Abstract
A high throughput, low volume microfluidic device has been designed to decouple the physical processes of protein crystal nucleation and growth. This device, called the Phase Chip, is constructed out of poly(dimethylsiloxane) (PDMS) elastomer. One of the Phase Chip’s innovations is to exploit surface tension forces to guide each drop to a storage chamber. We demonstrate that nanoliter water-in-oil drops of protein solutions can be rapidly stored in individual wells thereby allowing the screening of 1000 conditions while consuming a total of only 10 μg protein on a 20 cm2 chip. Another significant advance over current microfluidic devices is that each well is in contact with a reservoir via a dialysis membrane through which only water and other low molecular weight organic solvents can pass, but not salt, polymer, or protein. This enables the concentration of all solutes in a solution to be reversibly, rapidly, and precisely varied in contrast to current methods, such as the free interface diffusion or sitting drop methods, which are irreversible. The Phase Chip operates by first optimizing conditions for nucleation by using dialysis to supersaturate the protein solution, which leads to nucleation of many small crystals. Next, conditions are optimized for crystal growth by using dialysis to reduce the protein and precipitant concentrations, which leads small crystals to dissolve while simultaneously causing only the largest ones to grow, ultimately resulting in the transformation of many small, unusable crystals into a few large ones.
Keywords: microfluidics, PDMS, water permeation, high throughput, protein crystallization, phase diagram, nucleation, growth, Ostwald ripening
Introduction
It is necessary to crystallize a protein in order to reveal its three dimensional molecular structure by x-ray diffraction (McPherson 1999). Currently protein crystals are produced by trial and error methods, which necessitate exploring a large number of conditions consuming milligrams of protein. Non-microfluidic methods require about 1 μl of solution per trial (McPherson 1999), while microfluidic devices have reduced the volume per trial to 1 nl or less (Squires and Quake 2005; Zheng et al. 2005).
Reducing protein consumption, although important, is not the most pressing problem facing crystallographers. Rather, it is that crystallization, in general, is an activated process. Due to surface tension between the crystal and the fluid there is an energy barrier that prevents crystals below a certain size to grow (Debenedetti 1996). This barrier often is quite large for proteins (Berland et al. 1992), so to achieve a finite nucleation rate, protein solutions in crystallization conditions are highly supersaturated. However, under these circumstances both the nucleation and growth rate are high, leading to the formation of many small defect laden crystals that are unsuitable for x-ray diffraction. The conundrum facing the crystallographer is that while the nucleation of crystals requires high supersaturation, the converse is true to grow large, defect free crystals. Free interface diffusion, microbatch, and vapor diffusion are popular crystallization methods, which partially decouple the physical mechanisms of nucleation and growth (Chayen 2004; 2005). While these methods have been successfully implemented in microfluidics (Hansen et al. 2002; Hansen et al. 2004; Zheng et al. 2005), their drawback is that they rely on irreversible kinetic processes, which are difficult to control and optimize. Microdialysis (Smith and Delucas 1991; McPherson 1999; Lee and Cudney 2004) and crystal seeding (Bergfors 2003) are two methods practiced in protein crystallization that permit independent optimization of nucleation and growth. In microdialysis, several microliters of protein solution are sealed in a container by a semi-permeable membrane and subsequently submerged in a reservoir of fixed chemical potential. Microdialysis allows changing of solvent conditions so that nucleation and growth can be independently optimized. In the crystal seeding method, solution conditions are first highly supersaturated in order to nucleate many small crystals, or “seeds”. Then a few seeds are transferred to a solution of low supersaturation that is optimized for growth. However, these methods as currently practiced are not sufficiently controlled, nor are they conducive to high-throughput screening. To overcome these deficiencies we have developed a microfluidic implementation of dialysis and seeding, named the Phase Chip, which incorporates the attributes of high-throughput, precision, and low volume that are characteristics of microfluidics.
Results
The Phase Chip, shown in plan and section views in Figs. 1a and 1b, is a poly(dimethylsiloxane) (PDMS) device which utilizes hydrodynamic focusing to produce 1 nl drops of protein solution inside a continuous oil stream (Zheng et al. 2003; Squires and Quake 2005; Zheng et al. 2005). One of the Phase Chip’s innovations is to exploit surface tension forces to guide each drop to a storage chamber, or well, illustrated in Figure 1c. A drop in a well can adopt a spherical shape, minimizing its surface area and its surface energy. A drop that partially occupies both a channel and well will experience a gradient in surface energy, with the resulting force acting to drive and store the drop inside the well. As the wells exist as pockets on the sides of the channel, the enclosed, stored droplets are outside the flow stream and shielded from dislodgment by hydrodynamic forces.
