Abstract
Background and Purpose
Ultrasound transiently expands perflutren-lipid microspheres (µS), transmitting energy momentum to surrounding fluids. We report a pilot safety/feasibility study of ultrasound-activated µS with systemic tissue plasminogen activator (tPA).
Methods
Stroke subjects treated within 3 hours had abnormal Thrombolysis in Brain Ischemia (TIBI) residual flow grades 0 to 3 before tPA on transcranial Doppler (TCD). Randomization included Controls (tPA+TCD) or Target (tPA+TCD+2.8 mL µS). The primary safety end point was symptomatic intracranial hemorrhage (sICH) with worsening by ≥4 NIHSS points within 72 hours.
Results
Fifteen subjects were randomized 3:1 to Target, n=12 or Control, n=3. After treatment, asymptomatic ICH occurred in 3 Target and 1 Control, and sICH was not seen in any study subject. µS reached MCA occlusions in all Target subjects at velocities higher than surrounding residual red blood cell flow: 39.8±11.3 vs 28.8±13.8 cm/s, P<0.001. In 75% of subjects, µS permeated to areas with no pretreatment residual flow, and in 83% residual flow velocity improved at a median of 30 minutes from start of µS infusion (range 30 s to 120 minutes) by a median of 17 cm/s (118% above pretreatment values). To provide perspective, current study recanalization rates were compared with the tPA control arm of the CLOTBUST trial: complete recanalization 50% versus 18%, partial 33% versus 33%, none 17% versus 49%, P=0.028. At 2 hours, sustained complete recanalization was 42% versus 13%, P=0.003, and NIHSS scores 0 to 3 were reached by 17% versus 8%, P=0.456.
Conclusions
Perflutren µS reached and permeated beyond intracranial occlusions with no increase in sICH after systemic thrombolysis suggesting feasibility of further µS dose-escalation studies and development of drug delivery to tissues with compromised perfusion.
Keywords: microspheres, thrombolysis, stroke, occlusion, transcranial Doppler
Intravenous tissue plasminogen activator (tPA) remains the only approved therapy for acute ischemic stroke.1 Successful thrombolysis depends on tPA delivery to the thrombus via residual flow around acute arterial obstruction.2 Pulsed wave transcranial Doppler (TCD) can expose the thrombus-residual flow interface to a mechanical ultrasound pressure wave, and TCD monitoring can safely facilitate early recanalization in stroke subjects treated with systemic tPA.3 In the CLOTBUST (Combined Lysis of Thrombus with 2 MHz transcranial Ultrasound and Systemic TPA) trial, TCD monitoring for 2 hours after tPA bolus with 1 hour tPA infusion tripled the chance of complete and sustained recanalization.3 This early augmentation of reperfusion resulted in a trend toward favorable clinical recovery. Based on this trend, a pivotal trial to confirm efficacy of sonothrombolysis would require an estimated 600 subjects.3 Because this is a large sample for an acute stroke trial, CLOTBUST investigators started to explore further ways to augment early reperfusion that could reduce the sample size of the pivotal trial.
One such possibility could be gaseous microspheres (µS) with protective shells that were first engineered as contrast agents for ultrasound imaging.4–6 After gaseous µS are compressed by an ultrasonic pressure wave, the gas expands, and the spheres oscillate. Because µS have impedance much higher than red blood cells,7 they act like bright reflectors and send back stronger echoes useful for imaging. With expansion, they also transmit mechanical energy momentum to surrounding fluids accelerating residual flow and possibly producing “microangioplasties” to thrombus with transient µS expansion in size.8,9 Moreover, experimental data have shown that acoustic radiation could force µS through the thrombus and create numerous microscopic holes inside the clot.10 This can potentially facilitate thrombolysis and may aid ultrasound-targeted drug delivery. Recently, promising human studies were done with TCD-activated first generation gaseous µS as an adjunctive facilitator of thrombus breakup with systemic tPA therapy.11
Early generation µS are air-filled4,6 and tend to “bubble-up” in saline making continuous infusion difficult. They also have weak shell compositions that produce µS of variable and relatively large sizes that could decrease their ability to cross lung circulation and permeate through thrombus. Thus, the goal was to test the feasibility and safety of novel lipid coated µS containing C3F8 (perflutren) that are consistent in size (1 to 2 µm) and more stable in saline solution.12
Subjects and Methods
