Abstract
Background and Aims
Plant species from various taxa ‘escape’ from low oxygen conditions associated with submergence by a suite of traits collectively called the low oxygen escape syndrome (LOES). The expression of these traits is associated with costs and benefits. Thus far, remarkably few studies have dealt with the expected benefits of the LOES.
Methods
Young plants were fully submerged at initial depths of 450 mm (deep) or 150–240 mm (shallow). Rumex palustris leaf tips emerged from the shallow flooding within a few days, whereas a slight lowering of shallow flooding was required to expose R. acetosa leaf tips to the atmosphere. Shoot biomass and petiole porosity were measured for all species, and treatments and data from the deep and shallow submergence treatments were compared with non-flooded controls.
Key Results
R. palustris is characterized by submergence-induced enhanced petiole elongation. R. acetosa lacked this growth response. Upon leaf tip emergence, R. palustris increased its biomass, whereas R. acetosa did not. Furthermore, petiole porosity in R. palustris was twice as high as in R. acetosa.
Conclusions
Leaf emergence restores gas exchange between roots and the atmosphere in R. palustris. This occurs to a much lesser extent in R. acetosa and is attributable to its lower petiole porosity and therefore limited internal gas transport. Leaf emergence resulting from fast petiole elongation appears to benefit biomass accumulation if these plants contain sufficient aerenchyma in petioles and roots to facilitate internal gas exchange.
Key words: Submergence, emergence, enhanced shoot elongation, porosity, aerenchyma, Rumex, cost–benefit analysis, phenotypic plasticity
INTRODUCTION
A variety of specialized plant species are able to complete their life cycle in flood-prone environments such as lake shores, river floodplains, peat bogs and swamps. These species are occasionally exposed to elevated water levels and are therefore characterized by traits that enable them to cope with the resulting hampered gas exchange between flooded plant organs and the air. This slow rate of gas diffusion in water dramatically reduces oxygen and carbon dioxide influx, causing an imbalance in a plant's energy and carbon economy (Bailey-Serres and Voesenek, 2008; Jackson, 2008).
A suite of complementary traits, collectively called the low oxygen escape syndrome (LOES), facilitates escape from submergence stress in some species and thus avoids carbohydrate starvation and oxygen depletion (Gibbs and Greenway, 2003; Voesenek et al., 2006; Bailey-Serres and Voesenek, 2008; Jackson, 2008; Voesenek and Pierik, 2008). The LOES includes upward growth of leaves (hyponastic growth), petiole/stem elongation, thinner leaves, cuticles and cell walls, re-orientation of chloroplasts, leaf gas envelopes, aerenchyma and a barrier for radial oxygen loss (Colmer, 2003; Cox et al., 2003; Mommer et al., 2005b, 2007; Colmer and Pedersen, 2008). The elongation traits are initiated by ethylene that accumulates in submerged plant tissues (Musgrave et al., 1972; Kende et al., 1998; Vreeburg et al., 2005; Jackson, 2008). In the semi-aquatic plant Rumex palustris, ethylene accumulation is accompanied by an up-regulation of ethylene receptor and biosynthesis genes (Vriezen et al., 1997, 1999; Rieu et al., 2005) and a fast decline of the endogenous abscisic acid (ABA) concentration. This drop in ABA is regulated via a decrease in biosynthesis and an increase in catabolism (Benschop et al., 2005). Down-regulation of ABA is necessary for fast underwater elongation because normal levels of ABA inhibit an increase of the growth-promoting hormone gibberellin (Benschop et al., 2006). Next to this hormonal regulation, ethylene also directly causes an increase in the transcription of the cell-wall loosening gene RpEXPA1 and accumulation of Expansin proteins (Vreeburg et al., 2005). Finally, ethylene also induces a fast apoplastic acidification, which is presumably related to the pH optimum of Expansins between 3·5 and 4·5 (Vreeburg et al., 2005).
Selection for a specific trait such as flooding-induced shoot elongation in a given environment is an expression of the balance between involved costs and the benefits raised by such a trait. Setter and Laureles (1996) demonstrated that, in rice, elongation growth under water goes at the expense of survival if the plants are unable to emerge from deep water. This shows that costs are involved in underwater elongation and explains why submergence-induced shoot elongation is only selected for in environments with relatively shallow but prolonged flooding events (Voesenek et al., 2004). However, surprisingly little research attention has been given to the expected benefits of leaf emergence as a consequence of submergence-induced shoot elongation.
