Abstract
Background:
The links among smoking, inflammation, and cardiovascular disease (CVD) are well established. Several studies have demonstrated that quitting smoking reverses the risk of coronary heart disease within 5 to 10 years. However, the immediate effects of quitting smoking on inflammatory biomarkers associated with CVD risk have not been well described.
Methods:
In this pilot study, we examined a panel of circulating inflammatory biomarkers associated with CVD in “at-risk” women during the smoking cessation program. Forty-six women enrolled in a smoking cessation program consented to attend four study visits over 6 to 7 weeks. Health/medical information and blood were collected at each visit. Circulating levels of C-reactive protein (CRP), tumor necrosis factor (TNF), interleukin (IL)-6, soluble TNF receptor (sTNFR)-I, sTNFR-II, and soluble vascular cell adhesion molecule (sVCAM)-1 were measured, and changes between baseline levels (visit 1, while smoking) and visits 2 through 4 were determined.
Results:
Significant reductions in circulating levels of TNF, sTNFR-I, sTNFR-II, and sVCAM-1 were observed among participants over the course of the smoking cessation program. Serum levels of both IL-6 and CRP declined during the smoking cessation program; the changes were not statistically significant, however.
Conclusions:
These findings suggest there are rapid consequences of smoking cessation on inflammatory biomarkers in women at risk for CVD. Additional, larger studies including diverse smokers desiring to quit are required to confirm changes in “measurable milestones” that could serve as motivating factors to assist smokers to quit.
Smoking promotes enhanced production of proinflammatory molecules by numerous cell types1–6 and contributes to systemic inflammation with elevated levels of inflammatory biomarkers.7,8 Numerous studies8–16 have identified serum biomarkers (eg, C-reactive protein [CRP], interleukin [IL]-6, tumor necrosis factor [TNF], soluble TNF receptors [sTNFRs] I and II) that predict the risk of COPD9–11 and cardiovascular disease (CVD).8,12–16 By contrast, smoking cessation is associated with improved COPD17 and CVD risk/mortality.18,19 However, it is not clear whether the benefits of quitting smoking are achieved immediately or require years.20,21 The purpose of this pilot study was to investigate the effect of quitting smoking on serum inflammatory biomarkers associated with CVD in women at risk for CVD during smoking cessation.
Materials and Methods
Subjects
The institutional review board approved this study, and all subjects gave written informed consent prior to study procedures. Subjects were recruited from the North Shore-LIJ Health System “Quit for Keeps” smoking cessation program (a behavioral support program) between July 2005 and June 2007. Women smokers were enrolled in the study if they were at risk for CVD, which was defined as having one or more of the following: abdominal obesity; elevated cholesterol levels; high BP and/or history of heart disease; had smoked approximately 1 pack of cigarettes per day for the past year (with exhaled carbon monoxide [CO] concentration of > 15 ppm); were relatively healthy; and wanted to quit smoking. Subjects were excluded if they were receiving antiinflammatory agents (including oral corticosteroids), were currently pregnant or trying to conceive, were drinking beyond moderation, or were using other tobacco products.
Study Design
Subjects consented to attend multiple visits that overlapped with the Quit for Keeps program (6 to 8 weeks) [Table 1]. At visit 1, subjects (n = 46) were smoking and chose smoking cessation aids (eg, nicotine replacement therapy [NRT] and/or bupropion [Wellbutrin; GlaxoSmithKline; Research Triangle Park, NC]), which were provided free of charge), with the help of trained nurses. Subjects completed questionnaires and the Medical Outcomes Study 36-item short form (SF-36) [physical and mental health assessment] at visits 1 and 3. Height, weight, body mass index (BMI), BP, and smoking status (assessed using the measurement of expired CO and serum cotinine levels, and self-report) were recorded at each visit. Visit 2 was within 24 to 72 h of their last cigarette (and 1 week after visit 1), visit 3 was 1 to 2 weeks after visit 2, and visit 4 was 3 to 5 weeks later. Peripheral blood was collected at each visit; serum was isolated, aliquoted, and stored at −80°C until analyses of biomarkers and cotinine were performed.
Table 1.
—Description of Study Visits for Subjects Attending Smoking Cessation Program
| Study Visit | ||||
| Variables | 1 | 2 | 3 | 4 |
| Subjects, No. | 46 | 42 | 39 | 36 |
| Smoking | + | 0 | 0 | 0 |
| Cessation aids | 0 | + | + | + |
| Height | + | 0 | 0 | 0 |
| Weight | + | + | + | + |
| BP | + | + | + | + |
| Exhaled CO | + | + | + | + |
| SF-36 | + | 0 | + | 0 |
| Questionnaires | + | 0 | 0 | 0 |
| Review smoking history | + | + | + | + |
| Review medication history | + | + | + | + |
| Blood collection | + | + | + | + |
Study subjects attended four study visits over a 6- to 7-week smoking cessation program. + = yes or assessed; 0 = no or not assessed.
