Abstract
The galactolipids, mono- and digalactosyldiacylglycerol (DGDG), are the most common nonphosphorous lipids in the biosphere and account for 80% of the membrane lipids found in green plant tissues. These lipids are major constituents of photosynthetic membranes (thylakoids), and a large body of evidence suggests that galactolipids are associated primarily with plastid membranes in seed plants. A null-mutant of Arabidopsis (dgd1), which lacks the DGDG synthase (DGD1) resulting in a 90% reduction in the amount of DGDG under normal growth conditions, accumulated DGDG after phosphate deprivation up to 60% of the amount present in the wild type. This observation suggests the existence of a DGD1-independent pathway of galactolipid biosynthesis. The fatty acid composition of the newly formed DGDG was distinct, showing an enrichment of 16-carbon fatty acids in the C-1 position of the glycerol backbone of DGDG. Roots with their rudimentary plastids accumulated large amounts of DGDG after phosphate deprivation, suggesting that this galactolipid may be located in extraplastidic membranes. Corroborating evidence for this hypothesis was obtained directly by fractionation of subcellular membranes from leaf tissue and indirectly by lipid analysis of the phosphate-deprived fad3 mutant primarily deficient in extraplastidic fatty acid desaturation. The discovery of extraplastidic DGDG biosynthesis induced by phosphate deprivation has revealed a biochemical mechanism for plants to conserve phosphate. Apparently, plants replace phospholipids with nonphosphorous galactolipids if environmental conditions such as phosphate deprivation require this for survival.
One of the most powerful environmental stimuli affecting the overall glycerolipid composition of bacterial membranes is phosphate deprivation. The replacement of phospholipids by nonphosphorous lipids was discovered first in nonphotosynthetic bacteria (1) and later in those capable of photosynthesis (2, 3). Mutants of different photosynthetic bacteria lacking the acidic nonphosphorus sulfolipid sulfoquinovosyldiacylglycerol showed impaired growth only under phosphate-limited growth conditions (2, 4). This led to the hypothesis that sulfolipid can replace the acidic thylakoid phospholipid phosphatidylglycerol under these growth conditions (5). An inverse relationship between sulfolipid and phosphatidylglycerol as a function of phosphate availability was also observed for Arabidopsis (6). Experiments with the phosphate-deficient pho1 mutant of Arabidopsis (7) suggested that not only sulfolipid but also the amounts of digalactosyldiacylglycerol are increased after phosphate deprivation (8). Thus, it seemed reasonable to ask whether the substitution of phospholipids by nonphosphorous lipids is a more general phenomenon in plants.
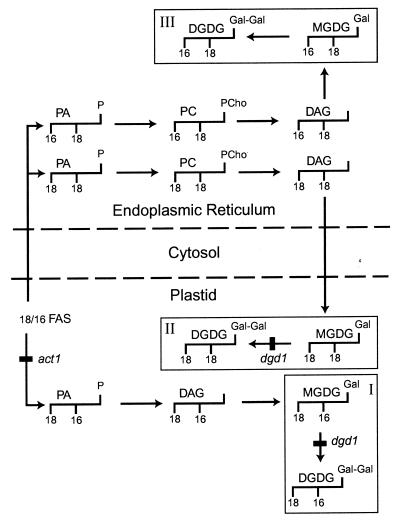
The nonphosphorous galactolipids mono- and digalactosyldiacylgycerol (MGDG and DGDG) constitute the bulk of membrane lipids in green tissues of seed plants where they are known to be located in the photosynthetic membranes (thylakoids) of the chloroplasts (9, 10). In many plants, including Arabidopsis, two pathways exist for the biosynthesis of these lipids, one involving only enzymes located in the plastid (Fig. 1 I), the other also requiring enzymes associated with the endoplasmic reticulum (ER) (Fig. 1 II). The plastid pathway gives rise to thylakoid lipids with 16-carbon fatty acids in the carbon-2 (C-2) position of the glycerol backbone, whereas lipids derived from the ER pathway contain 18-carbon fatty acids in this position (11, 12). Therefore, fatty acid analysis of thylakoid lipids provides a convenient tool to estimate the contribution of each pathway. In addition, the availability of mutants of Arabidopsis has proven very useful in the dissection of the different lipid biosynthetic pathways in plants. For example, the act1 mutant is deficient in the plastid glycerol-3-phosphate:acyl-ACP acyltransferase (Fig. 1), and as a consequence the plastidic pathway (Fig. 1 I) is impaired and 16-carbon fatty acid-containing lipids are decreased (13). The mgd1 mutant of Arabidopsis deficient in MGDG synthase activity revealed that MGD1 is responsible for the bulk of MGDG biosynthesis (14), whereas the dgd1 mutant phenotype suggested that the DGD1 gene product (Fig. 1) is responsible for over 90% of DGDG biosynthesis (15). Although the dgd1 mutant carries a stop codon in the center of the ORF and is presumably a null allele, residual DGDG is still present (16). We used this mutant as a starting point to search for a DGD1-independent pathway of galactolipid biosynthesis, which may be up-regulated after phosphate deprivation of the plant.
