Abstract
Fasting hyperglycemia in patients with type 2 diabetes mellitus (T2DM) is attributed to increased hepatic gluconeogenesis, which has been ascribed to increased transcriptional expression of phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase, catalytic (G6Pc). To test this hypothesis, we examined hepatic expression of these 2 key gluconeogenic enzymes in 2 rodent models of fasting hyperglycemia and in patients with T2DM. In rats, high-fat feeding (HFF) induces insulin resistance but a robust β-cell response prevents hyperglycemia. Fasting hyperglycemia was induced in the first rat model by using nicotinamide and streptozotocin to prevent β-cell compensation, in combination with HFF (STZ/HFF). In a second model, control and HFF rats were infused with somatostatin, followed by portal vein infusion of insulin and glucagon. Finally, the expression of these enzymes was measured in liver biopsy samples obtained from insulin sensitive, insulin resistant, and untreated T2DM patients undergoing bariatric surgery. Rats treated with STZ/HFF developed modest fasting hyperglycemia (119 ± 4 vs. 153 ± 6 mg/dL, P < 0.001) and increased rates of endogenous glucose production (EGP) (4.6 ± 0.6 vs. 6.9 ± 0.6 mg/kg/min, P = 0.02). Surprisingly, the expression of PEPCK or G6Pc was not increased. Matching plasma insulin and glucagon with portal infusions led to higher plasma glucoses in the HFF rats (147 ± 4 vs. 161 ± 4 mg/dL, P = 0.05) with higher rates of EGP and gluconeogenesis. However, PEPCK and G6Pc expression remained unchanged. Finally, in patients with T2DM, hepatic expression of PEPCK or G6Pc was not increased. Thus, in contrast to current dogma, these data demonstrate that increased transcriptional expression of PEPCK1 and G6Pc does not account for increased gluconeogenesis and fasting hyperglycemia in patients with T2DM.
Keywords: gluconeogenesis, insulin resistance, type 2 diabetes mellitus
It is well-established that fasting hyperglycemia is a function of increased endogenous glucose production (EGP) (1, 2). Several studies using a variety of methods have confirmed that the increase in EGP is due to increased gluconeogenesis, as opposed to hepatic glycogenolysis (3–6). Gluconeogenesis has long been known to be inhibited by insulin and activated by glucagon. Recently, the mechanisms by which these 2 hormones exert their opposing effects have been ascribed to an intricate web of transcriptional factors regulating the expression of 2 key gluconeogenic enzymes, phosphoenolpyruvate carboxykinase (PEPCK or PCK) and glucose-6-phosphatase (G6Pc).
In gluconeogenesis, PEPCK simultaneously decarboxylates and phosphorylates oxaloacetate into phosphoenolpyruvate, one of the earliest, rate-limiting steps in gluconeogenesis. The transcription of this gene is heavily regulated with the involvement of many transcriptional factors (e.g., FKHR1, HNF3, C/EBP) and other proteins (e.g., PGC1α, SIRT1, TRB3) (7–13). PEPCK1, the cytosolic form, has been reported to be up-regulated in diabetic rodent models, but these mice are either completely lacking insulin (e.g., following streptozotocin) or have increased plasma glucocorticoids, and thus do not accurately reflect the hormonal profile observed in patients with type 2 diabetes mellitus (T2DM) (14, 15).
G6Pc stands as the final gatekeeper for glucose efflux from the cell, catalyzing the last step of gluconeogenesis. The promoter for G6Pc has several elements in common with PEPCK (16, 17) and its expression has also been reported to be increased in some diabetic rodent models (18–21). However, the regulation of G6Pc has an added level of complexity. It is spatially discrete, localized to microsomes, with the catalytic subunit facing the ER lumen. Its activity depends on 3 transporters (T1–3), one to move G6P into the lumen and then two to allow the efflux of glucose and Pi out into the cytoplasm, and a “stabilizing protein” which confers Ca2+ responsiveness to the system (22).
We sought to test the hypothesis that the expression of these 2 key gluconeogenic enzymes increases in the setting of fasting hyperglycemia. Although high-fat fed (HFF) rodents have been used to study the pathogenesis of insulin resistance, the hyperglycemia seen in diabetes does not manifest, in part because of a compensatory β-cell response. And, as stated above, other commonly used rodent models of diabetes are not representative of T2DM due to the various genetic changes such as impaired leptin action and subsequently increased plasma glucocorticoid concentrations. Here, 2 rat models were used where the β-cell response was either prevented or reversed, allowing hyperglycemia to develop. First, the β-cell toxicity of streptozotocin (STZ) can be mitigated by pretreating rats with nicotinic acid, creating a modest β-cell defect (23). When high-fat fed, these rats were found to develop modest hyperglycemia with insulin, glucagon, and corticosterone concentrations similar to control rats. Second, hyperinsulinemia was acutely reversed in high-fat fed rats by using a combination of somatostatin with portal insulin and glucagon to match the concentrations of these hormones in control and HFF rats. Finally, to establish the relevance of our animal modes to patients with T2DM, we measured the expression of these enzymes from liver-biopsy specimens obtained from obese, insulin-sensitive, insulin-resistant, and T2DM patients undergoing bariatric surgery.
Results
Fasting Hyperglycemia in Rats Treated with Streptozocin and Fed a High-Fat Diet.