Analysis of Inflammatory Biomarkers
Serum CRP levels were determined using ultrasensitive nephelometry. Serum IL-6 and TNF levels were determined using high-sensitivity enzyme-linked immunosorbent assays; serum sTNFR-I, sTNFR-II, and soluble vascular cell adhesion molecule (sVCAM)-1 levels were determined by enzyme-linked immunosorbent assays (R&D Systems; Minneapolis, MN). All serum samples were analyzed in duplicate, and samples for each subject (visits 1 through 4) were on the same plate with standards. The coefficients of variation for interassay and intraassay variability for the inflammatory markers were between 1% and 5%, and 2% and 8%, respectively.
Statistical Analysis
Statistics were analyzed using a statistical software package (SAS/PC, version 9.1; SAS Institute; Cary, NC).
Inflammatory Markers
Repeated measures analysis of covariance (RMANCOVA), where time (visits 2, 3, and 4) was the within-subjects effect (and there was no between-subjects effect), was used to examine changes from baseline (at visit 1, while smoking) and visits 2 through 4 for all subjects who completed four visits. For each biomarker, the ratio of levels of that marker to baseline levels was calculated, and then the log transformation was used to meet the assumptions of the RMANCOVA model. CO levels at each visit and BMI (at baseline) were included as covariates to control for potential effects on biomarkers. Exhaled CO was used as a surrogate marker for smoking status because it significantly associated with self-reported smoking (and NRT interfered with serum cotinine assays). To measure the degree of correlation between the self-reported number of cigarettes smoked per day and CO levels, the Spearman correlation coefficient was calculated for visits 2 through 4 (range, 0.47 to 0.62). At visit 4, all subjects who reported not smoking exhaled ≤ 8 ppm CO. On finding a significant effect of time on biomarker status, pairwise comparisons of visits 2 through 4 were carried out (RMANCOVA). To determine whether the change from baseline was significant, the level of each marker at each visit was compared to baseline levels by testing whether the ratio of visit x-to-visit 1 differed from 1 using the t test within the RMANCOVA model. For both comparisons, a Bonferroni adjustment was used, such that these comparisons were considered significant with p < 0.0167. Additional models were examined (eg, using serum cotinine levels as a surrogate for smoking instead of expired CO levels), and the results did not differ qualitatively from the reported results.
Feelings of Wellness (SF-36)
RMANCOVA, in which the visit was the “within-subjects effect” (and there was no “between-subjects effect”), was used to examine the pattern of SF-36 scores during smoking cessation. In addition, a RMANCOVA model was used to test for associations between each marker and SF-36 scores (total, physical, and mental health scores). Baseline SF-36 scores (mental, physical, and total) for subjects who completed fewer than four visits were compared with those who completed four visits using the exact Mann-Whitney test.
Results
Characteristics of the Study Population
Of the 46 female participants (mean [± SD] age, 54.1 ± 9.1 years) who smoked, 36 (78.3%) completed four study visits and were included in the analyses. All subjects quit smoking at least 72 h prior to visit 2. Baseline characteristics are shown in Table 2. The baseline mean (± SD) SF-36 scores for physical and mental health were 72.0 ± 15.3 and 72.5 ± 13.9, respectively, and these values did not significantly change over the course of the study. Many subjects had multiple risk factors for CVD (in addition to smoking) [Table 2]. NRT (nicotine patch [14 or 21 mg], nicotine gum, nasal spray, inhalers, and lozenges [Cardinal Health; Dublin, OH]) and bupropion or NRT alone were used as smoking cessation aids (Fig 1).
Table 2.
—Baseline Characteristics of the Study Population
| Characteristics (n = 36) | Mean (SD) | Median (IQR) | % |
| Age, yr | 53.9 (9.1) | 54.4 (14.3) | |
| BMI, kg/m2 | 30.0 (5.8) | 29.4 (7.2) | |
| Systolic BP* | 130.2 (13.84) | 130.0 (19.0) | |
| Diastolic BP* | 77.5 (9.1) | 80.0 (12.0) | |
| Smoking status | |||
| Cigarettes smoked/d | 22.8 (5.7) | 20.0 (0.0) | |
| Pack-yr | 39.0 (13.53) | 38.0 (14.0) | |
| Total SF-36 score | 74.4 (14.0) | 76.4 (20.4) | |
| Physical health score | 72.0 (15.3) | 75.4 (25.8) | |
| Mental health score | 72.5 (13.9) | 73.8 (22.6) | |
| Ethnicity | |||
| White | 58 | ||
| African-American | 11 | ||
| Hispanic | 3 | ||
| Unknown | 28 | ||
| CVD risk factors | |||
| Elevated cholesterol | 61.1 | ||
| Hypertension* | 36.1 | ||
| Abdominal obesity | 19.4 | ||
| Coronary artery disease | 13.9 |
Subjects with high BP were receiving antihypertensive medications.
Figure 1.
