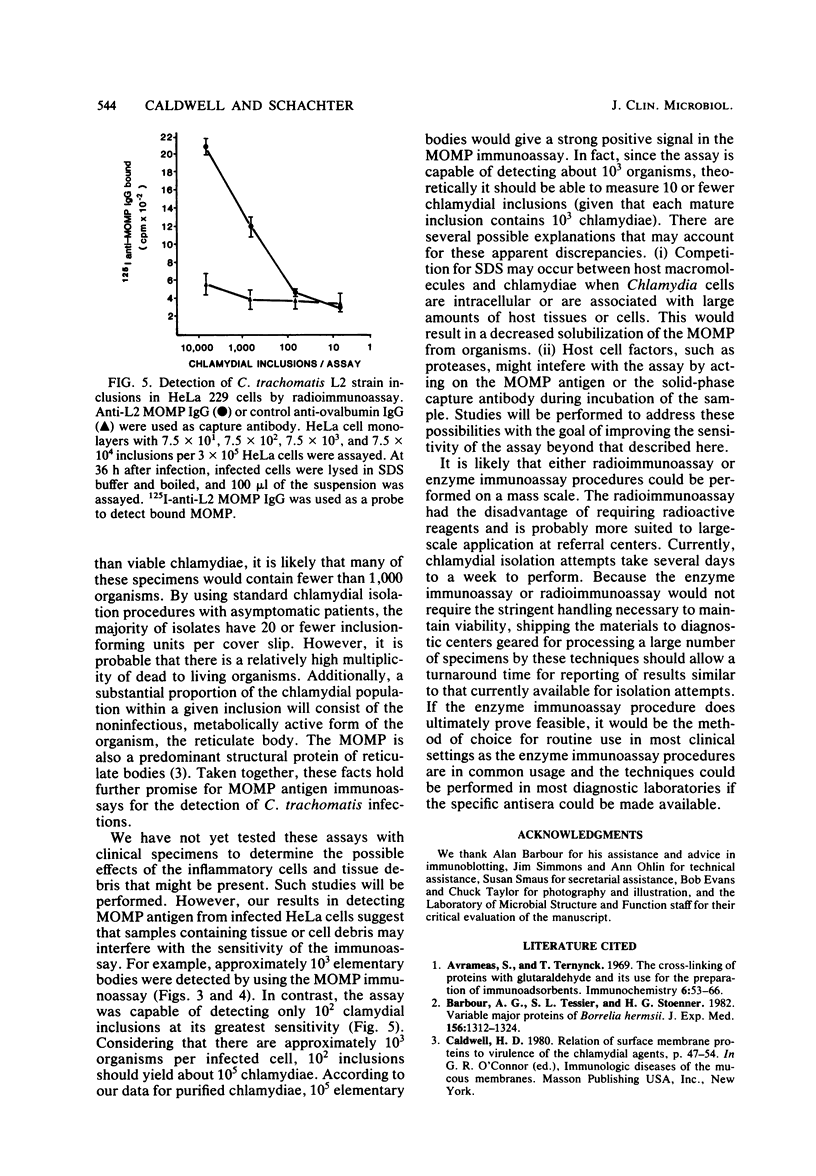
Abstract
The development of a solid-phase immunoassay for the detection of the 39,500-dalton major outer membrane protein of the Chlamydia trachomatis lymphogranuloma venereum serotype L2 is described. The test uses immunoadsorbent-purified rabbit anti-L2 major outer membrane protein immunoglobulin G (IgG) passively adsorbed to microtiter plates as a capture antibody. This same IgG antibody was either conjugated to horseradish peroxidase or radioiodinated with 125I and used as a probe to detect major outer membrane protein bound to immobilized IgG. At its greatest sensitivity, the test was capable of detecting 0.5 to 1 ng of purified major outer membrane protein, 5 X 10(3) elementary body inclusion-forming units, and approximately 100 C. trachomatis intracytoplasmic inclusions per assay.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S., Ternynck T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorbents. Immunochemistry. 1969 Jan;6(1):53–66. doi: 10.1016/0019-2791(69)90178-5. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Stoenner H. G. Variable major proteins of Borrellia hermsii. J Exp Med. 1982 Nov 1;156(5):1312–1324. doi: 10.1084/jem.156.5.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Kromhout J., Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981 Mar;31(3):1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Schachter J. Antigenic analysis of the major outer membrane protein of Chlamydia spp. Infect Immun. 1982 Mar;35(3):1024–1031. doi: 10.1128/iai.35.3.1024-1031.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Goding J. W. Conjugation of antibodies with fluorochromes: modifications to the standard methods. J Immunol Methods. 1976;13(3-4):215–226. doi: 10.1016/0022-1759(76)90068-5. [DOI] [PubMed] [Google Scholar]
- Grayston J. T., Wang S. New knowledge of chlamydiae and the diseases they cause. J Infect Dis. 1975 Jul;132(1):87–105. doi: 10.1093/infdis/132.1.87. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- Schachter J. Chlamydial infections (third of three parts). N Engl J Med. 1978 Mar 9;298(10):540–549. doi: 10.1056/NEJM197803092981005. [DOI] [PubMed] [Google Scholar]
- Stephens R. S., Tam M. R., Kuo C. C., Nowinski R. C. Monoclonal antibodies to Chlamydia trachomatis: antibody specificities and antigen characterization. J Immunol. 1982 Mar;128(3):1083–1089. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Yolken R. H. Enzyme immunoassays for the detection of infectious antigens in body fluids: current limitations and future prospects. Rev Infect Dis. 1982 Jan-Feb;4(1):35–68. doi: 10.1093/clinids/4.1.35. [DOI] [PubMed] [Google Scholar]