We designed a prospective open-label randomized clinical trial based on the CLOTBUST methodology that combines a standard 1-hour 0.9-mg/kg tPA infusion with 2 hours of continuous TCD monitoring.3 In brief, subjects presenting with acute ischemic stroke were screened for their eligibility for systemic thrombolysis within 3 hours of symptom onset according to the NINDS-rt-PA Stroke Study criteria.1 In addition, a fast-track diagnostic TCD was performed in the emergency department to detect the middle cerebral artery (MCA) occlusion.13 We used previously validated Thrombolysis in Brain Ischemia (TIBI) criteria for residual blood flow signals around MCA thrombus.14 At least 1 of the abnormal TIBI flow grades (absent, minimal, blunted or dampened) had to be present before tPA bolus in the affected MCA at depth range 30 to 65 mm with >30% velocity asymmetry to the contralateral MCA. TCD signs of flow diversion to branching vessels were considered supportive information for the diagnosis of the MCA occlusion.15
Institutional Review Board approvals were obtained at all participating centers, and all subjects or proxies signed an informed consent. Subjects who had MCA occlusions on TCD before tPA bolus were then randomized in a 3:1 ratio to Target (tPA +2 hours TCD +60 minutes infusion of 2.8 mL perflutren-lipid µS diluted in 100 cc normal saline) or Control (tPA +2 hours TCD). An intravenous line separate from tPA was used for µS infusion. Gravity macrodrip had to be used to administer µS because electronic pumps detect the presence of gas with µS solutions and hinder its administration.
Subjects were monitored with 100 M TCD (Spencer Technologies), and with Ez-Dop (Compumedics) when Power Motion Doppler (PMD) showed no detectable signals in one subject. Both units emitted pulsed ultrasound of 2 MHz frequency with power outputs below 720 mW as measured by hydrophone in a water tank.
Spectral waveforms from the depths of the worst detectable residual flow signals were obtained at 0, 30, 60, 90, and 120 minutes from treatment initiation using a standard monitoring headframe and protocol.3,16 Baseline and 120-minute TIBI grades were documented in the case report form while unblinded sonographers also evaluated and digitally stored the 30, 60, and 90 minutes waveforms. To determine recanalization on TCD, waveforms were interpreted by onsite investigators who were all certified in using the TIBI flow grading system before trial initiation. In addition, waveforms were transferred for an independent central evaluation (Andrew Demchuk, MD, Calgary, AB, Canada).
Similar to our previous reports,3,14,16,17 partial recanalization on TCD was diagnosed if the affected MCA segment with the worst residual flow grade pretreatment showed an improvement by 1 TIBI grade or more to TIBI grade 2 or 3. Complete recanalization was diagnosed if TIBI flow grades recovered to the highest grade 4 or 5 within 2 hours after treatment initiation.
To determine whether µS can reach MCA occlusion depths and permeate to areas of no detectable flow, PMD flow tracks were analyzed. PMD provides high resolution (3 mm gate length×33 gates) information about direction and intensity of flow signals sufficiently covering unilateral MCA depths (PMD display range 25 to 85 mm). PMD tracks were obtained simultaneously with spectral waveforms as part of diagnostic and monitoring TCD procedures. Still PMD images of µS traces were analyzed using high-resolution color pixel software (Microsoft Digital Image Editor, 2006). This protocol was validated internally before our analysis for consistency between the raters blinded to subject information. The intrarater and interrater reliability in the measurement of Motion-mode and Doppler parameters was assessed using interclass correlation coefficients. Measurements by 2 blinded investigators showed an inter- and intraobserver agreement for maximum single-gate µS duration and µS velocity of 0.81/0.87, and 0.84/0.90, respectively (interclass correlation coefficients, n=30 µS samples). Further details of this methodology are presented in a separate report.18
The primary safety end point was symptomatic intracranial hemorrhage (sICH) within 72 hours from treatment. Hemorrhage was considered symptomatic if the presence of intracranial blood diagnosed by repeated brain CT or MRI scanning was related in the opinion of the clinical investigator to the worsening of the neurological deficit by ≥4 NIHSS points. Secondary signal-of-efficacy end points were complete recanalization on TCD diagnosed as TIBI flow grade 4 or 5 at 2 hours of treatment, early clinical neurological recovery at 2 hours (a total NIHSS score 0 to 3 points or reduction by ≥10 NIHSS points), and clinical recovery at 24 hours (total NIHSS score 0 to 2 points).3 All scoring physicians were certified to use the NIHSS. Favorable clinical outcome at 3 months was defined as a modified Rankin score of 0 to 1 or a NIHSS score of 0 to 1. Mortality and functional recovery were documented at 3 months after symptom onset if subjects were available for follow-up.