Smoking cessation aids used by study subjects. Most study subjects used NRT (97%), the majority of individuals used NRT + bupropion (BUP), and 19% used NRT alone.
Approximately 85% of the subjects who completed four study visits quit smoking. Those subjects who relapsed (six subjects; 15%) and continued in the study reported smoking fewer cigarettes; when these six subjects were excluded from the analyses, the results were similar to those observed for all subjects who had attended four study visits. Several subjects (n = 10) did not attend visit 4 due to relapse. Two differences were noted between subjects who did not complete four visits and those who did. Women who completed three visits or fewer had the following: (1) significantly lower median quality-of-life (SF-36) scores (mental score, 61.8 [interquartile range (IQR), 31.4]; total score, 62.6 [IQR, 27.9]) than women who completed four visits (mental score, 73.8 [IQR, 22.6]; total score, 76.4 [IQR, 20.4]; p < 0.01 and p < 0.02, respectively); and (2) were less likely to use smoking cessation aids.
Serum Biomarkers Associated With CVD Decline During Smoking Cessation
Based on the evidence that circulating TNF is associated with cardiovascular mortality/risk,7,22 we examined serum TNF levels during smoking cessation. The mean baseline serum TNF concentration (1.213 ± 0.703 pg/mL) in our subjects was higher than TNF concentrations measured at later visits (Table 3). Overall, there was a decline in serum TNF concentrations over the course of the smoking cessation program among women who were at risk for CVD with a significant difference between circulating TNF concentrations observed at visits 2 and 3 (p < 0.0115) [Table 3].
Table 3.
—Inflammatory Markers Before and After Smoking Cessation
| Markers | Baseline Value | Visit | Mean Change From Baseline, % (95% CI) | p Value |
| TNF | 1.213 ± 0.703 pg/mL | V1 | 0 | |
| V2 | −14.5 (−49.8 to 45.5) | < 0.0115 (vsV2) | ||
| V3 | −27.7 (−57.6 to 23.0) | |||
| V4 | −20.2 (−53.1 to 35.9) | < 0.0001 (vs V4) | ||
| sTNFR-I | 2,667.9 ± 1,066.4 pg/mL | V1 | 0 | < 0.01 (vs V4) |
| V2 | −3.0 (−7.9 to 2.2) | < 0.0068 (vs V4) | ||
| V3 | −3.9 (−8.7 to 1.2) | |||
| V4 | −11.6 (−16.1 to −7.0) | < 0.0048 (vs V4) | ||
| sTNFR-II | 2,395.1 ± 993.1 pg/mL | V1 | 0 | NS (vs V4) |
| V2 | −10.4 (−18.9 to −1.0) | < 0.0021 (vs V4) | ||
| V3 | −3.2 (−12.3 to 6.9) | |||
| V4 | −13.7 (−21.8 to −4.7) | < 0.0001 (vs V4) | ||
| sVCAM-1 | 310.1 ± 119.3 ng/mL | V1 | 0 | < 0.0001 (vs V4) |
| V2 | −0.6 (−8.8 to 8.3) | < 0.0001 (vs V4) | ||
| V3 | −4.1 (−11.9 to 4.4) | |||
| V4 | −19.5 (−26.0 to −12.3) | NS | ||
| IL-6* | 3.314 ± 2.873 pg/mL | V1 | 0 | |
| V2 | 15.0 (−10.8 to 48.4) | |||
| V3 | 0.7 (−21.8 to 29.7) | |||
| V4 | −9.6 (−29.8 to 16.4) | NS | ||
| CRP* | 0.462 ± 0.519 mg/dL | V1 | 0 | |
| V2 | −46.5 (−75.7 to 18.0) | |||
| V3 | −54.4 (−79.1 to −0.3) | |||
| V4 | −19.1 (−63.0 to 76.8) |
Values are given as the mean ± SD, unless otherwise indicated. Baseline inflammatory marker levels and the mean percentage changes in inflammatory markers between each study visit 2 through 4 vs baseline (visit 1) are shown with the level of significance. CI = confidence interval; NS = not significant; V = visit.
Pairwise comparisons of visits 2 through 4 were not carried out due to nonsignificant findings.
Circulating sTNFR-I and sTNFR-II levels are significantly predictive indicators of coronary heart disease in women.12 Steady declines in serum sTNFR-I and sTNFR-II levels were observed after quitting smoking (p < 0.0121 and p < 0.0023, respectively) [Table 3]. The serum sTNFR-I level was lower at visit 4 than at visits 1, 2, and 3, and the average sTNFR-II level at visit 4 was significantly lower than at visits 1 and 3 (Table 3). The mean baseline serum sTNFR-I concentration in our subjects (2,667.9 ± 1066.4 pg/mL) was higher than the levels described for healthy women (1,267 ± 354 pg/mL), whereas the average baseline sTNFR-II level among our subjects (2,395.1 ± 993.1 pg/mL) was comparable to that of healthy control subjects.12
Endothelial cell activation is proposed to play a role in the development of coronary artery disease and smoking-induced endothelial cell dysfunction.14,23 We found a significant decrease in levels of serum sVCAM-1, a marker of endothelial cell activation, after quitting smoking (p < 0.0001). Serum sVCAM-1 levels were significantly lower at visit 4 than at visits 1, 2, and 3 (Table 3).