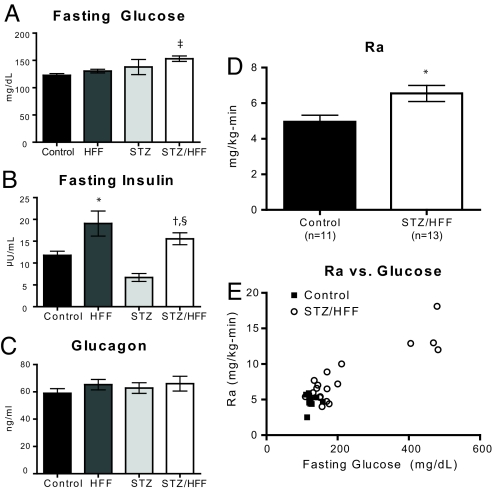
Although some rodent models of hyperglycemia have been reported to have increased expression of gluconeogenic enzymes, these models have increased corticosterone. We confirmed that this was the case in both Zucker Diabetic-Fatty (ZDF) (Fig. S1) and Goto-Kakizaki (GK) (Fig. S2) rats, as well as in db/db mice (Fig. S1). Thus, we sought other models in which to study the relation between fasting hyperglycemia and expression of gluconeogenic enzymes. Normal male Sprague–Dawley (SD) rats were either untreated or treated with a combination of streptozotocin (65 mg/kg) and nicotinic acid (170 mg/kg), followed by either 1 week of control chow (CONT) or HFF. In contrast to CONT rats or HFF, the STZ/HFF rats developed mild fasting hyperglycemia (Fig. 1A). The development of mild hyperglycemia in the STZ/HFF group occurred without any compensatory hyperinsulinemia, as in the HFF rats (Fig. 1B). Compared with the CONT rats, liver triglyceride content was 88% higher in the HFF group (P < 0.001vs. CONT) and 39% higher in the STZ/HFF (P < 0.05 vs. CONT, P < 0.01 vs. HFF). Plasma glucagon concentrations were similar in all 4 groups (Fig. 1C). Importantly, plasma corticosterone concentration was equivalent between the CONT and STZ/HFF groups (466 ± 64 vs. 438 ± 83 ng/mL, P = 0.79). Thus, the STZ/HFF group recapitulated some of the key features of T2DM, namely fasting hyperglycemia with inappropriately normal insulin and glucagon concentrations and without any increase in corticosterone. A small number (≈15%) of STZ/HFF-treated rats did develop more profound hyperglycemia (442 ± 17 mg/dL) and were considered separately for further subgroup analyses [STZ/HFF-very hyperglycemic (VH)]. These very hyperglycemic rats had weight loss (CONT: 318 ± 10 g vs. STZ/HFF: 314 ± 10 g vs. STZ/HFF-VH: 253 ± 16 g). Neither fasting plasma insulin (12.3 ± 1.6 μU/mL) nor plasma glucagon (53.17 ± 10 ng/mL) were significantly different in the very hyperglycemic rats.
Fig. 1.
Basal data for control and diabetic rats. Rats were either untreated or treated with a combination of Streptozocin and nicotinic acid followed by feeding with either a control chow or high-fat chow for 5 or 6 days. (A) Fasting plasma glucose. (B) Fasting plasma insulin. (C) Glucagon. (D) Ra glucose in control and STZ/HFF rats. (E) Rates of hepatic glucose production vs. plasma glucose concentration, including data from the very diabetic STZ/HFF rats. *, P < 0.05 vs. control; †, P < 0.01 vs. HFF; ‡, P < 0.001 vs. control, §, P < 0.01 vs. STZ.
Fasting hyperglycemia in the STZ/HFF rats was associated with a 30% increase in the rate of EGP (Fig. 1D). The very hyperglycemic STZ/HFF rats had much higher rates of EGP (14.0 ± 1.4 mg/kg/min), commensurate to their elevated fasting plasma glucose concentrations. Comparison of fasting plasma glucose concentrations to the rate of endogenous glucose production from all rats demonstrated a direct correlation (R2 of 0.8, P < 0.0001) similar to that observed in humans with T2DM (Fig. 1E). This relationship persists, even if the STZ/HFF-VH rats are excluded (R2 = 0.28, P = 0.006).
PEPCK and G6Pc Expression Are Not Increased in Mildly Hyperglycemic Rats.
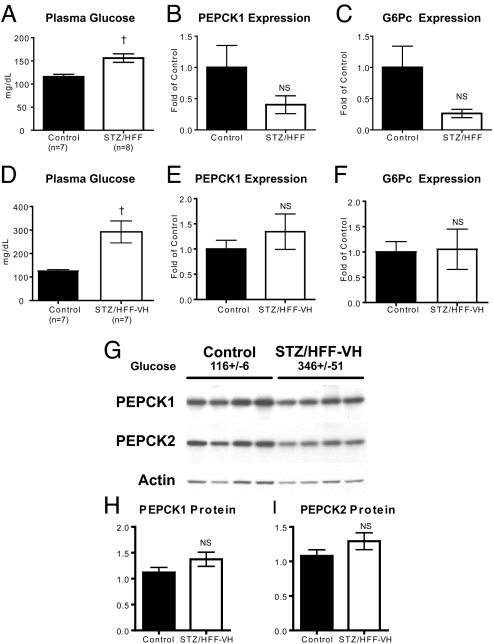
Fasting plasma glucose concentrations were higher in the STZ/HFF rats but surprisingly PEPCK1 (cytosolic) and G6Pc expression were not different (Fig. 2A–C). This was true even in the smaller cohort of STZ/HFF rats that developed more profound hyperglycemia (Fig. 2 D–F). There were also no changes in PEPCK2 (mitochondrial) expression (CONT: 1.0 ± 0.07 vs. STZ/HFF-VH: 0.98 ± 0.05, P = 0.83). These changes in PEPCK1 and PEPCK2 mRNA were confirmed with Western blots of liver extracts from these rats (Fig. 2 G and H).
Fig. 2.
PEPCK and G6Pc expression in diabetic rats. (A–C) Plasma glucose (A), PEPCK1 (B), and G6Pc (C) expression from a representative cohort of control and moderately diabetic rats. (D–F) Plasma glucose (D), PEPCK1 (E), and G6Pc (F) expression from a cohort of control and very diabetic rats. (G–I) PEPCK 1 and 2 Western blots for control and very diabetic rats. Plasma glucose for the rats from which the samples were taken is shown. †, P < 0.01 vs. control.
Because overnight fasting may have induced expression of the gluconeogenic enzymes in the CONT animals, any prior difference may have been minimized. To examine this possibility, additional experiments were performed where animals were fasted for only 6 hours before they were euthanized. After the 6-h fast, there were again clear differences in plasma glucose (CONT: 126 ± 3 vs. STZ/HFF: 179 ± 16, P = 0.005). Plasma insulin (CONT: 41.2 ± 5.6 μU/mL vs. 35.9 ± 10 μU/mL, P = 0.62) and plasma glucagon (55.9 ± 13.2 ng/mL vs. 39.4 ± 5.0 ng/mL, P = 0.27) were not different between the 2 groups. Despite the hyperglycemia in the STZ/HFF group, neither PEPCK1 (CONT: 1.0 ± 0.21 vs. HFF:0.72 ± 0.24, P = 0.4) nor G6Pc (1.0 ± 0.24 vs. STZ/HFF: 0.99 ± 0.28, P = 0.99) were altered. Thus, in these diabetic rats with clear increases in fasting plasma glucose concentrations and rates of endogenous glucose production, there was surprisingly no detectable increase in PEPCK or G6Pc expression. Given these unexpected results, a second rat model was developed to test this hypothesis.