To test the hypothesis that activation of perflutren-lipid µS with TCD during tPA infusion in stroke subjects is feasible, we planned to monitor safety (sICH) of µS activation in the sample size of 40 subjects. Although this predetermined sample size was not based on statistical power considerations, it was consistent with other pilot feasibility studies of novel medications performed in subjects with ischemic stroke. An independent Data Safety Monitoring Board (DSMB) was appointed to independently review all safety data generated during the study (see Acknowledgements) after every 10 subjects randomized to receive µS, or earlier if 2 symptomatic hemorrhages occurred in 10 subjects in the Target group, or overall symptomatic brain hemorrhage rate exceeded 15%. The study was terminated for administrative reasons before reaching its predetermined sample because a new formulation of µS was developed and a new dose-escalation study could be initiated. Study termination occurred without safety concerns and before primary and secondary analyses. Statistical analyses included descriptive statistics, χ2 test or Fisher’s exact test, unpaired t test, and Mann–Whitney U test as indicated.
Results
A total of 15 subjects were randomized to Target (n=12) or Controls (n=3) with no significant difference in pretreatment characteristics including age, NIHSS scores, depths of the worst residual flow signals location on TCD, and TIBI flow grades in the affected MCA’s (Table 1). One subject randomized to the Target group fell outside the window for treatment with µS and received target vessel insonation without concomitant administration of µS. In this case by the time informed was obtained and the patient was randomized, the subject was already outside the 3-hour window for intravenous thrombolytic treatment. Three subjects had distal MCA occlusions, whereas in 9 cases proximal MCA occlusions were identified on baseline TCD.
Table 1.
Baseline Subject Characteristics
| Pretreatment Parameter | Target n=12 | Control n=3 | P Value |
|---|---|---|---|
| Age, y | 75±13 | 58±33 | 0.178* |
| Median NIHSS score, range | 17 (9 to 28) | 24 (6 to 26) | 0.563** |
| Median residual flow depth, range (mm) | 51 (39 to 62) | 52 (51 to 56) | 0.384** |
| Mean TIBI grade | 1.5±0.8 | 0.7±0.6 | 0.117* |
Unpaired t test
Mann Whitney U test.
After treatment, a total of 4 asymptomatic hemorrhages were found: 3 in Target (25%) and 1 in Control (33%) subjects. Concerning the primary safety end point, no sICH was reported in any of the study subjects. This was confirmed on DSMB review of the data.
TCD recordings were evaluated as part of a secondary analysis. µS were detected at the depth of the worst residual flow signals on TCD reaching MCA occlusions in all Target subjects (Figure 1). µS were moving at velocities higher than surrounding residual red blood cell flow: 39.8±11.3 versus 28.8±13.8 cm/s, P<0.001 (Figure 2). Before any recanalization, µS permeated to areas with no detectable pretreatment residual flow in 9 Target subjects (75%) (Figure 3). Residual flow improvement was detected at a median of 30 minutes from the beginning of µS infusion (range 30 s to 120 minutes) with early augmentation of the residual flow velocity in 10 subjects (83%): median absolute increase 17 cm/s (median relative increase 118%) above pretreatment values.
