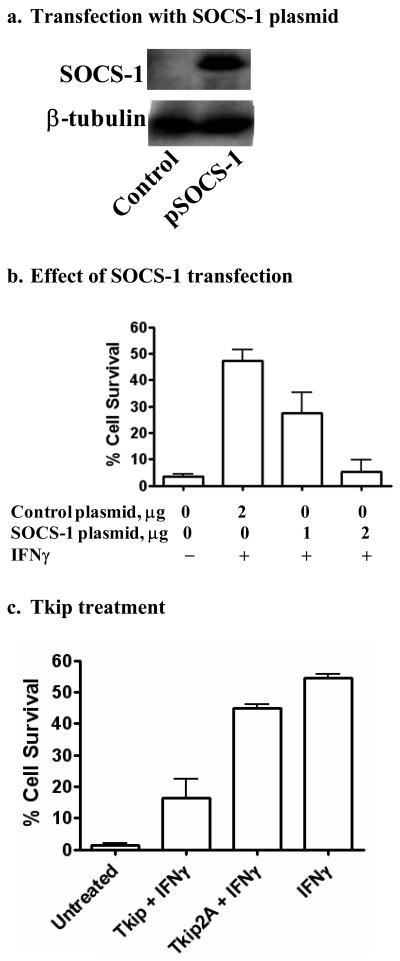
Overexpression of SOCS-1 in L929 fibroblasts results in inhibition of IFNγ induction of an antiviral state to HSV-1
As we have shown, HSV-1 infection of L929 fibroblasts does not cause significant induction of SOCS-1 and does not inhibit induction of an antiviral state by IFNγ. Accordingly, we transfected L929 cells with SOCS-1 expression plasmid (Figure 6a), and determined if this would cause a blockage of IFNγ induction of an antiviral state against HSV-1. As shown in Figure 6b, IFNγ induced an antiviral state in cells transfected with two μg of control plasmid, while one and two μg of SOCS-1 expression plasmid transfected cells showed a dose-dependent reduction in the ability of IFNγ to induce an antiviral state.
Figure 6.
Overexpression of SOCS-1 inhibits the antiviral activity of IFN-γ in HSV-1-infected L929 cells. (a). Transfection with cDNA expressing SOCS-1. L929 cells were transfected with SOCS-1 expression plasmid for one day. Cell extracts were then electrophoresed and probed with an antibody to SOCS-1 followed by stripping and probing with β-tubulin antibody as a control. (b). L929 fibroblasts were grown overnight in 12 well-plates to ∼90% confluence. Cells were transfected with the indicated amounts of control plasmid or pFLAG-SOCS-1 plasmid. Cells were incubated for 24 hours and treated with 100 U/ml IFNγ for 24 hours. Cells were washed once and infected with HSV-1 (syn 17+) at an moi of 0.1, and then incubated for 48 hours, washed with HBSS, fixed, and stained with crystal violet. Absorbance units of each well were calculated using Multi-Gauge software. (c). HSV-1-induced CPE is increased in L929 cells treated with a SOCS-1 peptide mimetic. L929 fibroblasts were grown overnight and treated with IFN-γ (100 U/ml) and Tkip (20 μM), or Tkip2A (20 μM) for 24 hours, after which HSV-1 (syn 17+) was added at an moi of 0.1. Cells were incubated for 48 hours, washed with HBSS, fixed and stained with crystal violet as in (a) above. Values are expressed as percent cell survival relative to mock-infected controls. Results are representative of duplicate wells of two independent experiments.
We have developed a small peptide mimetic of SOCS-1, WLVFFVIFYFFR, called tyrosine kinase inhibitor peptide or Tkip (Table 1) (29). Tkip specifically binds to the activation loop of JAK2 as per the SOCS-1 antagonist peptide pJAK2(1001-1013). Substitution of alanine for phenylalanine at positions 8 and 11 in Tkip, Tkip2A, abrogates binding to pJAK2(1001-1013) as well as inhibition of activation of STAT1α by IFNγ (data not shown). As a corollary to SOCS-1 transfection, we treated L929 cells with 100 U/ml of IFNγ and 20 μM of either Tkip or Tkip2A and determined the effect on HSV-1 infection. As shown in Figure 6c, Tkip but not Tkip2A significantly inhibited the ability of IFNγ to induce an antiviral state. Thus, the SOCS-1 mimetic Tkip had a similar effect on IFNγ treated L929 fibroblasts as did transfection with SOCS-1. These results mimicked those obtained with HEL-30 fibroblasts and support the data showing that induction of SOCS-1 in the latter by HSV-1 infection renders these cells refractory to the antiviral effects of IFNγ.