Figure 1.
Schematic representation of the three pathways of galactolipid biosynthesis discussed in the text. The glycerol backbones with the typical carbon length of fatty acids in the C-1 and C-2 positions are indicated to illustrate the main molecular species derived from each pathway. The head groups are always at the glycerol C-3 position. Three pools of galactolipids are indicated, representing the plastid-derived pool (I) in the plastid, the ER-derived pool in the plastid (II), and the ER-derived pool located in extraplastidic membranes (III). Pool III accumulates after phosphate deprivation. Reactions that are blocked in the act1 and dgd1 mutants are indicated. Broken lines separate plastid, cytosol, and ER. DAG, diacylglycerol; FAS, fatty acid synthase; Gal, galactose; PC, phosphatidylcholine; PCho, phosphocholine; PA, phosphatidic acid.
Materials and Methods
Plant Material.
Surface-sterilized seeds of Arabidopsis (A. thaliana, ecotype Col-2) and the multiple-times-backcrossed act1 (13), dgd1 (15), fad3 (17), and pho1 mutants (7), were germinated on 0.8% (wt/vol) agar-solidified MS medium (18) supplemented with 1% (wt/vol) sucrose. The seedlings were kept on agar for 10 days before transfer to pots containing a soil mixture, as described (15). Plants were grown in growth chambers (AR-75L, Percival Scientific, Boone, IA) under the light of a photosynthetic photon flux density (400–700 nm) of 75 μmol photons m−2⋅s−1. A 14-h light/10-h dark regime was applied. The day/night temperature was controlled at 23/18°C. All biochemical analyses were performed with leaves harvested at the rosette stage (6-week-old plants). For phosphate limitation experiments, sterile Arabidopsis medium (19) was used but at half concentration and containing 0.8% agarose, 1% sucrose, 20 mM Mes (pH 6.0), and different concentrations of KH2PO4 as indicated. Plants were grown for 10 days on 0.8% (wt/vol) agar-solidified MS medium supplemented with 1% (wt/vol) sucrose and then transferred to agar plates containing the phosphate-limited medium on which they continued to grow for another 10 days.
Phosphate Analysis.
Inorganic phosphate in leaf and root tissues was determined by the method of Ames (20). Roots were washed at least two times with water and stored at −70°C before analysis.
Lipid and Fatty Acid Analysis.
Harvested leaves were frozen immediately in liquid nitrogen, and lipids were extracted as previously described (15). Lipid extracts were analyzed on activated ammonium sulfate-impregnated silica gel TLC plates by using a solvent system of acetone/toluene/water (91:30:7, vol/vol/vol). Lipids were visualized with iodine vapor and identified by cochromatography with lipid extracts of known composition. For quantitative analysis, individual lipids were isolated from TLC plates and used to prepare fatty acid methyl esters. The methyl esters were quantified by GLC by using myristic acid as internal standard (15). The fatty acid composition at the C-1 and C-2 positions of the glycerol backbone was determined by lipase digestion according to Siebertz and Heinz (21), with modifications as described by Miquel et al. (22). Fatty acid values were corrected for the fatty acids present in the lipase preparation (EC 3.1.1.3, Rhizopus sp., Sigma).
Subcellular Fractionation.
Because dgd1 mutant thylakoids are highly unstable in vitro (23), we devised a simple and rapid protocol for the subfractionation of cell membranes and subsequent lipid analysis to preclude artifacts. Leaves (approximately 0.5 g fresh weight) were harvested directly into liquid nitrogen, stored at −70°C, and homogenized by grinding under liquid nitrogen by using a precooled mortar and pestle in cold buffer (0.35 M sorbitol/25 mM Hepes-KOH, pH 7.8/10 mM EDTA/0.2% fat-free BSA). The homogenates were filtered through two layers of wet miracloth (Calbiochem) and centrifuged at 800 × g for 1 min. The supernatant was transferred to a new tube. The pellets were gently resuspended in a small volume of cold wash buffer (10 mM Hepes-KOH, pH 7.8/0.35 M sorbitol/10 mM EDTA) and centrifuged at 800 × g for 1 min. The second supernatant was combined with the first in a glass tube. The pellet fraction containing mostly chloroplast membranes was immediately resuspended in 200 μl chloroform/methanol/formic acid (10:10:1, vol/vol/vol), followed by the addition of 100 μl 0.2 M H3PO4 and 1 M KCl. The combined supernatant fraction was centrifuged at 6,000 × g for 6 min to remove the chloroplast membranes further. The supernatant was transferred to a new glass tube, and lipids were extracted immediately by addition of two parts of chloroform/methanol/formic acid (10:10:1, vol/vol/vol). All steps were carried out at 4°C, and the time between homogenization and lipid extraction was kept to less than 10 min. Lipids were recovered in the chloroform phase, concentrated under a stream of N2, and lipid analysis was performed as described above.