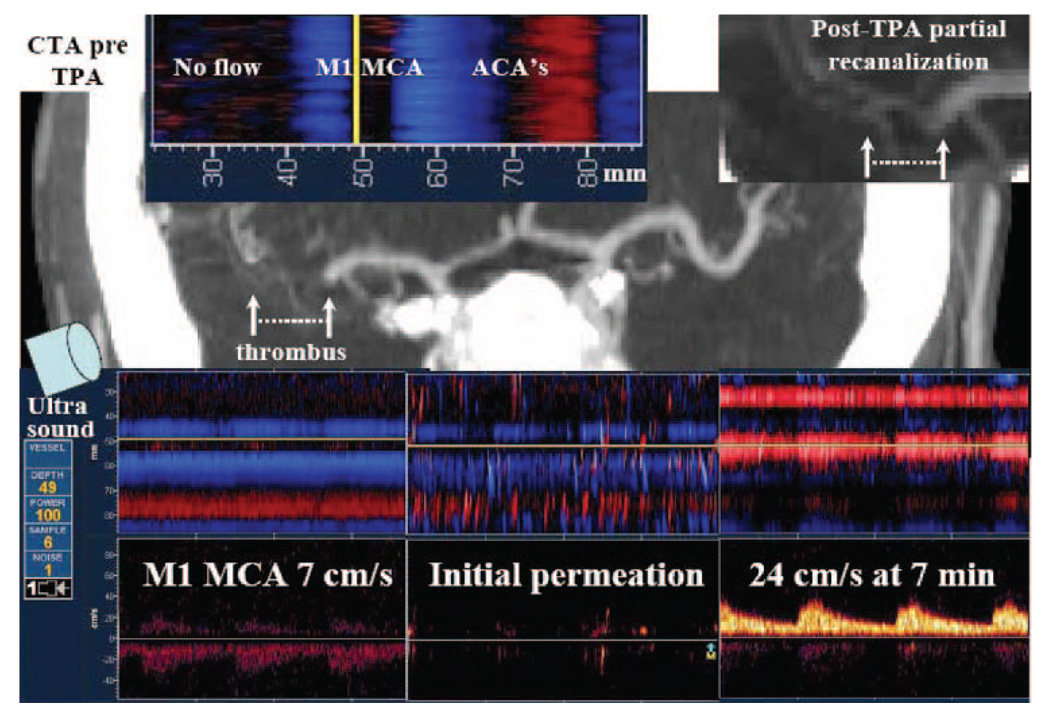
Figure 1.
Power motion Doppler flow tracks of acute MCA occlusion, µS arrival at thrombus location and early residual flow augmentation with partial recanalization. Upper images (left to right), Depth scale (mm) relative to pretreatment CTA showing areas of no detectable flow on PMD relative to thrombus location, and flow signals in opened parts of the MCA and the anterior cerebral artery (left). Posttreatment CTA inset shows partial recanalization (right). Bottom images (left to right), PMD and spectral Doppler recordings showing sluggish residual flow in the affected MCA (7 cm/s) at the time of tPA bolus (left); initial µS arrival at the thrombus and permeation beyond occlusion to areas with no detectable flow at 20 to 30 seconds from start of µS infusion (middle); and partial recanalization with velocity improvement 24 cm/s at 7 minutes after combined treatment initiation (right). Flow toward the probe is coded as red, whereas flow away from the probe is coded as blue. The blue band at a depth of 40 to 50 mm corresponds to MCA collateral retrograde flow. NIHSS score was 12 points pretreatment, 5 points at 2 hours, and 1 point at 24 hours.
Figure 2.
Calculation of µS propagation velocity on time-distance PMD flow tracks (upper image), and corresponding red blood cell velocities of the residual flow at the time of µS appearance on spectral Doppler (lower image). Vertical axis (upper image), depth of insonation (mm). Vertical axis (lower image), blood flow velocity (cm/s). Horizontal axis (both images), total recording time of 4 seconds. Dotted lines show measurements of distance, velocity, and time.
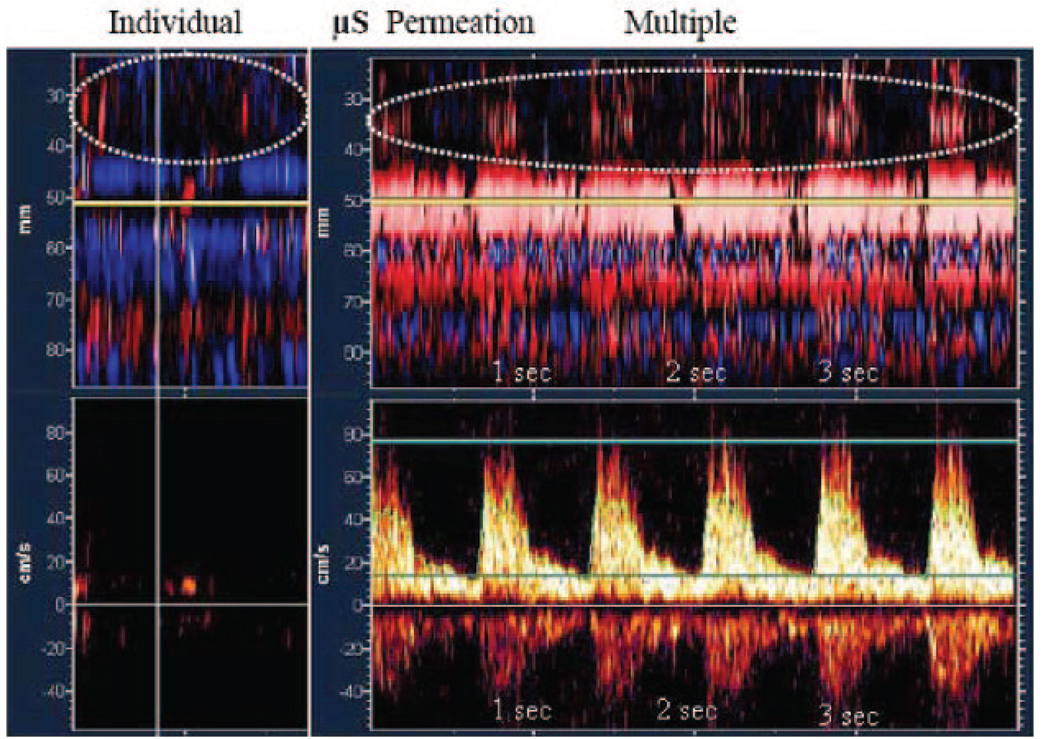
Figure 3.