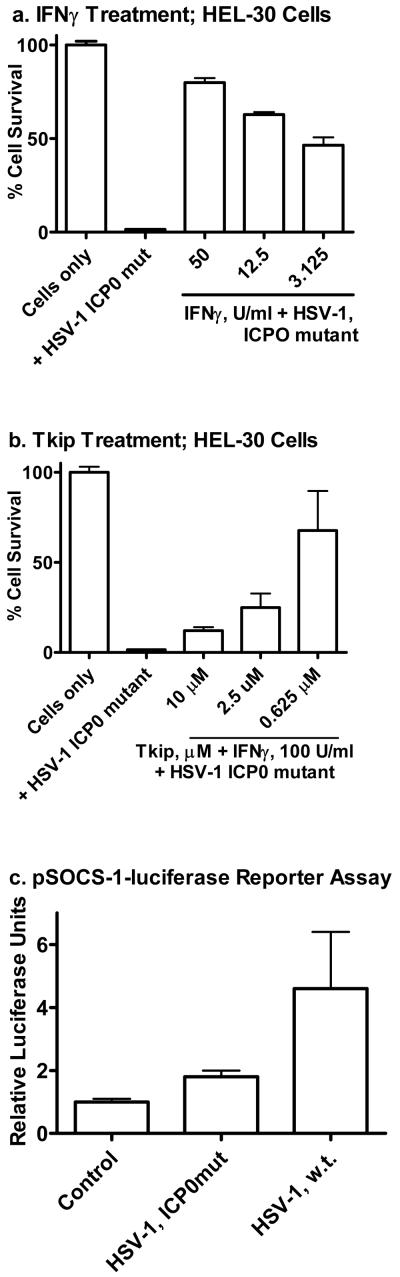
Reduction in SOCS-1 gene activation by HSV-1 ICP0 mutant in HEL-30 keratinocytes
ICP0 is an IE virulence protein that increases expression of HSV-1 genes in infected cells (31-33). One way that it functions is by blockage of histone deacetylation and/or increase in histone accetylation to facilitate HSV-1 gene expression (32, 33). It also causes degradation of host proteins that are involved in silencing HSV gene expression, such as the promyelocytic leukemia protein (32). To determine if ICP0 might play a role in HSV-1 refractiveness to IFNγ in HEL-30 keratinocytes, we infected the cells with HSV-1 syn17+ that had mutated and thus had a non-functional ICP0 (24). ICP0 mutated HSV-1 (ICP0mut, dl1403) was similarly lytic for HEL-30 cells as wild type virus, but unlike the wild type virus was inhibited by IFNγ (Figure 7a). Refractiveness to IFNγ was restored when the cells were treated with the SOCS-1 mimetic Tkip at the time of IFNγ treatment where 100 U /ml of IFNγ activity was significantly blocked by as little as 2.5 μM of SOCS-1 mimetic (Figure 7b). To assess as to how all this might be related to ICP0, SOCS-1, and IFNγ in HEL-30 cells, we transfected the cells with a luciferase reporter containing the SOCS-1 promoter (nucleotides -1577 to -3), infected the transfected cells with wild type HSV-1 and HSV-1 ICP0mut and compared them for reporter gene activation. As shown in Figure 7c, wild type HSV-1 was more than 2-fold more effective than ICP0mut in activation of the reporter gene at comparable levels of infectivity. It has been reported that ICP0 does not bind to DNA (34), but it obviously has an activation effect on the SOCS-1 gene in HEL-30 keratinocytes. Our observations suggest that ICP0 has a direct or indirect effect on SOCS-1 gene activation in keratinocytes either as per mechanisms indicated above with respect to its known function or in some cases it might function as a transcription/cotranscription factor.
Figure 7.
Reduction in SOCS-1 gene activation by HSV-1 ICP0 mutant in HEL-30 keratinocytes. (a). HEL-30 cells were cultured overnight in 24-well plates and treated with indicated amounts of IFNγ. Following treatment for 24 hours, cells were mock-infected or infected with HSV-1 at an moi of 0.1. Plates were incubated 48 hours, washed with PBS, fixed and stained with crystal violet. Absorbance units of each well were calculated using Multi-Gauge. Values are expressed as percent cell survival relative to mock-infected controls. Values are representative of duplicate wells of two independent experiments. (b). HEL-30 cells were cultured overnight in 24-well plates and treated with 100 U/ml IFN γ alone and with different concentrations of Tkip. Following treatment for 24 hours, cells were mock-infected or infected with HSV-1 ICP0 mutant at an moi of 0.1. Plates were incubated 48 hours, washed with PBS, fixed and stained with crystal violet and absorbance measured as in (a). There were statistically significant differences between IFNγ, Tkip, IFNγ + HSV-1 and Tkip + HSV-1 when compared to the untreated cells (P < 0.001) as determined by Mann-Whitney signed rank test. (c). HEL-30 cells were cultured overnight in 12-well plates and transfected with a luciferase reporter construct containing the full-length SOCS-1 promoter. Cells were incubated for 24 hours and then mock-infected or infected with HSV-1 or HSV ICP0 mutant at moi of 2 for 4 hours. Cell lysates were collected and luciferase activity was measured in a single-tube luminometer. Values given are expressed as luciferase units measured from the SOCS-1 reporter divided by luciferase units measured from a co-transfected constitutive reporter and subsequently normalized to medium controls. Values are representative of triplicate wells of the two independent experiments.