Results
Partial Suppression of the dgd1 Lipid Phenotype in a pho1 Mutant Background.
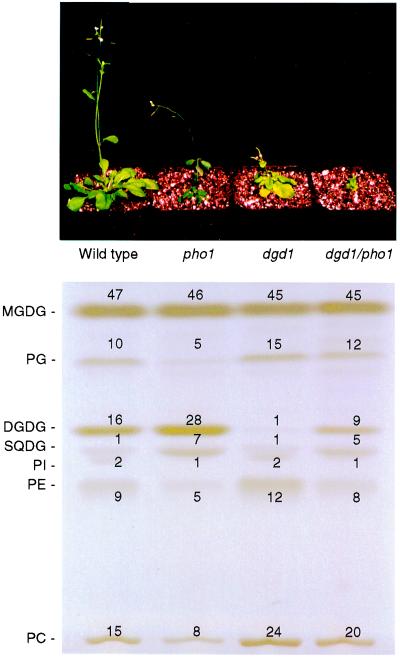
To investigate the possibility of DGD1-independent galactolipid biosynthesis in response to phosphate deprivation, we crossed the DGDG-deficient dgd1 mutant and the phosphate-deprived pho1 mutant of Arabidopsis. As expected from the recessive mutations, the F1 progeny showed wild-type lipid composition. Of 198 F2 plants analyzed for altered lipid composition, 44 plants were similar in phenotype to dgd1 and 39 plants to pho1, but no putative double mutants were recovered. Because both mutations are located on chromosome 3 within less than 10 cM, double mutants were expected to be underrepresented. To recover the double mutant, we selected 15 homozygous dgd1 F2 plants, a few of which we expected to be heterozygous for pho1, and screened 54 F3 descendants each for decreased phospholipid amounts that are typical for homozygous pho1 plants. Among these 15 F2 lines, three were found that segregated approximately 25% F3 plants with lowered amounts of phospholipids. These were assumed to be the dgd1/pho1 homozygous plants. In addition, the amount of inorganic phosphate per fresh weight was found to be reduced to about 10% and 12% compared with wild type and dgd1, respectively, as would be expected for a homozygous pho1 mutant. These homozygous dgd1/pho1 mutants were much more stunted than either parent (Fig. 2) but were viable on soil and produced flowers and seeds. Bolting lagged behind by 5–7 days as compared with wild type.
Figure 2.
Appearance (Top) and lipid composition (Bottom) of Arabidopsis homozygous wild type and pho1, dgd1, and dgd1/pho1 mutant lines grown for 6 weeks on soil. Lipids were separated on activated ammonium sulfate-impregnated silica gel TLC plates and visualized with iodine vapor. The lipids were identified by cochromatography with lipid extracts of known composition. In addition, glycolipids were verified by specific staining with α-naphthol reagent. The numbers at the bands indicate the relative proportion (mol%) of the respective lipids. Abbreviations not mentioned in the legend to Fig. 1. PE, phosphatidylethanolamine; PG, phosphatidylglycerol; PI, phosphatidylinositol; SQDG, sulfoquinovosyldiacylglycerol.
The lipid compositions of the double mutant, the single mutants, and the wild type are shown in Fig. 2. Although the homozygous dgd1 mutant contained less than 10% of wild-type amounts of DGDG, the dgd1/pho1 double mutant showed an increase in the relative amount of DGDG to 60% of wild-type levels. In the pho1 mutant, DGDG accumulated to approximately 160% of wild-type amounts. Phospholipids generally decreased in the pho1 mutant lines, whereas sulfolipid increased as observed previously (6, 8). The precursor of DGDG, MGDG, was present at similar levels in all four tested lines. Although the introduction of the pho1 mutation into the dgd1 mutant background caused an increase in DGDG content to approximately 60% of the amount found in the wild type, none of the photosynthetic parameters known to be altered in the dgd1 mutant (23–25) was restored (data not shown). Because partial restoration of photosynthetic defects after the ectopic expression of DGD1 in the dgd1 mutant, which resulted in intermediate amounts of DGDG, was observed (data not shown), we concluded that the DGDG formed after phosphate deprivation was either different in structure or was not as accessible to the components of the photosynthetic membrane as the DGDG derived from DGD1-mediated biosynthesis.
DGDG Formed After Phosphate Deprivation Is Not Derived from the Plastid Pathway.
