Summary
Recent studies have uncovered the molecular basis of self-avoidance and tiling, two fundamental principles required for the formation of neural circuits. Both of these wiring strategies are established through homophilic repulsion between Dscam proteins expressed on opposing cell surfaces. In Drosophila, Dscam1 mediates self-avoidance whereas Dscam2 mediates tiling. In contrast, phenotypes in the retina of the DSCAM mutant mouse indicate that DSCAM functions in both self-avoidance and tiling. These findings suggest that homophilic recognition molecules that have classically been defined as adhesive, may also function as repulsive cues, and that Dscam proteins specialize in this function.
Introduction
The complexity of neuronal wiring is daunting. In the human brain, for instance, it is estimated that 1012 neurons make 1015 connections! How these connections are specified during development remains a central issue in neurobiology. Molecular cues expressed on the surface of axons and dendrites mediate interactions between them. In addition to promoting association between pre- and post-synaptic neurons, cell surface molecules play other roles in patterning local circuitry within the developing neuropil. Among these are tiling and self-avoidance, two poorly understood patterning mechanisms described some 25 years ago. Tiling refers to the process of establishing non-overlapping synaptic domains between the same class of neurons. Self-avoidance refers to the tendency of neurites from the same cell to repel one another thereby promoting branch segregation and the establishment of uniform receptive fields.
Tiling was first described in the vertebrate retina. In the early 1980s, Boycott and colleagues described the dendritic organization of retinal ganglion cells (RGCs) in the cat. There are an estimated 20-30 different RGC subclasses in the cat retina based on functional characteristics and some classes of cells can be identified using specific markers. It has been proposed that visual information received at any point on the retina should be available to all cell classes and thus, each RGC subclass should completely cover the retina. Indeed, Wassle et al. observed that ON- and OFF-α-RGCs “achieve a uniform and independent coverage of the retina.” Furthermore, they observed that dendritic fields from cells of the same type did not overlap and proposed that interactions between neighboring cells likely shaped these domains[1].
Experimental evidence for interactions between processes of adjacent dendritic fields came from studies by Perry and Linden working in the rat retina. By surgically removing subsets of ganglion cells at birth, they were able to assess the effect this had on neighboring dendrites later in development. They found that dendrites from cells near the depleted region oriented towards and extended their arbors into the vacant area[2]. These observations were the first description of the phenomenon that came to be known as “tiling” and is defined as complete, but non-overlapping coverage of synaptic fields. Like tiles on a kitchen floor, each cell of the same class has a synaptic field that fills the area between adjacent cells. In contrast, the processes of different classes of neurons readily overlap with one another. Homotypic repulsion between cells of the same class would provide a simple mechanism to promote tiling.
Homotypic repulsion would also provide a simple model for another basic, though until recently, largely unappreciated wiring principle called self-avoidance. The concept of self-avoidance arose from studies by Kramer and Stent in the giant Amazon leech, Haementeria ghilianii. They observed that sister branches of the same mechanosensory neuron innervating the body wall did not overlap with each other, but did overlap with branches from different cells. Andy Kramer recounted this discovery in a recent correspondence with the authors of this review. “Thus it appeared that from the beginning of development, a cell's branching patterns were governed by some kind of self-avoidance rule, because there was no obvious stage when isoneuronal branches overlapped and later withdrew. We conjectured that self-avoidance was necessary to ensure maximal dispersion of sensory endings into the cell's available skin territory.” Kramer tested his self-avoidance theory using surgical manipulations in the embryonic leech. When he crushed a pioneering branch of a neuron so that it could not develop, secondary branches from the same cell widened their boundaries and overtook the area vacated by the pioneer. Kramer and Stent proposed two different models to explain self-avoidance. The first was based on correlated activity patterns between branches of the same neuron and the second invoked molecular cues that gave each neuron its own identity[3].