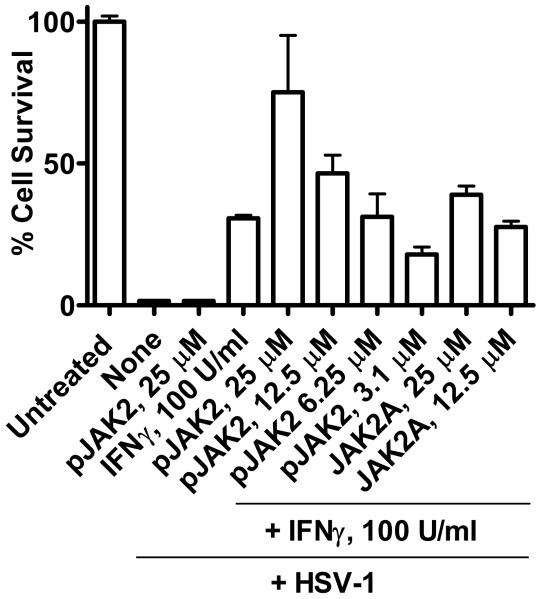
SOCS-1 antagonist synergizes with IFNγ in protection of macrophage cell line RAW264.7 against HSV-1
SOCS-1 acts at several sites in activation of Toll-like receptors (TLRs) in antigen presenting cells such as macrophages, perhaps to prevent an over-response of the innate immune system (18-20). We therefore determined the ability of SOCS-1 antagonist to enhance the anti-HSV-1 effects of IFNγ in the RAW264.7 macrophage cell line. Infection of RAW264.7 cells with HSV-1 at an moi of 0.1 resulted in 100% lysis in 48 hours in 96-well cultures as per Figure 8. IFNγ at 100 U/ml resulted in 30% protection. SOCS-1 antagonist pJAK2(1001-1013) at 25 μM did not protect against HSV-1. However, the combination of pJAK2(1001-1013) at 25 μM and IFNγ at 100 U/ml resulted in 75% protection. pJAK2(1001-1013) with alanine substitutions for tyrosines at positions 1007 and 1008 was much less effective at enhancement of IFNγ activity. Thus, the SOCS-1 antagonist enhanced IFNγ effects against HSV-1 in a macrophage cell line. Such enhancement reflects the linkage between SOCS-1 and the negative regulation of TLR signaling (18-20).
Figure 8.
pJAK2(1001-1013) synergizes with IFNγ to protect RAW264.7 murine macrophages against HSV-1. Murine macrophage cell line RAW264.7 was treated with IFNγ, pJAK2(1001-1013), IFNγ and different concentrations of pJAK2(1001-1013), or IFNγ and different concentrations of an alanine substituted mutant pJAK2(1001-1013)2A, followed by infection with HSV-1 at an moi of 0.1. Plates were incubated 48 hours, washed with PBS, fixed and stained with crystal violet. Absorbance units of each well were calculated using Multi-Gauge. Values are expressed as percent cell survival relative to mock-infected controls. Values are representative of duplicate wells of two independent experiments. There were statistically significant differences between different concentrations of pJAK2 peptide and IFNγ when compared to untreated cells (P < 0.001) as determined by Mann-Whitney signed rank test.
DISCUSSION
SOCS are a relatively recently discovered family of intracellular proteins that play an essential role in the regulation of JAK/STAT signal transduction as well as signaling via other tyrosine kinases such as MAL of TLR2 and 4 in innate and adaptive immune responses (18). There are eight members of this family, which share common functional sites that define these proteins. SOCS-1, SOCS-3, and perhaps SOCS-5 share the KIR functional sites that may define them as a subgroup within the SOCS family (20). For SOCS-1, this consists of a large SH2 domain, an N-terminal 12 amino acid sequence called extended SH2 (ESS), an additional N-terminal region called KIR, and a C-terminal SOCS box domain. SH2, ESS, and KIR are involved in binding to the activation loop of JAK2, and in fact, we have shown that the KIR region alone can bind to the JAK2 and TYK2 activation loops and inhibit their tyrosine kinase activity (27). The SOCS box, which is shared by all the SOCS proteins, is involved in ubiquitination of proteins targeted for proteasomal degradation (20). Thus, SOCS-1 regulates JAK kinase activity by direct binding and inhibition of function and by shuttling JAKs to proteasomal degradation.
