Abstract
Hypothalamic growth hormone (GH)-releasing hormone (GHRH) regulates the release of GH from the pituitary gland. The receptors for GHRH (GHRH-R) are expressed predominantly in the pituitary. Recent evidence demonstrates that splice variants of the GHRH receptor are also expressed in several nonpituitary tissues, both normal and tumoral, as well as in cancer cell lines. The aim of this study was to investigate the expression of the splice variant 1 (SV-1) of GHRH-R in colorectal cancer (CRC). Seventy patients who underwent partial colectomy for CRC were enrolled in the study. Immunohistochemical expression of SV-1 was studied in paraffin-embedded sections of patient tumor tissue. A cytoplasmic supranuclear expression of SV-1 was observed in CRC as well as in the normal colon mucosa. Tumor grade and pathological stage were negatively correlated with expression of SV-1 (P = 0.012 and P = 0.013, respectively). CRCs metastatic to the liver showed a lower expression of SV-1 than did primary tumors, but this difference was not statistically significant. Kaplan–Meier and Cox univariate survival analyses indicated an improved survival time in patients with high SV-1 compared with those with low GHRH-R expression, but this difference was not statistically significant. The immunohistochemical expression of SV-1 seems to be a favorable prognostic factor in CRC.
INTRODUCTION
Colorectal cancer (CRC) is globally the third most common malignancy among adults, and the fourth leading cause of cancer-related deaths after lung cancer (1,2). Recent studies indicate the involvement of growth factors in the tumorigenesis of various human tumors, including breast, uterine, endometrium, ovary, prostate, and lung as well as gastroen-teropancreatic cancers (2–4).
Growth hormone (GH)-releasing hormone (GHRH) is a 40–44 amino acid hypothalamic peptide that regulates the secretion of GH from the anterior pituitary gland (5). In addition to having neuroendocrine action, GHRH acts directly on a variety of peripheral tissues and tumors and induces cell proliferation (6). These effects of GHRH and its antagonists are mediated in many tumors by a splice variant of GHRH-receptor (GHRH-R) designated SV-1. SV-1 differs from the pituitary GHRH-R in a small portion of its amino-terminal region. Many antagonistic analogs of GHRH have been synthesized (2,5,7,8). These analogs inhibit tumor growth by blocking GH and hepatic secretion of insulinlike growth factor 1 (IGF-1), as well as by suppressing the synthesis of tumoral autocrine/paracrine IGF-1 and/or IGF-II (8–11), and by blocking the effects of autocrine GHRH on tumors.
The aim of the present work was to study the immunohistochemical expression of SV-1 in surgical specimens of human CRCs and investigate the prognostic significance of this expression.
MATERIAL AND METHODS
Patients and Material
Included in the study were 70 patients, 39 males and 31 females, median age 71 (42–91) years, who underwent partial colectomy for CRC of median size 5 cm (range 0.9–14 cm). Tumors were graded according to the WHO grading system; 6 of 70 (8.5%) of the CRC tumors were well differentiated, 44 of 70 (63%) were moderately differentiated, and 20 of 70 (28.5%) were low differentiated adenocarcinomas. Of the 70 CRCs, 10 (14%) were confined to the mucosa and the submucosa, 6 (9%) had invaded the muscle wall without penetrating it, and 54 (77%) had infiltrated the pericolic fat. Regional lymph-node metastases were observed in 37 of 70 (53%) cases. Liver metastasis was reported in 10 of 68 patients (15%); the data on liver metastasis were insufficient for 2 patients. The median follow-up period, available for 45 patients, was 53 months (range, 1–64 months). Death occurred in 22 of 45 patients (49%). The median overall survival was 23 months (range 1–58 months) (Table 1, Figures 1–5).
Table 1.
Distribution of SV-1 and other numerical clinicopathological variables of the study.