This paper presents the results of an experimental study on the benefits of leaf emergence from initial submergence expressed in terms of biomass accumulation. Two Rumex species were selected for study. The results demonstrate that elongation-mediated leaf tip emergence stimulates biomass accumulation in R. palustris. However, artificial emergence of leaf tips does not enhance growth of submerged R. acetosa, a species that by itself is unable to resurface after submergence This difference in the ability of the two species to benefit from leaf emergence is hypothesized to be related to a higher capacity for internal gas diffusion in R. palustris than in R. acetosa.
MATERIALS AND METHODS
Seeds of Rumex palustris and R. acetosa were sown on polyethylene beads floating in water in a transparent container and allowed to germinate for 10 d at 70 µmol m−2 s−1 photosynthetically active radiation (12 h light at 25 °C and 12 h dark at 20 °C). Seedlings were then transplanted to 175-mL pots containing a 2 : 1 mixture of potting soil and river sand, supplemented with 40 mL nutrient solution (for mineral components see Cox et al., 2003). Plants were grown on pots for 25 d at 200 µmol m−2 s−1 (16 h light, 8 h dark, relative humidity 70 %, 21 °C). After 3 d under glass to prevent dehydration, pots of transplanted seedlings were placed on irrigation mats that were automatically watered twice a day (0700 and 1900 h). Fifteen days after potting, the plants received a further 20 mL of nutrient solution and submergence treatments were started 25 d after potting.
The aim was to determine the extent to which plants benefit from restoring aerial contact with the longest leaves through enhanced petiole elongation. Therefore, the biomass of fully submerged plants was compared with that of plants which were initially fully submerged but able to regain contact with the aerial environment by means of accelerated petiole extension. Previous work on submergence responses has revealed that biomass accumulation shows a good correlation with fitness traits such as survival (Van Eck et al., 2006). To this end, plants were completely submerged in glass tanks. However, half of the plants were submerged to a depth of 450 mm, which is too deep for R. palustris to re-emerge through petiole elongation (deep submergence). The remaining plants were submerged in 160 mm (R. acetosa) or 240 mm (R. palustris) of water (shallow submergence). This shallow depth was outgrown in 5 d through submergence-induced petiole elongation by R. palustris, but not by R. acetosa, which fails to elongate underwater. Therefore, to observe the effect of re-emergence in R. acetosa it was necessary to lower the water level by 30 mm to expose the longest leaves to the air after 5 d. This allowed us to study whether this species could also benefit from restored contact with the atmosphere. In both species the laminae of 2–3 leaves thus re-emerged after 5 d in the shallow submergence treatment, whereas all leaves remained submerged for the entire 21 d in the deeper submergence treatment.
All submergence treatments lasted 21 d and were paralleled by plants that remained in aerated non-flooded control conditions. At the start of treatments (t = 0) and again 3 weeks later plants were harvested and petiole porosity, maximum petiole length and shoot dry weight were determined. Furthermore, plant height was recorded every 2–3 d during the 21-d experiment. Petiole porosity measurements were made as described by Raskin (1983) and the equations defined in Thomson et al. (1990). Each treatment for both species was carried out with ten replicate plants and the entire experiment was performed twice with comparable outcomes. Measurements made after 21 d of treatment were analysed with a one-way ANOVA and Tukey's post-hoc comparison using the SPSS 14 software package. Biomass data were ln transformed to meet the ANOVA requirements (equal variances and normal distribution of the data).