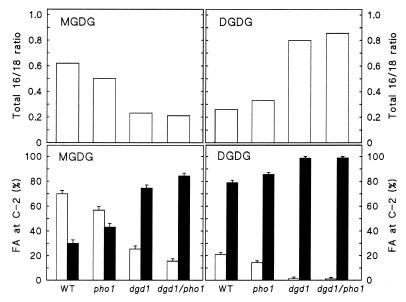
A detailed fatty acid compositional analysis of galactolipid molecular species can provide clues regarding their biosynthetic origin, because different molecular species are predominant in galactolipids derived from the plastid or the ER pathways as summarized in Fig. 1 I and II. Although the degree of saturation of each fatty acid bound in galactolipids constitutes an additional level of complexity, the most crucial information with regard to the origin of galactolipids can be deduced from the ratio of 16- to 18-carbon fatty acids and their relative position on the glycerol backbone (Fig. 3). The ratio of total 16- to 18-carbon fatty acids decreased in MGDG in the two dgd1 mutant lines, indicating a larger contribution of the ER pathway to MGDG biosynthesis (Fig. 1 II). This assumption was also confirmed by positional analysis, showing an increase in 18-carbon fatty acids in the glycerol C-2 position of MGDG in the dgd1 mutants. By contrast, the ratio of total 16- to 18-carbon fatty acids in DGDG increased in pho1 and to a larger extent in the two dgd1 mutant lines (Fig. 3 Upper). Being aware of only the plastidic and ER pathways (Fig. 1 I and II) and without further positional analysis, one would conclude that the plastid pathway gives rise to DGDG in the two dgd1 mutant lines. However, positional analysis revealed that the 16-carbon fatty acids are virtually excluded from the C-2 position of the glycerol backbone of DGDG found in plants with the dgd1 mutant background. This result suggested a DGDG structure different from that of galactolipids normally derived from the plastid pathway. These are characterized by the presence of 16-carbon fatty acids in the glycerol C-2 position (Fig. 1 I).
Figure 3.
Fatty acid composition of MGDG and DGDG in the wild-type and pho1, dgd1, and dgd1/pho1 mutant lines. (Upper) Ratio of total 16- to 18-carbon fatty acids in each lipid. (Lower) Relative abundance (mol%) of 16- (open bars) and 18-carbon fatty acids (closed bars) at the glycerol C-2 position of MGDG and DGDG. The proportion of fatty acids (FA) in the C-2 position of the glycerol backbone was determined from the lyso derivatives after digestion of the lipids with Rhizopus sp. lipase. Values represent the means of at least three measurements. The standard error is indicated.
Corroborating independent evidence that DGDG biosynthesis under phosphate deprivation must be caused by a pathway other than the plastid pathway (Fig. 1 I) was provided by our analysis of lipids of the act1 mutant grown under phosphate-limited growth conditions (data not shown). The act1 mutation effectively blocks the plastid pathway of galactolipid biosynthesis (13), yet the act1 mutant showed the same increase in DGDG content under phosphate-limited growth conditions as was observed for the pho1 mutant line.
DGDG Accumulates in Leaves and Roots with Decreasing Phosphate Availability.
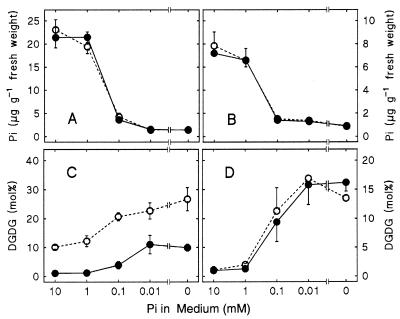
To verify independently that the changes in the lipid pattern in pho1 and dgd1/pho1 were solely because of phosphate depletion and not secondary genetic defects in the pho1 mutant background, wild type and dgd1 mutant were grown on agar-solidified medium containing different concentrations of phosphate. At the same time, we were able to examine the lipid composition in roots, a nonphotosynthetic tissue with a different ratio of plastidic to extraplastidic membranes than leaves. As the content of inorganic phosphate decreased in entire plants (Fig. 4 A and B), the relative amount of DGDG increased in both leaves and roots (Fig. 4 C and D). Although in leaves of wild type and dgd1 mutant the DGDG content increased in parallel starting from different values, the same change in DGDG content with an increase of up to 10-fold was observed for roots of the wild type and the dgd1 mutant after phosphate deprivation. In roots that are well supplied with phosphate, DGDG was usually present in trace amounts. However, it accounted for 15% of all of the lipids in roots when plants were grown for 10 days in the absence of phosphate. Rudimentary plastids are typically present in roots and represent only a minor fraction of cell membranes in nongreen tissues. Thus, the discovery of a large amount of DGDG in phosphate-deprived roots immediately suggested that this newly formed galactolipid may be associated with extraplastidic membranes.
Figure 4.
Changes in the total amounts of inorganic phosphate (A and B) and relative amounts of DGDG (C and D) in leaves (A and C) and roots (B and D) of wild-type and the dgd1 mutant of Arabidopsis with decreasing phosphate concentration in the nutrient medium. Open symbols denote wild-type and closed symbols dgd1 mutant values. The means of at least four measurements are shown.
Extraplastidic Membranes Are Enriched in DGDG After Phosphate Deprivation.