Figure 1.
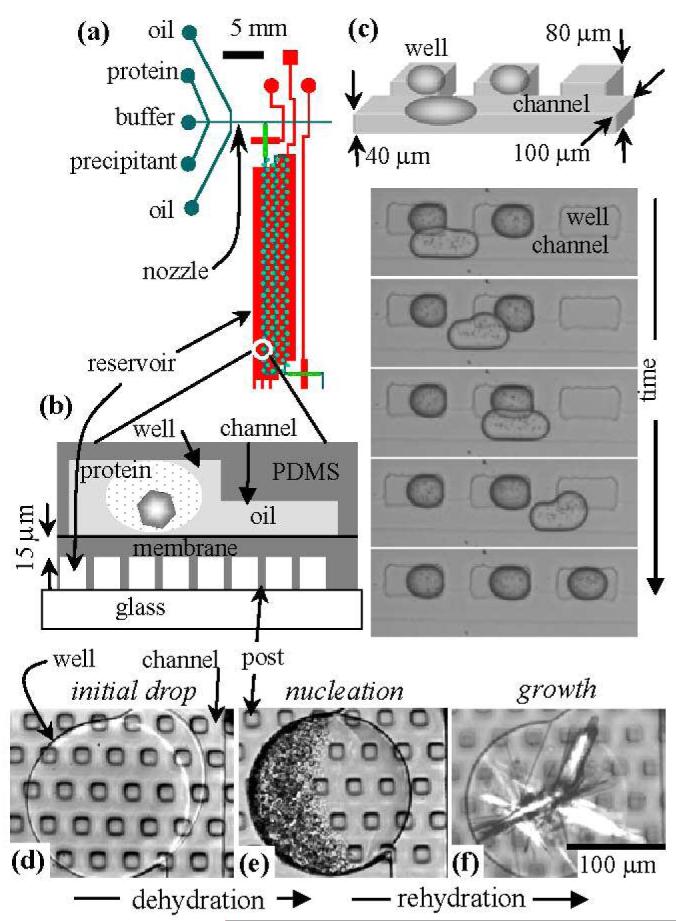
(a) Plan view of the Phase Chip. One reservoir (red) located underneath 100 wells (green circles) is shown here. There are five such sections on the chip. (b) Vertical section of a storage well, channel, reservoir and dialysis membrane. The device is constructed from two PDMS layers and subsequently sealed together (Squires and Quake 2005). In the upper, thick (5 mm) layer, there are flow channels and storage wells. In the lower, thin (40 μm) layer, there is a reservoir, sealed by a 15 μm thick PDMS membrane, The reservoir is formed by spin coating a 40 μm thick layer PDMS over a 25 μm high photoresist mold (Squires and Quake 2005). (c) Photographs of surface tension guided storage of aqueous drops into rectangular wells, without a reservoir. (d-f) Protein crystallization with reversible dialysis. The photographs are of a single 300 μm diameter circular well that contains protein solution. The channel is on the right side of the well. The square posts are 30 μm wide and support a 15 μm thick PDMS membrane, which forms the bottom of the well. (d) A stable protein solution of xylanase slightly overfills the well. (e) Protein gelation occurred after the reservoir was filled with 5M NaCl. (f) The reservoir was filled with pure water, which rehydrated the precipitate, transforming them into crystals.
Drops sequentially fill the wells, with the first drop going into the first well. Subsequent drops pass over all filled wells, entering the first empty well. To prevent coalescence of the drops during the loading process, surfactants must be added to the bulk, continuous phase. When dry air, or a concentrated salt solution, is introduced into the reservoir, water permeates from the protein drops through the membrane into the reservoir. The flux (J) of water through a PDMS membrane to dry air is with D the diffusion constant of water in PDMS, , c the solubility of water in PDMS, , and l the thickness of the membrane (Watson and Baron 1995). This leads to a water flow of about 2 nl/hr per 100 × 100 μm surface area of PDMS membrane of thickness l = 15 μm. Thus a drop in a well will shrink and completely evaporate in about one hour. If pure water is introduced in the reservoir, then the chemical potential gradient is reversed and water flows from the reservoir to the drop, thereby diluting each component as the drops swell. A thorough analysis of water transport in the Phase Chip is forthcoming. J[mol/m2s] = -D▽c = D[m2/s]c[mol/m3]/ l[m]D = 2 × 10-9 [m2/s]c = 30[mol/m3] Our strategy for high-throughput protein crystallization is to first formulate a combinatoric sequence of protein solutions of different salt and protein concentrations (Zheng et al. 2003) and subsequently store these drops in wells. Next, these protein drops are concentrated by introducing air into the reservoir. Protein solutions are notorious for their large region of metastability (Berland et al. 1992) and high supersaturations are required to nucleate crystals. Such conditions often lead to the creation of a large number of small crystals or small drops of protein gel. We regard this material as seeds and subsequently change reservoir conditions by introducing pure water into the reservoir. Water permeates from the reservoir into the protein solution and reduces the protein and salt concentrations, which lowers the chemical potential difference between the protein in solution and in the crystal. This shifts the nucleation barrier causing the smaller nuclei to dissolve and the larger crystals to grow, thereby transforming many small defected crystals into a few, large high quality crystals (Debenedetti 1996).