PMD flow tracks show individual and multiple perflutren-lipid µS permeation to areas with no detectable residual flow pretreatment (dotted circles). Upper images demonstrate µS appearance at shallow depths (25 to 40 mm) corresponding to the distal MCA areas that are distal to the residual flow signals on spectral Doppler (lower images). Spectral waveforms were obtained at 50 mm depth (horizontal yellow line). Vertical axis, depth in mm (upper images) and velocity in cm/s (lower images). Horizontal axis, time in seconds.
To provide perspective, recanalization rates as determined by onsite investigators in the present study were compared to previously published data from the CLOTBUST trial3 (Table 2). The rates of any recanalization within 2 hours after TPA bolus were (TPA+TCD+µS versus TPA in CLOTBUST): complete 50% (6/12) versus 18% (11/63), partial 33% (4/12) versus 33% (21/63), none 17% (2/12) versus 49% (31/63), (Pearson’s χ2=7.134, df=2, P=0.028). The rates of complete within 2 hours after TPA bolus were 50% in the present study (TPA+TCD+µS) and 18% in the control (TPA alone) group of CLOTBUST (P=0.023; Fisher’s exact test). Among concurrent Controls in the present study (n=3), none reached complete recanalization, 2 achieved partial recanalization, and 1 had no recanalization. As compared to the CLOTBUST data, sustained complete recanalization rate at 2 hours after tPA bolus was 42% with TPA+TCD+µS (5/12), 38% TPA+TCD (24/63), and 13% TPA (8/63) (Pearson’s χ2=11.832, df=2, P=0.003). An independent central evaluation of sonograms identified the need for development of a new set of TIBI flow grades definitions that will adjust to flow enhancement with microspheres. Sustained complete recanalization was shown in 2 of 3 cases of distal MCA occlusions (66%), whereas 3 subjects reached complete sustained recanalization in the subgroup of proximal MCA occlusions (25%).
Table 2.
Recanalization Rates
| Recanalization Within 2 Hours | tPA+US+µS n=12 | tPA (CLOTBUST) n=63 |
|---|---|---|
| Complete recanalization | 6/12 (50%) | 11/63 (18%) |
| Partial recanalization | 4/12 (33%) | 21/63 (21%) |
| No recanalization | 2/12 (17%) | 31/63 (49%) |
| Pearson 2×3 chi-square=7.134, df=2, P=0.028 | ||
| Sustained complete recanalization at 2 hours | 5/12 (42%) | 8/63 (13%) |
| Pearson chi-square=11.832, df=2, P=0.003 |
A total NIHSS score of 0 to 3 points at 2 hours was 17% for TPA+TCD+µS (2/12), 14% for TPA+TCD (9/63), and 8% of subjects for TPA (5/63), Pearson’s χ2=1.569, df=2, P=0.456. Reduction by ≥10 NIHSS points at 2 hours occurred in 17% with TPA+TCD+µS (2/12), 14% with TPA+TCD (9/63), and 13% of subjects with TPA (8/63; Pearson’s χ2=0.160, df=2, P=0.923).
Mortality (Target versus Control) was 25% (4/12) versus 33% (1/3), range 4 to 84 days. A total of 6 subjects completed the 3 month follow-up by the time of study termination. Favorable outcome was reached by 2 of 5 surviving Target subjects (40%), whereas functional outcome data were not available in 2 surviving Control subjects.