RESULTS
Two Rumex species differ in flooding-induced petiole elongation
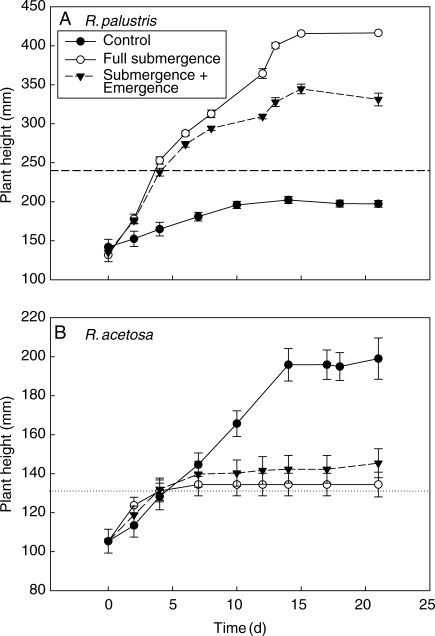
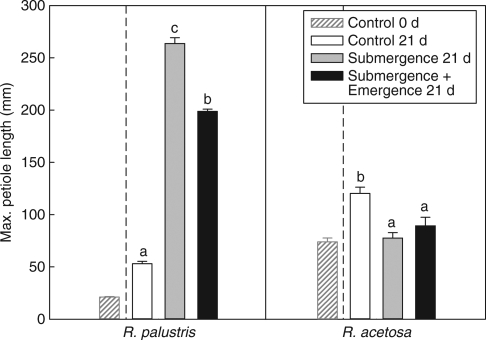
Maximum plant height was used as a plant trait to describe the maximum shoot elongation capacity to flooding regimes. R. palustris, a species from environments with long-lasting floods, responded to both complete and partial submergence with a strong increase in maximum plant height. The largest maximum height was attained upon complete submergence. This resulted in leaf emergence from the shallow flood (240 mm depth) after 4 d of submergence (Fig. 1A). In contrast, R. acetosa, a species from rarely flooded field sites, is characterized by a lower final plant height during both submergence treatments compared with control, non-submerged conditions (Fig. 1B). The increased maximum plant height of submerged R. palustris plants results from a strong increase in maximum petiole length and involves only a marginal contribution of leaf lamina elongation (data not shown). The lack of increased petiole elongation upon submergence and an earlier cessation of elongation growth as compared with control conditions in R. acetosa explains the low plant height in submerged plants of this species (Fig. 2A). In both species the total number of leaves did not differ between partial and complete submergence and was lower than in the non-submerged control plants (Table 1).
Fig. 1.
Changes in shoot height of (A) Rumex palustris and (B) R. acetosa during 21 d of submergence. Dotted lines indicate the depth of the water in the shallow submergence treatment, and in the case of R. acetosa this indicates the level after lowering it to 130 mm after 5 d to expose leaf tips to the air. Plants of R. palustris were allowed to emerge above the water level by fast underwater extension. Controls were non-submerged plants grown in well-drained soil. Data are means ± s.e. (n = 8–10).
Fig. 2.
Maximum petiole length of Rumex palustris and R. acetosa at the end of 21 d of submergence as described in Fig. 1. Control values at the start of the treatments (control t = 0) are shown for comparison. Data are means ± s.e. (n = 8–10). Within each bar graph, means with different letters are statistically significantly different (P ≤ 0·05).
Table 1.
Total leaf number per plant for non-submerged control plants, for plants that were submerged for 5 d and then re-emerged and for plants that remained fully submerged throughout the experiment of Rumex palustris and R. acetosa after 21 d of treatment
| Species | Control | Submergence + emergence | Submergence |
|---|---|---|---|
| R. palustris | 37·2 ± 0·9 | 12·3 ± 0·7 | 12·9 ± 0·5 |
| R. acetosa | 23·7 ± 1·8 | 13·1 ± 1·5 | 9·5 ± 1·1 |
Values are means ± s.e. (n = 8–10).
Biomass accumulation and aerenchyma
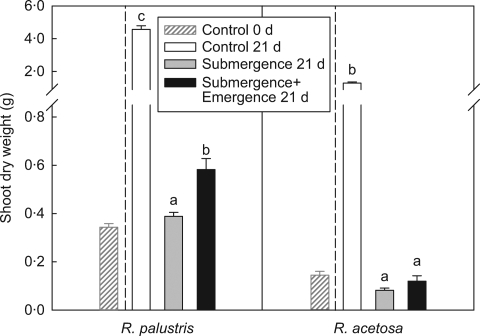
Rumex palustris plants exposed to 21 d of complete submergence neither lost nor gained shoot dry weight. However, when leaf parts of R. palustris emerged from the water surface a significant increase in shoot dry weight was observed as compared with plants that remained completely submerged. Such an increase was not observed in R. acetosa (Fig. 3). A possible benefit of elongation-induced leaf emergence is increased inward diffusion of oxygen and carbon dioxide from the atmosphere and enhanced outward diffusion of accumulated gases to the air. The internal diffusion depends on the porosity of the petioles. When petiole porosity was measured under control and submerged conditions for both species, porosity in R. palustris was twice that of R. acetosa. Furthermore, in neither species was petiole porosity enhanced by submergence for 21 d (Table 2).