In distinction to all other crystallization methods, the concentration of the solutes of the protein solution can be readily measured and reversibly controlled as a function of time on the Phase Chip. By temporally varying the reservoir conditions a wide variety of dynamic paths through the phase diagram can be generated and thus the Phase Chip promises to be a useful platform for the systematic study of the role of supersaturation kinetics on nucleation and growth of protein crystals.
Figure 1 illustrates the decoupling of nucleation and crystal growth in the Phase Chip. In order to obtain statistics on the crystallization process all drops contained the same protein and buffer. Because the droplets are identical there was no need to stabilize the droplets against coalescence The protein, xylanase was prepared in non-crystallizing conditions. After the wells were filled (Fig. 1d), a 5M NaCl solution was introduced into the reservoir, leading to permeation of water out of the protein solution. Over the period of a few hours, the protein solution in the droplets was concentrated to approximately 20 mg/ml, at which point the protein solution became unstable and formed numerous drops of a dense protein gel (Fig 1e). Since neither salt, PEG, or protein is permeable in PDMS as water leaves or enters the drop the ratio of solute concentrations stays constant and the solute concentrations are inversely proportional to the volume of the drop. The drops are confined such their height remains constant so a measurement of the area of the drop suffices to determine the solute concentration. The gel is a non-equilibrium state often observed in highly supersaturated protein solutions (Muschol and Rosenberger 1997). Next, the reservoir solution was changed to pure water, which caused water to flow from the reservoir to the protein solution and lowered the degree of supersaturation. Under these conditions nucleation theory predicts that large crystals grow at the expense of small ones (Debenedetti 1996). A photograph, taken five days later showed that the protein gel had transformed into needle shaped crystals (Fig 1f). Similar events occurred in 90 out of 100 wells.
Discussion
We have manufactured microfluidic devices designed to decouple the physical processes of protein crystal nucleation and growth. The Phase Chip can store 1 nl drops in wells at a density of 400/cm2 with an independent dialysis reservoir for each 100 wells and can screen 1000 crystallization conditions while consuming only 1 μL of protein solution. Incorporating a dialysis membrane on-chip presents several advantages for protein crystallization. First, protein crystallization is a non-equilibrium process so it makes sense to have dynamic control over the key thermodynamic variable; concentration. The Phase Chip, with its ability to reversibly control protein and precipitant concentrations, renders varying concentration as convenient as varying temperature. Second, by varying the water content of each drop we can explore many different crystallization conditions in the same drop. Third, the ability to reversibly grow and dissolve protein crystals can be exploited to salvage defective crystals and transform small crystals into large ones through repetitive recrystallization cycles. Finally, we have demonstrated that by cycling the protein concentration we can first formulate stable protein solutions, next induce nucleation and then grow large protein crystals. Because the PDMS membranes are thin and the protein drops are small, the diffusion times are short and dialysis is quick. For these reasons, the Phase Chip promises to be a faster, better, and cheaper method for protein crystallization.
Materials and methods
Drop formation
After the drops are formed, they are confined in a flow channel, which has a rectangular cross section of 100 μm width and 40 μm height. The device is designed such that the channels flatten and elongate the drop. Wells, located to the side of the channel with typical dimensions of 300 μm length and 80 μm depth, are deeper than the flow channel and are spaced 500 μm apart. In Figure 1(c), the oil is hexadecane (Aldrich) and the surfactant is Span80 at 2% w/v (Aldrich).