Although serum levels of both IL-6 and CRP are considered to be predictors of cardiovascular events in some studies,12 others have reported either weak or no association between IL-6 and CRP values and CVD15,16 or CVD severity.24 In this study, we found an insignificant reduction in serum IL-6 and CRP levels during smoking cessation.
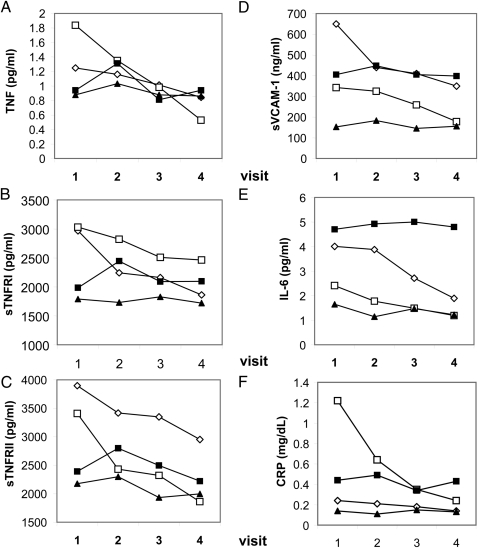
We observed variable responses among subjects. Some subjects showed steady declines in serum biomarker levels over the course of the smoking cessation program (Fig 2). Others exhibited either no pattern or no change in circulating biomarker levels (Fig 2).
Figure 2.
Individual subject patterns of inflammatory mediators during the smoking cessation program. Individual subject patterns of serum inflammatory mediators (associated with increased risk of CVD) during the smoking cessation process (visits 1 through 4) are shown for TNF (A), sTNFR-I (B), sTNFR-II (C), sVCAM-1 (D), IL-6 (E), and CRP (F). Open symbols denote mediator values for individual subjects (taking NRT + bupropion) whose inflammatory mediators declined over the smoking cessation program; closed symbols denote values for individual subjects where no changes in inflammatory mediator levels were observed.
Discussion
Smoking has deadly consequences. The long-term health benefits of smoking cessation for every age group are indisputable. The ultimate goal of smoking cessation programs is to assist smokers in quitting smoking and remaining smoke free. A very recent study25 reported that informing smokers of the “age and health” of their lungs (based on spirometric assessment) significantly improved quit rates. We propose a similar program focused on improving cardiovascular health for smokers. To develop a smoking cessation program centered on a “healthy heart,” it is necessary to identify measurable early biomarkers (in men and women) associated with cardiovascular risk that are sensitive to change with smoking cessation. Once established, these biomarkers can be assessed before and during smoking cessation. Quantifiable information reflecting cardiovascular health may act as positive reinforcement for those trying to quit and remain smoke free. Similarly, biomarkers (indicative of lung dysfunction) could be determined for smokers with compromised lung function to establish smoking cessation programs focused on “healthy lungs.”
Several studies13,26,27 have reported elevated levels of inflammatory biomarkers among smokers vs those for nonsmokers. Others21,28 have described a decline in inflammatory biomarker levels at some point after quitting smoking. Twenty years is required to reverse CRP, fibrinogen, and fibrin levels of smokers to levels found in nonsmokers.21 Similarly, 10 years is required to observe a 50% reduction in inflammatory/hemostatic markers after quitting smoking.29 These improvements in biomarker scores translate to a decline in cardiovascular risks ranging between 5 and 10 years for reducing the risk of myocardial infarctions to that of nonsmokers.30,31 Likewise, a recent report19 showed that women gained 61% of the full benefit of quitting smoking with regard to coronary disease mortality within 5 years of quitting.
To our knowledge, this is the first study to examine serum biomarkers associated with inflammation in women smokers at risk for CVD during smoking cessation. Based on our experience with smokers who complained of physical feelings that mimicked flu-like symptoms immediately after quitting, we hypothesized that smoking cessation might produce an initial spike in levels of inflammatory markers (immediately after quitting) reminiscent of “serum-sickness” with elevated circulating cytokine levels found after experimental administration of endo-toxin. This undesirable physical feeling would be an obstacle to quitting. In several subjects, we observed a slight (insignificant) increase in biomarker levels at visit 2 (compared to baseline). These data suggest the following: (1) there is no sharp inflammatory “peak” after quitting smoking; (2) the immediate rise was not found because blood was not sampled within a narrow time frame of quitting; (3) NRT (used by 97%) blunted inflammatory responses; and/or (4) variable responses to smoking cessation occur and a much larger population is required to examine these variable responses.