Acute Reversal of Hyperinsulinemia in HFF Rats Results in Hyperglycemia.
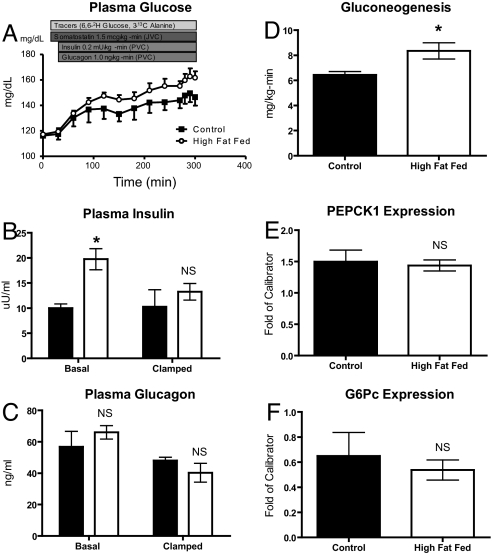
As stated previously, HFF alone fails to yield hyperglycemia in rodents, in part because of hyperinsulinemia. Hyperinsulinemia was acutely reversed by using an infusion of somatostatin to suppress endogenous islet hormone secretion followed by replacement portal vein glucagon and insulin. Following a 4.5-h infusion protocol in CONT and HFF rats, the HFF rats became hyperglycemic relative to the CONT rats during the last 30 min of the infusion (Fig. 3A; CONT: 147 ± 5 mg/dL vs. HFF: 161 ± 4 mg/dL, P = 0.05). This hyperglycemia developed as plasma insulin concentrations dropped in the HFF group to those seen in the CONT group (Fig. 3B). Plasma glucagon concentrations remained at basal levels throughout the infusion protocol (Fig. 3C).
Fig. 3.
UPortal infusion studies in control and HFF rats. Triply catheterized rats (portal vein, jugular vein, and carotid artery) were subjected to either 3 days of control chow or high-fat feeding before studies. (A) Infusion protocol and time-course of plasma glucose. (B) Plasma insulin at basal and steady states. (C) Plasma glucagon at basal and steady state conditions. (D) Gluconeogenesis. (E) PEPCK1 expression. (F) G6Pc expression. *, P < 0.05.
In these experiments, glucose production was determined as previously described by using an infusion of [6,6-2H] glucose. Additionally, [3-13C] alanine was infused to measure the contribution of gluconeogenesis to glucose production (24). The infusion of [3-13C] alanine results in 13C-labeling of intrahepatic trioses (i.e., 3-phosphoglycerate) and hexoses (i.e., glucose-6-phosphate). The percent of gluconeogenesis is determined by a product/precursor approach, dividing the enrichment of G6PM+1 by twice the enrichment of 3PGM+1 (i.e., G6PM+1/(2*3PGM+1). By measuring the enrichment of intrahepatic triose pools, this technique avoids problems of label dilution that complicated past techniques using alanine to measure gluconeogenesis (25).
Using these isotopic techniques in combination with the portal hormone infusion, endogenous glucose production was 17% higher in the HFF rats compared with CONT rats (7.6 ± 0.2 vs. 8.9 ± 0.3, P < 0.05). The rate of gluconeogenesis was 30% higher in the HFF rats compared with controls (Fig. 3D). Thus, by acutely reversing the hyperinsulinemia in HFF rats, increases in gluconeogenesis and EGP led to hyperglycemia. PEPCK1 and G6P expression was assessed from the livers of these rats. Again, despite the increases in gluconeogenesis, there was no increase in the expression of these gluconeogenic enzymes (Fig. 3 E and F).
Hepatic Expression of Gluconeogenic Enzymes Is Not Increased in Patients with T2DM.
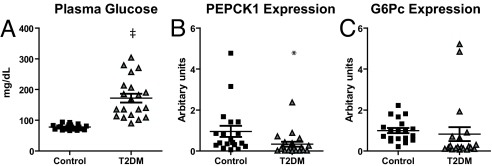
To determine whether or not the changes observed in these 2 discrete rat models were applicable to humans, liver mRNA was obtained from patients who underwent weight-loss surgery. The patients were classified into 3 groups: nondiabetic, insulin sensitive (IS); nondiabetic, insulin resistant (IR); and T2DM subjects who were not on medication (Table 1). The distinction between the IS and IR groups was made on basis of homeostatic model assessment (HOMA), with a value of <1.5 for the IS group and >13 for the IR group. Plasma glucose concentration was significantly higher in the T2DM group, as compared with the IS and IR groups (Table 1). Despite the clear hyperglycemia in the T2DM group, there was no difference in expression of PEPCK1 or G6Pc (Table 1). PEPCK2 has a greater abundance in human liver, in contrast to rat liver where PEPCK1 is predominant (26). However, there were no differences in PEPCK2 expression in the livers of IS and T2DM patients (IS: 1.0 ± 0.5 vs. DM: 1.7 ± 0.7, P = 0.42). These initial observations were confirmed in a second group of patients, focusing on IS and T2DM patients who were not on any medication (Table 2). As shown in Fig. 4, despite the hyperglycemia in the T2DM subjects, the expression of PEPCK1, PEPCK2, and G6Pc were not increased. These data suggest that the hyperglycemia seen in patients with T2DM cannot be attributed to increased expression of these key gluconeogenic enzymes.
Table 1.
Data for human subjects 1
| IS (n = 10) | IR (n = 8) | DM (n = 8) | |
|---|---|---|---|
| Age, years | 50 ± 3 | 43 ± 5 | 47 ± 3 |
| Gender, male/female | 1/9 | 2/6 | 3/5 |
| BMI, kg/m2 | 50.6 ± 4.3 | 57.4 ± 3 | 51.8 ± 2.9 |
| Plasma glucose, mg/dL | 83 ± 3 | 95 ± 5 | 172 ± 18*† |
| Plasma insulin, μU/mL | 5.1 ± 0.6 | 68.4 ± 3.7* | 32.6 ± 4.3*† |
| HOMA | 1.0 ± 0.1 | 16.0 ± 1.0* | 13.8 ± 2.1* |
| PEPCK1 expression, relative to IS | 1.0 ± 0.08 | 1.0 ± 0.08 | 0.6 ± 0.3 |
| G6Pc Expression, relative to IS | 1.0 ± 0.13 | 1.2 ± 0.12 | 0.5 ± 0.13† |
*, P < 0.05 vs. IS
†, P < 0.05 vs. IR
Table 2.
