Abstract
The human selenoproteome is composed of ~25 selenoproteins, which cotranslationally incorporate selenocysteine, the 21st amino acid. Selenoprotein expression requires an unusual translation mechanism, as selenocysteine is encoded by the UGA stop codon. SECIS-binding protein 2 (SBP2) is an essential component of the selenocysteine insertion machinery. SBP2 is also the only factor known to differentiate among selenoprotein mRNAs, thereby modulating the relative expression of the individual selenoproteins. Here, we show that expression of SBP2 protein varies widely across tissues and cell types examined, despite previous observations of only modest variation in SBP2 mRNA levels. This discrepancy between SBP2 mRNA and protein levels implies translational regulation, which is often mediated via untranslated regions (UTRs) in regulated transcripts. We have identified multiple sequences in the SBP2 3’ UTR that are highly conserved. The proximal short conserved region is GU rich and was subsequently shown to be a binding site for CUG-BP1. The distal half of the 3’ UTR is largely conserved, and multiple proteins interact with this region. One of these proteins was identified as HuR. Both CUG-BP1 and HuR are members of the Turnover and Translation Regulatory RNA-Binding Protein family (TTR-RBP). Members of this protein family are linked by the common ability to rapidly effect gene expression through alterations in the stability and translatability of target mRNAs. The identification of CUG-BP1 and HuR as factors that bind to the SBP2 3’ UTR suggests that TTR-RBPs play a role in the regulation of SBP2, which then dictates the expression of the selenoproteome.
Keywords: selenoprotein, RNA-binding, CUG-BP1, HuR
The importance of selenium as a micronutrient in human health is becoming increasingly apparent. Most dietary selenium is utilized as the amino acid selenocysteine, which is cotranslationally incorporated into a small subset of proteins called selenoproteins. The human selenoproteome is comprised of ~25 different proteins.1 While the function of many selenoproteins is unknown, those of known function include the glutathione peroxidases, the thioredoxin reductases and the deiodinases, all of which catalyze oxidation-reduction reactions within cells.2,3 Selenoproteins have been implicated in multiple health issues including cancer prevention, immunity, infertility, atherosclerosis, and SEPN1-related myopathy.4–6 Selenoprotein expression is essential for development as disruption of the selenocysteine tRNA gene in mice is embryonic lethal.7
The synthesis of selenoproteins is complex, as the insertion of selenocysteine requires the translational recoding of the UGA stop codon. This redefinition requires an RNA stem-loop structure, the Selenocysteine Insertion Sequence (SECIS), which is located in the 3’ untranslated region (UTR) of selenoprotein messages in eukaryotes.8 Several trans factors are also required, including a dedicated elongation factor (EFSec)9,10, which binds to selenocysteine-charged tRNA11, as well as SECIS-binding protein 2 (SBP2), which binds to SECIS elements and promotes selenocysteine insertion.12,13 For a more detailed discussion, refer to reviews of selenocysteine insertion.14–16
SBP2 is the critical determinant for the expression of the selenoproteome. SBP2 was the first factor identified in the UGA-recoding pathway that can discriminate among the various SECIS elements.17,18 This differential binding of SBP2 to the SECIS elements mediates the relative expression levels of individual selenoproteins. The interaction of SBP2 with the SECIS is absolutely required for selenocysteine insertion, and disruption of the interaction results in a loss of selenoprotein expression.13 This is clearly evident in one type of SepN-related myopathy that is caused by a single point mutation in the SECIS of Selenoprotein N.19 This mutation completely abolishes SBP2 binding, resulting in the loss of Selenoprotein N expression and leading to the myopathy. Conversely, a point mutation in the SBP2 protein, instead of the SECIS RNA, was linked to impaired thyroid hormone function20, due to reduced activity of the deiodinase selenoproteins. Structure-function studies revealed that this mutation selectively impaired the RNA-binding activity of SBP2, resulting in a loss of expression of a subset of selenoproteins.17
SBP2 is a limiting factor for selenoprotein synthesis in vitro13 and in vivo21, and the amount of SBP2 protein varies greatly from one cell type to another. As SBP2 is essential for selenoprotein production13, it is important to examine how the expression of this protein is controlled. It was previously found that SBP2 mRNA levels across somatic tissues are fairly constant13, yet the SECIS-binding activity of SBP2 varies greatly across the same tissues.12 Given this discrepancy between SBP2 mRNA and activity levels, we have hypothesized that the expression of SBP2 may be regulated at the translational level.13
Translation of mRNAs can be temporally and spatially regulated, and this regulation can be either global or transcript-specific. Translational regulation provides the cell with the ability to rapidly respond to current conditions without requiring the lag time or energy input required for de novo RNA synthesis or complete mRNA turnover to alter the gene expression profile. Global regulation is often mediated through the modification of translation initiation factors, such as the phosphorylation of eukaryotic initiation factor 2α.22 In contrast, message-specific regulation is often driven by RNA-binding proteins, although recently microRNAs have also been demonstrated to play a role in translational regulation (reviewed in 23). Message-specific regulation usually occurs in the UTRs of mRNAs, and is more often mediated by the 3’ UTR (reviewed in 24). The preferential use of 3’ UTRs as control regions may be due to their relatively longer length. Interestingly, the average length of the 3’ UTR also appears to correlate with organism complexity.24,25 Translational regulation by RNA-binding proteins can either enhance or inhibit the expression of the target protein. A protein that enhances the expression of one transcript may inhibit another, and this regulation is often carefully orchestrated through the interaction of several proteins. This underscores the complex interplay of the various RNA-binding proteins during translational regulation. One group of proteins that is highly involved in this process is the Turnover and Translation Regulatory RNA-binding Proteins (TTR-RBPs). They are a heterogeneous group of proteins that were originally linked together because of their similar functions in affecting both the stability and translation of target mRNAs.26
This study is the first step in investigating the translational regulation of SBP2, a critical protein in the selenoprotein biosynthesis pathway. Here we report the identification of several regions in the SBP2 3’UTR that are highly conserved across species, suggesting they play an important regulatory role. We also show that two different TTR-RBPs interact with two of these conserved regions.
Results
SBP2 protein levels vary across cell types
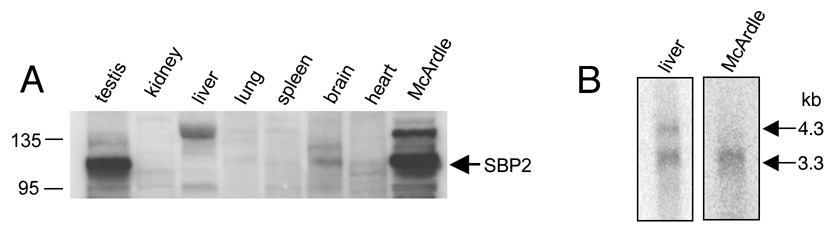
SBP2 is a critical determinant for expression of the selenoproteome. When SBP2 was first identified in this lab, we showed that the levels of SBP2 SECIS-binding activity varied dramatically across tissues with the strongest activity detected in testis and McArdle 7777 rat hepatoma cell extracts and low or undetectable levels in the rat somatic tissues.12 Subsequently, after cloning the SBP2 cDNA and performing Nothern blots, we found that these large differences in protein activity were not explained by variations in RNA levels.13 This was suggestive of a “disconnect” between SBP2 RNA and SBP2 activity levels. Here, we directly examined the protein levels across multiple tissues by Western blotting. As shown in Figure 1A, SBP2 is highly expressed in both testis and McArdle 7777 cell extracts, while expression is low or under the limit of detection in the remaining somatic rat tissues, which correlates with the previously published SBP2 activity profile.
Figure 1. SBP2 protein levels vary.