| Median quartile value
|
||||||
|---|---|---|---|---|---|---|
| Variable | n | Mean ± SE | Range | 25th | 50th | 75th |
| SV-1 | 125.4 ± 11.9 | 0–299 | 5.0 | 122.5 | 221.2 | |
| Patient age, years | 70 | 69.4 ± 1.3 | 42–91 | 62.0 | 71.0 | 77.0 |
| Tumor size, cm | 70 | 5.21 ± 0.26 | 0.90–14.00 | 3.50 | 5.00 | 6.25 |
| Follow-up, months | 45 | 26.7 ± 3.5 | 1.0–58.0 | 12.0 | 23.0 | 41.0 |
| Overall survival, months | 45 | 42.2 ± 2.7 | 1.0–64.0 | 23.0 | 53.0 | 57.0 |
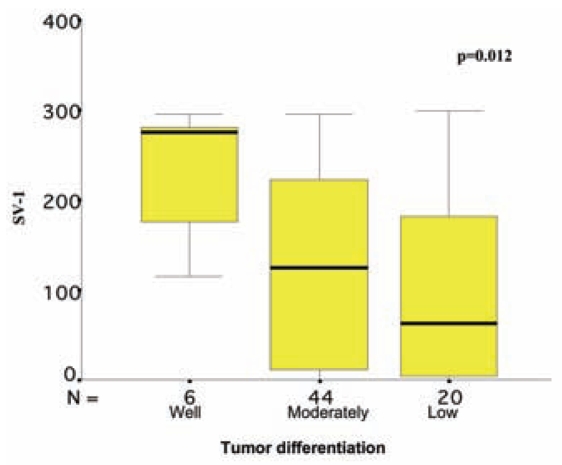
Figure 1.
Correlation between SV-1 immunohistochemical expression and colorectal cancer differentiation. The box plot lines display the 5th, 25th, 50th, 75th, and 95th percentiles. The bold lines represent the median value (50th percentile) for each patient’s cohort. P value was calculated with the Kruskal–Wallis test.
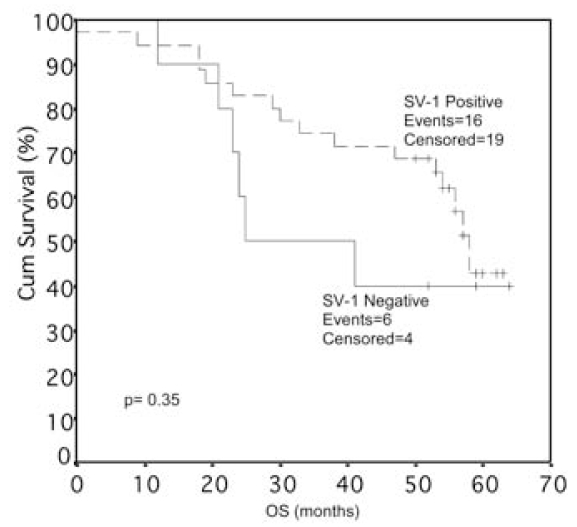
Figure 5.
Correlation between SV-1 immunohistochemical expression in colorectal cancer and overall survival (OS) of the patients. Cum, Cumulative.
Immunohistochemical Methods
A rabbit antiserum to SV-1 batch number JH 2317/5 raised in the laboratory of AV Schally’s group was used (12). The immunohistochemical staining was performed on 3-μm–thick paraffin sections of tissues fixed in buffered formalin. Staining procedures included de-paraffinization in warm xylene for 5 min with two changes of xylene at room temperature, followed by rehydration by transfer through graded alcohols and then rinsing with distilled water. The Trilogy antigen retrieval system (Cell Marque, Rocklin, CA, USA) was used for 1 h to expose the antigen epitopes. After being kept for 20 min at room temperature and then rinsed with distilled water, the sections were placed in 3% H202 for 10 min in darkness. Then the sections were washed with tap water, dipped twice for 5 min in Tris buffer saline (TBS), incubated with the SV-1 polyclonal antibody (1:3000) for 30 min, rinsed with TBS for 10 min, and then incubated with the Envision detection system peroxidase/DAB+, Rabbit/ Mouse (Dako Cytomation, Glostrup, Denmark) for 30 min. This procedure was followed by rinsing with TBS for 10 min, incubation in diaminobenzidine solution for 10 min at room temperature, and rinsing with tap water. The sections were then counterstained with hematoxylin, dehydrated, cleared in xylene, and mounted. We performed negative controls by omitting the primary antibody or by replacing the antibody with nonimmune serum. With both control preparations no immunohistochemical staining occurred, suggesting the specificity of our immunohistochemical staining.
Evaluation of Immunohistochemical Staining
Cytoplasmic, mainly supranuclear SV-1 immunohistochemical expression dominated the absorptive cells of normal colon mucosa. Goblet cells also showed a cytoplasmic, mainly supranuclear staining, whereas most mucin droplets remained unstained. The immunostaining in CRCs was also cytoplasmic, and was evaluated as grade 1, weak intensity with sparse distribution; grade 2, moderate intensity with dense distribution or strong intensity with sparse distribution; and grade 3, strong intensity with dense distribution. The percentage of each expression was measured in every tumor section and multiplied by 1, 2, and 3, respectively. A final SV-1 immunoexpression score between 0 and 300 for each case was obtained from the sum of the three products.