Clinical data for human subjects 2
| IS (n = 20) | T2DM (n = 20) | |
|---|---|---|
| Age, years | 42.75 ± 1.508 | 45.50 ± 2.432 |
| Gender, male/female | 6/14 | 7/13 |
| BMI, kg/m2 | 54.1 ± 3.1 | 48.2 ± 1.8 |
| Plasma glucose, mg/dL | 77.8 ± 1.9 | 172 ± 14.6* |
| Plasma insulin, μU/mL | 5.2 ± 0.3 | 39.0 ± 5.0* |
| HOMA | 1.0 ± 0.07 | 15.6 ± 2.4* |
*, P < 0.0001 vs. IS.
Fig. 4.
Gluconeogenic gene expression in livers of additional human subjects. (A) Plasma glucose. (B) PEPCK1 expression. (C) G6Pc expression. ‡, P < 0.0001 vs. control; *, P < 0.05 vs. control
Discussion
Fasting plasma glucose measurements are recommended as the best screening test for diabetes (27). Although it is a cornerstone of clinical management and marks the progression along a spectrum of disease, there are still fundamental questions regarding the molecular mechanisms leading to fasting hyperglycemia. It is well-established that increased fasting plasma glucose concentrations in patients with poorly controlled T2DM can mostly be ascribed to an increase in rates of EGP. Furthermore, by using 13C magnetic resonance spectroscopy to noninvasively measure rates of net hepatic glycogenolysis and gluconeogenesis, Magnusson et al. (4) found that increased rates of fasting EGP in patients with T2DM could entirely be attributed to increased gluconeogenesis. These findings have subsequently been confirmed by other methods (5, 6).
Fasting hyperglycemia and gluconeogenesis are controlled by the opposing actions of insulin and glucagon. Although the decline in β-cell function (28) is often invoked as a culprit for fasting hyperglycemia, glucagon also plays a role as postulated in the “bi-hormonal hypothesis” (29, 30). In humans, basal glucagon activates gluconeogenesis and mitigates insulin-stimulated hepatic glycogen synthesis (31). Moreover, patients with T2DM have an inappropriately high plasma glucagon concentration relative to their degree of glycemia (32–34). Thus, in the setting of impaired insulin action, from insulin resistance and decreased β-cell function, glucagon may promote gluconeogenesis and fasting hyperglycemia.
The bi-hormonal hypothesis has been extended to a cellular and molecular level with the discoveries of transcription factors and cofactors linking hormone action to the expression of gluconeogenic enzymes. Insulin inhibits key transcription factors such as FOXO1, FOXA2, and CBP. Glucagon may activate gluconeogenesis via factors such as CREB, PGC1α, TORC2, and TRB3. These links between hormone action and gluconeogenic gene transcription helped promote the view that increased expression of these enzymes was responsible for increased hepatic gluconeogenesis and fasting hyperglycemia in patients with poorly controlled T2DM. This prevailing view served as the hypothesis tested in this study.
Because most genetic rodent models of T2DM are associated with increased plasma glucocorticoid concentrations (35, 36), a well established transcriptional regulator of gluconeogenesis (37), we examined the role of gluconeogenic gene expression on hepatic gluconeogenesis and glucose production in 2 hyperglycemic rat models that would be free of this confounding effect. In rats, a high-fat diet alone will not lead to fasting hyperglycemia, in part due to compensatory β-cell hypersecretion of insulin. To dampen this compensatory β-cell response we used streptozotocin in combination with nicotinic acid to produce modest hyperglycemia. Despite the hyperglycemia, plasma insulin and glucagon concentrations were equivalent to control rats, which is similar to what is typically observed in patients with moderately to poorly controlled T2DM. Furthermore, in contrast to other commonly used rodent models of T2DM, plasma corticosterone levels were not increased in these hyperglycemic rats. Surprisingly, neither the expression of PEPCK or G6Pc was increased in this rat model of T2DM. There was also no difference in the expression of PEPCK or G6Pc even when we studied rats after only a 6-h fast to avoid any potential up-regulation of these enzymes in the control rats with the prolonged overnight fast. Moreover, we found no increase in the expression of these enzymes when we examined the most hyperglycemic subgroup of diabetic rats compared with the nondiabetic control rats.
These data are in marked contrast to previous studies in ZDF rats, where increased glucose production and expression of PEPCK have been observed (35). However, ZDF rats are known to have increased corticosterone, which may drive PEPCK expression (36). To confirm this, we measured basal plasma glucose, corticosterone, and hepatic PEPCK and G6Pc expression in both ZDF and db/db mice (Table S1 and Fig. S1). Although both of these models had increased expression of PEPCK, this was associated with increased plasma corticosterone concentrations. Another commonly used rat model, the inbred GK rat, has also been used as a model of T2DM. We observed that expression of PEPCK and G6Pc was markedly higher in the GK diabetic rats compared with the control Wistar-Kyoto (WK) rats (Fig. S2). However, as in the ZDF rats, we found plasma corticosterone concentrations were markedly higher in the GK rats compared with the WK rats. Similarly, high corticosterone values confound murine models of T2DM (38, 39). Thus, in contrast to the more commonly used models of T2DM, the STZ/HFF model does not have hypercorticosteronemia.
The negative findings of the first hyperglycemic rat model spurred us to test our hypothesis in a second hyperglycemic rat model. We acutely matched plasma insulin concentrations in control and high-fat fed rats by using somatostatin to suppress endogenous islet cell hormone secretion, followed by an infusion of insulin and glucagon into the portal vein. We found that after normalizing plasma insulin concentrations over the 4.5-h infusion, the high-fat fed rats developed higher fasting plasma glucose concentrations compared with the control chow-fed rats, which could be attributed to increased rates of endogenous glucose production and increased rates of hepatic gluconeogenesis, which is similar to what has been measured in patients with poorly controlled T2DM (4). However, despite higher plasma glucose concentrations and increased rates of gluconeogenesis, there was no detectable increase in either PEPCK or G6Pc mRNA expression.
To determine whether these rodent findings would translate to patients with T2DM, we measured the expression of mRNA in liver biopsy specimens obtained from nondiabetic insulin-sensitive and insulin-resistant patients, as well as untreated T2DM patients undergoing bariatric weight-loss surgery. As in the hyperglycemic rats, there were no increases in the mRNA for PEPCK1, PEPCK2, or G6Pc despite a marked increase in fasting plasma glucose concentration in the T2DM group. Thus, mechanisms other than increased transcription of these enzymes must be responsible for causing the increased rates of hepatic gluconeogenesis in patients with poorly controlled T2DM.
