Abstract
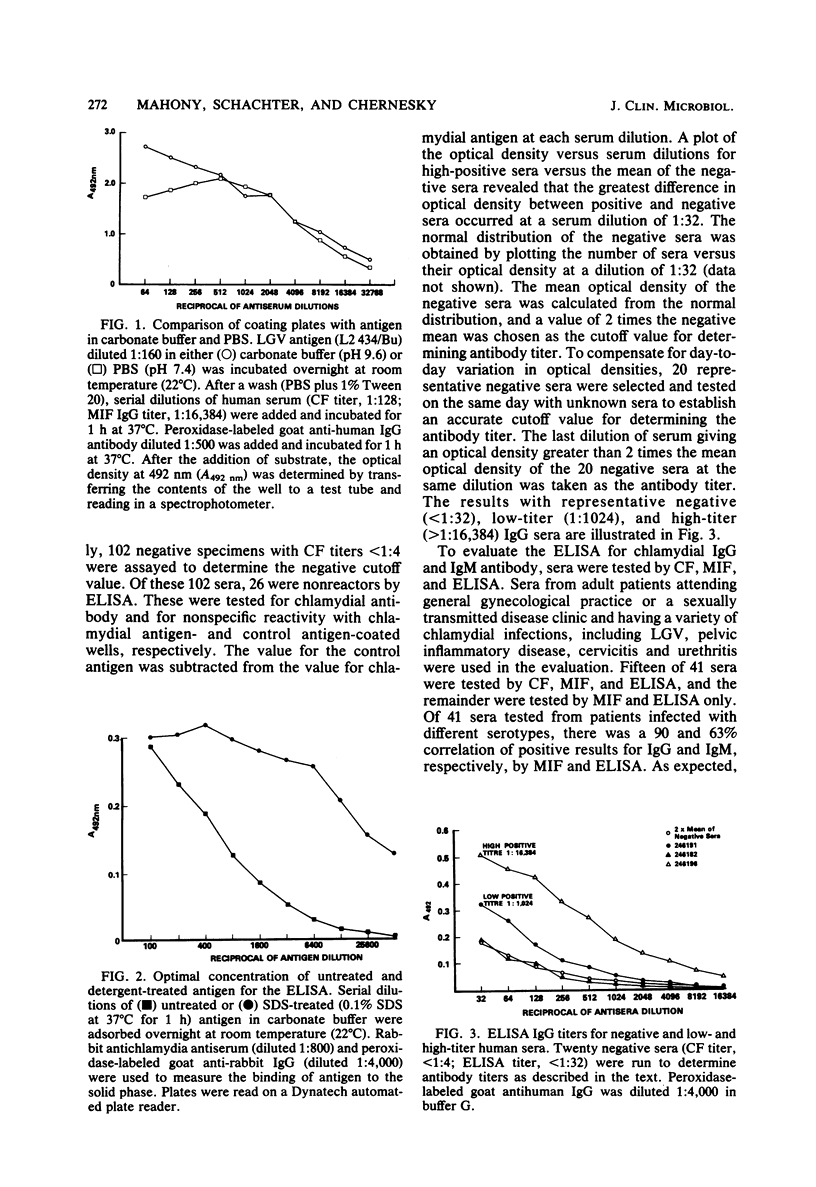
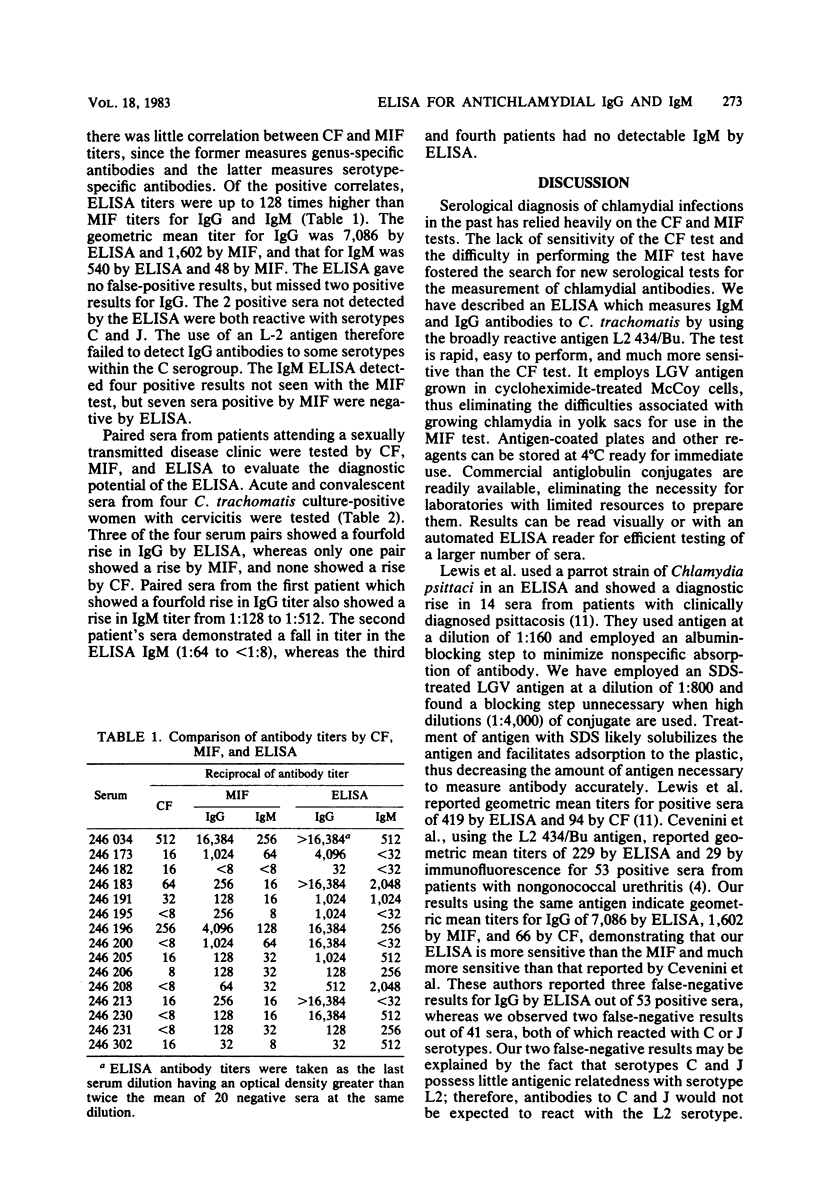
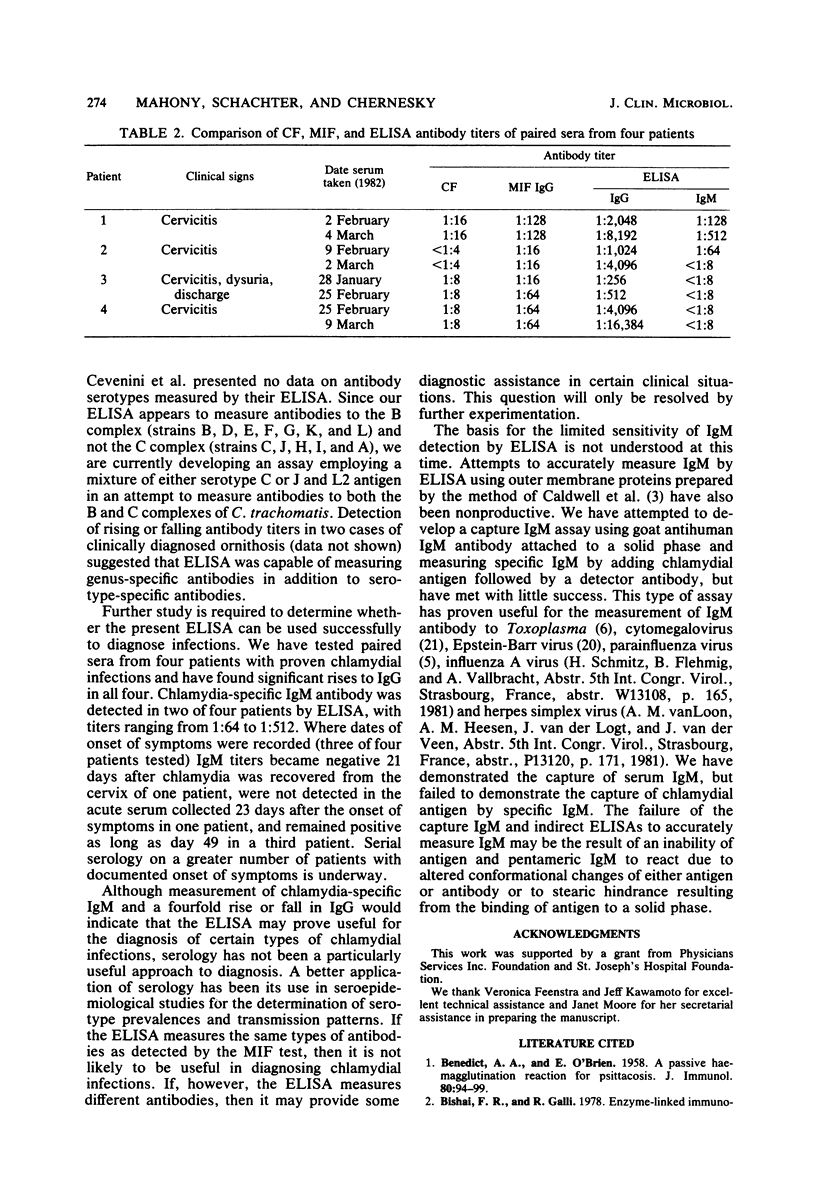
Chlamydia trachomatis causes a wide range of infections in adults and conjunctivitis and pneumonia in neonates. The complement fixation test for chlamydial antibody is broadly reactive, but possesses low sensitivity, whereas the microimmunofluorescence test is highly sensitive, but technically difficult to perform. A simple, rapid enzyme-linked immunosorbent assay (ELISA) has been developed for the measurement of immunoglobulin G (IgG) and IgM antibodies to C. trachomatis. Wells of microtiter plates were coated with Renografin-purified elementary bodies (serotype L2) grown in cycloheximide-treated McCoy cells, and serum antibody was detected with peroxidase-labeled goat antihuman IgG and IgM antibody. Of 41 sera tested from patients with lymphogranuloma venereum, pelvic inflammatory disease, cervicitis, or urethritis there was a 90 and 63% correlation of positive results for IgG and IgM, respectively, by microimmunofluorescence and ELISA. Of the positive correlates, ELISA titers were up to 128 times higher than microimmunofluorescence titers for IgG and IgM. The ELISA detected no false-positive results, but missed two positive results for IgG. Both of these sera were reactive against serotypes C and J, suggesting that the ELISA with LGV L2 antigen may not