The absolute critical importance of SOCS in regulating cytokines and other functions is underscored by the demonstration that homozygous knockout of the SOCS-1 gene in mice results in lethal neonatal inflammatory disease due in large part to unregulated IFNγ activity (37, 38). Thus, most JAK/STAT signaling that is related to innate and adaptive immunity also temporally activates genes such as SOCS-1 in order to mitigate against prolonged expression or overexpression of cytokines that might result in inflammatory damage to the individual. It would seem that this preemptive response to prevent overexpression of cytokines such as IFNγ, would be vulnerable to subversion by pathogens such as herpes viruses.
Our demonstration here that HSV-1 was refractory to IFNγ induction of an antiviral state in keratinocytes as compared to fibroblasts provides an explanation of why these cells play an important role in the epidermal route of infection. While infected fibroblasts did not show significant induction of SOCS-1 mRNA, HSV-1 infected keratinocytes showed significant levels of SOCS-1 mRNA as well as SOCS-1 protein. This resulted in blockage of IFNγ signal transduction as reflected by inhibition of STAT1α activation. Confirmation of HSV-1 activation of the SOCS-1 gene was provided by activation of the luciferase reporter fused to the full-length SOCS-1 promoter. Thus, HSV-1 refractiveness to IFNγ therapy in keratinocytes correlated with induction of SOCS-1, inhibition of STAT1α activation, and activation of the luciferase reporter fused to the full length SOCS-1 promoter.
We have developed a small peptide antagonist of SOCS-1 which was important in direct demonstration that HSV-1 induction of SOCS-1 in keratinocytes is responsible for the inability of IFNγ to induce an antiviral state in these cells. As indicated, we showed that the 12- amino acid KIR region of SOCS-1 bound to the activation loop of JAK2 phosphorylated at tyrosine 1007, pJAK2(1001-1013) (27). We reasoned that pJAK2(1001-1013) with a palmitate group for cell penetration would compete with JAK2 for SOCS-1 and thus block SOCS-1 inhibition of IFNγ activity in HSV-1 infected keratinocytes. Thus, pJAK2(1001-1013) synergized with IFNγ to induce a very strong antiviral state against HSV-1 in keratinocytes in a dose-dependent manner. Interestingly, pJAK2(1001-1013) alone also possessed antiviral activity in the keratinocytes. Various cells have been shown to constitutively produce very low levels of IFNβ, which interacts with IFNγ to enhance the antiviral state (39). In fact, these observations are consistent with our original demonstration of synergism between IFNα/IFNβ and IFNγ in “potentiation” of the antiviral response (40). The antiviral effects of pJAK2(1001-1013) were confirmed by SOCS-1 siRNA treatment of the cells. The antiviral properties of SOCS-1 peptide antagonist are not limited to effects on HSV-1 as we have also shown similar effects against vaccinia virus and encephalomyocarditis virus, two viruses quite different from HSV-1 (Manuscript in preparation).
The findings above with pJAK2(1001-1013), a SOCS-1 antagonist, are similar to findings of enhancement of the immune response to cancer cells and viral proteins with siRNA transfection of dendritic cells (DCs). Specifically, siRNA silencing of SOCS-1 in antigen-presenting DCs strongly enhanced antigen-specific antitumor immunity (41). Further, siRNA inhibition of SOCS-1 in HIV-1 gp120-pulsed bone marrow-derived DCs enhanced the immune response to the antigen (42). These studies concluded that SOCS-1 has an inhibitory effect on DCs in antigen presentation to T cells and that siRNA transfection of these cells inhibits this suppressive activity of SOCS-1. In this regard, our findings combined with the vaccine enhancement studies strongly suggest that suppression of SOCS-1 can enhance both innate and adaptive immunity.
Oncolytic viruses are currently being studied as potential anticancer therapeutics (43). The logic behind these studies is to reduce tumor burden by several mechanisms or effects, including direct cell lysis, disruption or destruction of tumor vasculature, as well as enhancement of antitumor immunity. Oncolytic HSV-1 (oHSV) mutants have been shown to possess promising antitumor activity in preclinical human tumor models along with significant tumor reduction in non-human primates (44). In a recent study of a panel of neuronal tumor cells, the ability of a oHSV-1 mutant (G207) to replicate in, and therefore lyse these tumor cells, correlated with the ability to induce SOCS-1 in the cells that were susceptible to the lytic activity (43). Poor induction resulted in correspondingly poor replication of oHSV, implying less effectiveness as an antitumor therapeutic. Transfection of cells with siRNA specific for SOCS-1 converted them to oHSV-1 resistance, supporting the importance of induction of SOCS-1 for oHSV-1 replication. These results are consistent with our HSV-1 results with keratinocytes.