This conclusion is contrary to the prevailing view that increased transcription of PEPCK1 (cytosolic), PEPCK2 (mitochondrial), or G6Pc is responsible for the increased rates of gluconeogenesis and glucose production observed in patients with poorly controlled T2DM. The discovery of proteins, whose activities are integrated within the complex promoter regions of these enzymes, suggested that increased transcription of these enzymes may in fact be the mechanism underlying increased gluconeogenesis. This high level of regulation has led PEPCK to be considered the key rate-controlling enzyme for gluconeogenesis. Yet, this role may not be aptly assigned to this enzyme. Burgess et al. (58), by using a variety of PEPCK-deficient mouse strains, report that the control coefficient for this enzyme was remarkably low for a rate-controlling enzyme: 0.18 as opposed to the ideal 1.0. They and others have proposed that PEPCK may play a role other than gluconeogenesis, such as regulation of the tricarboxylic acid cycle flux and generation of glycerol-3-phosphate to serve as a backbone for triglyceride synthesis (12, 40).
The regulation of G6Pc, which is poised to regulate the final efflux of glucose from the cell, is more complex. Its promoter region contains many elements in common with the PEPCK promoter (17, 41). Whereas insulin and glucocorticoids have been shown to regulate its expression, its transcriptional regulation by glucagon is less well-understood. Ichai et al. (42) reported that although glucagon does increase glucose-6-phosphate hydrolysis in vitro, this occurs out of proportion to an increase in G6Pc activity and in a cold-sensitive manner. The latter finding was ascribed to a hypothetical temperature-sensitive transport step. Recently, Sloop et al. (43) reported that 80–90% inhibition of the T1 transporter by antisense oligonucleotides in ob/ob mice normalized fasting glucose concentration. Moreover, knockdown of the transporter led to a dose-dependent decrease in plasma glucose concentrations, suggesting a tight coupling between microsomal transport and glycemia. In contrast, only small amounts of phosphatase activity are necessary for maintaining normal glucose production. This was demonstrated in G6Pc−/− mice, where adenoviral rescue with the G6Pc gene demonstrated that even a fraction of the phosphatase activity allowed mice to maintain normal plasma glucose (44). Thus, compared with the other potential mechanisms of regulation, transcription of the catalytic subunit may only have minimal impact on the overall activity of the hepatic G6Pase activity.
There are several other steps where gluconeogenesis may be regulated. Pyruvate carboxylase (PC) and fructose 1,6 bisphosphatase (FBPase) are also key enzymes in the pathway. In contrast to PEPCK and G6Pc, these enzymes are subject to allosteric regulation: PC by acetyl Co-A (45), FBPase by AMP and fructose 2,6-bisphosphate (46). Other possibilities for increased gluconeogenesis may include increased delivery of substrates such as amino acids (47, 48), and/or glycerol (49, 50).
In summary, in contrast to the prevailing dogma, these data demonstrate that increased expression of PEPCK and G6Pc are not responsible for the increased rates of hepatic gluconeogenesis and glucose production in patients with poorly controlled T2DM. Therefore, other cellular mechanisms must be sought in the pathogenesis of fasting hyperglycemia in T2DM.
Materials and Methods
Animals.
Normal male Sprague-Dawley (Harlan) rats weighing 275–300 g, housed in a 12-h dark/light cycle and allowed to acclimate to our facility for 5–7 days before use. They were either untreated or treated with a combination of nicotinamide (170 mg/kg i.p.) followed 15 min later by streptozotocin (65 mg/kg i.p.). Four to five days after treatment, rats underwent placement of jugular venous and carotid artery catheters. Rats were fed a normal rodent chow (Harlan-Teklad 2018: 77% CHO/5% fat/18% protein) or a high-fat diet containing 27% safflower oil (Dyets 112245: 26% CHO/59% fat/15% protein) for 5–6 days before study. For most of the studies, animals were fasted overnight (14–16 h) before study. In one study, to minimize the impact of the overnight fast, food was withdrawn for only 6 h (7 a.m. to 1 p.m.). At the time of study, some rats were very hyperglycemic (e.g., plasma glucose >300 mg/dL). These rats were classified in a separate group from the other diabetic animals. We also studied 3 strains of rodents with spontaneous diabetes. GK rats and their corresponding controls, WK rats, were obtained from Taconic Farms. ZDF/GmiCrl-fa/fa rats and their corresponding controls, ZDF/GmiCrl-fa/+ rats, were obtained from Charles River Labs. db/db mice (BKS.Cg-m +/+ Leprdb/J) and their corresponding controls db/+ (Heterozygous Leprdb +/+ m) were obtained from The Jackson Laboratory. These 3 animals were all on their regular diets and all were studied after an overnight fast. All studies were approved by the Institutional Animal Care and Use Committees at the Veteran's Affairs Medical Center, West Haven, CT and at Yale University School of Medicine.
Basal Rate of Glucose Production.
After an overnight fast, chronically catheterized rats received a primed/continuous infusion of [6,6 2H] glucose (prime: 3.0 mg/kg/min x 5 min; continuous: 0.3 mg/kg/min). Plasma blood samples were obtained at 10-min intervals between 90 and 120 min. At the end of the infusion, rats were euthanized and tissues harvested in situ with tongs precooled in liquid nitrogen. Additionally, samples of liver were placed in RNAlater for RNA extraction.
Portal Infusion of Insulin and Glucagon.
For these studies, 300–325-g male SD rats were purchased that were precatheterized with portal vein, jugular vein, and carotid artery catheters implanted (Charles River Labs). The rats were assigned to either control chow or a high-fat diet for 3 days. This short-term dietary intervention results specifically in hepatic steatosis and hepatic insulin resistance (51). After an overnight fast, the infusion study began with a 5-h, unprimed infusion of [3-13C] alanine (1.0 mg/kg/min), [6,6 2H] glucose (0.3 mg/kg/min) and somatostatin (1.5 μg/kg/min) through the jugular vein. After 30 min, a portal vein infusion of insulin (0.2 mU/kg/min) and glucagon (1.0 ng/kg/min) was begun (52, 53). Blood samples were obtained every 30 min from the carotid artery catheter to measure plasma glucose. Additional blood samples (100 μL) were obtained at times 0, 270, 280, 290, and 300 min to measure the atom percent enrichment of GlucoseM+1 and GlucoseM+2.