(A) 80 µg of protein extracts from various tissues was separated by SDS-PAGE, transferred to PVDF membrane and probed with an affinity-purified polyclonal SBP2 antibody. The predominant band of 120 kDa is the expected size of SBP2 and is indicated with an arrow. Multiple faint bands were also detected outside of this region and presumed to be non-specific background. The molecular weights are indicated in kDa. (B) Northen blotting of polyadenylated RNA from either McArdle 7777 rat hepatoma cells or liver tissue. Sizes of the two detected transcripts are indicated in kilobases.
In our previous RNA analysis, an abundant, testis-specific transcript of 2.5 kb was identified. As testis and McArdle 7777 cells both have high levels of SBP2 protein, we were interested to know if high levels of protein were attributable to the 2.5 kb transcript. In order to examine this possibility, Northern blotting was performed with McArdle 7777 cell RNA, using rat liver RNA as a control. The 4.3 and 3.3 kb transcripts were both detected in liver, as previously reported. These transcripts encode the same protein and differ only in the length of their 3’UTR. Only the 3.3 kb transcript was present in McArdle 7777 cells (Figure 1B), and the abundance was similar to that found in liver, which has much lower SBP2 protein levels. Thus, high level of SBP2 expression does not require the 2.5 kb transcript. This observed difference between the RNA and protein profiles suggests SBP2 is translationally regulated, therefore we began to look for cis-acting sequences and trans-acting factors involved in SBP2 expression.
Conserved elements are present in the 3’ UTR of SBP2
As UTRs are often sites of message-specific translational regulation25 and since regulatory elements are likely to be conserved across species, the 5’ and 3’ UTR sequences of the SBP2 were compared from human, rat, dog, cow and horse. In order to limit bias, only one primate and one rodent sequence were included. These sequences were aligned using the MUltiple Sequence Comparison by Log-Expectation (MUSCLE) program.27,28 When the 5’ UTR is the site of translational regulation, it is often longer than average and contains conserved motifs or structures.25 The 5’ UTR of SBP2 is relatively short (50–80 nt), where the average for a human gene is 210 nt and other mammals is 141 nt.25 All of the 5’ UTR sequences were GC-rich but were otherwise not conserved (data not shown).
In contrast, the 3’ UTR of SBP2 is approximately ten times longer than the 5’ UTR, which makes it more likely to contain elements involved in translational control of SBP2. Two regions of the 3’ UTR were identified as being enriched with conserved sequences (Figure 2). A short region of GU-rich sequence is conserved at the proximal end of the sequence (nt 15–24, with respect to the rat 3’ UTR sequence), while a larger section of conservation is present in the last 40% of the 3’UTR.
Figure 2. Alignment of SBP2 3’UTRs.
The sequences of the SBP2 3’UTR from rat, human, dog, cow and horse were obtained as described in Materials and Methods. Sequences are numbered with respect to first nucleotide of the UTR. The alignment was done using the MUSCLE program, available at http://www.ebi.ac.uk/Tools/muscle/index.html. Identical nucleotides are indicated with shaded boxes. The numbers above the rat sequence correspond to RNA sequences used in this study. The conserved GU-dinucleotide repeat motif is indicated with a line above it. Brackets indicate the 394–535 region where protein factors identified in this study interact.
The 3’ UTR of SBP2 interacts with proteins from Rabbit Reticulocyte Lysate S100 extracts
In order to investigate the interaction of the SBP2 3’UTR with protein factors, UV-crosslinking assays were done. A radiolabeled RNA transcript spanning the entire rat SBP2 3’ UTR (nt 1–632) was incubated with rabbit reticulocyte lysate (RRL) S100 extracts. The samples were then UV-irradiated to covalently link bound proteins to the radiolabeled RNA, digested with RNase A and separated by SDS-PAGE. As shown in Figure 3A, several proteins were crosslinked to the probe spanning the 3’ UTR. A prominent band was detected at ~50 kDa, while other bands are visible in the 35–40 and 60–70 kDa ranges.
Figure 3. A 50 kDa protein binds to nt 1–50 of the rat SBP2 3’UTR.
(A) A radiolabeled RNA probe spanning nt 1–632 of the rat SBP2 3’ UTR was used with RRL S100 lysate in UV-crosslinking assays. Several proteins that bound to this sequence were observed. Molecular weight markers are indicated in kDa. (B) UV-crosslinking of RRL S100 to RNA probes spanning either nt 1–200 or 1–100 of the 3’ UTR. The band of ~50 kDa is prominent, indicated by the black arrow. A faint band with a slightly lower molecular weight is indicated by the grey arrow. A higher molecular weight band is also detected, indicated by the asterisk. No other bands were observed in the regions cropped from this image. (C) RNAfold stemloop structure prediction in the rat 3’ UTR, nt 1–50. Regions A, B and C correspond to regions removed in deletion constructs. Circled nucleotides were targeted for mutagenesis. (D) Radiolabeled RNA probe spanning nt 1–50 was UV-crosslinked in the presence of increasing molar excess of unlabeled competitor RNA as indicated.
The GU-rich proximal element interacts with factors in RRL S100 extracts
Next we were interested in determining whether these interactions were dependent on either of the conserved regions of the 3’ UTR. First we focused on the GU-rich sequence at the proximal end of the 3’UTR of SBP2. In order to determine whether this conserved sequence could interact with protein factors, two smaller constructs (nt 1–200 and 1–100) were used as probes. As shown in Figure 3B, the black arrow indicates a prominent band at ~50 kDa that binds to these smaller probes, particularly the 1–100 construct. A second minor band is detected just below the major band, indicated by the grey arrow.
As shown in Figure 3C, RNAfold29 calculations for nt 1–100 predict a stemloop encompassing nucleotides 5–35 with the conserved GU sequence exposed in the loop region. Three deletions of five nucleotides were made across the predicted loop, creating a set of deletion mutants termed 1-50ΔA, 1-50ΔB, and 1-50ΔC, as indicated. These deletion mutants were then added to the UV-crosslinking assay as unlabeled competitor RNAs, using a 10 to 100 fold molar excess over the radiolabeled 1–50 probe. As indicated in Figure 3D, the unlabeled 1–50 RNA efficiently competes away the signal from the radiolabeled 1–50, as expected (lanes 2–4). In contrast, 1-50ΔA is only able to modestly compete for protein binding even at 100x molar excess (compare lanes 1, 4 and 7) and the 1-50ΔB mutant is not able to compete at any of the concentrations tested (compare lanes 1 with 8–10). The protein signal is competed away by the 1-50ΔC mutant, almost as effectively as the wildtype RNA (lanes 2–4). These results indicate that the binding of the ~50kDa protein requires sequences in the ΔA and ΔB regions, which encompasses the conserved GU-dinucleotide sequence.
CUG-BP1 interacts with the GU-rich motif in the 3’UTR
One ~50 kDa protein known to interact with GU-dinucleotide sequences is CUG Binding Protein 1 (CUG-BP1).30,31 CUG-BP1 is a member of the CUG-BP and ETR3-like factor (CELF) protein family 32. It is best known for its role in regulation of alternative splicing32–35, but has also been implicated in translational control.36–38 In order to investigate whether CUG-BP1 was the ~50 kDa protein interacting with the conserved GU-dinucleotide sequence, an in vitro transcription/translation system was employed. A synthetic RNA encoding human CUG-BP1 (hCUG-BP1) was used to express protein in RRL, and its synthesis was assessed both by 35S-Methionine labeling (Figure 4A) and western blotting (Figure 4B) using an anti-CUG-BP1 antibody. The protein was detected as a doublet with both methods, although the lower band in the western blot is somewhat obscured by the endogenous rabbit CUG-BP1 (rbCUG-BP1), indicated by an asterisk. CUG-BP1 is often detected as a doublet due to phosphorylation of the protein.39 The hCUG-BP1 is larger than the endogenous rbCUG-BP1 in the RRL due to the presence of two C-terminal epitope tags introduced during cloning.