Review
Progress in identifying the molecular determinants of both tiling and self-avoidance have come from recent studies in Drosophila. Dendrites of multiple dendritic (md) sensory neurons in the Drosophila larvae, like the leech axon branches studied by Kramer and Stent, follow the rules of self-avoidance. The dendritic arborization (da) neurons form a subset of the md neurons, reside on the body wall of the developing larvae, and comprise four discrete classes[4]. The dendritic fields elaborated by each class of cells exhibit self-avoidance behavior in that branches from the same cell rarely cross each other while dendrites from different classes of da neurons frequently cross. The molecular and genetic tools available in Drosophila provided a means to assess the genes required for self-avoidance in these cells.
If branches from the same cell avoid each other but not branches of other cells, then each neuron must have the ability to distinguish self from non-self. Given that self-avoidance is a general property of many different types of neurons, a diverse recognition system analogous to self-recognition in the vertebrate immune system must exist. What type of molecular cues could account for self-recognition? The immunoglobulin superfamily protein, Dscam1, was a good candidate for several reasons. First, the Dscam1 locus can generate tens of thousands of different recognition molecules through alternative splicing [5] and each neuron appears to express a unique combination of isoforms [6]. Second, each Dscam1 isoform mediates homophilic, but not heterophilic binding with other isoforms and thus, this single gene could generate the diversity required for self-recognition throughout the nervous system [7]. And finally, Dscam1 is required for the segregation of sister branches in mushroom body neurons, a process similar to self-avoidance[8]. These observations led to the proposal that Dscam1 promotes homophilic repulsion in vivo [9] [7]. Note that in previous publications we referred to this Dscam paralog as Dscam. As there are four paralogs in Drosophila we now refer to the family as Dscams, and the first Dscam gene described in flies[5] as Dscam1.
How does the molecular diversity of Dscam1 provide each neuron with a unique identity? One could imagine at least two different ways that this could be accomplished. Each neuron could express a different Dscam1 isoform, or alternatively, each neuron could express a different set of Dscam1 isoforms. Although both of these scenarios would confer a discrete cell surface identity upon each neuron, the former scenario requires a complex alternative splicing mechanism, whereas the latter can be achieved through stochastic expression of many Dscam1 isoforms within each cell. Expression studies in mushroom body neurons (as well as several other subclasses) argue that many isoforms are expressed in each neuron and that the expression pattern is largely random[6]. In addition, genetic studies demonstrated that the type of isoform expressed in each MB neuron is not important; rather, what is crucial is that an individual MB neuron expresses different isoforms from its neighbors[9-11].
These findings led three independent groups to test whether Dscam1 is required for self-avoidance in da neurons. Larvae homozygous mutant for Dscam1 exhibited self-avoidance defects in da neurons. To address whether Dscam1 is required cell autonomously in da neurons, single Dscam1 mutant cells were generated in an otherwise wild type background using the MARCM technique (Box 2). The morphology of Dscam1 mutant da neurons was strikingly different from that of wild type cells. Instead of having well separated dendritic arbors, mutant neurites frequently crossed and fasciculated with each other. Importantly, dendritic growth was not significantly affected. Neurites still branched and grew normally, but sister neurites failed to segregate from one another. Through this analysis it was concluded that all four classes of da neurons require Dscam1 for self-avoidance[12-14].
Box 2. Mosaic Analysis with a Repressible Cell Marker (MARCM).
MARCM is a technique that allows the investigator to analyze single mutant cells in an otherwise wild type background[20]. It exploits a recombinase to promote mitotic recombination and thus to generate homozygous mutant cells, and a transcriptional activator/repressor system to mark the mutant cell selectively with GFP. Reverse MARCM is identical to MARCM except that the wild type, but not the mutant cells are labeled[21]. This strategy allows the investigator to assess whether the mutation of interest is causing non-autonomous defects in wild type cells.