Metabolites and Hormones.
Plasma glucose was measured by the glucose oxidase method on a Beckman Glucose Analyzer II. Plasma insulin, glucagon, and corticosterone were measured by RIA (Millipore)
Analysis of Plasma Glucose by GC/MS.
To determine the enrichment of [6,6-2H] glucose in plasma, samples were deproteinized with 5 volumes of 100% methanol, dried, and derivatized with 1:1 acetic anhydride/pyridine to produce the pentacetate derivative of glucose. The atom percent of enrichment of GlucoseM+1 and GlucoseM+2 was measured by GC/MS analysis using a Hewlett-Packard 5890 gas chromatograph interfaced to a Hewlett-Packard 5971A mass-selective detector operating in the electron-ionization mode (54). GlucoseM+1 and GlucoseM+2 enrichments were determined from the ratio of m/z 201 to 200 and 202 to 200, respectively.
Analysis of Phosphorylated Intermediates by Liquid Chromatography-Tandem Mass Spectrometry.
Approximately 100 mg of ground, frozen liver were homogenized with 3 mL iced-cold methanol/water (vol/vol;1:1). Homogenates were centrifuged at 1,500 × g for 15 min at 4 °C. The water/methanol extracts were collected, evaporated to dryness, reconstituted with 300 μL water, and filtered with 5 K Nanosep (Pall) to remove macromolecules. This filtrate was used directly for LC/MS/MS analysis. LC/MS/MS measurements were performed on a bench-top PE-Sciex API 3000 triple quadruple mass spectrometer interfaced with electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI) sources, coupled to a PerkinElmer series 200 micropump liquid chromatography and an autosampler system. A porous graphitic carbon Hypercarb column (Thermo Electron) was used to separate different metabolites (55, 56) with ammonium acetate (10 mM) at a flow rate of 250 μL/min. 3-phosphoglycerate 13C enrichment was determined by measuring enrichment of ion pairs with an m/z of 185.1 and 79.0 and glucose 6-phosphate 13C enrichment was determined by measuring enrichment of ion pairs with an m/z of 289 and 96.9.
Calculations.
Endogenous glucose production was calculated by using Steele's equation in steady state (24). To calculate the contribution of gluconeogenesis to EGP, the following equations was used:
The ratio of G6P to 3PG is used as the enrichments can be determined from a single liver sample permitting us to readily assess intrahepatic gluconeogenesis. The 2 is required in the denominator because each molecule of glucose is comprised of 2 labeled trioses. The rate of gluconeogenesis is the product of the percent of gluconeogenic and the rate of EGP.
Human Samples.
Human liver samples were obtained from patients undergoing bariatric surgery at the Geisinger Clinic (Danvillle, PA). Samples were obtained from intra-operative wedge biopsies of the liver obtained at a standard anatomic location and immediately placed in RNAlater (Qiagen) for subsequent storage at −80 °C. The Institutional Review Board at the Geisinger Medical Clinic approved the research protocol and all participants provided written informed consent.
Total RNA Preparation and Real-Time Quantitative RT-PCR Analysis.
Total RNA was extracted from liver samples stored in RNAlater by using the RNeasy kit (Qiagen). RNA was reverse-transcribed into cDNA by using Stratascript Reverse Transcriptase (Stratagene). The abundance of transcripts for PEPCK1 (cytosolic), PEPCK2 (mitochondrial), and G6Pase was assessed by real time PCR using a SYBR Green detection system (Stratagene). For each run, samples were run in duplicates for both the gene of interest and actin. The expression data for each gene of interest and actin were normalized for the efficiency of amplification, as determined by a standard curve included on each run (57).
Western Blotting.
Liver proteins were extracted in homogenization buffer (50 mM Hepes, 150 mM NaCL, 1 mM EDTA, 2 mM Na3VO4, 20 mM Na4P2O7, 100 mM NaF, 1%TritonX-100, 2 mM PMSF, 20 μg/mL Aprotinin, 1 mg/mL Leupeptin and Pepstatin) and protein concentration determined by the Bradford method (Bio-Rad). Equal amounts of protein were resolved by SDS/PAGE and electroblotted onto a polyvinylidene difluoride membrane (DuPont) by using a semidry transfer cell (Bio-Rad). After blocking for 2h at room temperature in TBST containing 5% (wt/vol) nonfat dried milk, and then incubated overnight with polyclonal sheep anti-PEPCK1 antibody (a kind gift of Daryl Granner, Vanderbilt University, Nashville, TN). After further washings, membranes were incubated with horseradish peroxidase-conjugated secondary antibody and visualized by ECL (Amersham). These blots were stripped and reblotted with anti-PEPCK2 antibody (AbCam) and antiactin antibody.
Statistical Analysis.
Statistical analysis of the data was performed by using GraphPad Prism 5.0. All data are expressed as mean ± standard error of the mean. For comparisons of 2 groups, Student's t test was used. For comparison of >2 groups, ANOVA was performed followed by posthoc with Tukey's Multiple Comparison test to determine significance between groups.
Supplementary Material
Acknowledgments.
We thank Todd May, Yanna Kosover, and Aida Groszman for technical assistance with these studies. These studies were funded by a Veteran's Affairs Merit Review Award and Grants R01 DK-40936, R01 DK-49230, P30 DK-45735, and UL1 RR024139 from the United States Public Health Service.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812547106/DCSupplemental.