The accumulation of large amounts of DGDG in nonphotosynthetic tissues prompted us to fractionate plastidic and extraplastidic membranes from leaves of phosphate-deprived plants to determine their lipid composition. To avoid artifacts caused by degradation of lipids in crude membrane fractions, which we previously observed to be particularly severe for dgd1 samples (23), a simple and fast procedure based on differential centrifugation was designed. Among different centrifugation regimes tested, a maximum of chloroplast membranes revealing very low contamination with extraplastidic membranes was present in the pellet fraction after a low-speed centrifugation (800 × g). As a marker for extraplastidic membranes, we used phosphatidylethanolamine (PE), which is known to be excluded from plastid membranes (26). To evaluate the contamination of extraplastidic membranes with chloroplast membranes, most likely chloroplast envelopes, we also included MGDG in the analysis, which was present in constant amounts during the different phosphate treatments (Fig. 2). The chosen experimental approach and a combination of both, MGDG and PE, as markers provided a clear result with regard to DGDG localization as shown in Table 1. Very little PE is associated with the chloroplast pellet fraction, but its relative amount in the supernatant fraction is high. The reverse distribution is found for MGDG, although it is not as stringent as for PE. This may be a result of the presence of chloroplast envelope membranes containing MGDG in the supernatant. Comparing the relative amounts of DGDG to those of MGDG and PE, it is apparent that DGDG is highly enriched after phosphate deprivation in the supernatant, which represents primarily the extraplastidic membrane fraction. The relative proportion of DGDG in the supernatant fraction increased from 4.5% to 31.1% after phosphate deprivation in the wild-type and from 1.3% to 18.1% in the dgd1 mutant. This enrichment is even more drastically reflected in the ratios of DGDG-to-MGDG and DGDG-to-PE (Table 1).
Table 1.
Enrichment of DGDG in the extraplastidic membrane fraction
| Plant | Pi | Fraction | DGDG | MGDG | PE | D/M | D/P |
|---|---|---|---|---|---|---|---|
| WT | + | Pellet | 19.6 | 52.2 | 1.6 | 0.38 | 12.25 |
| + | SN | 4.5 | 13.5 | 26.3 | 0.34 | 0.17 | |
| − | Pellet | 28.0 | 50.4 | 0.9 | 0.56 | 31.11 | |
| − | SN | 31.1 | 16.8 | 11.2 | 1.85 | 2.78 | |
| dgd1 | + | Pellet | 1.9 | 52.4 | 2.2 | 0.04 | 0.86 |
| + | SN | 1.3 | 12.8 | 29.0 | 0.10 | 0.04 | |
| − | Pellet | 6.1 | 52.4 | 2.5 | 0.12 | 2.44 | |
| − | SN | 18.1 | 10.5 | 19.0 | 1.72 | 0.95 |
Lipids were isolated from different subcellular fractions of wild type and dgd1 mutant grown in medium with 1 mM or no added phosphate (Pi). The mean of three independent lipid measurements is given in mol%, and standard errors were <15% for values >10 or <30% for values <10. D/M, ratio of DGDG/MGDG; D/P, ratio of DGDG/PE; SN, supernatant; WT, wild type.
To obtain independent corroborating evidence for the presence of DGDG in extraplastidic membranes after phosphate deprivation, we took advantage of the fad3 mutant of Arabidopsis (17). This mutant is deficient in the 18:2 fatty acid desaturase associated with the ER resulting in increased amounts of 18:2 and decreased amounts of 18:3 fatty acids. Most importantly, the mutation primarily affects the fatty acid composition of lipids in extraplastidic membranes. Because 18:2 containing diacylglycerol moieties are thought to return from the ER to the plastid, where they are further desaturated by plastidic 18:2 fatty acid desaturases (27), chloroplast membrane lipids derived from the ER pathway are not affected by a mutation in the ER 18:2 fatty acid desaturase gene fad3. Thus we anticipated that the DGDG formed after phosphate deprivation in the fad3 mutant should show a strong increase in the 18:2- to 18:3-fatty acid ratio only if it is located in extraplastidic membranes. This effect should be particularly obvious in roots that are most enriched in DGDG after phosphate deprivation (Fig. 3) and that contain primarily extraplastidic membranes. As shown in Table 2, there is a strong effect in accordance with the fad3 desaturase defect on the fatty acid composition of DGDG (reduction of 18:3 and increase in 18:2 fatty acids) in phosphate-deprived roots of the fad3 mutant. On the other hand, there is virtually no effect of the fad3 mutation on DGDG in phosphate-sufficient roots. The overall increase in the amount of DGDG in roots was the same for wild-type and fad3 mutant. These results are in agreement with an extraplastidic location of a large fraction of DGDG synthesized in response to phosphate deprivation, but a predominant plastid association of DGDG when phosphate is plentiful in the medium.
Table 2.