Statistical Analysis
Because the distribution of SV-1 expression levels in the CRC patients was not Gaussian, analysis of the differences in the two or three groups of patients was performed with the nonparametric Mann–Whitney U test or the Kruskal–Wallis test, respectively. We assessed the relationships between different variables by using the Spearman correlation coefficient. The X-tile algorithm was used to generate an optimal cutpoint for SV-1, because it has no established cutpoints regarding its expression in CRC. After we corrected for the use of minimum P-value statistics, the X-tile software yielded an optimal cutoff, equal to the 25th percentile, with a calculated Monte Carlo P value < 0.05. We also performed survival analyses by constructing Kaplan–Meier curves. Differences between curves were evaluated by use of the log-rank test.
RESULTS
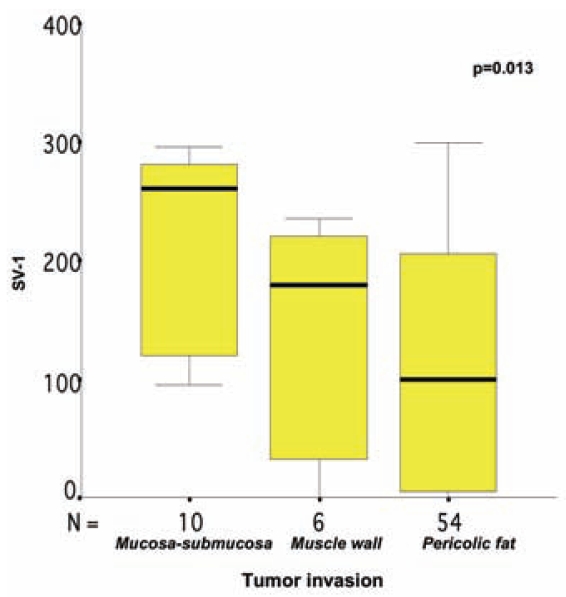
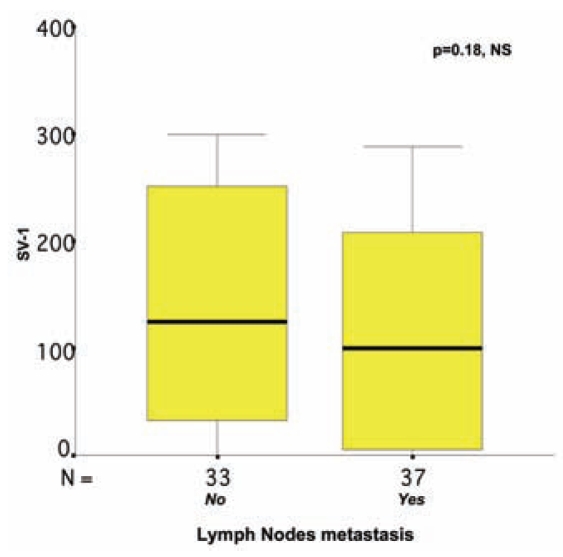
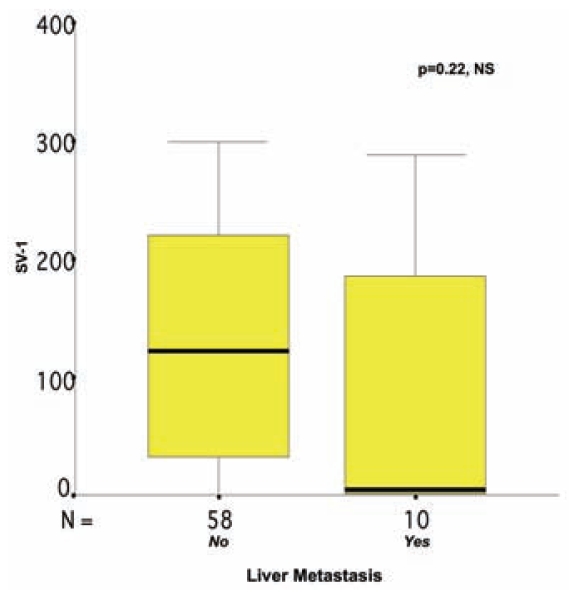
Usually, more than one SV-1 immunohistochemical expression grade was observed in each tumor section studied, and a score between 0 and 300 was obtained for each case (Figures 6–8). The SV-1 expression was correlated with tumor differentiation, tumor invasiveness, lymph node metastasis, liver metastasis, and patient survival. A higher median immunohistochemical expression was observed in well-differentiated CRCs than in low-differentiated ones. The median immunohistochemical expression of moderately differentiated tumors lay between that of the other two groups. The differences among the three groups were statistically significant (P = 0.012) (Figure 1). CRCs confined to the mucosa and submucosa showed a statistically significant higher median SV-1 expression than those invading the muscle coat. Tumors with pericolic fat invasion showed the lowest median SV-1 expression (P = 0.013) (Figure 2). Lymph node metastasis did not show a correlation with SV-1 expression (Figure 3). Liver metastasis was clearly associated with lower SV-1, but this difference was not statistically significant (Figure 4). Kaplan–Meier and Cox univariate survival analyses showed that patients with high SV-1 expression had higher survival rates than those with low GHRH-R expression, but the difference was not statistically significant (Figure 5). Our results suggest that if more patients were enrolled in the study, the differences regarding liver metastasis and survival would be significant. It is important to