The Dscam1 loss of function analysis in da neurons argued that dendrites from the same cell that express the same isoforms repel each other. If this were the case, then neurons with overlapping dendritic fields engineered to express the same Dscam1 isoform, should also avoid each other. To test this, a single isoform of Dscam1 was expressed in all da neurons and phenotypes were assessed in class I and class III neurons that normally have overlapping dendritic fields. When both class I and class III neurons overexpressed the same Dscam1 isoform, their dendritic fields segregated from one another and no longer overlapped[12-14]. It is noteworthy that in these gain of function experiments the da neurons still expressed a diverse array of Dscam1 isoforms. Presumably, the expression level of the ectopic isoform was sufficient to disguise the Dscam1 identity conferred upon these neurons by the endogenous gene and to induce repulsion.
The argument that that Dscam1 mediates self-avoidance through a homophilic repulsive mechanism was supported by two additional studies. In the first, Matthews et al. visualized interactions between processes of two different cells overexpressing the same isoform of Dscam1 using real-time confocal microscopy. Neurites expressing the same Dscam1 isoform were observed making contact with each other and then rapidly withdrawing from the encounter. In a second line of evidence, reported by the same group, it was shown that when the cytoplasmic domain of Dscam1 was replaced with GFP, neurites expressing the same Dscam1 isoform interacted with each other, but failed to withdraw[13]. Thus, removing the cytoplasmic domain of Dscam1, in effect, trapped a contact-dependent repulsion intermediate, arguing that the cytoplasmic domain transduces a signal essential for repulsion. In summary, these findings argue that Dscam1 provides the molecular basis of self-avoidance in flies.
Does Dscam1 also mediate tiling? Class IV da neurons exhibit tiling properties in addition to self-avoidance. Given that class IV neurons are likely to express Dscam1 isoforms in a stochastic fashion, Dscam1 would not be expected to mediate tiling. Consistent with this, Dscam1 mutant class IV neurons maintained their ability to tile even though they lacked self-avoidance[12-14]. Since tiling and self-avoidance are proposed to work through similar mechanisms (i.e. contact-dependent repulsion), other Dscam1-related recognition molecules were good candidates for tiling receptors. There are three other Dscam genes in Drosophila (Dscam2-4) and two in vertebrates (DSCAM and DSCAM-L). Although these genes encode proteins with extracellular domains organized identically to Dscam1, neither the fly paralogs nor their vertebrate counterparts undergo extensive alternative splicing. All Dscam proteins mediate homophilic binding, but none show heterophilic interactions with other family members.
To address whether other Dscam family members contribute to wiring the nervous system, we analyzed mutations in the Dscam2 gene. Class IV da neurons had normal morphology and tiling in the absence of Dscam2 (unpublished data). However, Dscam2 was found to be required for tiling of a subset of neurons in the visual system [15].
The medulla region of the fly visual system is a laminated structure somewhat similar to the inner plexiform layer (IPL) of the vertebrate visual system. Both structures are devoid of cell bodies, and axons and dendrites of different classes of cells form connections in discrete layers. In addition to layering, the medulla is organized along an orthogonal dimension into ∼750 columns. Each column contains ∼50 different cell types including axons from two photoreceptor neurons (R7 and R8) and five lamina neurons (L1-L5) which have a periodicity of one per column. The axons from these cells make synaptic connections at specific layers within each column, but not with target cells in neighboring columns. Thus, these axon terminals are tiled across specific layers in the medulla.
Tiling of L1 neurons requires Dscam2[15]. Single mutant L1 axons, generated using the MARCM technique, targeted to the correct layer within the appropriate column, but then extended laterally and invaded the L1 layer of neighboring columns. Using reverse MARCM, where wild type lamina neurons are labeled and mutant lamina neurons are not (Box 2), unidirectional tiling phenotypes were observed in wild type L1 axons[15]. This demonstrated that Dscam2 homophilic interactions are necessary for columnar restriction of L1. In addition, the unidirectional nature of the wild type defect suggested that Dscam2 homophilic interactions between adjacent columns, rather than within the same column, were critical for tiling. These data are consistent with a model where Dscam2-dependent homophilic repulsion provides the molecular basis for tiling of L1 axons.