Discussion
Our study showed no increased risk of symptomatic ICH in subjects who received intravenous tPA with perflutren-lipid µS activated with a single beam 2 MHz TCD. Although rates of asymptomatic hemorrhagic transformation were higher than previously reported in a multi-center Phase IV study of tPA,19 it was likely attributable the fact that our study included subjects with more severe strokes, all of whom had MCA occlusions. In these subjects, asymptomatic hemorrhagic transformation is considered a marker of potentially nutritious tPA-associated reperfusion particularly if clinical improvement is noticed despite appearance of blood on brain imaging.20
Our study parallels findings by Molina et al who showed safe enhancement of thrombolysis with earlier generation microbubbles also activated by TCD.11 Our study is in contrast to findings by Larrue et al21 who also used earlier generation microbubbles (Levovist, Schering AG) but activated these microbubbles with duplex ultrasound transducers that have higher energy levels than TCD. Although Larrue et al also showed no instances of sICH, they noticed much higher rates of asymptomatic brain hemorrhages at no signal of efficacy with their methodology of microbubble activation. Notably, the use of duplex technology to enhance tPA activity without microbubbles in another study also resulted in lower recanalization rates at higher rates of brain hemorrhages.22 However, numerous methodological differences between these studies should be taken into account, when interpreting the discrepant findings. For one, Eggers et al included more severely affected patients suffering from main-stem MCA (proximal M1 segment) occlusions with no residual flow (TIBI 0).22 Also, recanalization rate by TCCD was differently defined in the study by Eggers et al22 in comparison to CLOTBUST or the present report. Finally, recanalization status was assessed by MRA and not by ultrasound (TCD or TCCD) in the report by Larrue et al.21
Interestingly, in 75% of our study population µS were detected in areas with no pretreatment flow. This finding may indicate that perflutren-containing µS can reach and permeate beyond intracranial occlusions. However, these results need to be interpreted with caution because ultrasound contrast enhancer can increase measured peak systolic velocity by about 20%. Thus, it may be argued that residual flow improvement detection may be explained partly by enhanced reflection and not by improvement of flow itself. Also, we did not measure the increase of flow by µS in healthy subjects to compare the results of individuals with patent vessels with those of study subjects. Further studies are currently underway by our group to detect and quantify the effect of µS on blood flow in healthy subjects.
Our study has limitations, namely a small sample size and limited follow-up. Because of the small numbers in each study group, potential differences in age or baseline NIHSS may not reach statistical significance. However, they may affect the calculated results concerning functional outcome. Location of occlusion was similar in both groups and therefore we assume that recanalization rates may be more informative than NIH change. Our results should be taken with caution because they were obtained at centers with expertise in sono-thrombolysis. Given operator-dependency of TCD, future multi-center studies should provide training of sonographers in assessment and monitoring of acute stroke subjects. Moreover, the ultrasound diagnosis of partial recanalization on the basis of improvement of 1 TIBI flow grade may lead to false-positive results, because small flow aberrations or improved flow attributable to changing quality of flow detection may give an erroneous impression of TIBI flow improvement by just 1 grade. Of note, though, that this is the reason we selected complete and not partial recanalization as a secondary outcome parameter. Another limitation of the present report is related to the absence of regular follow-up for mortality and functional recovery. Because of the limited number of subjects included in the control group (n=3), we were unable to perform any statistical comparisons between the 2 groups. Therefore we decided to compare the active group of the present study (TPA+US+µS) to the control group of CLOTBUST (TPA alone). Thus, the reported posthoc analyses were not predetermined, and this should be taken into account when interpreting our results. Finally, our study was not powered on any specific signal of efficacy. We selected a predetermined sample size of 40 patients similar to other pilot feasibility trials on ultrasound-enhanced thrombolysis.21–23
Our study, nonetheless, shows the feasibility of administering a new generation of µS in acute stroke subjects. Yet, a further dose-escalation study is needed because it is unclear whether more µS delivered to thrombus during tPA infusion will safely facilitate thrombolysis in a dose-dependent manner. A multi-center dose-escalation controlled randomized trial is being completed to address this issue.