Total amount of DGDG and degree of saturation of 18-carbon fatty acids in DGDG in roots of wild type and fad3 mutant grown in the presence (+) and absence (−) of 1 mM phosphate (Pi)
| Plant | Pi | DGDG | Fatty acid
|
||||
|---|---|---|---|---|---|---|---|
| 18∶0 | 18∶1 | 18∶2 | 18∶3 | 18∶2/18∶3 | |||
| WT | + | 1.7 ± 0.1 | 9.7 ± 0.8 | 6.7 ± 3.1 | 10.8 ± 4.9 | 18.4 ± 4.4 | 0.59 |
| − | 16.6 ± 2.7 | 4.2 ± 0.7 | 4.5 ± 1.1 | 17.3 ± 0.8 | 26.6 ± 3.1 | 0.66 | |
| fad3 | + | 1.8 ± 0.4 | 6.1 ± 1.8 | 8.3 ± 3.2 | 16.4 ± 2.2 | 31.0 ± 4.5 | 0.52 |
| − | 18.1 ± 1.6 | 7.5 ± 2.9 | 4.5 ± 3.4 | 40.2 ± 7.6 | 9.1 ± 0.9 | 4.42 | |
The mean of three experiments (mol% of total fatty acids) ± standard error is shown. WT, wild type.
Discussion
Phosphate Deficiency Induces an ACT1/DGD1-Independent Pathway for Galactolipid Biosynthesis.
One of the most powerful environmental stimuli affecting the complex lipid composition of plant membranes is a limitation in the supply of inorganic phosphate. Enticed by our previous findings of a reduction in phospholipid content and a concomitant increase in nonphosphorus lipids in Arabidopsis (6, 8), we initiated a study to understand the underlying regulatory and biochemical principles. The model plant Arabidopsis provides a rich source for mutants with defects in different aspects of metabolism, and we attempted to take full advantage of the availability of these mutants to address this problem by using a combination of genetical and biochemical approaches. To apply constant phosphate deprivation to plants, we used the pho1 mutation (7) and to deconvolute the complex pathways of galactolipid biosynthesis, we took advantage of the dgd1 mutation (15). Combining both mutations into one line led to the discovery that pho1 apparently restores the biosynthesis of DGDG in the dgd1 genetic background. This is a surprising result because the dgd1 mutation is characterized by a stop codon in the coding region of DGD1 leading to a 90% reduction in DGDG content (16). This newly formed DGDG was identified by cochromatography with authentic standards and stained identical to the DGDG commonly present in Arabidopsis. However, its fatty acid composition was different from DGDG normally found in Arabidopsis, with increased amounts of 16-carbon fatty acids in the C-1 position of the glycerol backbone (Fig. 3). The fatty acid composition is considered to be diagnostic for the origin of DGDG from either the plastid or the ER pathways (Fig. 1 I and II). Based on the exclusion of 16-carbon fatty acids from the C-2 position of glycerol, it can be inferred that this DGDG is derived from the ER pathway. This conclusion is supported further by the fact that the same molecular species of DGDG was formed in the phosphate-deprived act1 mutant (data not shown), in which the plastid pathway of galactolipid biosynthesis is disrupted (13). Therefore, phosphate deprivation induces the biosynthesis of DGDG by a DGD1/ACT1-independent pathway not previously observed, which led us to modify the pathway scheme for the biosynthesis of DGDG as shown in Fig. 1. The existence of multiple or subpools of galactolipids in addition to the commonly accepted “prokaryotic” (plastid-derived) and “eukaryotic” (ER-derived) pools (Fig. 1 I and II) has been postulated recently for different plants based on positional fatty acid analysis (28) but not in the context of phosphate-deprived plants. With regard to MGDG, we observed a strong decrease in the relative amount of 16-carbon fatty acids in the glycerol C-2 position, from 70% in the wild type to 15% in dgd1/pho1, with no detectable changes in the overall proportion of MGDG in phosphate-deprived plants (Fig. 3). This would suggest an increased contribution of the ER pathway also for this lipid and, therefore, a general redirection of galactolipid synthesis from the plastid to the ER pathway in phosphate-deprived plants.
Galactolipid Accumulates in Extraplastidic Membranes on Phosphate Deprivation.
There are a few reports in the literature describing the presence of galactolipids in extraplastidic membranes (29, 30). These were based solely on fractionation of subcellular membranes isolated from either protoplast or intact plant tissues. Because of questions regarding the extent of unavoidable crosscontamination of different membrane fractions during preparation, these results have been largely ignored. Here, we provide four independent indirect or direct lines of evidence, which strongly suggest that a large portion of DGDG synthesized after phosphate deprivation is found in extraplastidic membranes: first, DGDG accumulated in large amounts in phosphate-deprived roots, which typically have rudimentary plastids. Because we are not aware of any evidence indicating there is a large increase in plastid membranes relative to extraplastidic membranes in roots under these conditions, this observation suggests an extraplastidic location of at least some of the newly formed DGDG. Second, with regard to different photosynthetic parameters altered in the dgd1 mutant, DGDG present in the dgd1 mutant after phosphate deprivation could not substitute for DGDG formed through the DGD1 pathway, such as DGDG in transgenic dgd1 plants expressing a wild-type DGD1 cDNA (data not shown). This was because of either the altered fatty acid composition of DGDG formed after phosphate deprivation (Fig. 3) or the extraplastidic localization of most of this DGDG. Third, fractionation of plastidic and extraplastidic membranes from phosphate-sufficient and -deficient wild-type and dgd1 plants by using MGDG and PE as markers for plastidic and extraplastidic membranes showed a strong enrichment of DGDG in the extraplastidic membrane fraction. However, it should be pointed out that there was also an increase in the relative DGDG content in the chloroplast fraction of phosphate-deprived wild-type and dgd1 plants (Table 1). This finding suggests that DGDG synthesized in phosphate-deprived plants may not be associated exclusively with extraplastidic membranes. A reason for this may be that DGDG replaces phosphatidylcholine (PC), which is associated with plastid membranes as well, but which is restricted to the outer envelope of chloroplasts (31). As a matter of fact, phosphate-sufficient plants lacking the plastid pathway contain considerable amounts of DGDG with 16-carbon fatty acids in the glycerol C-1 position, the same as observed in phosphate-deprived Arabidopsis, which is presumably located in the plastid in these plants (32). Fourth, a strong change in the ratio of 18:2- to 18:3-carbon fatty acids in DGDG of the phosphate-deprived fad3 mutant (Table 2) suggests an extraplastidic location of the lipid, because the fad3 mutation is thought to affect primarily extraplastidic lipids (17). Together, these four independent experimental strategies combining biochemical, genetical, and physiological approaches provide overwhelming evidence for the location of a major portion of the DGDG synthesized in Arabidopsis deprived of phosphate in extraplastidic membranes.