clarify that most of the CRC patients who underwent surgery in our hospital were referred to us from rural areas of Greece. Follow-up of these patients was performed by various primary care services locally. Consequently, we have no precise information regarding follow-up and time-to-progression for most of our patients, with the exception of time-to-death (final outcome) documented by death certificates. Thus, although our results suggest that GHRH-R expression is a favorable prognostic factor in CRC, we were unable to analyze the association of progression-free survival with SV-1 immunohistochemical expression.
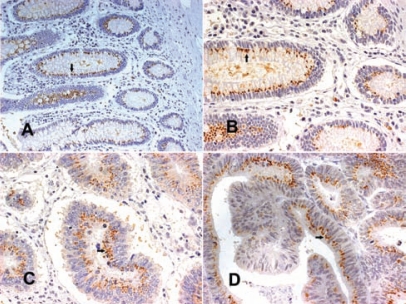
Figure 6.
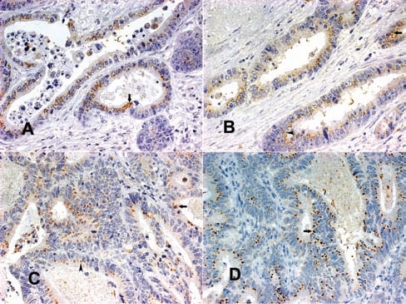
(A, B) Normal colon mucosa with supranuclear SV-1 immunohistochemical expression (arrows); (C, D) well-differentiated colorectal cancers with extended grade 3 SV-1 immunohistochemical expression (arrows) (all magnifications 400×, except A 200×) 118 × 88 mm (300 × 300 DPI).
Figure 8.
(A) Moderately differentiated colorectal cancer with an area with grade 3 SV-1 immunohistochemical expression (arrow) and an area with grade 2 SV-1 immunohistochemical expression (arrowhead); (B) moderately differentiated colorectal cancer with an area with grade 2 SV-1 immunohistochemical expression (arrow) and an area with grade 1 SV-1 immunohistochemical expression (arrowhead); (C) normal colon mucosa with supranuclear SV-1 immunohistochemical expression (arrow) and adjacent low-differentiated colorectal cancer with no SV-1 immunohistochemical expression (arrowhead); (D) low-differentiated colorectal cancer with pericolic fat invasion and with small foci with grade 2 (arrow) and grade 1 (arrowhead) SV-1 immunohistochemical expression (All magnifications 400×, except C 100×] 118 × 88 mm (300 × 300 DPI).
Figure 2.
Correlation between SV-1 immunohistochemical expression and colorectal cancer invasiveness. The box plot lines display the 5th, 25th, 50th, 75th, and 95th percentiles. The bold lines represent the median value (50th percentile) for each patient’s cohort. P value was calculated with the Kruskal–Wallis test.
Figure 3.
Correlation between SV-1 immunohistochemical expression and lymph nodes metastasis in colorectal cancer. The box plot lines display the 5th, 25th, 50th, 75th, and 95th percentiles. The bold lines represent the median value (50th percentile) for each patient’s cohort. P value was calculated with the Mann–Whitney U test. NS, nonsignificant.
Figure 4.