Figure 4. CUG-BP1 interacts with GU-rich sequence in the 3’ UTR.
(A) CUG-BP1 mRNA was in vitro translated in the presence of 35S-Met. An aliquot of the translation reaction was resolved by SDS-PAGE and the labeled protein was detected with a PhosphorImager. Molecular weight markers are indicated in kDa (B) Unlabeled translation reactions were analyzed by immunoblotting with an α-CUG-BP1 antibody. The first lane corresponds to the control reaction without RNA (−), while the second lane contains RRL that is expressing human CUG-BP1 (hCUG-BP1) (+).The two bands corresponding to hCUG-BP1 are indicated with arrows. The asterisk indicates the endogenous rabbit CUG-BP1 in the RRL. (C) UV-crosslinking of the translation reactions from panel B with the radiolabeled 1–100 nt RNA. The band corresponding to hCUG-BP1 binding is indicated with an arrow. The other observed bands due to endogenous proteins in the RRL. (D) UV-crosslinking of the translation products in the absence (−) or presence (+) of hCUG-BP1 to the radiolabeled 1–100, 1-100ΔB and 1-100U→A RNAs. Binding of CUG-BP1 to the 1–100 RNA is indicated with a black arrowhead. The reduction or loss of binding, with the 1-100ΔB and 1-100U→A RNAs respectively, is indicated with empty arrowheads.
Binding activity was assessed using UV-crosslinking, comparing the binding profile of unprogrammed RRL with RRL that expressed tagged human CUG-BP1. Two proteins from the RRL bound to the 1–100 probe, one of which is likely to be the endogenous rbCUG-BP1 (Figure 4C). Despite the presence of high levels of endogenous rbCUG-BP1 detected by western blotting (Figure 4B), the binding of the exogenous hCUG-BP1 to the 1–100 probe was clearly detected (Figure 4C). Interestingly, the presence of the hCUG-BP1 resulted in a decrease in binding of the lowest band of the endogenous RRL protein.
Next, the requirement for the conserved GU-dinucleotide sequence for CUG-BP1 binding was tested using two deletion mutants. The 1-100ΔB removes a portion of the GU-rich sequence in the middle of the predicted loop region and 1-100U→A replaces six adenine residues with uracils across the dinucleotide region, as indicated in Figure 3C. As shown in Figure 4D, binding is clearly detected with the wildtype 1–100 probe, while the binding is strongly reduced in the 1-100ΔB context and is no longer detectable with the 1-100U→A probe. We were interested in determining if this binding could occur in the absence of other proteins, however, while recombinant CUG-BP1 was successfully purified from E. coli, inherent solubility issues precluded its use under our assay conditions.
As a complementary approach to the overexpression studies, endogenous CUG-BP1 was immunodepleted from RRL. An anti-myc antibody was used as an isotype control for the immunodepletion. As shown by western blotting (Figure 5A), the CUG-BP1 protein is efficiently depleted by its cognate antibody, but not by the isotype control. The depleted supernatants were then tested in UV-crosslinking studies. As shown in Figure 5B, depletion of CUG-BP1 results in a loss of the ~50 kDa crosslinked band, while other crosslinked proteins are still detected, indicated by the black arrowheads. These two approaches confirm that CUG-BP1 interacts with the conserved GU-dinucleotide repeats in the 3’ UTR of SBP2.
Figure 5. Endogenous CUG-BP1 in RRL interacts with the conserved GU-rich sequence.
(A) Western blot of immunodepleted RRL, using either the isotype control α-myc antibody or cognate α-CUG-BP1 antibody. Molecular weight markers are indicated in kDa. (B) UV-crosslinking of radiolabeled 1–100 RNA with the immunodepleted RRL from panel A. The loss of endogenous CUG-BP1 binding is indicated with an empty arrowhead. The filled arrowheads indicate interactions that remain after CUG-BP1 depletion.
Several factors interact with the large conserved region in the distal portion of the 3’UTR
The distal end of the SBP2 3’UTR is highly conserved, despite the presence of large non-conserved regions preceding it. When a radiolabeled probe encompassing nt 394–641 of the rat SBP2 3’UTR was used in UV-crosslinking assays with either nuclear or cytoplasmic lysates from McArdle 7777 cells or RRL, several bands were detected (Figure 6, lanes 2–4). Two prominent bands were detected in the nuclear lysate in the range of 60–75 kDa, and a cluster of multiple bands between 34–42 kDa. Proteins from the cytoplasmic lysates were also detected in two groups of proteins from 34–42 kDa and 52–72 kDa. Fewer bands were detected in the RRL lane, but they were of similar size ranges to those observed in the cytoplasmic extract (compare lanes 3 and 4).
Figure 6. Several proteins bind to the conserved distal region of the SBP2 3’UTR.

Radiolabeled RNAs spanning nt 394–641, 394–535 and 521–641 were UV-crosslinked to McArdle 7777 cell nuclear extract (N), cytoplasmic extract (C) or RRL. Products were analyzed by SDS-PAGE and visualized by autoradiography using a PhosphorImager. Molecular weight markers are indicated in kDa.
In order to narrow the regions of interaction, two shorter probes were also tested (nt 394–535 and 521–641). The probe spanning 394–535 recapitulates the binding profile of the longer probe (Figure 6, compare lanes 2 and 6, lanes 3 and 7, lanes 4 and 8). In contrast, the binding profile to the 521–641 probe was different, with only a few faint bands observed (Figure 6, compare lanes 2 and 10, lanes 3 and 11, lanes 4 and 12). This indicates that most of the protein-RNA interactions are occurring in the region of 394–535. These results were confirmed by UV-crosslinking using the full length 394–641 as radiolabeled probe and either 394–535 or 521–641 as cold competitor RNA. As shown with the nuclear extracts (Figure 7A), the binding of the two larger proteins (indicated by the arrows), and the multiple smaller proteins (indicated by the bracket), are effectively competed away by a 10 fold molar excess of 394–535 RNA. In contrast, very little competition is observed even at 50x molar excess of 521–641 RNA. This was also observed when the cytoplasmic lysate was used as the protein source (Figure 7B). The prominent band at ~65 kDa indicated by an arrow is efficiently competed away by the 394–535 competitor, as are the multiple smaller bands around ~42 kDa (indicated by a bracket) and another single band at ~34 kDa.
Figure 7. Crosslinking of proteins to nt 394–535 is specific.
Radiolabeled 394–641 RNA was used in UV-crosslinking in the presence of increasing molar excess of unlabeled 394–535 or 521–641 RNA as indicated. (A) McArdle 7777 nuclear lysates were the protein source. (B) McArdle 7777 cell cytoplasmic lysates were the protein source. Proteins that are competed away by the 394–535 RNA but not the 521–641 RNA are indicated with arrows and brackets. Molecular weight markers are indicated in kDa.
HuR interacts with the conserved sequence spanning nt 394–535 of the 3’ UTR of SBP2
In an attempt to identify the proteins that are interacting with this distal conserved sequence, biotinylated 394–535 RNA was incubated with lysate and RNA-protein complexes were extracted with streptavidin beads. As multiple bands were detected with both the nuclear and cytoplasmic lysates in the UV-crosslinking, the nuclear lysates were initially used as the protein source, with cytoplasmic lysates to be investigated in future experiments. After extensive washing, the bound proteins were separated on an SDS-PAGE gel, and visualized by Coomassie staining. Several bands spanning a molecular weight range of 30 kDa to 130 kDa were excised for identification by mass spectrometry.