measure antibodies to serotypes within the C serogroup. The IgM ELISA detected 7 negative and 4 positive results not detected by the microimmunofluorescence test. Of four paired sera examined by ELISA, three showed a fourfold rise in IgG antibody titer, and one showed a twofold rise. Further evaluation of this ELISA will be required to determine how useful it will be in seroepidemiological studies and as a diagnostic tool.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENEDICT A. A., O'BRIEN E. A passive hemagglutination reaction for psittacosis. J Immunol. 1958 Feb;80(2):94–99. [PubMed] [Google Scholar]
- Bishai F. R., Galli R. Enzyme-linked immunosorbent assay for detection of antibodies to influenza A and B and parainfluenza type 1 in sera of patients. J Clin Microbiol. 1978 Dec;8(6):648–656. doi: 10.1128/jcm.8.6.648-656.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Kromhout J., Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981 Mar;31(3):1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertl H. C., Gerlich W., Koszinowski U. H. Detection of antibodies to Sendai virus by enzyme-linked immunosorbent assay (ELISA). J Immunol Methods. 1979;28(1-2):163–176. doi: 10.1016/0022-1759(79)90338-7. [DOI] [PubMed] [Google Scholar]
- Franco E. L., Walls K. W., Sulzer A. J. Reverse enzyme immunoassay for detection of specific anti-Toxoplasma immunoglobulin M antibodies. J Clin Microbiol. 1981 May;13(5):859–864. doi: 10.1128/jcm.13.5.859-864.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon F. B., Harper I. A., Quan A. L., Treharne J. D., Dwyer R. S., Garland J. A. Detection of Chlamydia (Bedsonia) in certain infections of man. I. Laboratory procedures: comparison of yolk sac and cell culture for detection and isolation. J Infect Dis. 1969 Oct;120(4):451–462. doi: 10.1093/infdis/120.4.451. [DOI] [PubMed] [Google Scholar]
- HAHON N., COOKE K. O. FLUORESCENT CELL-COUNTING NEUTRALIZATION TEST FOR PSITTACOSIS. J Bacteriol. 1965 Jun;89:1465–1471. doi: 10.1128/jb.89.6.1465-1471.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewis V. J., Thacker W. L., Engelman H. M. Indirect hemagglutination test for chlamydial antibodies. Appl Microbiol. 1972 Jul;24(1):22–25. doi: 10.1128/am.24.1.22-25.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis V. J., Thacker W. L., Mitchell S. H. Enzyme-linked immunosorbent assay for chlamydial antibodies. J Clin Microbiol. 1977 Nov;6(5):507–510. doi: 10.1128/jcm.6.5.507-510.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårdh P. A. An overview of infectious agents of salpingitis, their biology, and recent advances in methods of detection. Am J Obstet Gynecol. 1980 Dec 1;138(7 Pt 2):933–951. doi: 10.1016/0002-9378(80)91084-4. [DOI] [PubMed] [Google Scholar]