References
- 1.Maggs DG, et al. Metabolic effects of troglitazone monotherapy in type 2 diabetes mellitus. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;128:176–185. doi: 10.7326/0003-4819-128-3-199802010-00002. [DOI] [PubMed] [Google Scholar]
- 2.Gastaldelli A, et al. Separate contribution of diabetes, total fat mass, and fat topography to glucose production, gluconeogenesis, and glycogenolysis. J Clin Endocrinol Metab. 2004;89:3914–3921. doi: 10.1210/jc.2003-031941. [DOI] [PubMed] [Google Scholar]
- 3.Hundal R, et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49:2063–2069. doi: 10.2337/diabetes.49.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magnusson I, Rothman DL, Katz LD, Shulman RG, Shulman GI. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J Clin Invest. 1992;90:1323–1327. doi: 10.1172/JCI115997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wajngot A, et al. Quantitative contributions of gluconeogenesis to glucose production during fasting in type 2 diabetes mellitus. Metabolism. 2001;50(1):47–52. doi: 10.1053/meta.2001.19422. [DOI] [PubMed] [Google Scholar]
- 6.Kunert O, et al. Measurement of fractional whole-body gluconeogenesis in humans from blood samples using 2H nuclear magnetic resonance spectroscopy. Diabetes. 2003;52:2475–2482. doi: 10.2337/diabetes.52.10.2475. [DOI] [PubMed] [Google Scholar]
- 7.Du K, Herzig S, Kulkarni RN, Montminy M. TRB3: A tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science. 2003;300:1574–1577. doi: 10.1126/science.1079817. [DOI] [PubMed] [Google Scholar]
- 8.Jurado LA, Song S, Roesler WJ, Park EA. Conserved amino acids within CCAAT enhancer-binding proteins (C/EBP(alpha) and beta) regulate phosphoenolpyruvate carboxykinase (PEPCK) gene expression. J Biol Chem. 2002;277:27606–27612. doi: 10.1074/jbc.M201429200. [DOI] [PubMed] [Google Scholar]
- 9.Koo SH, et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–1111. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- 10.Nakae J, Kitamura T, Silver DL, Accili D. The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J Clin Invest. 2001;108:1359–1367. doi: 10.1172/JCI12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Brien RM, et al. Hepatic nuclear factor 3- and hormone-regulated expression of the phosphoenolpyruvate carboxykinase and insulin-like growth factor-binding protein 1 genes. Mol Cell Biol. 1995;15:1747–1758. doi: 10.1128/mcb.15.3.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.She P, et al. Mechanisms by which liver-specific PEPCK knockout mice preserve euglycemia during starvation. Diabetes. 2003;52:1649–1654. doi: 10.2337/diabetes.52.7.1649. [DOI] [PubMed] [Google Scholar]
- 13.Yoon JC, et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413:131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- 14.Robinson SW, Dinulescu DM, Cone RD. Genetic models of obesity and energy balance in the mouse. Annu Rev Genet. 2000;34:687–745. doi: 10.1146/annurev.genet.34.1.687. [DOI] [PubMed] [Google Scholar]
- 15.Veneziale CM, Donofrio JC, Nishimura H. The concentration of P-enolpyruvate carboxykinase protein in murine tissues in diabetes of chemical and genetic origin. J Biol Chem. 1983;258:14257–14262. [PubMed] [Google Scholar]
- 16.Argaud D, et al. Regulation of rat liver glucose-6-phosphatase gene expression in different nutritional and hormonal states: Gene structure and 5′-flanking sequence. Diabetes. 1996;45:1563–1571. doi: 10.2337/diab.45.11.1563. [DOI] [PubMed] [Google Scholar]
- 17.Vander Kooi BT, et al. The glucose-6-phosphatase catalytic subunit gene promoter contains both positive and negative glucocorticoid response elements. Mol Endocrinol. 2005;19:3001–3022. doi: 10.1210/me.2004-0497. [DOI] [PubMed] [Google Scholar]
- 18.Burchell A, Cain DI. Rat hepatic microsomal glucose-6-phosphatase protein levels are increased in streptozotocin-induced diabetes. Diabetologia. 1985;28:852–856. doi: 10.1007/BF00291077. [DOI] [PubMed] [Google Scholar]
- 19.Haber BA, et al. High levels of glucose-6-phosphatase gene and protein expression reflect an adaptive response in proliferating liver and diabetes. J Clin Invest. 1995;95:832–841. doi: 10.1172/JCI117733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Z, Barrett EJ, Dalkin AC, Zwart AD, Chou JY. Effect of acute diabetes on rat hepatic glucose-6-phosphatase activity and its messenger RNA level. Biochem Biophys Res Commun. 1994;205:680–686. doi: 10.1006/bbrc.1994.2719. [DOI] [PubMed] [Google Scholar]
- 21.Mosseri R, Waner T, Shefi M, Shafrir E, Meyerovitch J. Gluconeogenesis in non-obese diabetic (NOD) mice: In vivo effects of vandadate treatment on hepatic glucose-6-phoshatase and phosphoenolpyruvate carboxykinase. Metabolism. 2000;49:321–325. doi: 10.1016/s0026-0495(00)90132-x. [DOI] [PubMed] [Google Scholar]
- 22.Waddell ID, Burchell A. Transverse topology of glucose-6-phosphatase in rat hepatic endoplasmic reticulum. Biochem J. 1991;275:133–137. doi: 10.1042/bj2750133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masiello P, et al. Experimental NIDDM: Development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes. 1998;47:224–229. doi: 10.2337/diab.47.2.224. [DOI] [PubMed] [Google Scholar]
- 24.Wolfe RR, Chinkes DL. 2nd Ed. Hoboken, NJ: Wiley-Liss; 2005. Isotope Tracers in Metabolic Research: Principles and Practice of Kinetic Analysis; p. vii. [Google Scholar]
- 25.Kelleher JK. Gluconeogenesis from labeled carbon: Estimating isotope dilution. Am J Physiol. 1986;250:E296–E305. doi: 10.1152/ajpendo.1986.250.3.E296. [DOI] [PubMed] [Google Scholar]
- 26.Modaressi S, et al. Molecular cloning, sequencing and expression of the cDNA of the mitochondrial form of phosphoenolpyruvate carboxykinase from human liver. Biochem J. 1996;315:807–814. doi: 10.1042/bj3150807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anonymous. Screening for type 2 diabetes. Diabetes Care. 2004;27:S11–S14. doi: 10.2337/diacare.27.2007.s11. [DOI] [PubMed] [Google Scholar]
- 28.Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003;46:3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- 29.Unger RH. Glucagon physiology and pathophysiology in the light of new advances. Diabetologia. 1985;28:574–578. doi: 10.1007/BF00281991. [DOI] [PubMed] [Google Scholar]