The induction of SOCS-1 by HSV-1 in keratinocytes is similar to HSV-1 induction of SOCS-3 in the amniotic cell line FL, where such induction inhibited type I IFN activation of JAK2/STAT to induce an antiviral state (16). Another example of induction of SOCS-1 and reduction of IFN mediated antiviral activity, involved the influenza A virus strain (45). All of this points to the importance of controlling the induction of SOCS-1 or SOCS-3 by viruses as a means of controlling viral pathogenesis. The direct inhibition of virus growth in cells by the SOCS-1 antagonist as well as its ability to synergize with IFNγ and the IFNγ mimetic to inhibit HSV-1 replication in keratinocytes is thus of importance to negate and control viral infections. Although not presented here, pJAK2(1001-1013) is able to bind to the KIR region of SOCS-3, similar to its ability to bind to the KIR region of SOCS-1 (manuscript in preparation). Thus, pJAK2(1001-1013) should be effective as an antagonist of SOCS-3. The implication of this is that SOCS antagonists such as pJAK2(1001-1013) potentially possess broad antiviral activity, which would not be the case for siRNA in terms of specificity and possibly issues of practicality of use as an antiviral drug.
The resistance of wild type HSV-1 to IFNγ in HEL-30 cells is dependent on ICP0, since the ICP0 mutant was inhibited by IFNγ similar to the inhibition of wild type virus in fibroblasts. ICP0 is an HSV-1 IE protein that negates the silencing of virus gene by infected cells (31-33). The mechanism of this negation is thought to primarily involve blockage of histone deacetylation and/or increase in histone acetylation (32, 33). ICP0 also causes degradation of host proteins such as the promyelocytic leukemia protein (32). We have shown here that induction of SOCS-1 is the mechanism of HSV-1 resistance to IFNγ in the keratinocytes. In this regard the HSV-1 ICP0 mutant was less effective at activation of a luciferase reporter gene driven by the SOCS-1 promoter than the wild type virus. The question arises as to whether the activation of a host gene such as SOCS-1 by ICP0 occurs indirectly as per the mechanism above or by transcription/cotranscription mechanisms. ICP0 is not known to bind to DNA (34), but this has not been extensively examined with respect to host genes.
HSV-1 infection of the macrophage cell line RAW264.7 was not optimally inhibited by IFNγ compared to fibroblasts, since 6 U/ml of IFNγ in fibroblasts inhibited virus replication as effectively as 100 U/ml in RAW264.7 macrophages. The SOCS-1 antagonist synergized with IFNγ to enhance the IFNγ anti-HSV-1 effect in the macrophages, which suggests that innate immunity via TLR signaling is dampened by SOCS-1, which occurs at multiple stages, including JAK2 and TYK2 IFN signaling (18), as well as MyD88/MAL signaling (19). Thus, the SOCS-1 antagonist should enhance the innate immune response as well as the adaptive immune response against HSV-1.
As a complement to the results of inhibiting SOCS-1, we were able to convert fibroblasts to a phenotype similar to that of keratinocytes by either transfection of L929 cells with SOCS-1 plasmid or by treatment of the cells with the SOCS-1 mimetic Tkip. This provides additional evidence that negative regulation of SOCS-1 opens up a potentially transformational approach to enhancement of host defense against infections and cancer.
Strategies for treatment of recurrent HSV-1 disease have mainly focused on development of vaccines, an approach with mixed results (7). By understanding the interplay between HSV-1-infected neurons and associated IFN-γ-producing CD8+T cells, effective strategies for maintaining viral latency may evolve. Because IFN-γ is critical for maintaining HSV-1 latentcy (8), IFN-γ mimetics may provide therapeutic benefit to those suffering recurrent HSV-1 infections. Our results using a peptide inhibitor of SOCS-1, pJAK2(1001-1013) in a cell line that is refractory to treatment with IFNγ suggests that suppression of SOCS-1 is an effective method of increasing the efficacy of the antiviral effects of IFNγ. It is tempting to speculate that treatment of HSV-1-infected individuals with IFN-γ mimetic alone or in conjunction with pJAK2 may reduce both virus-induced mortality and morbidity. The effects of these peptide mimetics in thwarting HSV-1 reactivation from latency in a murine model of HSV-1 would provide strong support for this contention.