In conclusion, stroke subjects with MCA occlusions receiving infusion of perflutren lipid µS during systemic thrombolysis did not have an increased risk of sICH suggesting the feasibility of further studies. Perflutren-containing µS reached and permeated beyond intracranial occlusions. These findings are intriguing and are addressed in detail in a separate PMD data analysis.18 The ability of µS to permeate vessel segments distal to the occlusion may provide a rationale for development of µS-aided sono-thrombolysis and µS-based drug delivery to tissues with compromised perfusion.
Acknowledgments
Data Safety Monitoring Board: J. Donald Easton, MD (Chairman), Joseph F. Polak, MD, MPH, and Barbara Tilley, PhD.
Footnotes
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
Disclosures
Drs Alexandrov and Grotta were supported by the National Institute of Neurological Disorders and Stroke (NINDS) grants 1K23NS02229-01 and 1P50NS044227. As sponsor, ImaRx Therapeutics Inc covered study related expenses and provided perflutren-lipid µS to study sites. Dr Alexandrov served as consultant to ImaRx. All clinical investigators who enrolled and monitored subjects at participating sites (Houston, Phoenix, Wilmington) are listed as coauthors. Dr Sharma received financial grant for his fellowship training from the National Healthcare Group and National University Hospital, Singapore. Dr Tsivgoulis is recipient of a neurosonology fellowship grant from the Neurology Department, Eginition Hospital, University of Athens School of Medicine, Athens, Greece. Dr Lao received fellowship grant from the Neurology Department of Saint Thomas Hospital and Tan Yan Kee Foundation, Manila, Philippines. Dr Sierzenski received grant support from ImaRx Therapeutics Inc. Dr Grotta received grant support from ImaRx Therapeutics, Inc.
References
- 1.The NINDS rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Blinc A, Kennedy SD, Bryant RG, Marder VJ, Francis CW. Flow through clots determines the rate and pattern of fibrinolysis. Thromb Haemost. 1994;71:230–235. [PubMed] [Google Scholar]
- 3.Alexandrov AV, Molina CA, Grotta JC, Garami Z, Ford SR, Alvarez-Sabin J, Montaner J, Saqqur M, Demchuk AM, Moye LA, Hill MD, Wojner AW. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351:2170–2178. doi: 10.1056/NEJMoa041175. [DOI] [PubMed] [Google Scholar]
- 4.De Jong N, Ten Cate FJ. Principles and recent developments in ultrasound contrast agents. Ultrasonics. 1991;29:324–330. doi: 10.1016/0041-624x(91)90030-c. [DOI] [PubMed] [Google Scholar]
- 5.Feinstein SB, Shah PM. Advances in contrast two-dimensional echocardiography. Cardiovasc Clin. 1986;17:95–102. [PubMed] [Google Scholar]
- 6.Burns PN. Ultrasound contrast agents in radiological diagnosis. Radiol Med (Torino) 1994;87:71–82. [PubMed] [Google Scholar]
- 7.Moehring MA, Klepper JR. Pulse Doppler ultrasound detection, characterization and size estimation of emboli in flowing blood. IEEE Trans Biomed Eng. 1994;41:35–44. doi: 10.1109/10.277269. [DOI] [PubMed] [Google Scholar]
- 8.Xie F, Tsutsui JM, Lof J, Unger EC, Johanning J, Culp WC, Matsunaga T, Porter TR. Effectiveness of lipid microbubbles and ultrasound in declotting thrombosis. Ultrasound Med Biol. 2005;31:979–985. doi: 10.1016/j.ultrasmedbio.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Shi WT, Forsberg F, Vaidyanathan P, Tornes A, Ostensen J, Goldberg BB. The influence of acoustic transmit parameters on the destruction of contrast microbubbles in vitro. Phys Med Biol. 2006;51:4031–4045. doi: 10.1088/0031-9155/51/16/010. [DOI] [PubMed] [Google Scholar]
- 10.Everbach EC, Francis CW. Cavitational mechanisms in ultrasound-accelerated thrombolysis at 1 MHz. Ultrasound Med Biol. 2000;26:1153–1160. doi: 10.1016/s0301-5629(00)00250-7. [DOI] [PubMed] [Google Scholar]