We initially focused on proteins with known RNA-binding activity for further analysis. These included heterogeneous ribonucleoprotein A1 (hnRNP A1), heterogeneous ribonucleoprotein AB (hnRNP AB), heterogeneous ribonucleoprotein F (hnRNP F), Glyceraldehyde 3-phosphate dehydrogenase (GAPDH), embryonic lethal abnormal vision like-1 (ELAV1, more commonly known as HuR) and NonO/p54nrb. Antibodies were obtained for these candidate proteins and used to confirm the presence of the proteins in the nuclear lysates by western blotting (data not shown). Unfortunately, attempts to immunodeplete these proteins from the nuclear lysate to use in UV-crosslinking were unsuccessful (data not shown). As an alternative, RNA electrophoretic mobility shift assays (REMSA) were performed. In this method, protein lysates were incubated with radiolabeled probe and the protein-RNA complexes were separated on a non-denaturing polyacrylamide gel. Bound RNA will show a decreased electrophoretic mobility compared to unbound probe. As shown in Figure 8A, several protein-RNA complexes were detected upon the addition of nuclear lysate to the 394–535 probe, along with a decrease in the amount of free probe (compare lanes 1 and 2).
Figure 8. HuR interacts with the 394–535 region of the SBP2 3’UTR.
REMSA with radiolabeled 394–535 RNA incubated with nuclear lysate (A), or cytoplasmic lysate (B), and antibodies to candidate RNA-binding proteins. Complexes were analyzed on a non-denaturing polyacrylamide gel, and visualized by autoradiography using a PhosphorImager. The reduced mobility of a complex in lane 7 from the addition of the HuR antibody is indicated with an arrow. (C) REMSA with radiolabeled 394–535 RNA incubated with His-HuR, partially purified from RRL. Complexes were analyzed on a non-denaturing polyacrylamide gel, and visualized by autoradiography using a PhosphorImager. The HuR-RNA complex is indicated with an arrow. The reduced mobility of a complex from the addition of the HuR antibody is indicated with an arrowhead. The asterisk represents a slowly migrating conformation of probe only. (D) Radiolabeled 394–535 RNA was used in UV-crosslinking in the absence or presence of 50x molar excess of unlabeled 394–535 or 521–641 RNA as indicated. The HuR band is indicated with an arrow, while the arrowhead indicates and unidentified protein band (see text). Molecular weight markers are indicated in kDa.
In order identify which proteins are involved in the detected complexes, we used “supershift” assays. When an antibody to an RNA-binding protein is added to the incubation reaction, the addition of the antibody can further reduce the mobility of the complex resulting in a “supershift”. One caveat to interpreting these experiments is that while a change in profile indicates the interaction of the target protein with RNA, the absence of an effect of antibody addition on the profile is not conclusive. A negative result may indicate the target protein is not in one of the complexes, but it may also be that the antibody does not recognize the protein when it is bound to the RNA. As shown in Figure 8A, the addition of the hnRNP A1, -AB, -F, GAPDH and NonO antibodies did not change the profile of the RNA-protein complexes (compare lanes 3–6, 8 with lane 2). In contrast, the addition of the HuR antibody caused a clear upward shift of the lowest RNA-protein complex, as indicated by the black arrow (Figure 8A, lane 7).
The profile of RNA-protein complexes was different when the REMSA was done using the cytoplasmic lysate as the protein source (compare Figure 8A lane 2 with Figure 8B lane 2). Once again the addition of most of the antibodies did not influence the pattern of RNA-protein complexes (compare Figure 8B lanes 3–6, 8 with lane 2). Addition of the HuR antibody, however, completely shifted the lowest complex upward, in both the nuclear and cytoplasmic contexts. Given these results, and the fact that HuR protein falls within the 34–42 kDa molecular weight range defined above as having specific interactions with the 394–535 region, the tentative identification of HuR was encouraging. One caveat to these results is that this antibody can interact with the other Hu antigens. Although HuB, HuC and HuD are primarily neuronally expressed, this experiment could not formally exclude the possibility that one of these other family members was interacting with our region of interest. In order to confirm that HuR was indeed the protein interacting with the 394–535 region, His-tagged HuR protein was expressed in RRL and enriched using magnetic beads. This partially purified protein was then tested in REMSA assays. As shown in Figure 8C, a protein-RNA complex is detected upon addition of the recombinant HuR. The mobility of the complex is not affected by a control antibody (α-myc), however, the complex was completely shifted upon addition of the HuR antibody. The specificity of this interaction was tested using in vitro crosslinking to the 32P-labeled 394–535 probe in the presence or absence of unlabeled competitor RNA. Two bands were detected in this assay in the absence of competitor RNA. The lower band of ~44 kDa corresponds to HuR, with the increase in molecular weight due to the two epitope tags introduced into the protein (Figure 8D, arrow). Addition of 50x molar excess of competitor RNA was able to reduce the intensity of the upper band, by approximately half in both cases, showing the non-specific binding activity of this protein (Figure 8D, arrowhead). Similarly, 50x molar excess of the 521–641 RNA was unable to specifically compete the HuR signal, as the ratio between HuR and the nonspecific band is maintained. In contrast, addition of 50x molar excess of the 394–535 RNA completely abolished the detection of the HuR band. Taken together, the mass spectrometry data and our in vitro studies indicate that HuR is one of the factors that interact with a conserved region in the 3’UTR of SBP2.
Discussion
SBP2 is considered to be the master regulator of selenoprotein synthesis, thus understanding the expression of this protein is necessary to advance our knowledge of the expression of the selenoproteome. The lack of correlation between SBP2 mRNA and protein levels in somatic cells raises the possibility that SBP2 is translationally regulated.12,13 This is an area of investigation that had not been previously explored. Our study is the first to propose that conserved elements in the 3’ UTR may be involved in the translational regulation of SBP2 mRNA. We identify two regions of conservation in the 3’UTR of SBP2 and show that these regions are involved in protein-RNA interactions. Two of the proteins involved in these interactions were successfully identified as CUG-BP1 and HuR, which are both part of the TTR-RBP group of RNA-binding proteins and have been implicated in many steps in RNA metabolism.
CUG-BP1 is one of the founding members of the CELF family of proteins.32 While CUG-BP1 was originally named for its ability to interact with (CUG)8 repeats, a preferential affinity for GU-dinucleotide repeats was subsequently identified in a yeast three-hybrid system31 and for UGU-rich sequences using in vitro selection studies.40 The CUG-BP1 binding site we identified in the 3’UTR of SBP2 is also rich in GU-dinucleotides, similar to these previously identified sequences. CUG-BP1 can be found in both the nucleus and the cytoplasm, and it has been suggested that it shuttles between these compartments.39 With respect to function, CUG-BP1 is best known for its activity as a regulator of alternative splicing32–35, but it has also been implicated in RNA stability41, deadenylation42, 43 and translational control.36–38
HuR is a member of the ELAV-like family of proteins, which are also known as the Hu antigens.44 HuR was originally recognized as an AU-rich element (ARE) binding protein, involved in the recognition of the AUUUA pentamer45 and stabilization of ARE-containing mRNAs.46 However, a study that examined RNA sequences obtained from co-immunoprecipitation of endogenous HuR-RNA complexes found an enrichment of targets with a 17–20 base long U-rich motif.47 The region of the SBP2 3’ UTR that is bound by HuR contains a comparable motif (nt 471–487, with respect to the rat sequence, Fig. 2). HuR has also been implicated in multiple steps in RNA metabolism including splicing, export, mRNA turnover and translational control.44,48 HuR is primarily nuclear but is capable of shuttling between the nucleus and cytoplasm46 and will redistribute in response to various cell stimuli.49 In our study, HuR binding to the 3’ UTR of SBP2 was detected in lysates from both subcellular compartments. Cytoplasmic HuR is associated with export of ARE-containing mRNA, stabilization of mRNA and translational control.50
The relative abundance, subcellular localization and interactions of regulatory RNA-binding proteins will dictate the expression profile of their target mRNAs. We analyzed protein lysates from mouse and rat tissues for HuR and CUG-BP1 expression by immunoblotting. No correlations could be drawn among the expression of these proteins and SBP2 protein levels. However, both HuR and CUG-BP1 are nucleo-cytoplasmic shuttling proteins, and regulated by phosphorylation. Therefore, it is possible that analysis of whole cell extracts may miss correlations.