Correlation between SV-1 immunohistochemical expression and liver metastasis in colorectal cancer. The box plot lines display the 5th, 25th, 50th, 75th, and 95th percentiles. The bold lines represent the median value (50th percentile) for each patient’s cohort. P value was calculated by the Mann–Whitney U test. NS, nonsignificant.
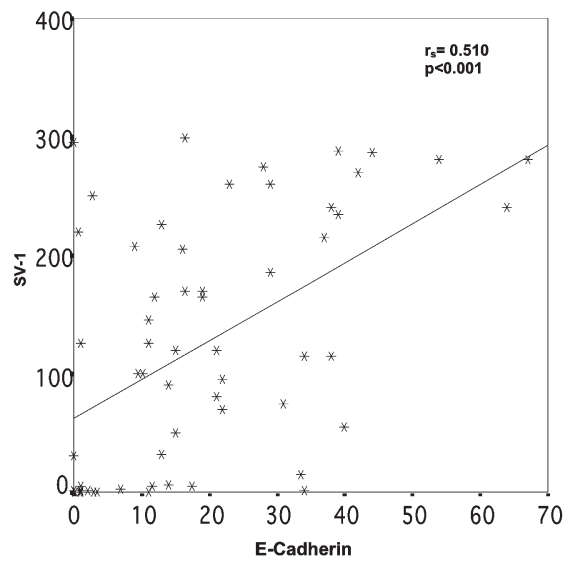
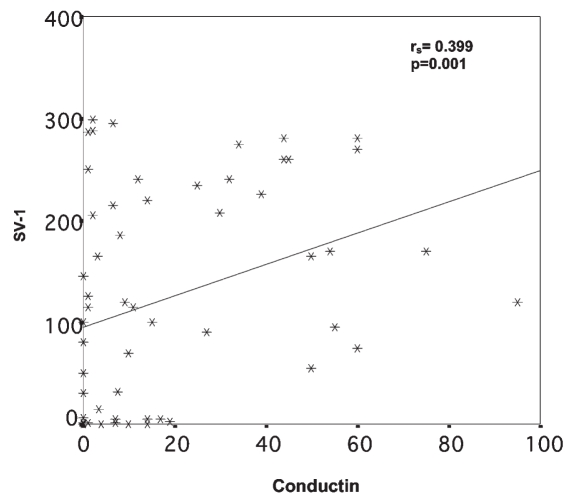
Comparison of GHRH-R (SV-1) immunohistochemical expression with the expression of the cell proliferation marker Ki-67, the apoptosis index (Tunnel), E-cadherin, β-catenin, conductin, clusterin, and survivin showed positive correlations only between SV-1 and E-cadherin (P < 0.001) and SV-1 and conductin (P = 0.001) (Figures 9 and 10).
Figure 9.
Relation between SV-1 and E-cadherin immunohistochemical expression. rs = Spearman correlation coefficient.
Figure 10.
Relation between SV-1 and conductin immunohistochemical expression. rs = Spearman correlation coefficient.
DISCUSSION
The only reliable method for curing colorectal adenocarcinomas is surgical resection. Unfortunately, one-third of patients who undergo a surgical resection with curative intent develop recurrent disease, and many CRCs are detected at a late stage when surgery cannot cure the disease. Chemotherapy alone and chemotherapy combined with radiotherapy are not highly effective against disseminated disease (2). Disease progression in patients with CRC may be associated with the presence of a locally advanced disease and with micrometastasis or macrometastasis in various organs, which are critical events for the prognosis of CRC patients (13). Therefore, new treatment modalities are needed.
Various findings support the involvement of IGF-I and IGF-II in the growth of CRC. The incidence of colon cancer is increased in persons with acromegaly, suggesting that excessive secretion of GH or IGF-I may be a contributing factor in this disease (8). GHRH antagonists can inhibit tumor growth indirectly through suppression of the endocrine GH–IGF-I axis and also by direct action on the tumor cells expressing SV-1 (7,14–19). The indirect mechanism is important for those cancers that depend on IGF-I as a growth factor. GHRH antagonists inhibit the release of GH from the pituitary, leading to suppression of hepatic IGF-I production and a decrease in levels of IGF-I in serum (2,7,11). SV-1 may inhibit the synthesis of tumoral autocrine/paracrine IGF-1 and/or IGF-II (7–11). GHRH antagonists also inhibit the autocrine production of IGF-I and/or IGF-II in many cancers, apparently through a direct action on the malignant cells (7). IGF-II, like IGF-I, is a potent mitogen for many cancers, and the suppression of its production would inhibit tumor growth. Unlike IGF-I, the synthesis of IGF-II does not depend on serum GH levels. In addition, IGF-I bioavailability depends on the expression of urokinase-type plasminogen activator and IGF-binding proteins in cancer cells and surrounding tissues (14,20–29). Furthermore, GHRH antagonists can inhibit proliferation by blocking the action of autocrine/paracrine GHRH by a direct effect that may not involve IGFs, a finding that suggests a promising approach to treat many tumors, including metastatic CRC (4,7).