Acknowledgments
This work was supported by the National Institutes of Health Grants 5R01NS51245 and 2R01AI056152 to H.M.J., Wright State University Research Foundation, Cellular immunology Fund 550527 to NJB and Sigma Xi Grant in Aid of Research to KGF. We thank S. Mohammad Haider for peptide synthesis.
Abbreviations used in this paper
- JAK
Janus kinase
- IFNγ
Interferon gamma
- SOCS
suppressors of cytokine signaling
- KIR
kinase inhibitory region
- Tkip
tyrosine kinase inhibitor peptide
Footnotes
DISCLOSURES
The authors have no conflict of interest.
REFERENCES
- 1.Roizman BD, Knipe M, Whitley RJ. Herpes Simplex Viruses. In: Knipe DM, Howley PM, editors. Fields Virology. 3rd Lippincott, Williams, and Wilkins; Philadelphia, PA: 2007. pp. 2501–2602. [Google Scholar]
- 2.Diefenbach RJ, Miranda-Saksena M, Douglas MW, Cunningham AL. Transport and egress of herpes simplex virus in neurons. Rev. Med. Virol. 2008;18:35–51. doi: 10.1002/rmv.560. [DOI] [PubMed] [Google Scholar]
- 3.Koelle DM, Corey L. Herpes Simplex: Insights on pathogenesis and possible vaccines. Ann. Rev. Med. 2008;59:381–395. doi: 10.1146/annurev.med.59.061606.095540. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham AL, Diefenbach RJ, Miranda-Saksena M, Bosnjak L, Kim M, Jones C, Douglas MW. The cycle of human herpes simplex virus infection: virus transport and immune control. J. Infect. Dis. 2006;194:S11–S18. doi: 10.1086/505359. [DOI] [PubMed] [Google Scholar]
- 5.Mikloska Z, Danis VA, Adams S, Lloyd AR, Adrian DL, Cunningham AL. In vivo production of cytokines and beta (C-C) chemokines in human recurrent herpes simplex lesions-do herpes simplex virus-infected keratinocytes contribute to their production? J Infect Dis. 1998;177:827–838. doi: 10.1086/515236. [DOI] [PubMed] [Google Scholar]
- 6.Knickelbein JE, Khanna KM, Yee MB, Baty CJ, Kinchington PR, Hendricks RL. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science. 2008;322:268–271. doi: 10.1126/science.1164164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheridan BS, Knickelbein JE, Hendricks RL. CD8+ T cells and latent herpes simplex virus type 1: keeping the peace in sensory ganglia. Expert Opin Biol Ther. 2007;7:1323–1331. doi: 10.1517/14712598.7.9.1323. [DOI] [PubMed] [Google Scholar]
- 8.Decman V, Kinchington PR, Harvey SA, Hendricks RL. Gamma interferon can block herpes simplex virus type 1 reactivation from latency, even in the presence of late gene expression. J. Virol. 2005;79:10339–10347. doi: 10.1128/JVI.79.16.10339-10347.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mossman K. Analysis of anti-interferon properties of the herpes simplex virus type 1 ICP0 protein. Methods Mol Med. 2005;116:195–205. doi: 10.1385/1-59259-939-7:195. [DOI] [PubMed] [Google Scholar]
- 10.Arduino PG, Porter SR. Herpes Simplex Virus type I infection: Overview on relevant clinic-pathological features. J. Oral Pathol. Med. 2008;37:107–121. doi: 10.1111/j.1600-0714.2007.00586.x. [DOI] [PubMed] [Google Scholar]
- 11.Patel AR, Romanelli P, Roberts B, Kirsner RS. Treatment of herpes simplex virus infection: rationale for occlusion. Adv. Skin Wound Care. 2007;20:408–412. doi: 10.1097/01.ASW.0000280199.58260.62. [DOI] [PubMed] [Google Scholar]
- 12.Burgos JS, Serrano-Saiz E, Sastre I, Valdivieso F. ICP47 mediates viral neuroinvasiveness by induction of TAP protein following intravenous inoculation of herpes simplex virus 1 in mice. J. Neurovirol. 2006;12:420–427. doi: 10.1080/13550280601009546. [DOI] [PubMed] [Google Scholar]