Functional antagonism was recently demonstrated for HuR and ELAV-type RNA-binding protein 3 (ETR-3, also known as CUG-BP2), a member of the CELF family that is closely related to CUG-BP1. These two proteins translationally regulate cyclooxygenase 2 RNA, where ETR-3 is an inhibitor of expression and HuR promotes translation.51 While CUG-BP1 and ETR-3 have overlapping binding activities, ETR-3 was not detected in RRL by western blotting (data not shown) and was not further tested in this study. ETR-3 is present in McArdle 7777 cell extracts (data not shown) however, so it is possible that it is also able to interact with the 3’ UTR of SBP2.
CUG-BP1 and HuR are clearly not the only factors binding to the SBP2 3’UTR, however the appearance of both factors is interesting, since they have been grouped together in the TTR-RBP family.50 Members of this diverse group are united in their ability to mediate rapid changes in both the stability and translational state of target mRNAs. The effect of any one TTR-RBP on a target mRNA cannot be predicted, as the effects are often transcript-specific. Furthermore mRNA turnover and translation can be simultaneously regulated, and this regulation can be either positive or negative. Layered on top of this is the complex interplay between TTR-RBPs that bind to the same target mRNA, which can be cooperative or antagonistic depending on the context. Many TTR-RBPs are also able to influence the expression levels of the group members through binding to its own and/or other TTR-RBP mRNAs.26 Thus, the function and expression of this group of proteins is highly interconnected. Coordinated posttranscriptional regulation of multiple genes is also one of the central tenets of the RNA operon theory52, which proposes that groups of specific RNA-binding proteins regulate functionally related mRNAs as subpopulations. The presence of multiple regulatory elements in a single gene allows for regulation as part of more than one RNA-operon.53 This combinatorial association of elements results in exquisite control of differential expression profiles in response to cell stimuli.
In this study we have identified two protein-RNA interactions in the 3’ UTR of SBP2. Just as there are additional proteins to be identified, other regions of the RNA are likely to be important. Within the large distal conserved region, the HuR protein bound within nt 394–535, but the conservation continues to the end of the UTR, and protein binding was observed in this region. Interestingly, the sequences at the very end of the 3’ UTR, flanking the polyadenylation signal, contain two imperfectly conserved copies of the UUAUUUA (U/A)(U/A) nonamer associated with RNA instability (nt 622–654 in the context of the rat sequence, Fig. 2).54, 55 Each sequence contains at least one consensus nonamer, and one other copy that may or may not perfectly match consensus. This region was not examined in this study, but it does add further circumstantial evidence that SBP2 may be a regulated transcript, and that its expression is influenced by the 3’ UTR.
While both CUG-BP1 and HuR clearly interact with the 3’ UTR of SBP2, the functional implications of these interactions are unclear, and a myriad of possibilities exist. The discrepancy between SBP2 mRNA and protein levels identified in previous studies implies that regulation of protein expression is occurring.12,13 The expression of SBP2 appears to be tightly regulated in a cell type and tissue-specific manner, yet the control of SBP2 translation has not been examined. In this study, we begin to investigate the possible translational regulation of SBP2 mRNA by identifying conserved regions within its 3’ UTR and potential regulatory proteins that bind to them. The identification of TTR-RBP proteins as factors that interact with these regions is intriguing, as the effects of these proteins can be either positive or negative on multiple steps of mRNA metabolism. Given that the functions and activities of this family of proteins are heavily interconnected, future studies will attempt to delineate the intricate RNA-protein and protein-protein interactions governing the function of the 3’UTR of SBP2.
Materials and Methods section
Sequences
The DNA sequences of the SBP2 3’ UTR from human (NM_024077), rat (NM_024002.1), dog (XM_533552), and cow (XM_588488.4) were obtained from GenBank. The sequence for the horse SBP2 3’ UTR was obtained from the domestic horse genomic sequence (EquCab 2.0, LOC100062130). Alignments were performed using the MUltiple Sequence Comparison by Log-Expectation (MUSCLE) software, which is available at http://www.ebi.ac.uk/Tools/muscle/index.html.
SBP2 3’UTR Constructs
Rat SBP2 3’UTR PCR fragments were generated using Pfu polymerase (Stratagene) and cloned into pcDNA3.1/V5-His-TOPO (Invitrogen). Deletion and point mutants were created using the QuikChange Site-Directed Mutagenesis Kit (Stratagene). All constructs were verified by DNA sequencing. Primer sequences used for cloning are listed in Table 1.
Table 1.
Primers for cloning and RNA synthesis
| UTRFor | 5′ | gaagagttct | tgcctgtgtg | ||
| SBP23′end | 5′ | ctaacaaata | agccctcttg | c | |
| 100Rev | 5′ | gtcacataaa | tacaccaggt | ||
| 200Rev | 5′ | cccacgggag | tgtctgggtg | ||
| 394For | 5′ | cgagaattcg | tgctgccaag | gat | |
| 521For | 5′ | cccgaattcc | agtttggtat | ctac | |
| 535Rev | 5′ | cccaagcttg | tagataccaa | actg | |
| 641Rev | 5′ | cccaagcttc | tctttattct | aac | |
| 1-50ΔA | 5′ | ctacctcccc | atccttaccc | aaaacacaga | aggcaagaac |
| tcttccccta | tagtgagtcg | tattaatttc | gat | ||
| 1-50ΔB | 5′ | ctacctcccc | atccttaccc | aaaaccacac | aggcaagaac |
| tcttccccta | tagtgagtcg | tattaatttc | gat | ||
| 1-50ΔC | 5′ | ctacctcccc | atccttaccc | acagacacac | aggcaagaac |
| tcttccccta | tagtgagtcg | tattaatttc | gat | ||
| 1-100ΔBUp | 5′ | gagttcttgc | cagtgtggtt | ttgggtaagg | atg |
| 1-100ΔBLow | 5′ | catccttacc | caaaaccaca | ctggcaagaa | ctc |
| 1-100U-AUp | 5′ | gaagagttct | tgccagagag | acagagtttt | gggtaaggat gg |
| 1-100U-ALow | 5′ | ccatccttac | ccaaaactct | gtctctctgg | caagaactct tc |
In vitro transcription
Synthetic RNA was synthesized from either EcoRV linearized plasmids, or DNA oligos containing a T7 promoter site for the nt 1–50 constructs, using T7 RNA polymerase, and 7.5 mM NTPs for 3 hours at 37°C. The transcription reactions were treated with DNAse I for 20 minutes and then phenol:chloroform extracted. The aqueous phase was passed through a Micro Bio-Spin P30 column according to manufacturer’s instructions (BioRad). Radiolabeled 3’ UTR probes were synthesized from linearized templates with T7 RNA polymerase using 1 mM GTP, 1 mM ATP, 1 mM CTP, 0.005 mM UTP and 25 µCi of 32P-labeled UTP for 3 hours at 37°C. Probes were DNAse I treated and cleaned as above, heated to 95°C for 2 minutes and slow cooled to room temperature. Biotinylated RNA was made as above using 5 mM GTP, 5 mM ATP, 5 mM CTP, 4 mM UTP and 1.5 mM biotin-16-UTP (Epicentre Biotechnologies).