We further hypothesized that an early spike in circulating levels of inflammatory mediators after quitting smoking would be followed by steady declines. This proposed decrease is based on previous reports showing that smoking enhances systemic inflammation,7,8 quitting is associated with declines in inflammatory biomarker levels,21,28 and that nicotine (used to relieve cravings) exerts antiinflammatory effects in patients with ulcerative colitis32–34 and in experimental models of systemic inflammation (eg, endotoxemia,35 sepsis,36 and ischemia37,38).
Based on data obtained from 36 subjects at risk for CVD who completed four visits, we found significant changes in serum levels of TNF, sTNFR-I, sTNFR-II, and sVCAM-1 from baseline (Table 3). Although no significant changes in serum levels of CRP and IL-6 levels were observed (Table 3), serum CRP levels declined slightly during smoking cessation. Our observations are consistent with a report showing that a significant reduction in serum CRP levels among heavy smokers following smoking cessation requires 5 to 9 years; 20 years are needed to reverse serum CRP levels to those found among never-smokers.21 Together, these findings suggest that CRP, a stable downstream inflammatory marker, may not be useful as an early milestone for smokers. CRP synthesis is determined by levels of IL-6 and, to a lesser extent, of TNF,39 which are elevated among smokers40,41 and have potential prognostic value in predicting cardiovascular health.42 We observed a significant decrease in serum TNF levels after quitting smoking but no significant decline in IL-6 levels (Table 3).
The physiologic response of circulating TNF is mediated through TNFR-I and TNFR-II, which are shed from circulating leukocytes during inflammation (sTNFR-I and sTNFR-II). sTNFRs, significant markers of coronary heart disease for women,12 act as slow-release reservoirs of bioactive TNF and extend its half-life.43 Thus, circulating sTNFR levels reflect a systemic pan-inflammatory state better than individual short-lived cytokine levels and should be better predictors of inflammation than TNF.43 Interestingly, a recent study44 implicates sTNFR-I and sTNFR-II in the pathogenesis of COPD. Together with our observations, these findings suggest that quitting smoking could improve inflammatory responses systemically and within the lung.
Our quit rate was 65% and 83.3%, respectively, among all subjects and subjects who completed visit 4. The major differences between subjects who completed four visits vs those who completed three or fewer visits were their SF-36 scores and use of smoking cessation aids. Our results suggest that higher mental and physical SF-36 scores are associated with a greater likelihood of attending classes, increased use of smoking cessation aids, and quitting smoking. Thus, smokers with low SF-36 scores may need additional support to quit or might want to postpone quitting until their SF-36 scores are elevated.
This study has several limitations. Although it was designed and funded to be a pilot study enrolling women only, the most critical limitation was the small sample size. This, in part, was related to US Food and Drug Administration approval of vareni-cline (Chantix; Pfizer; New York, NY) in May 2006. Although we had a smaller than anticipated enrollment, most of our subjects (97%) used NRT, and 78% of the subjects completed four visits. SF-36 scores did not change significantly with biomarker levels during the smoking cessation program. The SF-36 form is recommended for the assessment of general health,45 but a major disadvantage is that it may not be sensitive to short-term changes in feelings of wellness experienced by our subjects who were relatively healthy. In addition, the smokers might have reduced smoking prior to visit 1, which might have reduced baseline values. Assessment of smoking status based on exhaled CO concentrations and self-report is another limitation. The serum cotinine level was measured throughout the study, but values were confounded by NRT (and for ethical reasons subjects were encouraged to continue their NRT). It is important to note that variable responses in inflammatory markers were observed. Variability might be associated with ethnicity, health status, smoking cessation aids (although 97% of our subjects used NRT), genetics, and age. Areas of future investigation include determining the sources of variability in inflammatory responses during smoking cessation and whether this variability influences successful long-term smoking cessation.
CVD is the most common cause of preventable death among adult Americans. Smoking as few as one to four cigarettes to one pack per day significantly increases the risk of fatal coronary heart disease by more than twofold and fivefold, respectively.46 Each year more women die from CVD than men.47 Although numerous CVD risk factors have been identified, poor implementation of programs to reduce these risk factors in women has been widely documented.48,49 Because of these factors and the reported gender differences in smoking cessation rates, with women having less success than men,50,51 we included women who were at risk for CVD in our study.51 Thus, until a similar study can be repeated with a much larger, more diverse population, our results can be only generalized to women at risk for CVD.
The development of successful smoking cessation programs for men and women is critical to reduce the number of deaths associated with smoking. We propose the identification of a panel of inflammatory biomarkers that could be used as measurable milestones for persons quitting smoking in a smoking cessation program focused on improving cardiovascular health for smokers who are at risk. This quantifiable information reflecting cardiovascular health may serve as positive reinforcement for those trying to quit smoking and remain smoke free. Likewise, a panel of markers could be established for smokers with abnormal lung function.