Fig. 3.
Final shoot dry weight of Rumex palustris and R. acetosa after 21 d of submergence as described in Fig. 1. Controls were non-submerged plants grown in well-drained soil. Control values at the start of the treatments (control t = 0) are shown for comparison. Data are means ± s.e. (n = 8–10). Within each bar graph, means with different letters are statistically significantly different (P ≤ 0·05).
Table 2.
Petiole porosity (% by volume) in non-submerged and fully submerged plants of Rumex palustris and R. acetosa after 21 d underwater
| Species | Control | Submergence |
|---|---|---|
| R. palustris | 24·1 ± 0·3 | 23·4 ± 0·7 |
| R. acetosa | 10·7 ± 0·4 | 9·4 ± 0·3 |
Values are means ± s.e. (n = 4–8).
DISCUSSION
Enhanced petiole and/or stem elongation is an important plant trait in the so-called LOES (Bailey-Serres and Voesenek, 2008; Jackson, 2008). It brings leaves to the water surface and it is particularly relevant to survival in shallow, long-lasting floods (Voesenek et al., 2004). The present study shows that leaf emergence of R. palustris results in a significant increase of shoot dry weight compared with fully submerged plants. Furthermore, emergence of R. acetosa, a species with lower petiole porosity, does not lead to increased biomass accumulation.
Leaf emergence above the water line can be expected to promote gas exchange between submerged plant organs and the atmosphere. This can have a number of potential benefits. First, inward diffusion of oxygen will aerate potentially anoxic tissues such as apical and central stellar zones and relieve the energy crisis (Armstrong et al., 1999; Colmer, 2003; Gibbs and Greenway, 2003; Fukao and Bailey-Serres, 2004). Secondly, this influx may enhance the oxygen concentration in plant zones characterized by so-called micro-aerobic conditions (0·1–0·75 % oxygen). These micro-aerobic conditions are thought to be responsible for the generation of reactive oxygen species and thus peroxidation of lipid membranes (Santosa et al., 2007). A third benefit of leaf emergence is enhanced potential for photosynthesis as a result of access to atmospheric CO2, which will be discussed in the next section. A final potential benefit would be the ventilation of accumulated gaseous components such as ethylene, which, at high concentration, interferes with normal root growth (Visser et al., 1997; Visser and Pierik, 2007). These benefits all depend on fast gas diffusion. The rate of gas diffusion in submerged plant organs is inversely related to path length, loss of oxygen along the path and tortuosity of the air channels, and positively correlated with the porosity of the organ (Armstrong et al., 1994; Colmer, 2003). Petiole porosity was found to be much higher in petioles of flood-tolerant R. palustris than in those of R. acetosa. The low petiole porosity in R. acetosa is therefore predicted to hamper fast gas exchange between emerged leaf parts and the root system, thereby preventing significant benefits from leaf emergence. This is further strengthened by the very low root porosity in R. acetosa (3–9 % by volume; Laan et al., 1989; Visser et al., 1996) compared with R. palustris (24–37 %; Visser et al., 1996). The limited internal aeration this low porosity inevitably brings about will probably restrict aerobic respiration especially in roots and rhizomes and this, in turn, would suppress root growth, nutrient uptake and root-dependent processes of the shoot. This conceptual picture highlights the capacity for internal aeration as a major determinant of submergence tolerance and one that underpins the effectiveness of other traits, such as shoot elongation. This is in agreement with a recent study by Mommer et al. (2006) on the importance of various ecophysiological traits in determining submergence tolerance in a variety of species. It was found that capacity for internal aeration is a strong determinant of submergence tolerance (Mommer et al., 2006). They also proposed that a high porosity may have coevolved with the ability for submergence-induced shoot elongation given that the coupling of the two traits appears to maximize the chances of survival by aerating the whole plant effectively.