Northen blotting
Two µg of polyadenylated RNA from McArdle 7777 cells or rat liver was probed with a 32P-labeled cDNA encompassing nucleotides 1238–3240 of rat SBP2. The experiment was performed as previously described.56
In vitro translation
For the in vitro translation of hCUG-BP1, 0.25 µg of in vitro transcribed RNA was used in a 25 µL in vitro translation reaction using rabbit reticulocyte lysate (RRL), according to manufacturer’s suggestion (Promega). Translations were done in the presence and absence of 35S-Methionine. A 2 µl aliquot of the 35S-Methionine reaction was resolved on a sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) and visualized with a PhosphorImager screen. In order to assess non-radioactive protein synthesis, 1.0 µl of the translation reaction was resolved on a sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE), and analyzed by immunoblotting. His-tagged HuR was partially purified from the translation extracts using the MagZ kit (Promega) according to manufacturer’s instructions.
Western blotting
Proteins were transferred to ImmunoBlot polyvinylidene fluoride (PVDF) membrane (BioRad). The SBP2 antibody was previously described.13 The primary antibody used was α-CUG-BP1 (3B1) mouse monoclonal antibody (SantaCruzBiotechnology, Inc.), and the secondary was α-mouse-HRP (Jackson Immunochemicals). Proteins were detected using Immobilon Chemiluminescent HRP detection substrate (Millipore), and exposure to Biomax MR film (Kodak).
UV-crosslinking
Crosslinking of the S100 extracts was performed as previously described.12 Crosslinking with either the RRL (Promega) or McArdle 7777 cell extracts was done as follows. Extracts were incubated with 20 fmol 32P-labeled synthetic RNA (and unlabeled RNA in competition experiments) for 30 min at 37°C in a final volume of 20 µl containing 100 mM potassium chloride (KCl), 10 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) pH 7.9, 12.5% glycerol, 0.25 mM dithiothreitol (DTT), 0.1 mM ethylene-diamine-tetra-acetic acid (EDTA), 0.75 mM magnesium chloride (MgCl2) and 0.25 µg tRNA. The reactions were exposed to UV light (254 nm, 125 mJoules) for 10 minutes, followed by treatment with 10 µg RNase A (Fermentas) for 60 minutes at 37°C. Samples were analyzed by electrophoresis on 12% SDS-PAGE. The gels were dried and proteins were visualized with a PhosphorImager screen, followed by exposure to BioMax MR film.
Immunodepletion
In order to immunodeplete CUG-BP1, 200 µL of RRL was incubated with 1 mg of the α-CUG-BP1 (3B1) mouse monoclonal antibody for 1.5 hours at 4°C. As an isotype control, 1 mg of α-myc antibody (Invitrogen) was used in a parallel reaction. A 60 µL aliquot of MagnaBind Protein G magnetic beads (Pierce) was washed three times in wash buffer (10 mM tris-(hydroxymethyl)aminomethane (TRIS), 150 mM sodium chloride (NaCl), 0.05% (v/v) nonoidet- P40) for each reaction. The washed beads were added to the samples and incubated while rotating for 3 hours at 4°C to allow the protein-antibody-bead complexes to form. After this incubation, the samples were placed on a magnetic stand to capture the complexes. The supernatant was removed and used for downstream assays.
Preparation of Cell Extracts
RRL S100 was a kind gift from Dr. Bill Merrick, Case Western Reserve University. McArdle 7777 rat hepatoma cells were purchased from ATCC (CRL-1601). For preparation of nuclear and cytosolic extract, McArdle 7777 cells were incubated with 2 packed cell volumes (PCV) of Buffer A (10 mM HEPES pH 7.4, 10 mM KCl, 0.75 mM MgCl2, 0.5 mM DTT, Complete EDTA-free protease inhibitor cocktail tablet (Roche Diagnostics) for 15 minutes on ice. Cells were lysed 5 times using a 25-gauge needle and the extract was centrifuged at 14000 rpm. The supernatant was removed and saved for further processing into cytosolic extracts (see below). The pellet was resuspended in 0.5 PCV of Buffer B (20 mM HEPES pH 7.8, 25% glycerol, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT) with 20 mM KCl, and followed by drop-wise addition of one half PCV of Buffer B with 1.2 M KCl. After incubation on ice for 30 min, the extract was centrifuged at 14000 rpm at 4°C and taken as the nuclear extract. The previously retained supernatant was mixed with 1/10 volume of Dignam Buffer D (0.3 M HEPES pH 7.4, 1.4 M KCl, 30 mM MgCl2) and centrifuged at 14000 rpm for 20 minutes at 4°C. The new supernatant was taken as the cytosolic extract. Protein concentrations were determined using Bio-Rad Protein Assay Reagent using IgG as a standard.
Biotinylated RNA Pulldown
A reaction containing 2 µg of biotinylated 394–641 RNA, 20 µL of nuclear extract, 40 µL 5x Binding buffer (700 mM KCl, 50 mM HEPES pH 7.9, 25% glycerol, 5 mM DTT and 1.65 mg/ml tRNA) and nuclease-free water to a final volume of 200 µL was incubated for 30 minutes at 37°C to allow protein-RNA complexes to form. A control reaction without RNA was also done. After the incubation, 100 µL of magnetic streptavidin beads (Roche) that had been washed once in 1x Binding buffer, were added to each reaction. The samples were incubated with rotation for 30 minutes at room temperature. The protein-RNA complexes were extracted using a magnetic stand, washed five times with 50 µL 1x Binding buffer, and resuspended in 20 uL 1x Binding buffer. An equal volume of protein loading dye was added and the samples were boiled to remove the proteins from the beads. The proteins were then resolved on a 10% SDS-Page gel and stained with Coomassie Blue Dye. Bands that were differential between the control and experimental lanes were excised and sent for identification using a ThermoFisher LTQ ion trap mass spectrometer system at the Lerner Research Institute Proteomics Laboratory.
RNA Electrophoretic Mobility Shift Assay (REMSA)
Ten micrograms of protein lysate was incubated in the binding buffer in the presence or absence of the antibody for 20 minutes. The tested antibodies included α-HuR (3A2), a generous gift from Dr. Hua Lou (Case Western Reserve University), α-hnRNP A1(sc-10029, Santz Cruz Biotechnology, Inc.), α-hnRNP F (sc-10045, Santa Cruz Biotechnology, Inc.), α-NONO (APR40715, Aviva Systems Biology), α-hnRNP AB(APR41036, Aviva Systems Biology) and α-GAPDH (G9545, Sigma-Aldrich). The final REMSA binding buffer concentrations were 140 mM KCl, 10 mM HEPES pH 7.9, 5% glycerol, 1 mM DTT and 0.33 mg/ml tRNA. The reaction was further supplemented with 15 µg salmon sperm DNA to reduce non-specific interactions from the lysate. Labeled probe (20 fmol) was then added and the reactions were incubated at 37°C for an additional 20 minutes and complexes were resolved on 5% non-denaturing polyacrylamide gels. The gels were dried and the appearance of complexes was analyzed on a PhosphorImager, followe by exposure to BioMax MR film.