Permeation of water
To perform reversible permeation of water from the stored drops of protein solution, the bottom of the wells are constructed from a thin PDMS membrane (15 μm thick) that is slightly permeable to water (Watson and Baron 1995; Randall and Doyle 2005), but impermeable to proteins and salts. The other side of the membrane contains a 100 nl reservoir, through which flows either dry air or an aqueous salt solution. This produces a chemical potential gradient between the protein solution stored in the well and the reservoir.
Crystallization and Ostwald ripening of Xylanase
The protein, xylanase (Hampton Research, HR7-104), was dialyzed against 0.4M potassium sodium tartrate tetrahydrate (Hampton Research, Crystal Screen HR2-110) and the initial protein concentration was 15.3 mg/ml. Xylanase does not crystallize under these conditions. For this experiment the oil was a 10:1 mixture of 3M Fluorinert FC-43 and Tridecafluoro-1-octanol from Aldrich. Fluorinated oils are reputed to be inert and not cause proteins to denature. However, drops do not form stable emulsions in this oil; drops merged and broke apart as they passed over occupied wells. Because we used multiple drops of the same composition it was not necessary to use a surfactant to stabilize the droplets against emulsion failure.
Supplementary Material
Acknowledgments
SF acknowledges support from the NSF Major Research Instrumentation program, DMR-0420921, the NIH-NIGMS, R21 PA-03-1000, and the CIMS at Harvard University (now the Center for Nanoscale Science).
Footnotes
e-mail: fraden@brandeis.edu
REFERENCES
- Bergfors T. Seeds to crystals. J. Struct. Biol. 2003;142:66–76. doi: 10.1016/s1047-8477(03)00039-x. [DOI] [PubMed] [Google Scholar]
- Berland CR, Thurston GM, Kondo M, Broide ML, Pande J, Ogun O, Benedek GB. Solid liquid-phase boundaries of lens protein solutions. Proc. Natl. Acad. Sci. 1992;89:1214–1218. doi: 10.1073/pnas.89.4.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chayen NE. Turning protein crystallisation from an art into a science. Curr. Op. Struct. Biol. 2004;14:577–583. doi: 10.1016/j.sbi.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Chayen NE. Methods for separating nucleation and growth in protein crystallisation. Prog. Biophys. Mol. Biol. 2005;88:329–337. doi: 10.1016/j.pbiomolbio.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Debenedetti PG. Metastable liquids: Concepts and principles. Princeton University Press; Princeton, NJ: 1996. p. 411. [Google Scholar]
- Hansen CL, Skordalakes E, Berger JM, Quake SR. A robust and scalable microfluidic metering method that allows protein crystal growth by free interface diffusion. Proc. Natl. Acad. Sci. 2002;99:16531–16536. doi: 10.1073/pnas.262485199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen CL, Sommer MOA, Quake SR. Systematic investigation of protein phase behavior with a microfluidic formulator. Proc. Natl. Acad. Sci. 2004;101:14431–14436. doi: 10.1073/pnas.0405847101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SSJ, Cudney R. A modified microdialysis button for use in protein crystallization. J. Appl. Cryst. 2004;37:504–505. [Google Scholar]
- McPherson A. Crystallization of biological macromolecules. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1999. p. 586. [Google Scholar]
- Muschol M, Rosenberger F. Liquid-liquid phase separation in supersaturated lysozyme solutions and associated precipitate formation/crystallization. J. Chem. Phys. 1997;107:1953–1962. [Google Scholar]
- Randall GC, Doyle PS. Permeation-driven flow in poly(dimethylsiloxane) microfluidic devices. Proc. Natl. Acad. Sci. 2005;102:10813–10818. doi: 10.1073/pnas.0503287102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HW, Delucas LJ. A method for programmable control of reservoir concentrations for protein crystal growth. J. Cryst. Growth. 1991;110:137–141. [Google Scholar]
- Squires TM, Quake SR. Microfluidics: Fluid physics at the nanoliter scale. Rev. Modern Physics. 2005;77:977–1026. [Google Scholar]
- Watson JM, Baron MG. Precise static and dynamic permeation measurements using a continuous-flow vacuum cell. J. Membrane Sci. 1995;106:259–268. [Google Scholar]
- Zheng B, Gerdts CJ, Ismagilov RF. Using nanoliter plugs in microfluidics to facilitate and understand protein crystallization. Curr. Op. Struct. Biol. 2005;15:548–555. doi: 10.1016/j.sbi.2005.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Roach LS, Ismagilov RF. Screening of protein crystallization conditions on a microfluidic chip using nanoliter-size droplets. JACS. 2003;125:11170–11171. doi: 10.1021/ja037166v. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