In our investigation of SV-1 expression in human CRC, we observed cytoplasmic supranuclear expression in CRC tissue as well as in the normal colon mucosa. Because SV1 acts as a membrane receptor, in a manner analogous to that of GHRH receptor, our findings may be attributable to SV1 activation by other peptides that share structural similarities with GHRH, such as the vasoactive intestinal peptide and pituitary adenylcyclase activating polypeptide. It is noteworthy that cytoplasmic immunohistochemical expression has also been observed in other neoplasms (18,19). We were able to demonstrate that SV-1 was present in most of our cases. Among the 70 CRC patients examined, 63 had tissue samples that were positive for SV-1 immunoreactivity, and only 7 had samples that were not positive for SV-1. Well-differentiated CRCs and those confined to the mucosa and submucosa had higher immunohistochemical expression. Low-differentiated tumors and those with pericolic fat invasion showed the lowest immunohistochemical expression (P = 0.012 and P = 0.013, respectively). We observed obviously lower SV-1 expression in CRCs with liver metastasis than in nonmetastatic tumors, although these results were not statistically significant. Furthermore, higher SV-1 expression was associated with better survival, and it is evident that the inclusion of more patients in the study would lead to statistically significant results. According to our results, SV-1 expression seems to be a favorable prognostic factor in CRC.
Chatzistamou et al. demonstrated the immunohistochemical expression of GHRH and SV-1 in breast cancer patients, providing evidence that an autocrine stimulatory loop may operate between GHRH and SV-1 in primary cancers (18). In a recent study of melanomas, Chatzistamou et al. found increased SV-1 expression in melanoma compared with dysplastic nevi, demonstrating the involvement of SV-1 in melanoma pathogenesis (19). Busto et al. who studied mRNA, reported the presence of an autocrine/paracrine stimulatory loop based on GHRH and SV-1 receptors in human pancreatic, colorectal, and gastric cancers. These authors also suggested that the finding of SV-1 in human cancers provides an approach to an antitumor therapy based on the blockade of this receptor by specific GHRH antagonists (4).
In our study, the positive correlation of SV-1 and E-cadherin, as well as conductin expression (P < 0.001 and P = 0.001 respectively) is in accordance with results of studies in cancer cell lines showing that changes in the structure or in the expression of the components of the E-cadherin/catenin complex result in the suppression of cell–cell adhesion and in the expression of an invasive phenotype (30–32). The majority of colon tumors develop because of tumor-suppressor gene APC mutations that lead to Wnt/β-catenin signaling activation and subsequent transcription of target genes, including conductin/Axin2 (33). Our study provides the first evidence that SV-1 is immunoexpressed in CRC.
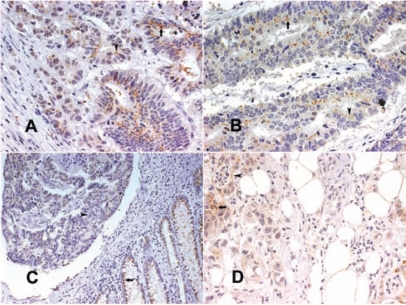
Figure 7.
(A) Well-differentiated colorectal cancer with an area with grade 3 SV-1 immunohistochemical expression (arrow); (B) well-differentiated colorectal cancer with an area with grade 3 SV-1 immunohistochemical expression (arrow) and an area with grade 2 GHRH-R immunohistochemical expression (arrowhead); (C) moderately differentiated colorectal cancer with an area with grade 2 SV-1 immunohistochemical expression (arrow) and an area with grade 1 GHRH-R immunohistochemical expression (arrowhead); (D) moderately differentiated colorectal cancer with extended grade 2 SV-1 immunohistochemical expression (arrow) (all magnifications 400×] 117 × 88 mm (300 × 300 DPI).