Tiling of other axon terminals in the medulla (e.g. R7, R8 and L2) is independent of Dscam2. Thus, there must be other tiling receptors and likely other mechanisms for achieving tiling in the fly visual system. Indeed, tiling of R7 photoreceptor neurons requires the autocrine activation of the TGFβ/activin pathway and a yet to be identified paracrine signal from adjacent R7 terminals [16].
Recent genetic studies argue that the role of Dscam proteins in self-avoidance and tiling are evolutionarily conserved. Burgess and colleagues identified defects in the organization of retinal amacrine cell neurites in a DSCAM mutant mouse, consistent with a role in both processes [17]. Amacrine cells make synaptic connections with retinal ganglion cells in specific layers within the inner plexiform layer. Like the dendrites of retinal ganglion cells described by Boycott and colleagues, amacrine cells of the same class extend processes into discrete layers and their synaptic fields are tiled across the retina. In addition, sister branches from the same neuron seldom overlap suggesting that these neurons also exhibit self-avoidance. DSCAM is selectively expressed in two classes of amacrine cells, tyrosine hydroxylase and nitric oxide synthase positive cells, which arborize in different layers. In DSCAM mutants, tiling and self-avoidance were disrupted selectively in these cells, causing fasciculation of processes from the same and from different cells of the same class within a given layer. Tiling and self-avoidance in other classes of amacrine cells were unaffected.
These findings argue that Dscam proteins provide neurons with a recognition system that promotes the establishment of local circuitry through repulsive interactions. All or at least the vast majority of neurons in Drosophila express Dscam1. As each cell is proposed to express an essentially random collection of isoforms, all cells can use Dscam1 for self-avoidance, but this mode of expression precludes its use in tiling. A second Dscam, Dscam2, promotes this function, at least in a subset of cells. By contrast, it is the cell-type specific expression of a single DSCAM protein that allows it to promote both self-avoidance and tiling in a subclass of amacrine cells in the vertebrate retina. Interestingly, recent evidence suggests that vertebrate DSCAMs may also play an adhesive function in promoting layer specific targeting (Box 3). Thus, repulsion may not be an intrinsic feature of DSCAM signaling, but may reflect the intracellular signaling pathways operating downstream from activated DSCAM. Whether fly Dscams function adhesively in some developmental contexts remains an intriguing possibility.
Box 3. Dscam Proteins and Synaptic Matching.
In addition to its roles in self-avoidance and tiling, vertebrate DSCAM has also been implicated in layer-specific targeting. In the IPL of the chick retina, DSCAM and DSCAM-L are expressed in non-overlapping populations of amacrine and retinal ganglion cells that form synaptic contacts with each other in specific layers. Interestingly, cells that express the same DSCAM target to the same layer, suggesting that the DSCAMs may be instructive targeting cues. Consistent with this, knocking down DSCAM in retinal ganglion cells causes abnormal targeting and misexpressing DSCAM in cells that do not normally express this protein causes them to target to DSCAM-positive layers[22]. Thus, DSCAM proteins may have different functions in different developmental contexts.
Conclusion
In summary, self-avoidance and tiling are patterning mechanisms contributing to the assembly of precise neural circuits in both vertebrates and invertebrates. Dscam proteins play a critical role in self-avoidance and tiling by promoting recognition and subsequent repulsion between specific processes of either the same cell or the same class of cells. While Dscam1 diversity allows the products of one gene to, in principle, regulate self-avoidance in all fly neurons, tiling in flies and both tiling and self-avoidance in vertebrates must rely on proteins other than Dscams. What are these proteins? One possibility is that, like Dscams, other proteins originally characterized as homophilic adhesion molecules may act in a repulsive fashion in vivo.
Box 1. Homophilic Repulsion.