An important benefit of leaf emergence is related to photosynthesis. When still fully submerged R. palustris can already fix some external carbon dioxide, using a variety of submergence-induced leaf acclimations (e.g. thinner cuticles and cell walls) that decrease the diffusion resistance for CO2 and increase the affinity for CO2 and thus enhance underwater maximum rates of photosynthesis (Mommer et al., 2005b). Underwater photosynthesis in R. palustris (Mommer et al., 2005b) will result in enhanced internal oxygen levels, thereby increasing the efficiency of respiration. The combination of these two benefits probably prevented a decline in shoot dry weight observed in R. palustris during 21 d of complete submergence. However, much higher rates of photosynthesis would presumably be possible in leaves that re-emerge from the submerged environment and restart aerial photosynthesis. The net gain in biomass by R. palustris plants that emerged from the water by petiole extension is probably the combined result of the occurrence of aerial photosynthesis in the emerged leaves and enhanced aerobic respiration elsewhere in the plant. Internal transport of carbon dioxide to still submerged parts of leaves may also benefit from the faster ingress of carbon dioxide by virtue of the low porosity/low diffusion resistance pathway. The expected increase in the rate of photosynthesis in R. acetosa when emerging a few leaves into an aerated environment did not lead to an increase in shoot dry weight. This suggests that the lack of biomass increase in this species is strongly determined by the poor capacity of this species for internal gas diffusion related to its low overall tissue porosity.
The developmental context of the present study is as follows. Plasticity for petiole elongation in R. palustris incorporates an ability for fast extension growth under stressful submerged conditions, a prime example of adaptive phenotypic plasticity. A similar adaptive effect has also been shown for shoot elongation responses to shade imposed by competing neighbours, where the ability to elongate and outgrow adjacent plants stimulates growth in dry matter and reproduction in dense stands (Schmitt et al., 1995; Schmitt, 1997). Interestingly, shoot elongation responses to submergence resemble elongation responses to shade imposed by competing neighbours and both involve a key role for the plant hormone ethylene (Pierik et al., 2005; Mommer et al., 2005a). The picture is emerging of ethylene as an essential modifier of a variety of plant growth adjustments to environmental fluctuations (Pierik et al., 2007) and thus a key mediator of adaptive phenotypic plasticity.
In summary, the data presented indicate that, in R. palustris, leaf emergence arising from fast underwater petiole elongation restores gain in dry mass, this being the probable outcome of improved gas exchange between the atmosphere and the plant. This will contribute to the high survival rates and more seed production (Voesenek et al., 1992) that is denied to plants that are fully submerged for long periods. However, even if closely related R. acetosa were capable of regaining contact with the air by means of fast petiole elongation (which it is not), this by itself would not ensure that growth in dry matter would ensue. This is because its low tissue porosity would hinder restoration of the rapid gas exchange needed to sustain basic energy-generating processes in the remaining submerged lower sections of the plant. This impediment will, ultimately, accelerate plant death even when parts of leaves were emerged above the water surface (Voesenek et al., 1992).
ACKNOWLEDGEMENTS
We thank Diederik Keuskamp for help with porosity measurements and Dr Liesje Mommer and Professor Michael B. Jackson for helpful comments on earlier versions of this paper. This research was funded by the Earth and Life Sciences foundation of the Netherlands Organisation for Scientific Research (VENI grant no. 86306001 to R.P.).