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
We declare that the authors have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Levin B, Brooks D, Smith RA, Stone A. Emerging technologies in screening for colorectal cancer: CT colonography, immunochemical fecal occult blood tests, and stool screening using molecular markers. CA Cancer J Clin. 2003;53:44–55. doi: 10.3322/canjclin.53.1.44. [DOI] [PubMed] [Google Scholar]
- 2.Schally AV, Szepeshazia K, Nagya A, Comaru-Schally AM, Halmosa G. New approaches to therapy of cancers of the stomach, colon and pancreas based on peptide analogs. Cell Mol Life Sci. 2004;61:1042–68. doi: 10.1007/s00018-004-3434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chatzistamou I, Schally AV, Pafiti A, Kiaris H, Koutselini H. Expression of growth hormone-releasing hormone in human primary endometrial carcinomas. Eur J Endocrinol. 2002;147:381–6. doi: 10.1530/eje.0.1470381. [DOI] [PubMed] [Google Scholar]
- 4.Busto R, et al. The expression of growth hormone-releasing hormone (GHRH) and splice variants of its receptor in human gastroen-teropancreatic carcinomas. Proc Natl Acad Sci U S A. 2002;99:11866–71. doi: 10.1073/pnas.182433099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varga JL, et al. Increased activity of antagonists of growth hormone-releasing hormone substituted at positions 8, 9, and 10. Proc Natl Acad Sci U S A. 2004;101:1708–13. doi: 10.1073/pnas.0307288101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiaris H, Koutsilieris M, Kalofoutis A, Schally AV. Growth hormone-releasing hormone and extra-pituitary tumorigenesis: therapeutic and diagnostic applications of growth hormone-releasing hormone antagonists. Expert Opin Investig Drugs. 2003;12:1385–94. doi: 10.1517/13543784.12.8.1385. [DOI] [PubMed] [Google Scholar]
- 7.Schally AV, Varga JL, Engel JB. Antagonists of growth-hormone-releasing hormone: an emerging new therapy for cancer. Nat Clin Pract Endocrinol Metab. 2008;4:33–43. doi: 10.1038/ncpendmet0677. [DOI] [PubMed] [Google Scholar]
- 8.Schally AV, et al. Hypothalamic hormones and cancer. Front Neuroendocrinol. 2001;22:248–91. doi: 10.1006/frne.2001.0217. [DOI] [PubMed] [Google Scholar]
- 9.Christodoulou C, et al. Expression of growth hormone-releasing hormone (GHRH) and splice variant of GHRH receptors in normal mouse tissues. Regul Pept. 2006;136:105–8. doi: 10.1016/j.regpep.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Kanashiro CA, et al. Inhibition of mutant p53 expression and growth of DMS-153 small cell lung carcinoma by antagonists of growth hormone-releasing hormone and bombesin. Proc Natl Acad Sci U S A. 2003;100:15836–41. doi: 10.1073/pnas.2536558100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khandwala HM, McCutcheon IE, Flyvbjerg A, Friend KE. The effects of insulin-like growth factors on tumorigenesis and neoplastic growth. Endocr Rev. 2000;21:215–44. doi: 10.1210/edrv.21.3.0399. [DOI] [PubMed] [Google Scholar]
- 12.Toller GL, et al. Development of a polyclonal antiserum for the detection of the isoforms of the receptors for human growth hormone-releasing hormone on tumors. Proc Natl Acad Sci U S A. 2004;101:15160–5. doi: 10.1073/pnas.0406348101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsouma A, et al. Circulating tumor cells in colorectal cancer: detection methods and clinical significance. Anticancer Res. 2008;28:3945–60. [PubMed] [Google Scholar]
- 14.Kiaris H, et al. Ligand-dependent and -independent effects of GHRH receptor splice variant 1. Proc Natl Acad Sci U S A. 2003;100:9512–7. doi: 10.1073/pnas.1533185100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaylinn BD. Growth hormone releasing hormone receptor. Receptors Channels. 2002;8:155–62. [PubMed] [Google Scholar]
- 16.Plonowski A, et al. Expression of growth hormone-releasing hormone (GHRH) and splice variants of GHRH receptors in human experimental prostate cancers. Peptides. 2002;23:1127–33. doi: 10.1016/s0196-9781(02)00043-8. [DOI] [PubMed] [Google Scholar]