Acknowledgements
This work was funded by the NIH/NHLBI (HL29582) (DMD) and a postdoctoral Fellowship from the American Heart Association, Ohio Valley Affiliate (CAG). We thank Dr. Hua Lou and Dr. Bill Merrick, both from Case Western Reserve University for kindly supplying reagents used in this work. We would also like to acknowledge Dr. Mike Kinter and Dr. Belinda Willard from the Lerner Research Institute Proteomics Laboratory for the mass spectrometry analysis. We thank Dr. Paul R. Copeland for critical review of this manuscript.
Abbreviations
- SEPN1
selenoprotein N1
- SECIS
selenocysteine insertion sequence
- SBP2-SECIS
binding protein 2
- UTR
untranslated region
- TTR-RBPs
turnover and translation regulatory RNA-binding proteins
- CUG-BP1
CUG-binding protein 1
- CELF
CUG-BP and ETR-3 like factor
- RRL
rabbit reticulocyte lysate
- REMSA-RNA
electrophoretic mobility shift assay
References
- 1.Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigo R, Gladyshev VN. Characterization of mammalian selenoproteomes. Science. 2003;300:1439–1443. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- 2.Gromer S, Eubel JK, Lee BL, Jacob J. Human selenoproteins at a glance. Cell Mol Life Sci. 2005;62:2414–2437. doi: 10.1007/s00018-005-5143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Driscoll DM, Chavatte L. Finding needles in a haystack. In silico identification of eukaryotic selenoprotein genes . EMBO reports. 2004;5:140–141. doi: 10.1038/sj.embor.7400080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moghadaszadeh B, Petit N, Jaillard C, Brockington M, Roy SQ, Merlini L, Romero N, Estournet B, Desguerre I, Chaigne D, Muntoni F, Topaloglu H, Guicheney P. Mutations in SEPN1 cause congenital muscular dystrophy with spinal rigidity and restrictive respiratory syndrome. Nature genetics. 2001;29:17–18. doi: 10.1038/ng713. [DOI] [PubMed] [Google Scholar]
- 5.Rayman MP. The importance of selenium to human health. Lancet. 2000;356:233–241. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- 6.Ferreiro A, Ceuterick-de Groote C, Marks JJ, Goemans N, Schreiber G, Hanefeld F, Fardeau M, Martin JJ, Goebel HH, Richard P, Guicheney P, Bonnemann CG. Desmin-related myopathy with Mallory body-like inclusions is caused by mutations of the selenoprotein N gene. Annals of neurology. 2004;55:676–686. doi: 10.1002/ana.20077. [DOI] [PubMed] [Google Scholar]
- 7.Bosl MR, Takaku K, Oshima M, Nishimura S, Taketo MM. Early embryonic lethality caused by targeted disruption of the mouse selenocysteine tRNA gene (Trsp) Proceedings of the National Academy of Sciences of the United States of America. 1997;94:5531–5534. doi: 10.1073/pnas.94.11.5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berry MJ, Banu L, Chen YY, Mandel SJ, Kieffer JD, Harney JW, Larsen PR. Recognition of UGA as a selenocysteine codon in type I deiodinase requires sequences in the 3' untranslated region. Nature. 1991;353:273–276. doi: 10.1038/353273a0. [DOI] [PubMed] [Google Scholar]
- 9.Tujebajeva RM, Copeland PR, Xu XM, Carlson BA, Harney JW, Driscoll DM, Hatfield DL, Berry MJ. Decoding apparatus for eukaryotic selenocysteine insertion. EMBO reports. 2000;1:158–163. doi: 10.1093/embo-reports/kvd033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fagegaltier D, Hubert N, Yamada K, Mizutani T, Carbon P, Krol A. Characterization of mSelB, a novel mammalian elongation factor for selenoprotein translation. The EMBO journal. 2000;19:4796–4805. doi: 10.1093/emboj/19.17.4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee BJ, Worland PJ, Davis JN, Stadtman TC, Hatfield DL. Identification of a selenocysteyl-tRNA(Ser) in mammalian cells that recognizes the nonsense codon, UGA. The Journal of biological chemistry. 1989;264:9724–9727. [PubMed] [Google Scholar]
- 12.Copeland PR, Driscoll DM. Purification, redox sensitivity, and RNA binding properties of SECIS-binding protein 2, a protein involved in selenoprotein biosynthesis. The Journal of biological chemistry. 1999;274:25447–25454. doi: 10.1074/jbc.274.36.25447. [DOI] [PubMed] [Google Scholar]
- 13.Copeland PR, Fletcher JE, Carlson BA, Hatfield DL, Driscoll DM. A novel RNA binding protein, SBP2, is required for the translation of mammalian selenoprotein mRNAs. The EMBO journal. 2000;19:306–314. doi: 10.1093/emboj/19.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann PR, Berry MJ. Selenoprotein synthesis: a unique translational mechanism used by a diverse family of proteins. Thyroid. 2005;15:769–775. doi: 10.1089/thy.2005.15.769. [DOI] [PubMed] [Google Scholar]
- 15.Hatfield DL, Gladyshev VN. How selenium has altered our understanding of the genetic code. Molecular and cellular biology. 2002;22:3565–3576. doi: 10.1128/MCB.22.11.3565-3576.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caban K, Copeland PR. Size matters: a view of selenocysteine incorporation from the ribosome. Cell Mol Life Sci. 2006;63:73–81. doi: 10.1007/s00018-005-5402-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bubenik JL, Driscoll DM. Altered RNA binding activity underlies abnormal thyroid hormone metabolism linked to a mutation in selenocysteine insertion sequence-binding protein 2. The Journal of biological chemistry. 2007;282:34653–34662. doi: 10.1074/jbc.M707059200. [DOI] [PubMed] [Google Scholar]
- 18.Squires JE, Stoytchev I, Forry EP, Berry MJ. SBP2 binding affinity is a major determinant in differential selenoprotein mRNA translation and sensitivity to nonsense-mediated decay. Molecular and cellular biology. 2007;27:7848–7855. doi: 10.1128/MCB.00793-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allamand V, Richard P, Lescure A, Ledeuil C, Desjardin D, Petit N, Gartioux C, Ferreiro A, Krol A, Pellegrini N, Urtizberea JA, Guicheney P. A single homozygous point mutation in a 3'untranslated region motif of selenoprotein N mRNA causes SEPN1-related myopathy. EMBO reports. 2006;7:450–454. doi: 10.1038/sj.embor.7400648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dumitrescu AM, Liao XH, Abdullah MS, Lado-Abeal J, Majed FA, Moeller LC, Boran G, Schomburg L, Weiss RE, Refetoff S. Mutations in SECISBP2 result in abnormal thyroid hormone metabolism. Nature genetics. 2005;37:1247–1252. doi: 10.1038/ng1654. [DOI] [PubMed] [Google Scholar]
- 21.Low SC, Grundner-Culemann E, Harney JW, Berry MJ. SECIS-SBP2 interactions dictate selenocysteine incorporation efficiency and selenoprotein hierarchy. The EMBO journal. 2000;19:6882–6890. doi: 10.1093/emboj/19.24.6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dever TE. Gene-specific regulation by general translation factors. Cell. 2002;108:545–556. doi: 10.1016/s0092-8674(02)00642-6. [DOI] [PubMed] [Google Scholar]
- 23.Gebauer F, Hentze MW. Molecular mechanisms of translational control. Nature reviews. 2004;5:827–835. doi: 10.1038/nrm1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazumder B, Seshadri V, Fox PL. Translational control by the 3'-UTR: the ends specify the means. Trends in biochemical sciences. 2003;28:91–98. doi: 10.1016/S0968-0004(03)00002-1. [DOI] [PubMed] [Google Scholar]