LITERATURE CITED
- Armstrong J, Freen-Zobayed F, Blyth S, Armstrong W. Phragmites australis: effects of shoot submergence on seedling growth and survival and radial oxygen loss from roots. Aquatic Botany. 1999;64:275–289. [Google Scholar]
- Armstrong W, Strange ME, Cringle S, Beckett PM. Microelectrode and modelling study of oxygen distribution in roots. Annals of Botany. 1994;74:287–299. [Google Scholar]
- Bailey-Serres J, Voesenek LACJ. Flooding stress: acclimations and genetic diversity. Annual Review of Plant Biology. 2008;59:313–339. doi: 10.1146/annurev.arplant.59.032607.092752. [DOI] [PubMed] [Google Scholar]
- Benschop JJ, Jackson MB, Guhl K, Vreeburg RAM, Croker SJ, Peeters AJM, Voesenek LACJ. Contrasting interactions between ethylene and abscisic acid in Rumex species differing in submergence tolerance. Plant Journal. 2005;44:756–768. doi: 10.1111/j.1365-313X.2005.02563.x. [DOI] [PubMed] [Google Scholar]
- Benschop JJ, Bou J, Peeters AJM, Wagemaker N, Guhl K, Ward D, et al. Long-term submergence-induced elongation in Rumex palustris requires abscisic acid-dependent biosynthesis of gibberellin. Plant Physiology. 2006;141:1644–1652. doi: 10.1104/pp.106.082636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmer TD. Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant, Cell and Environment. 2003;26:17–36. [Google Scholar]
- Colmer TD, Pedersen O. Underwater photosynthesis and respiration in leaves of submerged wetland plants: gas films improve CO2 and O2 exchange. New Phytologist. 2008;177:918–926. doi: 10.1111/j.1469-8137.2007.02318.x. [DOI] [PubMed] [Google Scholar]
- Cox MCH, Millenaar FF, van Berkel YEMD, Peeters AJM, Voesenek LACJ. Plant movement. Submergence-induced petiole elongation in Rumex palustris depends on hyponastic growth. Plant Physiology. 2003;132:282–291. doi: 10.1104/pp.102.014548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao T, Bailey-Serres J. Plant responses to hypoxia – is survival a balancing act? Trends in Plant Science. 2004;9:449–456. doi: 10.1016/j.tplants.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Gibbs J, Greenway H. Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Functional Plant Biology. 2003;30:1–47. doi: 10.1071/PP98095. [DOI] [PubMed] [Google Scholar]
- Jackson MB. Ethylene-promoted elongation: an adaptation to submergence stress. Annals of Botany. 2008;101:229–248. doi: 10.1093/aob/mcm237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kende H, Van der Knaap E, Cho H-T. Deepwater rice: a model plant to study stem elongation. Plant Physiology. 1998;118:1105–1110. doi: 10.1104/pp.118.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laan P, Berrevoets MJ, Lythe S, Armstrong W, Blom CWPM. Root morphology and aerenchyma formation as indicators of the flood-tolerance of Rumex species. Journal of Ecology. 1989;77:693–703. [Google Scholar]
- Mommer L, de Kroon H, Pierik R, Bogemann GM, Visser EJW. A functional comparison of acclimation to shade and submergence in two terrestrial plant species. New Phytologist. 2005;167:197–206. doi: 10.1111/j.1469-8137.2005.01404.x. [DOI] [PubMed] [Google Scholar]
- Mommer L, Pons TL, Wolters-Arts M, Venema JH, Visser EJW. Submergence-induced morphological, anatomical, and biochemical responses in a terrestrial species affect gas diffusion resistance and photosynthetic performance. Plant Physiology. 2005;139:497–508. doi: 10.1104/pp.105.064725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mommer L, Lenssen JPM, Huber H, Visser EJW, de Kroon H. Ecophysiological determinants of plant performance under flooding: a comparative study of seven plant families. Journal of Ecology. 2006;94:1117–1129. [Google Scholar]
- Mommer L, Wolters-Arts M, Andersen C, Visser EJW, Pedersen O. Submergence-induced leaf acclimation in terrestrial species varying in flooding tolerance. New Phytologist. 2007;176:337–345. doi: 10.1111/j.1469-8137.2007.02166.x. [DOI] [PubMed] [Google Scholar]
- Musgrave A, Jackson MB, Ling E. Callitriche stem elongation is controlled by ethylene and gibberellin. Nature. 1972;238:93–96. [Google Scholar]
- Pierik R, Millenaar FF, Peeters AJM, Voesenek LACJ. New perspectives in flooding research: the use of shade avoidance and Arabidopsis thaliana. Annals of Botany. 2005;96:533–540. doi: 10.1093/aob/mci208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik R, Sasidharan R, Voesenek LACJ. Growth control by ethylene: adjusting phenotypes to the environment. Journal of Plant Growth Regulation. 2007;26:188–200. [Google Scholar]