Enzymes Involved in the Biosynthesis of Extraplastidic DGDG.
It must be emphasized that although much of the DGDG synthesized under conditions of phosphate deprivation is located in extraplastidic membranes, the enzymes involved in its biosynthesis must not necessarily be located in extraplastidic membranes (i.e., ER associated). This DGDG could also be synthesized in the outer chloroplast envelope and subsequently transferred to other membranes. Three putative MGDG synthase genes are known for Arabidopsis (33). Only one of these genes, MGD1, encodes a protein with a clearly recognizable chloroplast-targeting sequence. Similarly, DGD2, a gene encoding a protein with high similarity to DGD1, seems to lack a chloroplast transit peptide (16). Although clear evidence is not available at this time indicating that MGD2, MGD3, and DGD2 encode enzymes involved in galactolipid biosynthesis, these genes represent candidates for the biosynthesis of extraplastidic DGDG directly in the ER membranes. Because the fatty acid composition of DGDG synthesized after phosphate deprivation is very similar to that of PC (11), it seems likely that this DGDG is derived directly from PC in the ER. However, the biosynthetic mechanism may be different from that postulated for plastid galactolipid biosynthesis, which occurs by galactosylation of diacylglycerol to give rise to MGDG and by disproportioning of two MGDG molecules resulting in DGDG and diacylgycerol (34).
To Conserve Phosphate, Plants Rely Less Than Animals on Phospholipids.
The DGDG formed after phosphate deprivation is located in extraplastidic membranes like PC and has a similar fatty acid composition. Both lipids are neutral and bilayer forming. Thus, it is tempting to propose that DGDG synthesized in response to phosphate deprivation substitutes for PC in similar ways as sulfolipid substitutes for phosphatidylglycerol in chloroplast membranes (6, 8). Such a mechanism, the replacement of phospholipids with nonphosphorous glycolipids to adapt to phosphate-limited growth conditions, would be important for the survival of plants, which unlike animals cannot escape unfavorable environmental conditions. One-third of organic phosphate in Arabidopsis is bound normally in phospholipids (7), and conserving this phosphate may be an important survival strategy for plants. Photosynthesis requires extensive membrane systems in the chloroplast. Their function would be compromised easily under conditions of low phosphate availability if plants would rely solely on phospholipids as their main building blocks of membranes. During evolution, plants presumably avoided this potential impasse by maintaining and evolving their capability to synthesize nonphosphorous membrane lipids. It may be this evolutionary pressure that led to the fact that today nonphosphorous galactolipids represent the most abundant lipids in the biosphere.
Acknowledgments
We thank John Browse for helpful discussions. Financial support was provided by the United States Department of Energy (Grant DE-FG02–98ER20305).