- 25.Mignone F, Gissi C, Liuni S, Pesole G. Untranslated regions of mRNAs. Genome biology. 2002;3 doi: 10.1186/gb-2002-3-3-reviews0004. REVIEWS0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pullmann R, Jr, Kim HH, Abdelmohsen K, Lal A, Martindale JL, Yang X, Gorospe M. Analysis of turnover and translation regulatory RNA-binding protein expression through binding to cognate mRNAs. Molecular and cellular biology. 2007;27:6265–6278. doi: 10.1128/MCB.00500-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edgar RC. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004;5:113. doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hofacker IL. Vienna RNA secondary structure server. Nucleic Acids Res. 2003;31:3429–3431. doi: 10.1093/nar/gkg599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mori D, Sasagawa N, Kino Y, Ishiura S. Quantitative analysis of CUG-BP1 binding to RNA repeats. J Biochem. 2008;143:377–383. doi: 10.1093/jb/mvm230. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi N, Sasagawa N, Suzuki K, Ishiura S. The CUG-binding protein binds specifically to UG dinucleotide repeats in a yeast three-hybrid system. Biochemical and biophysical research communications. 2000;277:518–523. doi: 10.1006/bbrc.2000.3694. [DOI] [PubMed] [Google Scholar]
- 32.Ladd AN, Charlet N, Cooper TA. The CELF family of RNA binding proteins is implicated in cell-specific and developmentally regulated alternative splicing. Molecular and cellular biology. 2001;21:1285–1296. doi: 10.1128/MCB.21.4.1285-1296.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Savkur RS, Philips AV, Cooper TA. Aberrant regulation of insulin receptor alternative splicing is associated with insulin resistance in myotonic dystrophy. Nature genetics. 2001;29:40–47. doi: 10.1038/ng704. [DOI] [PubMed] [Google Scholar]
- 34.Gromak N, Matlin AJ, Cooper TA, Smith CW. Antagonistic regulation of alpha-actinin alternative splicing by CELF proteins and polypyrimidine tract binding protein. RNA (New York, NY. 2003;9:443–456. doi: 10.1261/rna.2191903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Charlet BN, Savkur RS, Singh G, Philips AV, Grice EA, Cooper TA. Loss of the muscle-specific chloride channel in type 1 myotonic dystrophy due to misregulated alternative splicing. Molecular cell. 2002;10:45–53. doi: 10.1016/s1097-2765(02)00572-5. [DOI] [PubMed] [Google Scholar]
- 36.Timchenko NA, Welm AL, Lu X, Timchenko LT. CUG repeat binding protein (CUGBP1) interacts with the 5' region of C/EBPbeta mRNA and regulates translation of C/EBPbeta isoforms. Nucleic Acids Res. 1999;27:4517–4525. doi: 10.1093/nar/27.22.4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Timchenko NA, Patel R, Iakova P, Cai ZJ, Quan L, Timchenko LT. Overexpression of CUG triplet repeat-binding protein, CUGBP1, in mice inhibits myogenesis. The Journal of biological chemistry. 2004;279:13129–13139. doi: 10.1074/jbc.M312923200. [DOI] [PubMed] [Google Scholar]
- 38.Iakova P, Wang GL, Timchenko L, Michalak M, Pereira-Smith OM, Smith JR, Timchenko NA. Competition of CUGBP1 and calreticulin for the regulation of p21 translation determines cell fate. The EMBO journal. 2004;23:406–417. doi: 10.1038/sj.emboj.7600052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts R, Timchenko NA, Miller JW, Reddy S, Caskey CT, Swanson MS, Timchenko LT. Altered phosphorylation and intracellular distribution of a (CUG)n triplet repeat RNAbinding protein in patients with myotonic dystrophy and in myotonin protein kinase knockout mice. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:13221–13226. doi: 10.1073/pnas.94.24.13221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marquis J, Paillard L, Audic Y, Cosson B, Danos O, Le Bec C, Osborne HB. CUG-BP1/ CELF1 requires UGU-rich sequences for high-affinity binding. Biochem J. 2006;400:291–301. doi: 10.1042/BJ20060490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vlasova IA, Tahoe NM, Fan D, Larsson O, Rattenbacher B, Sternjohn JR, Vasdewani J, Karypis G, Reilly CS, Bitterman PB, Bohjanen PR. Conserved GU-rich elements mediate mRNA decay by binding to CUG-binding protein 1. Molecular cell. 2008;29:263–270. doi: 10.1016/j.molcel.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moraes KC, Wilusz CJ, Wilusz J. CUG-BP binds to RNA substrates and recruits PARN deadenylase. RNA (New York, NY. 2006;12:1084–1091. doi: 10.1261/rna.59606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paillard L, Legagneux V, Beverley Osborne H. A functional deadenylation assay identifies human CUG-BP as a deadenylation factor. Biology of the cell / under the auspices of the European Cell Biology Organization. 2003;95:107–113. doi: 10.1016/s0248-4900(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 44.Hinman MN, Lou H. Diverse molecular functions of Hu proteins. Cell Mol Life Sci. 2008 doi: 10.1007/s00018-008-8252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Myer VE, Fan XC, Steitz JA. Identification of HuR as a protein implicated in AUUUA-mediated mRNA decay. The EMBO journal. 1997;16:2130–2139. doi: 10.1093/emboj/16.8.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fan XC, Steitz JA. Overexpression of HuR, a nuclear-cytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. The EMBO journal. 1998;17:3448–3460. doi: 10.1093/emboj/17.12.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lopez de Silanes I, Zhan M, Lal A, Yang X, Gorospe M. Identification of a target RNA motif for RNA-binding protein HuR. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:2987–2992. doi: 10.1073/pnas.0306453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brennan CM, Steitz JA. HuR and mRNA stability. Cell Mol Life Sci. 2001;58:266–277. doi: 10.1007/PL00000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doller A, Pfeilschifter J, Eberhardt W. Signalling pathways regulating nucleocytoplasmic shuttling of the mRNA-binding protein HuR. Cellular signalling. 2008 doi: 10.1016/j.cellsig.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 50.Abdelmohsen K, Kuwano Y, Kim HH, Gorospe M. Posttranscriptional gene regulation by RNA-binding proteins during oxidative stress: implications for cellular senescence. Biological chemistry. 2008;389:243–255. doi: 10.1515/BC.2008.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sureban SM, Murmu N, Rodriguez P, May R, Maheshwari R, Dieckgraefe BK, Houchen CW, Anant S. Functional antagonism between RNA binding proteins HuR and CUGBP2 determines the fate of COX-2 mRNA translation. Gastroenterology. 2007;132:1055–1065. doi: 10.1053/j.gastro.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 52.Keene JD, Tenenbaum SA. Eukaryotic mRNPs may represent posttranscriptional operons. Molecular cell. 2002;9:1161–1167. doi: 10.1016/s1097-2765(02)00559-2. [DOI] [PubMed] [Google Scholar]
- 53.Keene JD. RNA regulons: coordination of post-transcriptional events. Nat Rev Genet. 2007;8:533–543. doi: 10.1038/nrg2111. [DOI] [PubMed] [Google Scholar]
- 54.Zubiagaq AM, Belasco JG, Greenberg ME. The nonamer UUAUUUAUU is the key AU-rich sequence motif that mediates mRNA degradation. Molecular and cellular biology. 1995;15:2219–2230. doi: 10.1128/mcb.15.4.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lagnado CA, Brown CY, Goodall GJ. AUUUA is not sufficient to promote poly(A) shortening and degradation of an mRNA: the functional sequence within AU-rich elements may be UUAUUUA(U/A)(U/A) Molecular and cellular biology. 1994;14:7984–7995. doi: 10.1128/mcb.14.12.7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sambrook J, Russell DW. Molecular Cloning:a laboratory manual. Cold Spring Harbor, NY: Cold Spring Laboratory Press; 2001. [Google Scholar]