Acknowledgments
We acknowledge Dr. Martin Lesser for his assistance with the statistical analyses.
Abbreviations
- BMI
body mass index
- CO
carbon monoxide
- CRP
C-reactive protein
- CVD
cardiovascular disease
- IL
interleukin
- IQR
interquartile range
- NRT
nicotine replacement therapy
- RMANCOVA
repeated measures analysis of covariance
- SF-36
Medical Outcomes Study 36-item short form
- sTNFR
soluble tumor necrosis factor receptor
- sVCAM
soluble vascular cell adhesion molecule
- TNF
tumor necrosis factor
Footnotes
The work presented in this manuscript was the result of a Clinically Applied Research Grant awarded to Dr. Metz by the American Heart Association for improving cardiovascular risk in women through a smoking cessation program. In addition, Dr. Metz was funded by National Institutes of Health grant No. NIHRO1GM070727.
The authors have reported to the ACCP that no significant conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.org/site/misc/reprints.xhtml).
References
- 1.Mochida-Nishimura K, Surewicz K, Cross JV, et al. Differential activation of MAP kinase signaling pathways and nuclear factor-κB in bronchoalveolar cells of smokers and nonsmokers. Mol Med. 2001;7:177–185. [PMC free article] [PubMed] [Google Scholar]
- 2.Mio T, Romberger DJ, Thompson AB, et al. Cigarette smoke induces interleukin-8 release from human bronchial epithelial cells. Am J Respir Crit Care Med. 1997;155:1770–1776. doi: 10.1164/ajrccm.155.5.9154890. [DOI] [PubMed] [Google Scholar]
- 3.Matsumoto K, Aizawa H, Inoue H, et al. Eosinophilic airway inflammation induced by repeated exposure to cigarette smoke. Eur Respir J. 1998;12:387–394. doi: 10.1183/09031936.98.12020387. [DOI] [PubMed] [Google Scholar]
- 4.Chalmers GW, MacLeod KJ, Thomson L, et al. Smoking and airway inflammation in patients with mild asthma. Chest. 2001;120:1917–1922. doi: 10.1378/chest.120.6.1917. [DOI] [PubMed] [Google Scholar]
- 5.Walters MJ, Paul-Clark MJ, McMaster SK, et al. Cigarette smoke activates human monocytes by an oxidant-AP-1 signaling pathway: implications for steroid resistance. Mol Pharmacol. 2005;68:1343–1353. doi: 10.1124/mol.105.012591. [DOI] [PubMed] [Google Scholar]
- 6.Barbieri SS, Weksler BB. Tobacco smoke cooperates with interleukin-lβ to alter β-catenin trafficking in vascular endothelium resulting in increased permeability and induction of cyclooxygenase-2 expression in vitro and in vivo. FASEB J. 2007;21:1831–1843. doi: 10.1096/fj.06-7557com. [DOI] [PubMed] [Google Scholar]
- 7.Frohlich M, Sund M, Lowel H, et al. Independent association of various smoking characteristics with markers of systemic inflammation in men: results from a representative sample of the general population (MONICA Augsburg Survey 1994/95) Eur Heart J. 2003;24:1365–1372. doi: 10.1016/s0195-668x(03)00260-4. [DOI] [PubMed] [Google Scholar]
- 8.Koenig W, Sund M, Frohlich M, et al. C-Reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation. 1999;99:237–242. doi: 10.1161/01.cir.99.2.237. [DOI] [PubMed] [Google Scholar]
- 9.Karadag F, Kirdar S, Karul AB, et al. The value of C-reactive protein as a marker of systemic inflammation in stable chronic obstructive pulmonary disease. Eur J Intern Med. 2008;19:104–108. doi: 10.1016/j.ejim.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 10.Wouters EF, Groenewegen KH, Dentener MA, et al. Systemic inflammation in chronic obstructive pulmonary disease: the role of exacerbations. Proc Am Thorac Soc. 2007;4:626–634. doi: 10.1513/pats.200706-071TH. [DOI] [PubMed] [Google Scholar]
- 11.Groenewegen KH, Postma DS, Hop WC, et al. Increased systemic inflammation is a risk factor for COPD exacerbations. Chest. 2008;133:350–357. doi: 10.1378/chest.07-1342. [DOI] [PubMed] [Google Scholar]
- 12.Pai JK, Pischon T, Ma J, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351:2599–2610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- 13.Wannamethee SG, Tchernova J, Whincup P, et al. Plasma leptin: associations with metabolic, inflammatory and haemostatic risk factors for cardiovascular disease. Atherosclerosis. 2007;191:418–426. doi: 10.1016/j.atherosclerosis.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Demerath E, Towne B, Blangero J, et al. The relationship of soluble ICAM-1, VCAM-1, P-selectin and E-selectin to cardiovascular disease risk factors in healthy men and women. Ann Hum Biol. 2001;28:664–678. doi: 10.1080/03014460110048530. [DOI] [PubMed] [Google Scholar]