Homophilic repulsion is a multi-step process. In the first step, identical proteins on opposing cell surfaces bind to each other. This adhesive interaction activates signals that lead to repulsion between the two membranes. One of the signals communicated by homophilic binding dissociates the adhesive receptor complex. For Eph-ephrin heterophilic repulsion, two different mechanisms have been described. One involves the induction of a metalloprotease that cleaves the receptor complex from the cell surface, and the other involves internalization of the entire complex into one of the two opposing membranes[18,19]. Dscam1 homophilic repulsion likely proceeds through similar mechanisms. Signaling also leads to actin cytoskeleton rearrangements that allow the two cell surfaces to retract from one another.
Figure 1.
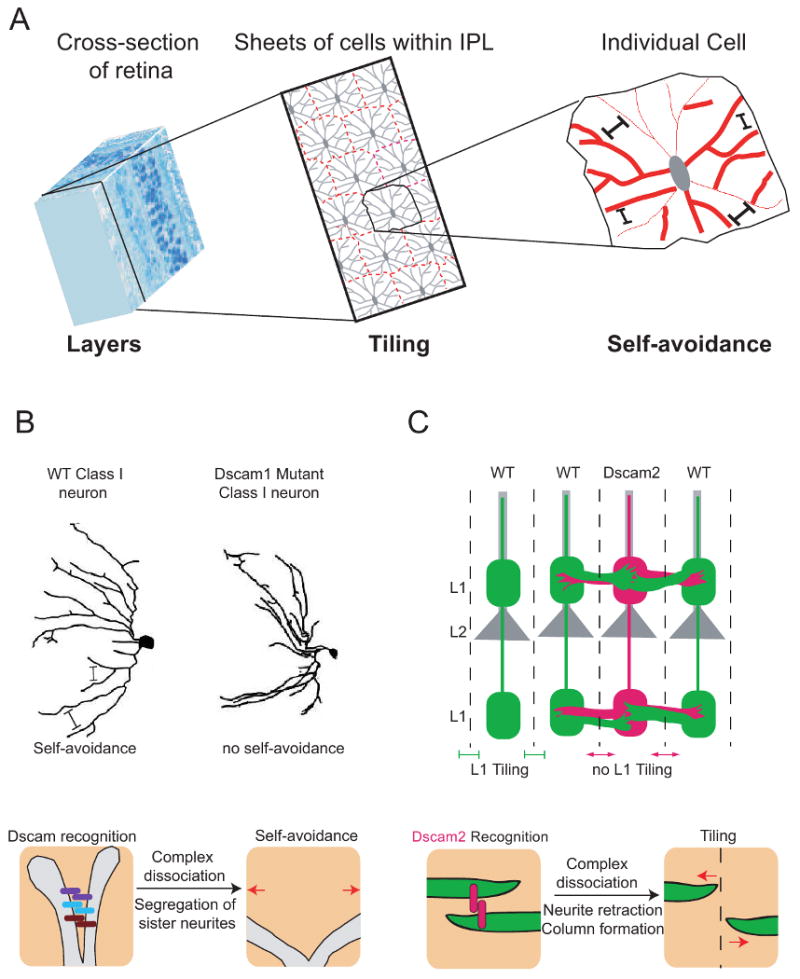
Tiling and self-avoidance are wiring strategies generated by a common homophilic repulsive mechanism. (A) Tiling and self-avoidance participate in neural circuit formation. (Left) A cross-section of the vertebrate retina illustrating its stratified organization. The section outlined in black passes through the inner plexiform layer (IPL). (Middle) Tiling of amacrine cells within the IPL. Each cell sends its processes to a specific layer where they form individual synaptic domains. Little overlap is seen between cells of the same class (indicated with dashed red lines). Tiling results in complete, but non-overlapping coverage of the retina. (Right) Individual amacrine cells exhibit self-avoidance. Branches from the same cell rarely cross each other permitting maximal coverage of each cell's synaptic domain. (B) Fly Dscam1 mediates self-avoidance. (Top) A wild type and a Dscam1 mutant classI da neuron are shown. Dscam promotes branch segregation through self-recogniton followed by homophilic repulsion. In the mutant cell, branches still form but they fail to effectively segregate from one another. (Bottom) The molecular mechanism of self-avoidance involves self-recognition and repulsion. As a neurite branches, each sister branch expresses the same set of Dscam1 isoforms. These isoforms bind homophilically and this transduces a signal to dissociate the receptor complex and initiate a repulsive response. (C) Dscam2 is a tiling receptor for L1 neurons. (Top) A schematic of the medulla showing four mature columns and lamina neurons L1 (green) and L2 (gray) in each column. Wild type L1 axons are tiled in a Dscam2-dependent manner if both of their neighboring columns express Dscam2. In contrast, Dscam2 mutant L1 axons (red) extend laterally and invade the L1 layer of neighboring columns. The lack of Dscam2 in the mutant L1 axon also causes a defect in neighboring wild type L1 axons because they are unable to participate in Dscam2 homophilic interactions. (Bottom) Dscam2 homophilic interactions restrict L1 axons to a single column. During pupal development, L1 neurites from neighboring columns (green) interact with each other as they explore their target area. Dscam2 homophilic interactions (red bars) initiate a series of events that lead to dissociation of the adhesive complex and the retraction of neurites back to their column of origin.