- Raskin I. A method for measuring leaf volume, density, thickness, and internal gas volume. HortScience. 1983;18:698–699. [Google Scholar]
- Rieu I, Cristescu SM, Harren FJM, Huibers W, Voesenek LACJ, Mariani C, Vriezen WH. RP-ACS1, a flooding-induced 1-aminocyclopropane-1-carboxylate synthase gene of Rumex palustris, is involved in rhythmic ethylene production. Journal of Experimental Botany. 2005;56:841–849. doi: 10.1093/jxb/eri078. [DOI] [PubMed] [Google Scholar]
- Santosa IE, Ram PC, Boamfa EI, Laarhoven LJJ, Reuss J, Jackson MB, Harren FJM. Patterns of peroxidative ethane emission from submerged rice seedlings indicate that damage from reactive oxygen species takes place during submergence and is not necessarily a post-anoxic phenomenon. Planta. 2007;226:193–202. doi: 10.1007/s00425-006-0457-z. [DOI] [PubMed] [Google Scholar]
- Schmitt J. Is photomorphogenic shade avoidance adaptive? Perspectives from population biology. Plant, Cell and Environment. 1997;20:826–830. [Google Scholar]
- Schmitt J, McCormac AC, Smith H. A test of the adaptive plasticity hypothesis using transgenic and mutant plants disabled in phytochrome-mediated elongation responses to neighbors. The American Naturalist. 1995;146:937–953. [Google Scholar]
- Setter TL, Laureles EV. The beneficial effect of reduced elongation growth on submergence tolerance of rice. Journal of Experimental Botany. 1996;47:1551–1559. [Google Scholar]
- Thomson CJ, Armstrong W, Waters I, Greenway H. Aerenchyma formation and associated oxygen movement in seminal and nodal roots of wheat. Plant, Cell and Environment. 1990;13:395–403. [Google Scholar]
- Van Eck WHJM, Lenssen JPM, van de Steeg HM, Blom CWPM, de Kroon H. Seasonal dependent effects of flooding on plant species survival and zonation: a comparative study of 10 terrestrial grassland species. Hydrobiologia. 2006;565:59–69. [Google Scholar]
- Visser EJW, Pierik R. Inhibition of root elongation by ethylene in wetland and non-wetland plant species and the impact of longitudinal ventilation. Plant, Cell and Environment. 2007;30:31–38. doi: 10.1111/j.1365-3040.2006.01601.x. [DOI] [PubMed] [Google Scholar]
- Visser EJW, Blom CWPM, Voesenek LACJ. Flooding-induced adventitious rooting in Rumex: morphology and development in an ecological perspective. Acta Botanica Neerlandica. 1996;45:17–28. [Google Scholar]
- Visser EJW, Nabben RHM, Blom CWPM, Voesenek LACJ. Elongation by primary lateral roots and adventitious roots during conditions of hypoxia and high ethylene concentrations. Plant, Cell and Environment. 1997;20:647–653. [Google Scholar]
- Voesenek LACJ, Pierik R. Plant stress profiles. Science. 2008;320:880–881. doi: 10.1126/science.1158720. [DOI] [PubMed] [Google Scholar]
- Voesenek LACJ, Vandersman AJM, Harren FJM, Blom CWPM. An amalgamation between hormone physiology and plant ecology – a review on flooding resistance and ethylene. Journal of Plant Growth Regulation. 1992;11:171–188. [Google Scholar]
- Voesenek LACJ, Rijnders JHGM, Peeters AJM, Van de Steeg HMV, de Kroon H. Plant hormones regulate fast shoot elongation under water: from genes to communities. Ecology. 2004;85:16–27. [Google Scholar]
- Voesenek LACJ, Colmer TD, Pierik R, Millenaar FF, Peeters AJM. How plants cope with complete submergence. New Phytologist. 2006;170:213–226. doi: 10.1111/j.1469-8137.2006.01692.x. [DOI] [PubMed] [Google Scholar]
- Vreeburg RAM, Benschop JJ, Peeters AJM, Colmer TD, Ammerlaan AHM, Staal M, et al. Ethylene regulates fast apoplastic acidification and expansin A transcription during submergence-induced petiole elongation in Rumex palustris. Plant Journal. 2005;43:597–610. doi: 10.1111/j.1365-313X.2005.02477.x. [DOI] [PubMed] [Google Scholar]
- Vriezen WH, vanRijn CPE, Voesenek LACJ, Mariani C. A homolog of the Arabidopsis thaliana ERS gene is actively regulated in Rumex palustris upon flooding. Plant Journal. 1997;11:1265–1271. doi: 10.1046/j.1365-313x.1997.11061265.x. [DOI] [PubMed] [Google Scholar]
- Vriezen WH, Hulzink R, Mariani C, Voesenek LACJ. 1-aminocyclopropane-1-carboxylate oxidase activity limits ethylene biosynthesis in Rumex palustris during submergence. Plant Physiology. 1999;121:189–195. doi: 10.1104/pp.121.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]