- 15.Cesari M, Penninx BW, Newman AB, et al. Inflammatory markers and cardiovascular disease (The Health, Aging and Body Composition [Health ABC] Study) Am J Cardiol. 2003;92:522–528. doi: 10.1016/s0002-9149(03)00718-5. [DOI] [PubMed] [Google Scholar]
- 16.Tuomisto K, Jousilahti P, Sundvall J, et al. C-reactive protein, interleukin-6 and tumor necrosis factor α as predictors of incident coronary and cardiovascular events and total mortality: a population-based, prospective study. Thromb Haemost. 2006;95:511–518. doi: 10.1160/TH05-08-0571. [DOI] [PubMed] [Google Scholar]
- 17.Celli BR. Update on the management of COPD. Chest. 2008;133:1451–1462. doi: 10.1378/chest.07-2061. [DOI] [PubMed] [Google Scholar]
- 18.Mohiuddin SM, Mooss AN, Hunter CB, et al. Intensive smoking cessation intervention reduces mortality in high-risk smokers with cardiovascular disease. Chest. 2007;131:446–452. doi: 10.1378/chest.06-1587. [DOI] [PubMed] [Google Scholar]
- 19.Kenfield SA, Stampfer MJ, Rosner BA, et al. Smoking and smoking cessation in relation to mortality in women. JAMA. 2008;299:2037–2047. doi: 10.1001/jama.299.17.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ockene IS, Miller NH. Cigarette smoking, cardiovascular disease, and stroke: a statement for healthcare professionals from the American Heart Association. American Heart Association Task Force on Risk Reduction. Circulation. 1997;96:3243–3247. doi: 10.1161/01.cir.96.9.3243. [DOI] [PubMed] [Google Scholar]
- 21.Wannamethee SG, Lowe GD, Shaper AG, et al. Associations between cigarette smoking, pipe/cigar smoking, and smoking cessation, and haemostatic and inflammatory markers for cardiovascular disease. Eur Heart J. 2005;26:1765–1773. doi: 10.1093/eurheartj/ehi183. [DOI] [PubMed] [Google Scholar]
- 22.Van Den Biggelaar AH, De Craen AJ, Gussekloo J, et al. Inflammation underlying cardiovascular mortality is a late consequence of evolutionary programming. FASEB J. 2004;18:1022–1024. doi: 10.1096/fj.03-1162fje. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez MA, Selwyn AP. Endothelial function, inflammation, and prognosis in cardiovascular disease. Am J Med. 2003;115(suppl):99S–106S. doi: 10.1016/j.amjmed.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Rifai N, Joubran R, Yu H, et al. Inflammatory markers in men with angiographically documented coronary heart disease. Clin Chem. 1999;45:1967–1973. [PubMed] [Google Scholar]
- 25.Parkes G, Greenhalgh T, Griffin M, et al. Effect on smoking quit rate of telling patients their lung age: the Step2quit randomised controlled trial. BMJ. 2008;336:598–600. doi: 10.1136/bmj.39503.582396.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madsen C, Nafstad P, Eikvar L, et al. Association between tobacco smoke exposure and levels of C-reactive protein in the Oslo II Study. Eur J Epidemiol. 2007;22:311–317. doi: 10.1007/s10654-007-9121-6. [DOI] [PubMed] [Google Scholar]
- 27.Lofdahl JM, Wahlstrom J, Skold CM. Different inflammatory cell pattern and macrophage phenotype in chronic obstructive pulmonary disease patients, smokers and non-smokers. Clin Exp Immunol. 2006;145:428–437. doi: 10.1111/j.1365-2249.2006.03154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammett CJ, Prapavessis H, Baldi JC, et al. Variation in blood levels of inflammatory markers related and unrelated to smoking cessation in women. Prev Cardiol. 2007;10:68–75. doi: 10.1111/j.1520-037x.2007.05957.x. [DOI] [PubMed] [Google Scholar]
- 29.Negri E, La Vecchia C, D'Avanzo B, et al. Acute myocardial infarction: association with time since stopping smoking in Italy; GISSI-EFRIM investigators—Gruppo Italiano per lo Studio della Sopravvivenza nell'infarto—Epidemiologia dei Fattori di Rischio dell'Infarto Miocardico. J Epidemiol Community Health. 1994;48:129–133. doi: 10.1136/jech.48.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dobson AJ, Alexander HM, Heller RF, et al. How soon after quitting smoking does risk of heart attack decline? J Clin Epidemiol. 1991;44:1247–1253. doi: 10.1016/0895-4356(91)90157-5. [DOI] [PubMed] [Google Scholar]
- 31.Samet JM. The 1990 Report of the Surgeon General: the health benefits of smoking cessation. Am Rev Respir Dis. 1990;142:993–994. doi: 10.1164/ajrccm/142.5.993. [DOI] [PubMed] [Google Scholar]