Acknowledgments
We would like to acknowledge Wes Grueber for schematics of the md neurons and Andy Kramer for communicating his historical perspective of self-avoidance. S.L.Z is an investigator of the Howard Hughes Medical Institute. This work was also supported by the NIH (S.L.Z). S.S.M. was supported by the Cellular Neurobiology training grant from the NIH (NS07101).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wassle H, Peichl L, Boycott BB. Dendritic territories of cat retinal ganglion cells. Nature. 1981;292:344–345. doi: 10.1038/292344a0. [DOI] [PubMed] [Google Scholar]
- 2.Perry VH, Linden R. Evidence for dendritic competition in the developing retina. Nature. 1982;297:683–685. doi: 10.1038/297683a0. [DOI] [PubMed] [Google Scholar]
- 3.Kramer AP, Stent GS. Developmental arborization of sensory neurons in the leech Haementeria ghilianii. II. Experimentally induced variations in the branching pattern. J Neurosci. 1985;5:768–775. doi: 10.1523/JNEUROSCI.05-03-00768.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grueber WB, Jan LY, Jan YN. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development. 2002;129:2867–2878. doi: 10.1242/dev.129.12.2867. [DOI] [PubMed] [Google Scholar]
- 5.Schmucker D, Clemens JC, Shu H, Worby CA, Xiao J, Muda M, Dixon JE, Zipursky SL. Drosophila Dscam is an axon guidance receptor exhibiting extraordinary molecular diversity. Cell. 2000;101:671–684. doi: 10.1016/s0092-8674(00)80878-8. [DOI] [PubMed] [Google Scholar]
- 6.Neves G, Zucker J, Daly M, Chess A. Stochastic yet biased expression of multiple Dscam splice variants by individual cells. Nat Genet. 2004;36:240–246. doi: 10.1038/ng1299. [DOI] [PubMed] [Google Scholar]
- 7.Wojtowicz WM, Flanagan JJ, Millard SS, Zipursky SL, Clemens JC. Alternative splicing of Drosophila Dscam generates axon guidance receptors that exhibit isoform-specific homophilic binding. Cell. 2004;118:619–633. doi: 10.1016/j.cell.2004.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J, Zugates CT, Liang IH, Lee CH, Lee T. Drosophila Dscam is required for divergent segregation of sister branches and suppresses ectopic bifurcation of axons. Neuron. 2002;33:559–571. doi: 10.1016/s0896-6273(02)00570-6. [DOI] [PubMed] [Google Scholar]
- 9.Zhan XL, Clemens JC, Neves G, Hattori D, Flanagan JJ, Hummel T, Vasconcelos ML, Chess A, Zipursky SL. Analysis of Dscam diversity in regulating axon guidance in Drosophila mushroom bodies. Neuron. 2004;43:673–686. doi: 10.1016/j.neuron.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 10.Hattori D, Demir E, Kim HW, Viragh E, Zipursky SL, Dickson BJ. Dscam diversity is essential for neuronal wiring and self-recognition. Nature. 2007;449:223–227. doi: 10.1038/nature06099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Ma X, Yang JS, Zheng X, Zugates CT, Lee CH, Lee T. Transmembrane/juxtamembrane domain-dependent Dscam distribution and function during mushroom body neuronal morphogenesis. Neuron. 