- 13.Goldsmith K, Chen W, Johnson DC, Hendricks RL. Infected cell protein (ICP) 47 enhances herpes simplex virus neurovirulence by blocking the CD8+ T cell response. J. Exp. Med. 1998;187:341–348. doi: 10.1084/jem.187.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chee AV, Roizman B. Herpes simplex virus 1 gene products occlude the interferon signaling pathway at multiple sites. J. Virol. 2004;78:4185–4196. doi: 10.1128/JVI.78.8.4185-4196.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halford WP, Weisend C, Grace J, Soboleski M, Carr DJ, Balliet JW, Imai Y, Margolis TP, Gebhardt BM. ICP0 antagonizes STAT1-dependent repression of herpes simplex virus: Implications for the regulation of viral latency. Virology J. 2006;3:44–49. doi: 10.1186/1743-422X-3-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edison KM, Hobbs WE, Manning BJ, Carlson P, DeLuca NA. Expression of herpes simplex virus ICP0 inhibits the induction of interferon-stimulated genes by viral infection. J. Virol. 2002;76:2180–2191. doi: 10.1128/jvi.76.5.2180-2191.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yokota S, Yokosawa N, Okabayashi T, Suzutani T, Miura S, Jimbow K, Fujii N. Induction of suppressor of cytokine signaling-3 by Herpes Simplex Virus type 1 contributes to inhibition of interferon signaling pathway. J. Virol. 2004;78:6282–6286. doi: 10.1128/JVI.78.12.6282-6286.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat. Rev. Immunol. 2007;7:454–465. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- 19.Mansell A, Smith R, Doyle SL, Gray P, Fenner JE, Crack PJ, Nicholson SE, Hilton DJ, O'Neill LA, Hertzog PJ. Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nat. Immunol. 2006;7:148–155. doi: 10.1038/ni1299. [DOI] [PubMed] [Google Scholar]
- 20.Croker BA, Kiu H, Nicholson SE. SOCS regulation of JAK/STAT signaling pathway. Sem. Cell Dev. Biol. 2008;19:414–422. doi: 10.1016/j.semcdb.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yasukawa H, Misawa H, Sakamoto H, Masuhara M, Sasaki A, Wakioka T, Ohtsuka S, Imaizumi T, Matsuda T, Ihle JN, Yoshimura A. The JAK-binding protein JAB inhibits Janus tyrosine kinase activity through binding in the activation loop. EMBO J. 1999;18:1309–1320. doi: 10.1093/emboj/18.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dorsky DI, Crumpacker CS. Drugs five years later: acyclovir. Ann. Intern. Med. 1987;107:859–874. doi: 10.7326/0003-4819-107-6-859. [DOI] [PubMed] [Google Scholar]
- 23.Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J. Infect. Dis. 2002;185:45–52. doi: 10.1086/338231. [DOI] [PubMed] [Google Scholar]
- 24.Stow ND, Stow EC. Isolation and characterization of a Herpes Simplex Virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J. Gen. Virol. 1986;67:2571–2585. doi: 10.1099/0022-1317-67-12-2571. [DOI] [PubMed] [Google Scholar]
- 25.Szente BE, Weiner IJ, Jablonsky MJ, Krishna NR, Torres BA, Johnson HM. Structural requirements for agonist activity of a murine interferon-gamma peptide. J. Interferon Cytokine Res. 1996;16:813–817. doi: 10.1089/jir.1996.16.813. [DOI] [PubMed] [Google Scholar]
- 26.Thiam K, Loing E, Verwaerde C, Auriault C, Gras-Masse H. IFN-gamma-derived lipopeptides: influence of lipid modification on the conformation and the ability to induce MHC class II expression on murine and human cells. J. Med. Chem. 1999;42:3732–3736. doi: 10.1021/jm991025f. [DOI] [PubMed] [Google Scholar]
- 27.Waiboci LW, Ahmed CM, Mujtaba MG, Flowers LO, Martin JP, Haider MI, Johnson HM. Both the suppressor of cytokine signaling 1 (SOCS-1) kinase inhibitory region and SOCS-1 mimetic bind to JAK2 autophosphorylation site: implications for the development of a SOCS-1 antagonist. J. Immunol. 2007;178:5058–5068. doi: 10.4049/jimmunol.178.8.5058. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed CM, Johnson HM. IFN-gamma and its receptor subunit IFNGR1 are recruited to the IFN-gamma-activated sequence element at the promoter site of IFN-gamma-activated genes: evidence of transactivational activity in IFNGR1. J. Immunol. 2006;177:315–321. doi: 10.4049/jimmunol.177.1.315. [DOI] [PubMed] [Google Scholar]
- 29.Flowers LO, Johnson HM, Mujtaba MG, Ellis MR, Haider SM, Subramaniam PS. Characterization of a peptide inhibitor of Janus kinase 2 that mimics suppressor of cytokine signaling 1 function. J. Immunol. 2004;172:7510–7518. doi: 10.4049/jimmunol.172.12.7510. [DOI] [PubMed] [Google Scholar]
- 30.Lucet IS, Fantino E, Styles M, Bamert R, Patel O, Broughton SE, Walter M, Burns CJ, Treutlein H, Wilks AF, Rossjohn J. The structural basis of Janus kinase 2 inhibition by a potent and specific pan-Janus kinase inhibitor. Blood. 2006;107:176–183. doi: 10.1182/blood-2005-06-2413. [DOI] [PubMed] [Google Scholar]