- Quinn T. C., Goodell S. E., Mkrtichian E., Schuffler M. D., Wang S. P., Stamm W. E., Holmes K. K. Chlamydia trachomatis proctitis. N Engl J Med. 1981 Jul 23;305(4):195–200. doi: 10.1056/NEJM198107233050404. [DOI] [PubMed] [Google Scholar]
- Schachter J. Chlamydial infections (third of three parts). N Engl J Med. 1978 Mar 9;298(10):540–549. doi: 10.1056/NEJM197803092981005. [DOI] [PubMed] [Google Scholar]
- Schachter J., Grossman M., Holt J., Sweet R., Spector S. Infection with Chlamydia trachomatis: involvement of multiple anatomic sites in neonates. J Infect Dis. 1979 Feb;139(2):232–234. doi: 10.1093/infdis/139.2.232. [DOI] [PubMed] [Google Scholar]
- Schmitz H. Detection of immunoglobulin M antibody to Epstein-Barr virus by use of an enzyme-labeled antigen. J Clin Microbiol. 1982 Aug;16(2):361–366. doi: 10.1128/jcm.16.2.361-366.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz H., von Deimling U., Flehmig B. Detection of IgM antibodies to cytomegalovirus (CMV) using an enzyme-labelled antigen (ELA). J Gen Virol. 1980 Sep;50(1):59–68. doi: 10.1099/0022-1317-50-1-59. [DOI] [PubMed] [Google Scholar]
- Wang S. P., Grayston J. T., Alexander E. R., Holmes K. K. Simplified microimmunofluorescence test with trachoma-lymphogranuloma venereum (Chlamydia trachomatis) antigens for use as a screening test for antibody. J Clin Microbiol. 1975 Mar;1(3):250–255. doi: 10.1128/jcm.1.3.250-255.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