Abbreviations
- MGDG
monogalactosyldiacylgycerol
- DGDG
digalactosyldiacylgycerol
- ER
endoplasmic reticulum
- C-2
carbon-2
- PE
phosphatidylethanolamine
- PC
phosphatidylcholine
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.180320497.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.180320497
References
- 1.Minnikin D E, Abdolrahimzadeh H, Baddiley J. Nature (London) 1974;249:268–269. doi: 10.1038/249268a0. [DOI] [PubMed] [Google Scholar]
- 2.Benning C, Beatty J T, Prince R C, Somerville C R. Proc Natl Acad Sci USA. 1993;90:1561–1565. doi: 10.1073/pnas.90.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benning C, Huang Z H, Gage D A. Arch Biochem Biophys. 1995;317:103–111. doi: 10.1006/abbi.1995.1141. [DOI] [PubMed] [Google Scholar]
- 4.Güler S, Seeliger A, Härtel H, Renger G, Benning C. J Biol Chem. 1996;271:7501–7507. doi: 10.1074/jbc.271.13.7501. [DOI] [PubMed] [Google Scholar]
- 5.Benning C. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:53–75. doi: 10.1146/annurev.arplant.49.1.53. [DOI] [PubMed] [Google Scholar]
- 6.Essigmann B, Güler S, Narang R A, Linke D, Benning C. Proc Natl Acad Sci USA. 1998;95:1950–1955. doi: 10.1073/pnas.95.4.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poirier Y, Thoma S, Somerville C, Schiefelbein J. Plant Physiol. 1991;97:1087–1093. doi: 10.1104/pp.97.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Härtel H, Essigmann B, Lokstein H, Hoffmann-Benning S, Peters-Kottig M, Benning C. Biochim Biophys Acta. 1998;1415:205–218. doi: 10.1016/s0005-2736(98)00197-7. [DOI] [PubMed] [Google Scholar]
- 9.Browse J, Somerville C. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:467–506. [Google Scholar]
- 10.Joyard J, Marechal E, Miege C, Block M A, Dorne A J, Douce R. In: Lipids in Photosynthesis: Structure, Function and Genetics. Siegenthaler P-A, Murata N, editors. Dordrecht, The Netherlands: Kluwer; 1998. pp. 21–52. [Google Scholar]
- 11.Browse J, Warwick N, Somerville C R, Slack C R. Biochem J. 1986;235:25–31. doi: 10.1042/bj2350025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heinz E, Roughan G. Plant Physiol. 1983;72:273–279. doi: 10.1104/pp.72.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kunst L, Browse J, Somerville C. Proc Natl Acad Sci USA. 1988;85:4143–4147. doi: 10.1073/pnas.85.12.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jarvis P, Dörmann P, Peto C A, Lutes J, Benning C, Chory J. Proc Natl Acad Sci USA. 2000;97:8175–8179. doi: 10.1073/pnas.100132197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dörmann P, Hoffmann-Benning S, Balbo I, Benning C. Plant Cell. 1995;7:1801–1810. doi: 10.1105/tpc.7.11.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dörmann P, Balbo I, Benning C. Science. 1999;284:2181–2184. doi: 10.1126/science.284.5423.2181. [DOI] [PubMed] [Google Scholar]
- 17.Browse J, McConn M, James D, Jr, Miquel M. J Biol Chem. 1993;268:16345–16351. [PubMed] [Google Scholar]
- 18.Murashige T, Skoog F. Physiol Plant. 1962;15:473–497. [Google Scholar]
- 19.Estelle M A, Somerville C. Mol Gen Genet. 1987;206:200–206. [Google Scholar]
- 20.Ames B N. Methods Enzymol. 1966;8:115–118. [Google Scholar]
- 21.Siebertz H P, Heinz E. Z Naturforsch. 1977;32c:193–205. [Google Scholar]
- 22.Miquel M, Cassagne C, Browse J. Plant Physiol. 1998;117:923–930. doi: 10.1104/pp.117.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Härtel H, Lokstein H, Dörmann P, Grimm B, Benning C. Plant Physiol. 1997;115:1175–1184. doi: 10.1104/pp.115.3.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Härtel H, Lokstein H, Dörmann P, Trethewey R N, Benning C. Plant Physiol Biochem. 1998;36:407–417. [Google Scholar]
- 25.Reifarth F, Christen G, Seeliger A G, Dörmann P, Benning C, Renger G. Biochemistry. 1997;36:11769–11776. doi: 10.1021/bi9709654. [DOI] [PubMed] [Google Scholar]
- 26.Marechal E, Block M A, Dorne A-J, Joyard J. Physiol Plant. 1997;100:65–77. [Google Scholar]
- 27.Roughan P G, Slack C R. Annu Rev Plant Physiol. 1982;33:97–132. [Google Scholar]
- 28.Williams J P, Kahn M U. Plant Physiol Biochem. 1996;34:93–100. [Google Scholar]
- 29.Kogel K H, Ehrlich-Rogzinski S, Reisner H J, Sharon N. Plant Physiol. 1984;76:924–928. doi: 10.1104/pp.76.4.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshida S, Uemura M. Plant Physiol. 1986;82:807–812. doi: 10.1104/pp.82.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dorne A-J, Joyard J, Douce R. Proc Natl Acad Sci USA. 1990;87:71–74. doi: 10.1073/pnas.87.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Douce R, Joyard J. In: The Biochemistry of Plants. Stumpf P K, editor. Vol. 4. New York: Academic; 1980. pp. 321–362. [Google Scholar]
- 33.Miege C, Marechal E, Shimojima M, Awai K, Block M A, Ohta H, Takamiya K, Douce R, Joyard J. Eur J Biochem. 1999;265:990–1001. doi: 10.1046/j.1432-1327.1999.00801.x. [DOI] [PubMed] [Google Scholar]
- 34.Heemskerk J W M, Schmidt H, Hammer U, Heinz E. Plant Physiol. 1991;96:144–152. doi: 10.1104/pp.96.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]