2004;43:663–672. doi: 10.1016/j.neuron.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 12.Hughes ME, Bortnick R, Tsubouchi A, Baumer P, Kondo M, Uemura T, Schmucker D. Homophilic Dscam interactions control complex dendrite morphogenesis. Neuron. 2007;54:417–427. doi: 10.1016/j.neuron.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matthews BJ, Kim ME, Flanagan JJ, Hattori D, Clemens JC, Zipursky SL, Grueber WB. Dendrite self-avoidance is controlled by Dscam. Cell. 2007;129:593–604. doi: 10.1016/j.cell.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Soba P, Zhu S, Emoto K, Younger S, Yang SJ, Yu HH, Lee T, Jan LY, Jan YN. Drosophila sensory neurons require Dscam for dendritic self-avoidance and proper dendritic field organization. Neuron. 2007;54:403–416. doi: 10.1016/j.neuron.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]; These three papers (references 12-14) use both loss and gain of function experiments to demonstrate that Dscam1 mediates self-avoidance in da neurons through a homophilic repulsive mechanism.
- 15.Millard SS, Flanagan JJ, Pappu KS, Wu W, Zipursky SL. Dscam2 mediates axonal tiling in the Drosophila visual system. Nature. 2007;447:720–724. doi: 10.1038/nature05855. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper demonstrates that Dscam2 is a tiling receptor for L1 lamina neurons. Like Dscam1, Dscam2 uses homophilic repulsion as a mechanism for patterning. Dscam2 is the first tiling receptor to be identified.
- 16.Ting CY, Herman T, Yonekura S, Gao S, Wang J, Serpe M, O'Connor MB, Zipursky SL, Lee CH. Tiling of r7 axons in the Drosophila visual system is mediated both by transduction of an activin signal to the nucleus and by mutual repulsion. Neuron. 2007;56:793–806. doi: 10.1016/j.neuron.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuerst PG, Koizumi A, Masland RH, Burgess RW. Neurite arborization and mosaic spacing in the mouse retina require DSCAM. Nature. 2008;451:470–474. doi: 10.1038/nature06514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hattori M, Osterfield M, Flanagan JG. Regulated cleavage of a contact-mediated axon repellent. Science. 2000;289:1360–1365. doi: 10.1126/science.289.5483.1360. [DOI] [PubMed] [Google Scholar]
- 19.Zimmer M, Palmer A, Kohler J, Klein R. EphB-ephrinB bi-directional endocytosis terminates adhesion allowing contact mediated repulsion. Nat Cell Biol. 2003;5:869–878. doi: 10.1038/ncb1045. [DOI] [PubMed] [Google Scholar]
- 20.Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 21.Lee T, Winter C, Marticke SS, Lee A, Luo L. Essential roles of Drosophila RhoA in the regulation of neuroblast proliferation and dendritic but not axonal morphogenesis. Neuron. 2000;25:307–316. doi: 10.1016/s0896-6273(00)80896-x. [DOI] [PubMed] [Google Scholar]
- 22.Yamagata M, Sanes JR. Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature. 2008;451:465–469. doi: 10.1038/nature06469. [DOI] [PubMed] [Google Scholar]; This paper describes a role for DSCAM and DSCAM-L in promoting layer-specific targeting in the inner plexiform layers of the chick retina.