- 11.Molina CA, Ribo M, Rubiera M, Montaner J, Santamarina E, Delgado-Mederos R, Arenillas JF, Huertas R, Purroy F, Delgado P, Alvarez-Sabin J. Microbubble administration accelerates clot lysis during continuous 2-MHz ultrasound monitoring in stroke subjects treated with intravenous tissue plasminogen activator. Stroke. 2006;37:425–429. doi: 10.1161/01.STR.0000199064.94588.39. [DOI] [PubMed] [Google Scholar]
- 12.Unger EC, Porter T, Culp W, Labell R, Matsunaga T, Zutshi R. Therapeutic applications of lipid-coated microbubbles. Adv Drug Deliv Rev. 2004;56:1291–1314. doi: 10.1016/j.addr.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Alexandrov AV, Demchuk A, Wein T, Grotta JC. The yield of transcranial Doppler in acute cerebral ischemia. Stroke. 1999;30:1605–1609. doi: 10.1161/01.str.30.8.1604. [DOI] [PubMed] [Google Scholar]
- 14.Demchuk AM, Burgin WS, Christou I, Felberg RA, Barber PA, Hill MD, Alexandrov AV. Thrombolysis in Brain Ischemia (TIBI) TCD flow grades predict clinical severity, early recovery and mortality in intravenous TPA treated subjects. Stroke. 2001;32:89–93. doi: 10.1161/01.str.32.1.89. [DOI] [PubMed] [Google Scholar]
- 15.Demchuk AM, Christou I, Wein TH, Felberg RA, Malkoff M, Grotta JC, Alexandrov AV. Specific transcranial Doppler flow findings related to the presence and site of arterial occlusion with transcranial Doppler. Stroke. 2000;31:140–146. doi: 10.1161/01.str.31.1.140. [DOI] [PubMed] [Google Scholar]
- 16.Sugg RM, Pary JK, Uchino K, Baraniuk S, Shaltoni HM, Gonzales NR, Mikulik R, Garami Z, Shaw SG, Matherne DE, Moye LA, Alexandrov AV, Grotta JC. Argatroban tPA stroke study: study design and results in the first treated cohort. Arch Neurol. 2006;63:1057–1062. doi: 10.1001/archneur.63.8.1057. [DOI] [PubMed] [Google Scholar]
- 17.Tsivgoulis G, Saqqur M, Sharma VK, Lao AY, Hill MD, Alexandrov AV. CLOTBUST Investigators. Association of pretreatment blood pressure with tissue plasminogen activator-induced arterial recanalization in acute ischemic stroke. Stroke. 2007;38:961–966. doi: 10.1161/01.STR.0000257314.74853.2b. [DOI] [PubMed] [Google Scholar]
- 18.Sharma VK, Tsivgoulis G, Lao AY, Malkoff MD, Alexandrov AW, Alexandrov AV. Quantification of micro-bubbles appearance in brain vessels: implications for residual flow velocity measurements, dose calculations and potential drug delivery. Stroke. 2008;39:1476–1481. doi: 10.1161/STROKEAHA.107.501593. [DOI] [PubMed] [Google Scholar]
- 19.Albers GW, Bates VE, Clark WM, Bell R, Verro P, Hamilton SA. Intravenous tissue-type plasminogen activator for treatment of acute stroke: the Standard Treatment with Alteplase to Reverse Stroke (STARS) study. JAMA. 2000;283:1145–1150. doi: 10.1001/jama.283.9.1145. [DOI] [PubMed] [Google Scholar]
- 20.Molina CA. Hemorrhagic transformation: a foe that could be a friend? Intl J Stroke. 2006;1:226. doi: 10.1111/j.1747-4949.2006.00063.x. [DOI] [PubMed] [Google Scholar]
- 21.Larrue V, Viguier A, Arnaud C, Cognard C, Petit R, Rigal M, Cristini C, Vuillier F. Trancranial ultrasound combined with intravenous microbubbles and tissue plasminogen activator for acute ischemic stroke: a randomized controlled study. Stroke. 2007;38:472. Abstract. [Google Scholar]
- 22.Eggers J, Koch B, Meyer K, Konig I, Seidel G. Effect of ultrasound on thrombolysis of middle cerebral artery occlusion. Ann Neurol. 2003;53:797–800. doi: 10.1002/ana.10590. [DOI] [PubMed] [Google Scholar]
- 23.Daffertshofer M, Gass A, Ringleb P, Sitzer M, Sliwka U, Els T, Sedlaczek O, Koroshetz WJ, Hennerici MG. Transcranial low-frequency ultrasound-mediated thrombolysis in brain ischemia: increased risk of hemorrhage with combined ultrasound and tissue plasminogen activator: results of a phase II clinical trial. Stroke. 2005;36:1441–1446. doi: 10.1161/01.STR.0000170707.86793.1a. [DOI] [PubMed] [Google Scholar]