- 32.Pullan RD, Rhodes J, Ganesh S, et al. Transdermal nicotine for active ulcerative colitis. N Engl J Med. 1994;330:811–815. doi: 10.1056/NEJM199403243301202. [DOI] [PubMed] [Google Scholar]
- 33.Green JT, Thomas GA, Rhodes J, et al. Nicotine enemas for active ulcerative colitis: a pilot study. Aliment Pharmacol Ther. 1997;11:859–863. doi: 10.1046/j.1365-2036.1997.00220.x. [DOI] [PubMed] [Google Scholar]
- 34.Sandborn WJ, Tremaine WJ, Leighton JA, et al. Nicotine tartrate liquid enemas for mildly to moderately active left-sided ulcerative colitis unresponsive to first-line therapy: a pilot study. Aliment Pharmacol Ther. 1997;11:663–671. doi: 10.1046/j.1365-2036.1997.00208.x. [DOI] [PubMed] [Google Scholar]
- 35.Wang H, Liao H, Ochani M, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10:1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 36.Huston JM, Ochani M, Rosas-Ballina M, et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203:1623–1628. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernik TR, Friedman SG, Ochani M, et al. Cholinergic antiinflammatory pathway inhibition of tumor necrosis factor during ischemia reperfusion. J Vasc Surg. 2002;36:1231–1236. doi: 10.1067/mva.2002.129643. [DOI] [PubMed] [Google Scholar]
- 38.Yeboah MM, Xue X, Duan B, et al. Cholinergic agonists attenuate renal ischemia-reperfusion injury in rats. Kidney Int. 2008;74:62–69. doi: 10.1038/ki.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yudkin JS, Kumari M, Humphries SE, et al. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 40.Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265:621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeidel A, Beilin B, Yardeni I, et al. Immune response in asymptomatic smokers. Acta Anaesthesiol Scand. 2002;46:959–964. doi: 10.1034/j.1399-6576.2002.460806.x. [DOI] [PubMed] [Google Scholar]
- 42.Valgimigli M, Ceconi C, Malagutti P, et al. Tumor necrosis factor-α receptor 1 is a major predictor of mortality and new-onset heart failure in patients with acute myocardial infarction: the Cytokine-Activation and Long-Term Prognosis in Myocardial Infarction (C-ALPHA) study. Circulation. 2005;111:863–870. doi: 10.1161/01.CIR.0000155614.35441.69. [DOI] [PubMed] [Google Scholar]
- 43.Aderka D. The potential biological and clinical significance of the soluble tumor necrosis factor receptors. Cytokine Growth Factor Rev. 1996;7:231–240. doi: 10.1016/s1359-6101(96)00026-3. [DOI] [PubMed] [Google Scholar]
- 44.D'hulst AI, Bracke KR, Maes T, et al. Role of tumour necrosis factor-α receptor p75 in cigarette smoke-induced pulmonary inflammation and emphysema. Eur Respir J. 2006;28:102–112. doi: 10.1183/09031936.06.00059305. [DOI] [PubMed] [Google Scholar]
- 45.Haywood KL, Garratt AM, Fitzpatrick R. Quality of life in older people: a structured review of generic self-assessed health instruments. Qual Life Res. 2005;14:1651–1668. doi: 10.1007/s11136-005-1743-0. [DOI] [PubMed] [Google Scholar]
- 46.Willett WC, Green A, Stampfer MJ, et al. Relative and absolute excess risks of coronary heart disease among women who smoke cigarettes. N Engl J Med. 1987;317:1303–1309. doi: 10.1056/NEJM198711193172102. [DOI] [PubMed] [Google Scholar]
- 47.Heron MP, Smith BL. Deaths: leading causes for 2003. Natl Vital Stat Rep. 2007;55:1–92. [PubMed] [Google Scholar]
- 48.Goff DC, Jr, Bertoni AG, Kramer H, et al. Dyslipidemia prevalence, treatment, and control in the Multi-Ethnic Study of Atherosclerosis (MESA): gender, ethnicity, and coronary artery calcium. Circulation. 2006;113:647–656. doi: 10.1161/CIRCULATIONAHA.105.552737. [DOI] [PubMed] [Google Scholar]
- 49.Barnhart J, Lewis V, Houghton JL, et al. Physician knowledge levels and barriers to coronary risk prevention in women:survey results from the Women and Heart Disease Physician Education Initiative. Womens Health Issues. 2007;17:93–100. doi: 10.1016/j.whi.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 50.Bohadana A, Nilsson F, Rasmussen T, et al. Gender differences in quit rates following smoking cessation with combination nicotine therapy: influence of baseline smoking behavior. Nicotine Tob Res. 2003;5:111–116. doi: 10.1080/1462220021000060482. [DOI] [PubMed] [Google Scholar]
- 51.Perkins KA. Smoking cessation in women: special considerations. CNS Drugs. 2001;15:391–411. doi: 10.2165/00023210-200115050-00005. [DOI] [PubMed] [Google Scholar]