- 30.Unger RH. Role of glucagon in the pathogenesis of diabetes: The status of the controversy. Metabolism. 1978;27:1691–1709. doi: 10.1016/0026-0495(78)90291-3. [DOI] [PubMed] [Google Scholar]
- 31.Roden M, et al. The roles of insulin and glucagon in the regulation of hepatic glycogen synthesis and turnover in humans. J Clin Invest. 1996;97:642–648. doi: 10.1172/JCI118460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raskin P, Unger RH. Hyperglucagonemia and its suppression. Importance in the metabolic control of diabetes. N Engl J Med. 1978;299:433–436. doi: 10.1056/NEJM197808312990901. [DOI] [PubMed] [Google Scholar]
- 33.Basu R, Schwenk WF, Rizza RA. Both fasting glucose production and disappearance are abnormal in people with “mild” and “severe” type 2 diabetes. Am J Physiol. 2004;287:E55–E62. doi: 10.1152/ajpendo.00549.2003. [DOI] [PubMed] [Google Scholar]
- 34.Reaven GM, Chen YD, Golay A, Swislocki AL, Jaspan JB. Documentation of hyperglucagonemia throughout the day in nonobese and obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1987;64:106–110. doi: 10.1210/jcem-64-1-106. [DOI] [PubMed] [Google Scholar]
- 35.Munoz MC, et al. Effects of tungstate, a new potential oral antidiabetic agent, in zucker diabetic fatty rats. Diabetes. 2001;50:131–138. doi: 10.2337/diabetes.50.1.131. [DOI] [PubMed] [Google Scholar]
- 36.Livingstone DEW, et al. Understanding the role of glucocorticoids in obesity: Tissue-specific alterations of corticosterone metabolism in obese zucker rats. Endocrinology. 2000;141:560–563. doi: 10.1210/endo.141.2.7297. [DOI] [PubMed] [Google Scholar]
- 37.Imai E, et al. Characterization of a complex glucocorticoid response unit in the phosphoenolpyruvate carboxykinase gene. Mol Cell Biol. 1990;10:4712–4719. doi: 10.1128/mcb.10.9.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coleman DL, Burkart DL. Plasma corticosterone concentrations in diabetic (db) mice. Diabetologia. 1977;13:25–26. doi: 10.1007/BF00996323. [DOI] [PubMed] [Google Scholar]
- 39.Saito M, Bray GA. Diurnal rhythm for corticosterone in obese (ob/ob) diabetes (db/db) and gold-thioglucose-induced obesity in mice. Endocrinology. 1983;113:2181–2185. doi: 10.1210/endo-113-6-2181. [DOI] [PubMed] [Google Scholar]
- 40.Reshef L, et al. Glyceroneogenesis and the triglyceride/fatty acid cycle. J Biol Chem. 2003;278:30413–30416. doi: 10.1074/jbc.R300017200. [DOI] [PubMed] [Google Scholar]
- 41.Schmoll D, et al. Regulation of glucose-6-phosphatase gene expression by protein kinase b-alpha and the forkhead transcription factor FKHR: Evidence for insulin response unit-dependent and independent effects of insulin on promoter activity. J Biol Chem. 2000;275:36324–36333. doi: 10.1074/jbc.M003616200. [DOI] [PubMed] [Google Scholar]
- 42.Ichai C, et al. Glucose 6-phosphate hydrolysis is activated by glucagon in a low temperature-sensitive manner. J Biol Chem. 2001;276:28126–28133. doi: 10.1074/jbc.M010186200. [DOI] [PubMed] [Google Scholar]
- 43.Sloop KW, et al. Specific reduction of hepatic glucose 6-phosphate transporter-1 ameliorates diabetes while avoiding complications of glycogen storage disease. J Biol Chem. 2007;282:19113–19121. doi: 10.1074/jbc.M610759200. [DOI] [PubMed] [Google Scholar]
- 44.Zingone A, et al. Correction of glycogen storage disease type 1a in a mouse model by gene therapy. J Biol Chem. 2000;275:828–832. doi: 10.1074/jbc.275.2.828. [DOI] [PubMed] [Google Scholar]
- 45.McClure W, Lardy H, Kneife lH. Rat liver pyruvate carboxylase: Preparation, properties and cation specificity. J Biol Chem. 1971;246:3569–3578. [PubMed] [Google Scholar]
- 46.Meek DW, Nimmo HG. The allosteric properties of rat liver fructose-1,6-bisphosphatase. Biochem J. 1984;222:131–138. doi: 10.1042/bj2220131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chevalier S, et al. The greater contribution of gluconeogenesis to glucose production in obesity is related to increased whole-body protein catabolism. Diabetes. 2006;55:675–681. doi: 10.2337/diabetes.55.03.06.db05-1117. [DOI] [PubMed] [Google Scholar]
- 48.Krebs M, et al. Direct and indirect effects of amino acids on hepatic glucose metabolism in humans. Diabetologia. 2003;46:917–925. doi: 10.1007/s00125-003-1129-1. [DOI] [PubMed] [Google Scholar]
- 49.Previs SF, Cline GW, Shulman GI. A critical evaluation of mass isotopomer distribution analysis of gluconeogenesis in vivo. Am J Physiol. 1999;277:E154–E160. doi: 10.1152/ajpendo.1999.277.1.E154. [DOI] [PubMed] [Google Scholar]
- 50.Puhakainen I, Koivisto VA, Yki-Jarvinen H. Lipolysis and gluconeogenesis from glycerol are increased in patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1992;75:789–794. doi: 10.1210/jcem.75.3.1517368. [DOI] [PubMed] [Google Scholar]
- 51.Samuel VT, et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279:32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- 52.Cardin S, et al. Portal glucose infusion increases hepatic glycogen deposition in conscious unrestrained rats. J Appl Physiol. 1999;87:1470–1475. doi: 10.1152/jappl.1999.87.4.1470. [DOI] [PubMed] [Google Scholar]
- 53.Wei Y, Pagliassotti MJ. Hepatospecific effects of fructose on c-jun NH2-terminal kinase: Implications for hepatic insulin resistance. Am J Physiol. 2004;287:E926–E933. doi: 10.1152/ajpendo.00185.2004. [DOI] [PubMed] [Google Scholar]
- 54.Petersen KF, et al. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603–608. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buchholz A, Takors R, Wandrey C. Quantification of intracellular metabolites in Escherichia coli K12 using liquid chromatographic-electrospray ionization tandem mass spectrometric techniques. Anal Biochem. 2001;295:129–137. doi: 10.1006/abio.2001.5183. [DOI] [PubMed] [Google Scholar]
- 56.Vizan P, et al. Quantification of intracellular phosphorylated carbohydrates in HT29 human colon adenocarcinoma cell line using liquid chromatography-electrospray ionization tandem mass spectrometry. Anal Chem. 2007;79:5000–5005. doi: 10.1021/ac070170v. [DOI] [PubMed] [Google Scholar]
- 57.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burgess SC, et al. Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver. Cell Metab. 2007;5:313–320. doi: 10.1016/j.cmet.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.