- 31.Poon AP, Gu H, Roizman B. ICP0 and US3 protein kinase of herpes simplex virus 1 independently block histone deacetylation to enable gene expression. Proc. Natl. Acad. Sci. USA. 2006;103:9993–9998. doi: 10.1073/pnas.0604142103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu H, Roizman B. The two functions of Herpes Simplex Virus 1 ICP0, inhibition of silencing by the CoREST/REST/HDAC complex and degradation of PML, are executed in tandem. J. Virol. 2009;83:181–187. doi: 10.1128/JVI.01940-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cliffe AR, Knipe DM. Herpes Simplex Virus ICP0 promotes both histone removal and acetylation on viral DNA during lytic infection. J Virol. 2008;82:12030–12038. doi: 10.1128/JVI.01575-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sekulovich RE, Leary K, Sandri-Goldin RM. The herpes simplex virus type 1 alpha protein ICP27 can act as a trans-repressor or a trans-activator in combination with ICP4 and ICP0. J. Virol. 1988;62:4510–4522. doi: 10.1128/jvi.62.12.4510-4522.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobayashi T, Takaesu G, Yoshimura A. Mal-function of TLRs by SOCS. Nat. Immunol. 2006;7:123–124. doi: 10.1038/ni0206-123. [DOI] [PubMed] [Google Scholar]
- 36.Ahmad R, El Bassam S, Cordeiro P, Menezes J. Requirement of TLR2-mediated signaling for the induction of IL-15 gene expression in human monocytic cells by HSV-1. Blood. 2008;112:2360–2368. doi: 10.1182/blood-2008-02-137711. [DOI] [PubMed] [Google Scholar]
- 37.Marine JC, Topham DJ, McKay C, Wang D, Parganas E, Stravopodis D, Yoshimura A, Ihle JN. SOCS1 deficiency causes a lymphocyte-dependent perinatal lethality. Cell. 1999;98:609–616. doi: 10.1016/s0092-8674(00)80048-3. [DOI] [PubMed] [Google Scholar]
- 38.Starr R, Metcalf D, Elefanty AG, Brysha M, Willson TA, Nicola NA, Hilton DJ, Alexander WS. Liver degeneration and lymphoid deficiencies in mice lacking suppressor of cytokine signaling-1. Proc. Natl. Acad. Sci. USA. 1998;95:14395–14399. doi: 10.1073/pnas.95.24.14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takaoka A, Mitani Y, Suemori H, Sato M, Yokochi T, Noguchi S, Tanaka N, Taniguchi T. Cross talk between interferon-γ and −α/β signaling components in caveolar membrane domains. Science. 2000;288:2357–2360. doi: 10.1126/science.288.5475.2357. [DOI] [PubMed] [Google Scholar]
- 40.Fleischmann WR, Georgiades JA, Osborne LC, Johnson HM. Potentiation of interferon activity by mixed preparation of fibroblast and immune interferon. Infection and Immunity. 1979;26:248–253. doi: 10.1128/iai.26.1.248-253.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen L, Evel-Kabler K, Strube R, Chen SY. Silencing of SOCS1 enhances antigen presentation by dendritic cells and antigen-specific anti-tumor immunity. Nat. Biotechnol. 2004;22:1546–1553. doi: 10.1038/nbt1035. [DOI] [PubMed] [Google Scholar]
- 42.Song XT, Evel-Kabler K, Aldrich M, Gao F, Huang XF, Chen SY SY. An alternative and effective HIV vaccination approach based on inhibition of antigen presentation attenuators in dendritic cells. PLoS Med. 2006;3:e11. doi: 10.1371/journal.pmed.0030011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahller YY, Sakthivel B, Baird WH, Aronow BJ, Hsu Y, Cripe TP, Mehrian-Shai R. Molecular analysis of human cancer cells infected by an oncolytic HSV-1 reveals multiple upregulated cellular genes and a role for SOCS1 in virus replication. Cancer Gene Ther. 2008;15:733–741. doi: 10.1038/cgt.2008.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Todo T. Oncolytic virus therapy using genetically engineered simplex viruses. Front. Biosc. 2008;13:2060–2064. doi: 10.2741/2823. [DOI] [PubMed] [Google Scholar]
- 45.Pothlichet J, Chignard M, Si-Tahar M. Cutting edge: innate immune response triggered by influenza A virus is negatively regulated by SOCS1 and SOCS3 through a RIG-1/IFNAR1-dependent pathway. J. Immunol. 2008;180:2034–2038. doi: 10.4049/jimmunol.180.4.2034. [DOI] [PubMed] [Google Scholar]