- 17.Csernus VJ, Schally AV, Kiaris H, Armatis P. Inhibition of growth, production of insulin-like growth factor-II (IGF-II), and expression of IGF-II mRNA of human cancer cell lines by antagonistic analogs of growth hormone-releasing hormone in vitro. Proc Natl Acad Sci U S A. 1999;96:3098–103. doi: 10.1073/pnas.96.6.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chatzistamou I, et al. Immunohistochemical detection of GHRH and its receptor splice variant 1 in primary human breast cancers. Eur J Endocrinol. 2004;151:391–6. doi: 10.1530/eje.0.1510391. [DOI] [PubMed] [Google Scholar]
- 19.Chatzistamou I, Volakaki AA, Schally AV, Kiaris H, Kittas C. Expression of growth hormone-releasing hormone receptor splice variant 1 in primary human melanomas. Regul Pept. 2008;147:33–6. doi: 10.1016/j.regpep.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Koutsilieris M, et al. Urokinase-type plasminogen activator: a paracrine factor regulating the bioavailability of IGFs in PA-III cell-induced osteoblastic metastases. Anticancer Res. 1993;13:481–6. [PubMed] [Google Scholar]
- 21.Polychronakos C, Janthly U, Lehoux JG, Koutsilieris M. Mitogenic effects of insulin and insulin-like growth factors on PA-III rat prostate adenocarcinoma cells: characterization of the receptors involved. Prostate. 1991;19:313–21. doi: 10.1002/pros.2990190405. [DOI] [PubMed] [Google Scholar]
- 22.Koutsilieris M, et al. The molecular concept of prostate cancer. Cancer J. 1996;9(2):89–94. [Google Scholar]
- 23.Koutsilieris M, et al. A combination therapy of dexamethasone and somatostatin analog reintroduces objective clinical responses to LHRH analog in androgen ablation-refractory prostate cancer patients. J Clin Endocrinol Metab. 2001;86:5729–36. doi: 10.1210/jcem.86.12.8119. [DOI] [PubMed] [Google Scholar]
- 24.Koutsilieris M. Pathophysiology of uterine leiomyomas. Biochem Cell Biol. 1992;70:273–8. doi: 10.1139/o92-043. [DOI] [PubMed] [Google Scholar]
- 25.Koutsilieris M, Sourla A, Pelletier G, Doillon CJ. Three-dimensional type I collagen gel system for the study of osteoblastic metastases produced by metastatic prostate cancer. J Bone Miner Res. 1994;9:1823–32. doi: 10.1002/jbmr.5650091120. [DOI] [PubMed] [Google Scholar]
- 26.Mitsiades CS, Koutsilieris M. Molecular biology and cellular physiology of refractoriness to androgen ablation therapy in advanced prostate cancer. Expert Opin Investig Drugs. 2001;10:1099–115. doi: 10.1517/13543784.10.6.1099. [DOI] [PubMed] [Google Scholar]
- 27.Janvier R, Sourla A, Koutsilieris M, Doillon CJ. Stromal fibroblasts are required for PC-3 human prostate cancer cells to produce capillary-like formation of endothelial cells in a three-dimensional co-culture system. Anticancer Res. 1997;17:1551–7. [PubMed] [Google Scholar]
- 28.Koutsilieris M, Allaire-Michaud L, Fortier M, Lemay A. Mitogen(s) for endometrial-like cells can be detected in human peritoneal fluid. Fertil Steril. 1991;56:888–93. doi: 10.1016/s0015-0282(16)54660-3. [DOI] [PubMed] [Google Scholar]
- 29.Tsigkos S, Koutsilieris M, Papapetropoulos A. Angiopoietins in angiogenesis and beyond. Expert Opin Investig Drugs. 2003;12:933–41. doi: 10.1517/13543784.12.6.933. [DOI] [PubMed] [Google Scholar]
- 30.Truant SC, et al. E-Cadherin and β-catenin mRNA levels throughout colon cancer progression. J Surg Res. 2008;150:212–8. doi: 10.1016/j.jss.2007.12.800. [DOI] [PubMed] [Google Scholar]
- 31.Gavert N, Ben-Ze’ev A. β-Catenin signaling in biological control and cancer. J Cell Biochem. 2007;102:820–8. doi: 10.1002/jcb.21505. [DOI] [PubMed] [Google Scholar]
- 32.Onder TT, et al. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68:3645–54. doi: 10.1158/0008-5472.CAN-07-2938. [DOI] [PubMed] [Google Scholar]
- 33.Hadjihannas MV, et al. Aberrant Wnt/β-catenin signaling can induce chromosomal instability in colon cancer. Proc Natl Acad Sci U S A. 2006;103:10747–52. doi: 10.1073/pnas.0604206103. [DOI] [PMC free article] [PubMed] [Google Scholar]